Intervenciones para la prevención y el tratamiento de las afecciones renales en la púrpura de Schönlein‐Henoch (PSH)
Information
- DOI:
- https://doi.org/10.1002/14651858.CD005128.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 07 August 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Kidney and Transplant Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
-
Wattana Chartapisak: study selection, quality appraisal, data extraction, data analysis, writing original protocol, writing original review.
-
Sauwalak Opastrirakul: study selection, quality appraisal, data extraction, writing original protocol
-
Elisabeth Hodson: study selection, quality appraisal, data extraction, data analysis, writing original protocol, writing original review, updating review.
-
Narelle Willis: reviewing protocol and review, data analysis
-
Jonathan Craig: writing review, disagreement resolution
-
Deirdre Hahn: study selection, quality appraisal, data extraction, data analysis, writing of manuscript for update of review
Declarations of interest
-
Deirdre Hahn: nothing to declare
-
Elisabeth Hodson: nothing to declare
-
Narelle Willis: nothing to declare
-
Jonathan Craig: nothing to declare
Acknowledgements
-
We thank Dr Dudley, Dr Smith and Dr Tizard for additional information on their RCT. We thank Dr Ronkainen, Dr Nuutinen and Dr Mara Medeiros for additional information on their RCTs
-
We acknowledge the contributions made by authors Wattana Chartapisak and Sauwalak Opastrirakul to a previous version of this review
-
We would like to thank Drs Michael Dillon, Matti Nuutinen and Lesley Rees for their editorial advice during the preparation of this review
-
This work was presented in part at the 42nd Annual Scientific Meeting of the Australian and New Zealand Society of Nephrology (Melbourne 2006)
-
We would like to thank Sunny Wu for translating one paper.
Version history
| Published | Title | Stage | Authors | Version |
| 2023 Feb 28 | Interventions for preventing and treating kidney disease in IgA vasculitis | Review | Deirdre Hahn, Elisabeth M Hodson, Jonathan C Craig | |
| 2015 Aug 07 | Interventions for preventing and treating kidney disease in Henoch‐Schönlein Purpura (HSP) | Review | Deirdre Hahn, Elisabeth M Hodson, Narelle S Willis, Jonathan C Craig | |
| 2009 Jul 08 | Interventions for preventing and treating kidney disease in Henoch‐Schönlein Purpura (HSP) | Review | Wattana Chartapisak, Sauwalak Opastirakul, Elisabeth M Hodson, Narelle S Willis, Jonathan C Craig | |
| 2005 Jan 24 | Interventions for preventing and treating renal disease in Henoch‐Schönlein Purpura (HSP) | Protocol | Wattana Chartapisak, Sauwalak Opastirakul, Elisabeth M Hodson, Narelle S Willis, Jonathan C Craig | |
Differences between protocol and review
New methodology for assessing the risk of bias has replaced the quality assessment checklist. Studies of therapies with herbal treatments and non‐pharmacological interventions were excluded.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Adrenal Cortex Hormones [therapeutic use];
- Cyclophosphamide [therapeutic use];
- IgA Vasculitis [*complications];
- Immunosuppressive Agents [therapeutic use];
- Kidney Diseases [*drug therapy, etiology, *prevention & control];
- Platelet Aggregation Inhibitors [therapeutic use];
- Randomized Controlled Trials as Topic;
Medical Subject Headings Check Words
Child; Child, Preschool; Humans;
PICOs

Flow diagram of included and excluded study in Review

Risk of bias: Review authors' judgements about each methodological quality item presented as percentages across all included studies.
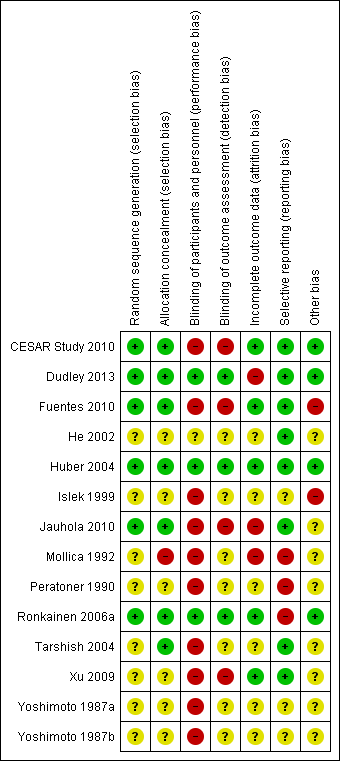
Risk of bias: Review authors' judgements about each risk of bias item for each included study

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 1 Persistent kidney disease at any time after treatment.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 2 Number of children with any continuing kidney disease at different time points.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 3 Any continuing kidney disease at different time points (study with high risk of bias excluded).

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 4 Number of children with kidney disease in first month/with kidney disease at follow‐up.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 5 Number developing severe kidney disease.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 6 Duration of kidney disease.

Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 7 Gastrointestinal complications requiring hospital admission.
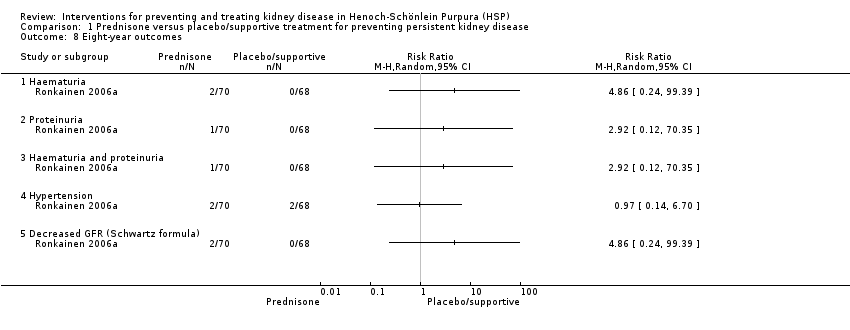
Comparison 1 Prednisone versus placebo/supportive treatment for preventing persistent kidney disease, Outcome 8 Eight‐year outcomes.

Comparison 2 Antiplatelet agents versus supportive treatment for preventing persistent kidney disease, Outcome 1 Kidney disease at any time.

Comparison 2 Antiplatelet agents versus supportive treatment for preventing persistent kidney disease, Outcome 2 Kidney disease at any time.

Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 1 Any kidney disease at 3 months after onset or relapse.

Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 2 Type of kidney disease at 3 months or more after onset or relapse.

Comparison 3 Heparin versus placebo for preventing persistent kidney disease, Outcome 3 Time to development of kidney disease.
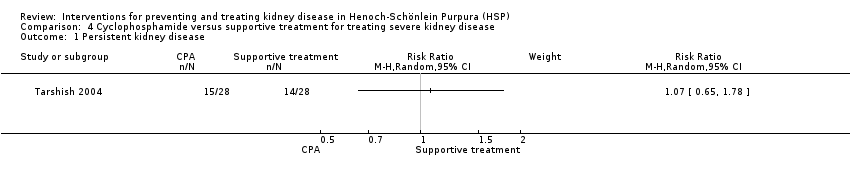
Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 1 Persistent kidney disease.

Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 2 Persistent severe kidney disease.

Comparison 4 Cyclophosphamide versus supportive treatment for treating severe kidney disease, Outcome 3 ESKD.

Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 1 Primary outcome: BVAS at 6 months.

Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 2 Secondary endpoints at 12 months.

Comparison 5 Cyclophosphamide + steroids versus steroids, Outcome 3 Adverse effects.
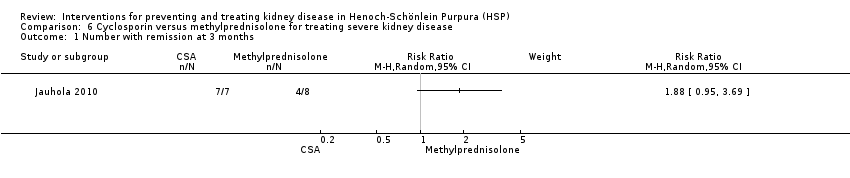
Comparison 6 Cyclosporin versus methylprednisolone for treating severe kidney disease, Outcome 1 Number with remission at 3 months.

Comparison 6 Cyclosporin versus methylprednisolone for treating severe kidney disease, Outcome 2 Number with remission at last follow‐up (mean 6.3 years).

Comparison 7 Mycophenolate mofetil versus azathioprine for treating severe kidney disease, Outcome 1 Remission of proteinuria at 1 year.
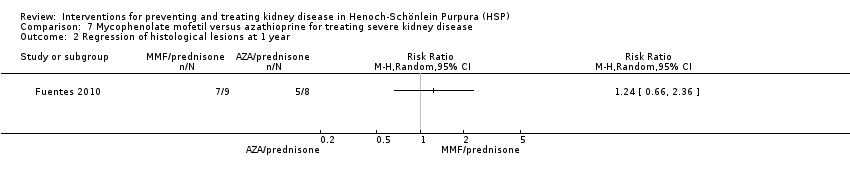
Comparison 7 Mycophenolate mofetil versus azathioprine for treating severe kidney disease, Outcome 2 Regression of histological lesions at 1 year.

Comparison 8 Fosinopril + supportive treatment versus supportive treatment, Outcome 1 Proteinuria.
| Prednisone versus placebo or supportive treatment for preventing persistent kidney disease in patients with Henoch‐Schönlein Purpura (HSP) | ||||||
| Patient or population: patients with HSP | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or supportive treatment | Prednisone | |||||
| Persistent kidney disease at any time after treatment | Study population | RR 0.74 | 746 (5) | ⊕⊕⊕⊝ | ||
| 143 per 1000 | 106 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 78 per 1000 | |||||
| Number of children with any continuing kidney disease at 3 months | Study population | RR 0.83 | 655 (4) | ⊕⊕⊕⊝ | ||
| 199 per 1000 | 165 per 1000 | |||||
| Moderate | ||||||
| 156 per 1000 | 129 per 1000 | |||||
| Number of children with any continuing kidney disease at 6 months | Study population | RR 0.51 | 379 (3) | ⊕⊕⊕⊝ | ||
| 100 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 53 per 1000 | 27 per 1000 | |||||
| Number of children with any continuing kidney disease at 12 months | Study population | RR 1.06 | 455 (3) | ⊕⊕⊝⊝ | ||
| 84 per 1000 | 89 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 111 per 1000 | |||||
| Any continuing kidney disease at 3 months (study with high risk of bias excluded) | Study population | RR 0.98 | 487 (3) | ⊕⊕⊕⊕ | ||
| 243 per 1000 | 238 per 1000 | |||||
| Moderate | ||||||
| 207 per 1000 | 203 per 1000 | |||||
| Any continuing kidney disease at 12 months (study with high risk of bias excluded) | Study population | RR 1.39 | 287 (2) | ⊕⊕⊕⊝ | ||
| 105 per 1000 | 146 per 1000 | |||||
| Moderate | ||||||
| 105 per 1000 | 146 per 1000 | |||||
| Number developing severe kidney disease | Study population | RR 1.58 | 418 (2) | ⊕⊕⊝⊝ | ||
| 14 per 1000 | 22 per 1000 | |||||
| Moderate | ||||||
| 17 per 1000 | 27 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Two studies had unclear or biased allocation concealment & were not blinded | ||||||
| Study | Timing of outcome | Haematuria | Proteinuria | Blood pressure | Kidney function |
| 1, 3 and 12 months | Any level on dipstick | UPC > 20 mg/mmol Dipstick for protein | Not defined | Not defined | |
| 1, 3, 6 and 12 months | ≥ 5 RBC/HPF or RBC casts | > 300 mg/L on dipstick | > 90th percentile for age and sex | Elevated Cr | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| 1, 3, 6 and 12 months | ≥ 10 RBC/HPF | ≥ 4 mg/m²/h | > 2 SD above normal | Cr ≥ 0.8 mg/dL/mm² | |
| During initial 12 months | > 5 RBC/mm² | Not defined | Not defined | Reduced GFR | |
| 1, 3 and 6 months | > 5 RBC/HPF | > 200 mg/L or urinary albumin > 30 mg/L | Not defined | Not defined | |
| 2 years | Not defined | Remission: UPC < 200 mg/mmol or daily urine protein < 40 mg/m²/d | Not defined | Not defined | |
| Mean follow‐up to 7 years | Addis Count > 30,000 RBC/h/m² or ≥ 1+ on dipstick ≥ 3 cells/HPF or > 2 RBC/mm³ | > 4 mg/h/m² or 2+ or more by dipstick Heavy proteinuria > 40 mg/h/m² | Not defined | GFR < 80 mL/min/1.73 m² ESKD | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Unclear | Not defined | Not defined | Not defined | Not defined | |
| Cr ‐ creatinine; ESKD ‐ end‐stage kidney disease; GFR ‐ glomerular filtration rate; HPF ‐ high power field; UPC ‐ urinary protein:creatinine ratio; RBC ‐ red blood cell | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent kidney disease at any time after treatment Show forest plot | 5 | 746 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.32] |
| 2 Number of children with any continuing kidney disease at different time points Show forest plot | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 One month | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.34, 1.84] |
| 2.2 Three months | 4 | 655 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.46, 1.52] |
| 2.3 Six months | 3 | 379 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.24, 1.11] |
| 2.4 Twelve months | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.38, 2.91] |
| 3 Any continuing kidney disease at different time points (study with high risk of bias excluded) Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 One month | 3 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.93] |
| 3.2 Three months | 3 | 487 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.70, 1.36] |
| 3.3 Six months | 2 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.23, 1.50] |
| 3.4 Twelve months | 2 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [0.75, 2.59] |
| 4 Number of children with kidney disease in first month/with kidney disease at follow‐up Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4.1 One month | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Three months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Six months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Number developing severe kidney disease Show forest plot | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.58 [0.42, 6.00] |
| 6 Duration of kidney disease Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Haematuria | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Proteinuria | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Gastrointestinal complications requiring hospital admission Show forest plot | 3 | 517 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.25, 1.23] |
| 8 Eight‐year outcomes Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 8.1 Haematuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 Proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.3 Haematuria and proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.4 Hypertension | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.5 Decreased GFR (Schwartz formula) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Kidney disease at any time Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Dipyridamole ± cyproheptadine in children without kidney disease at entry | 2 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.46, 2.95] |
| 1.2 Dipyridamole ± cyproheptadine in children with kidney disease at entry | 1 | 19 | Risk Ratio (M‐H, Random, 95% CI) | 0.92 [0.23, 3.72] |
| 2 Kidney disease at any time Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Aspirin versus supportive treatment | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any kidney disease at 3 months after onset or relapse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Type of kidney disease at 3 months or more after onset or relapse Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 Haematuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Proteinuria | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Nephrotic syndrome | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Time to development of kidney disease Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Persistent kidney disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Persistent severe kidney disease Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 ESKD Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: BVAS at 6 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 BVAS = 0 at 6 months | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Improvement in BVAS score | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Secondary endpoints at 12 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2.1 BP > 125/75 mm Hg | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 eGFR < 60 mL/min | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Proteinuria > 1 g/d | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 RAS blockers | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 Kidney function improvement > 50% | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.6 ESKD | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.7 Mortality | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse effects Show forest plot | 1 | Risk Difference (M‐H, Random, 95% CI) | Totals not selected | |
| 3.1 infection | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Newly diagnosed or deterioration in existing diabetes | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Depression/anxiety | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Alopecia | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Insomnia | 1 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number with remission at 3 months Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Number with remission at last follow‐up (mean 6.3 years) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Remission of proteinuria at 1 year Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Regression of histological lesions at 1 year Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proteinuria Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Complete remission of proteinuria < 150 mg/d | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Partial remission | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Minimal response/no response | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |

