Miel como tratamiento tópico para heridas
Resumen
Antecedentes
La miel es una solución de azúcar supersaturada y viscosa obtenida del néctar recogido y modificado por la abeja, Apis mellifera. La miel se utiliza desde la antigüedad como un remedio para tratar las heridas. La evidencia de estudios en animales y de algunos ensayos indica que la miel puede acelerar la cicatrización de heridas.
Objetivos
El objetivo de esta revisión fue evaluar los efectos de la miel en comparación con apósitos para heridas y tratamientos tópicos alternativos en la cicatrización de heridas agudas (p.ej., quemaduras, laceraciones) o crónicas (p.ej., úlceras venosas).
Métodos de búsqueda
Para esta actualización de la revisión, se realizaron búsquedas en el registro especializado del Grupo Cochrane de Heridas (Cochrane Wounds Group) (búsqueda 15 de octubre de 2014); Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials, CENTRAL) (The Cochrane Library 2014, número 9); Ovid MEDLINE (1946 hasta la semana 1 de octubre de 2014); Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations 13 de octubre de 2014); Ovid EMBASE (1974 hasta el 13 de octubre de 2014); y EBSCO CINAHL (1982 al 15 de octubre de 2014).
Criterios de selección
Se buscaron ensayos aleatorizados y cuasialeatorizados que evaluaron la miel como un tratamiento para cualquier tipo de herida aguda o crónica. No se impusieron restricciones en cuanto a la fuente, fecha de publicación o idioma. La cicatrización de la herida fue la variable principal de evaluación.
Obtención y análisis de los datos
Un autor de la revisión extrajo y resumió los datos de los ensayos elegibles con una planilla de extracción de datos y un segundo autor los verificó de forma independiente. Todos los datos han sido posteriormente verificados por dos autores más.
Resultados principales
Se identificaron 26 ensayos elegibles (total 3011 participantes). Tres ensayos evaluaron los efectos de la miel en heridas agudas menores, 11 evaluaron la miel en las quemaduras, 10 incluyeron a pacientes con diferentes heridas crónicas (dos en pacientes con úlceras venosas de las piernas), dos en pacientes con úlceras del pie diabético y ensayos individuales sobre heridas posoperatorias infectadas, lesiones por presión, leishmaniasis cutánea y gangrena de Fournier. Dos ensayos incluyeron a una población mixta de pacientes con heridas agudas y crónicas. La calidad de la evidencia varió entre diferentes comparaciones y resultados. La calidad de la evidencia se vio disminuida principalmente por el riesgo de sesgo, la imprecisión y, en algunos pocos casos, la inconsistencia.
Hay evidencia de alta calidad (dos ensayos, n = 992) de que los apósitos de miel cicatrizan las quemaduras de espesor parcial más rápidamente que los apósitos convencionales (DMP ‐4,68 días, IC del 95%: ‐5,09 a ‐4,28), aunque no está claro si hay una diferencia en las tasas de eventos adversos (evidencia de muy baja calidad) o de infección (evidencia de baja calidad).
Hay evidencia de calidad muy baja (cuatro ensayos, n = 332) de que las quemaduras tratadas con miel cicatrizan más rápidamente que las tratadas con sulfadiazina de plata (SSD, por sus siglas en inglés) (DMP ‐5,12 días, IC del 95%: ‐9,51 a ‐0,73) y evidencia de alta calidad de seis ensayos (n = 462) de que no hay ninguna diferencia en el riesgo general de cicatrización en seis semanas para la miel en comparación con SSD (RR 1,00; IC del 95%: 0,98 a 1,02), aunque se observó una reducción del riesgo general de eventos adversos con miel en relación con SSD. Hay evidencia de calidad baja (un ensayo, n = 50) de que la escisión temprana y el injerto cicatrizan las quemaduras de espesor parcial y total más rápidamente que la miel seguida del injerto según sea necesario (DMP 13,6 días, IC del 95%: 9,82 a 17,38).
Hay evidencia de calidad baja (dos ensayos, diferentes comparadores, n = 140) de que la miel cicatriza una población mixta de heridas agudas y crónicas más rápidamente que la SSD o los apósitos de azúcar.
La miel cicatrizó las heridas postoperatorias infectadas más rápidamente que los lavados antisépticos seguidos de gasa y se asoció con menos eventos adversos (un ensayo, n = 50, evidencia de calidad moderada, RR de cicatrización 1,69; IC del 95%: 1,10 a 2,61); úlceras de decúbito cicatrizadas más rápidamente que con la inmersión en solución salina (un ensayo, n = 40; evidencia de muy baja calidad, RR 1,41; IC del 95%: 1,05 a 1,90), y gangrena de Fournier cicatrizada más rápidamente que con la inmersión Eusol (un ensayo, n = 30; evidencia de muy baja calidad, DMP ‐8,00 días, IC del 95%: ‐6,08 a ‐9,92 días).
Los efectos de la miel en relación con los comparadores no están claros para: úlceras venosas de la pierna (dos ensayos, n = 476, evidencia de baja calidad); heridas agudas menores (tres ensayos, n = 213, evidencia de muy baja calidad); úlceras del pie diabético (dos ensayos, n = 93, evidencia de baja calidad); leishmaniasis (un ensayo, n = 100, evidencia de baja calidad); heridas crónicas mixtas (dos ensayos, n = 150, evidencia de baja calidad).
Conclusiones de los autores
Es difícil establecer conclusiones generales con respecto a los efectos de la miel como un tratamiento tópico para las heridas debido a la naturaleza heterogénea de las poblaciones de pacientes y los comparadores estudiados y la calidad principalmente baja de la evidencia. La calidad de la evidencia se vio disminuida principalmente debido al riesgo de sesgo y la imprecisión. La miel parece cicatrizar las quemaduras de espesor parcial más rápidamente que el tratamiento convencional (que incluyó una película de poliuretano, gasa de parafina, gasa impregnada con soframicina, lino estéril y exposición de las quemaduras) y las heridas postoperatorias infectadas más rápidamente que los antisépticos y la gasa. Más allá de estas comparaciones, la evidencia sobre las diferencias en los efectos de la miel y los comparadores es de calidad baja o muy baja y no forma una base consistente para la toma de decisiones.
PICOs
Resumen en términos sencillos
Miel como tratamiento tópico para las heridas agudas y crónicas
Se examinó la evidencia acerca de los efectos de la aplicación de miel en la cicatrización de cualquier clase de herida. Se encontraron 26 estudios con 3011 pacientes con diferentes tipos de heridas. La miel se comparó con muchos tratamientos diferentes en los estudios incluidos.
Las diferencias en los tipos de herida y los comparadores hacen imposible extraer conclusiones generales acerca de los efectos de la miel sobre la cicatrización de la herida. La evidencia para la mayoría de las comparaciones es de calidad baja o muy baja. Lo anterior se debió en gran parte a que se cree que los problemas con el diseño de algunos de los estudios hicieron que los resultados fuesen poco fiables, y para muchos resultados sólo se dispuso de escasa información. En algunos casos, los resultados de los estudios variaron considerablemente.
Hay evidencia de calidad alta de que la miel cicatriza las quemaduras de espesor parcial más rápidamente (cerca de cuatro a cinco días antes) que los apósitos convencionales. Hay evidencia de calidad moderada de que la miel es más efectiva que los antisépticos seguidos de gasa para la cicatrización de las heridas infectadas después de las cirugías.
No está claro si la miel es mejor o peor que otros tratamientos para las quemaduras, las heridas agudas y crónicas mixtas, las úlceras por presión, la gangrena de Fournier, las úlceras venosas de las piernas, las heridas agudas menores, las úlceras del pie diabético y la leishmaniasis, debido a que la mayoría de la evidencia existente es de calidad baja o muy baja.
La evidencia está actualizada hasta octubre de 2014.
Authors' conclusions
Summary of findings
| Honey compared with conventional dressings for minor acute wounds | ||||||
| Patient or population: patients with Minor acute wounds | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional dressings | Honey | |||||
| Complete healing (time to healing)(days) | The mean complete healing (time to healing) in the intervention groups was | 213 | ⊕⊝⊝⊝ | |||
| Adverse events | Study population | RR 1.19 | 82 | ⊕⊝⊝⊝ | ||
| 357 per 1000 | 425 per 1000 | |||||
| Infection | Study population | RR 0.91 | 151 | ⊕⊝⊝⊝ | ||
| 14 per 1000 | 13 per 1000 | |||||
| Costs | The mean cost of dressing materials per patient was 0.49 ZAR in the honey group and 12.06 ZAR in the control (hydrogel) group | 82 | ⊕⊝⊝⊝ | |||
| Quality of Life6 | Not reported | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias (one level): High risk of attrition bias in all three included studies | ||||||
| Honey compared with conventional dressings for burns | ||||||
| Patient or population: patients with Burns | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional dressings | Honey | |||||
| Complete healing (time to healing)(days) | The mean complete healing (time to healing) in the intervention groups was | 992 | ⊕⊕⊕⊕ | |||
| Adverse events | Study population | RR 0.56 | 992 | ⊕⊝⊝⊝ | ||
| 206 per 1000 | 115 per 1000 | |||||
| Negative wound swab | Study population | RR 1.31 | 92 | ⊕⊝⊝⊝ | ||
| 630 per 1000 | 826 per 1000 | |||||
| Costs5 | Not reported | Not estimable5 | N/A | N/A | ||
| Quality of life5 | Not reported | Not estimable5 | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to inconsistency (one level): High heterogeneity was detected with an I‐squared of 70% | ||||||
| Honey compared with silver sulfadiazine for burns | ||||||
| Patient or population: patients with Burns | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silver sulfadiazine | Honey | |||||
| Complete healing | Study population | RR 1.00 | 462 | ⊕⊕⊕⊕ | ||
| 1000 per 1000 | 1000 per 1000 | |||||
| Mean time to complete healing (days) | The mean time to complete healing in the intervention groups was | 332 | ⊕⊝⊝⊝ | |||
| Adverse events | Study population | RR 0.29 | 412 | ⊕⊕⊕⊕ | ||
| 413 per 1000 | 120 per 1000 | |||||
| Negative wound swab | Study population | RR 3.92 | 412 | ⊕⊝⊝⊝ | ||
| 236 per 1000 | 923 per 1000 | |||||
| Costs | The cost of dressing treatment per % TBSA affected was 0.75 PKR for honey and 10 PKR for silver sulfadiazine. | 50 | ⊕⊕⊝⊝ | |||
| Quality of Life6 | Not reported | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to inconsistency (two levels): Very high level of statistical heterogeneity (I squared of 93%) | ||||||
| Honey for venous leg ulcers | ||||||
| Patient or population: patients with Venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Honey | |||||
| Complete healing (time to healing) | Study population | HR 1.1 | 368 | ⊕⊕⊝⊝ | ||
| 497 per 1000 | 531 per 1000 | |||||
| Complete healing (proportion wounds healed) | Study population | RR 1.15 | 476 | ⊕⊕⊝⊝ | ||
| 460 per 1000 | 529 per 1000 | |||||
| Adverse events | Study population | RR 1.28 | 368 | ⊕⊕⊝⊝ | ||
| 464 per 1000 | 594 per 1000 | |||||
| Infection | Study population | RR 0.71 | 476 | ⊕⊕⊝⊝ | ||
| 221 per 1000 | 157 per 1000 | |||||
| Costs | The mean cost in the intervention group was | 368 | ⊕⊝⊝⊝ | The ICER was sensitive to the inclusion of hospitalisation costs. Hospitalisation unlikely related to treatment and when these were excluded the ICER was in favour of control. | ||
| Quality of Life | The mean PCS in the intervention group was | 368 | ⊕⊕⊕⊝ | |||
| Quality of Life | The mean MCS in the intervention groups was | 368 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias (one level): Unblinded outcome assessment | ||||||
Background
Description of the condition
Acute and chronic wounds are terms in regular use in clinical practice, yet definition of these terms has received little attention. Lazarus 1994 suggested acute wounds proceed through to healing "in an orderly and timely reparative process". Orderliness refers to the healing sequence of inflammation, angiogenesis, matrix deposition, wound contraction, epithelialisation, and scar remodelling. Timeliness is subjective, but refers to a healing time that could be reasonably expected. A chronic wound is, therefore, a wound where the orderly biological progression to healing has been disrupted and healing is delayed.
Description of the intervention
Honey is a viscous, supersaturated sugar solution derived from nectar gathered and modified by the honeybee, Apis mellifera. Honey contains approximately 30% glucose, 40% fructose, 5% sucrose, and 20% water, as well as many other substances, such as amino acids, vitamins, minerals and enzymes (Sato 2000). Honey has been used in wound care since ancient times and is frequently mentioned in early pharmacopeia, although more usually as an ingredient or carrier vehicle rather than a specific treatment. Dioscorides (40‐80 CE) often mentioned honey as a vehicle for carrying therapeutic agents in de materia medicis (Riddle 1985), and Hippocrates (460‐377 BCE), who is often cited as advocating honey for wound care, simply listed it as one of many ingredients in a multitude of unguents (Adams 1939). Probably the first deliberate advocacy of honey as a wound treatment was by the anonymous author of the Edwin Smith papyrus, an Egyptian surgical text written between 2600‐2200 BCE (Breasted 1930). A dressing made from honey and plant material was also recommended for treating burns in the London Medical Papyrus written around 1325 BCE (Trevisanato 2006). Other early medical customs, including Ayurvedic (Johnson 1992), Chinese (Fu 2001) and Roman traditions (Hajar 2002), also used honey in wound care.
How the intervention might work
Between 1996 and 2006 there was a surge in interest about honey as a wound treatment, with 40 case reports or series in 875 patients published (Jull 2008). Recent research has tended to concentrate on the antibacterial activity of the many different types of honey, rather than its effect on wound healing (Molan 1999). Manuka honey, a monofloral honey derived from the Leptospermum tree in New Zealand and Australia, has been of particular interest, as it has antibacterial activity independent of the effect of honey's peroxide activity and osmolarity (Molan 2001). The substance (or substances) responsible for this non‐peroxide activity has not been definitively identified, but has been termed Unique Manuka Factor (UMF). Manuka honey with a UMF rating has an antibacterial activity equivalent to a similar percentage of phenolic acid in solution. Recent research suggests methylglyoxal is the substance responsible for the non‐peroxide activity (Mavric 2008).
There is evidence from different animal models that honey may accelerate healing (Bergman 1983; Oryan 1998; Postmes 1997). Fifteen of the 16 controlled trials in five different animal models (mice, rat, rabbit, pig, and buffalo calf) found that honey‐treated incisional and excisional wounds, and standard burns, healed faster than control wounds (Jull 2008). In addition, a systematic review of honey as a wound dressing found seven randomised trials in humans, six in burns patients and one in infected post‐operative wounds (Moore 2001). Although the poor quality of the trial reports prevented any recommendations, the findings did suggest an effect in favour of honey.
Honey may exert multiple microscopic actions on wounds. It appears to draw fluid from the underlying circulation, providing both a moist environment and topical nutrition that may enhance tissue growth (Molan 1999). Histologically, honey appears to stimulate tissue growth in animal and human controlled trials, with earlier tissue repair noted (Bergman 1983; Subrahmanyam 1998), fewer inflammatory changes (Oryan 1998; Postmes 1997), and improved epithelialisation (Oryan 1998). Macroscopically, reports have also noted the debriding action of honey (Blomfield 1973; Efem 1988; Ndayisaba 1993; Subrahmanyam 1991).
Why it is important to do this review
Honey dressings are widely available and promoted as effective wound treatments. A systematic review of the evidence is therefore warranted as a basis for clinical and policy decision making. This version comprises a substantive update.
Objectives
To assess the effects of honey compared with alternative wound dressings and topical treatments on the healing of acute (e.g. burns, lacerations) and/or chronic (e.g. venous ulcers) wounds.
The publication of the first version of this review (Jull 2008a) was preceded by a published protocol (Jull 2004).
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi‐randomised controlled trials were included. Quasi‐randomised controlled trials are trials which use a quasi‐random allocation strategy, such as alternate days, date of birth, or hospital number.
Types of participants
Trials involving participants of any age with an acute or chronic wound were included. For the purposes of this review an acute wound was considered to be any of the following: burns, lacerations or other skin injuries resulting from minor trauma, and minor surgical wounds healing by primary or secondary intention. Chronic wounds were considered to be: skin ulcers of any type, pressure ulcers and infected wounds healing by secondary intention.
Types of interventions
The primary intervention was any formulation of honey topically applied by any means, alone or in combination with other dressings or components, to an acute or chronic wound. Eligible comparison interventions were dressings or other topical agents applied to the wound.
Types of outcome measures
Trials had to provide data on one of the primary outcomes listed below, and the unit of analysis had to be by participant (See "Differences between protocol and review" for further information about unit of analysis issues).
Primary outcomes
-
Time to complete wound healing;
-
proportion of participants with completely healed wounds.
Secondary outcomes
-
Incidence of adverse events;
-
length of hospital stay;
-
change in wound size:
-
incidence of infection;
-
cost;
-
quality of life.
Search methods for identification of studies
Electronic searches
For the search methods used in the original version of this review see Appendix 1.
For this second update we searched the following databases for reports of eligible randomised controlled trials:
-
The Cochrane Wounds Group Specialised Register (searched 15 October 2014);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 9);
-
Ovid MEDLINE (1946 to October Week 1 2014);
-
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations 13 October, 2014);
-
Ovid EMBASE (1974 to 13 October, 2014);
-
EBSCO CINAHL (1982 to 15 October, 2014).
The following search strategy was used in CENTRAL and adapted appropriately for other databases:
#1 MeSH descriptor Skin Ulcer explode all trees
#2 MeSH descriptor Pilonidal Sinus explode all trees
#3 MeSH descriptor Wounds, Penetrating explode all trees
#4 MeSH descriptor Lacerations explode all trees
#5 MeSH descriptor Burns explode all trees
#6 MeSH descriptor Wound Infection explode all trees
#7 MeSH descriptor Surgical Wound Dehiscence explode all trees
#8 MeSH descriptor Bites and Stings explode all trees
#9 MeSH descriptor Cicatrix explode all trees
#10 ((plantar or diabetic or heel* or foot or feet or ischaemic or ischemic or venous or varicose or stasis or arterial or decubitus or pressure or skin or leg or mixed or tropical or rheumatoid or sickle
cell) NEAR/5 (wound* or ulcer*)):ti,ab,kw
#11 (bedsore* or (bed NEXT sore*)):ti,ab,kw
#12 (pilonidal sinus* or pilonidal cyst*):ti,ab,kw
#13 (cavity wound* or sinus wound*):ti,ab,kw
#14 (laceration* or gunshot stab or stabbing or stabbed or bite*):ti,ab,kw
#15 ("burn" or "burns" or "burned" or scald*):ti,ab,kw
#16 (surg* NEAR/5 infection*):ti,ab,kw
#17 (surg* NEAR/5 wound*):ti,ab,kw
#18 (wound* NEAR/5 infection*):ti,ab,kw
#19 (malignant wound* or experimental wound* or traumatic wound*):ti,ab,kw
#20 (infusion site* or donor site* or wound site* or surgical site*):ti,ab,kw
#21 (skin abscess* or skin abcess*):ti,ab,kw
#22 (hypertrophic scar* or keloid*):ti,ab,kw
#23 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR
#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22)
#24 MeSH descriptor Honey explode all trees
#25 honey:ti,ab,kw
#26 (#24 OR #25)
#27 (#23 AND #26)
The search strategies for Ovid MEDLINE, Ovid EMBASE and EBSCO CINAHL can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision) (Lefebvre 2011). The Ovid EMBASE and EBSCO CINAHL searches were combined with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN) (SIGN 2008). There were no restrictions with respect to language, date of publication (taking into account searches from the original review) or study setting.
Searching other resources
For the initial review we contacted experts in the field, authors of the included trials and manufacturers of honey products for wound care (Comvita NZ Ltd and MediHoney Australia Pty Ltd), but did not repeat this for updates. The bibliographies of all obtained studies and review articles were searched for potentially eligible trials for both the initial review and the first update. No language or date restrictions were applied to the trials and both published and unpublished trials were sought.
Data collection and analysis
Selection of studies
Two authors (either AJ and NW, or for this update JD and NC) independently examined titles and abstracts of potentially relevant trials. Full text copies of all relevant trials, or trials that might be relevant to the review were obtained. The two review authors independently selected the trials using the inclusion criteria. Disagreements were resolved by discussion.
Data extraction and management
Data were extracted from included trials by one review author and recorded on a standardised form. The extracted data were independently reviewed for accuracy by a second review author and disagreements resolved by discussion. All data have subsequently been verified by a third (MW) and fourth author (NC). If the data from the trial report were inadequate, or ambiguous, additional information was sought from the trial authors. We collected data on the topics listed below:
-
Author; title; source of reference.
-
Study setting.
-
Study design.
-
A priori sample size calculation; sample size.
-
Inclusion/exclusion criteria.
-
Age of participants; sex of participants.
-
Wound type.
-
Intervention and comparison.
-
Outcomes.
-
Withdrawals and reason for withdrawal.
-
Funding source.
-
Co‐interventions
Assessment of risk of bias in included studies
For the first update, one review author (SD) assessed each included study using the Cochrane Collaboration tool for assessing risk of bias (Higgins 2011), and this assessment was checked by a second (AJ) review author. For this second update two more pairs of review authors/editors checked risk of bias (either MW and JD or JD and NC) and reviewed by the lead review author (AJ). This tool addresses six specific domains, namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues, such as extreme baseline imbalance (see Appendix 3 for details of criteria on which the judgement was based). Blinding and completeness of outcome data were assessed for each outcome separately. We completed a risk of bias table for each eligible study and discussed any disagreement amongst all review authors to achieve a consensus.
We present an assessment of risk of bias using a 'risk of bias summary figure', which presents all of the judgements in a cross‐tabulation of study by entry. This display of internal validity indicates the weight the reader may give the results of each study.
Data synthesis
Where trials were sufficiently alike in terms of population and comparison interventions, their results were combined. Mean differences (MD) and 95% confidence intervals (95% CI) were reported for continuous outcomes, and risk ratio (RR) and 95% confidence intervals (95% CI) were reported for dichotomous variables. Statistical heterogeneity was tested by comparing Cochran's Q statistic and the chi‐squared distribution. Heterogeneity was assumed with P values of less than 0.1 (Higgins 2011). In addition, the I2 statistic was used to determine the percentage of variation due to heterogeneity rather than chance (Higgins 2003), and any sources of heterogeneity were explored. Where significant statistical heterogeneity was present, a random‐effects model was used when combining trials (Ioannidis 2008).
Summary of findings
The evidence was summarised in summary of findings tables using the approach of the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) (Langendam 2013). This approach assesses the quality of the body of evidence per comparison and outcome, taking into account five factors: risk of bias across all studies reporting that outcome; indirectness of population, interventions and outcomes, across all studies reporting the outcome; inconsistency amongst studies; imprecision (taking into account the optimum information size and the confidence intervals) and publication bias. The results are reported below according to condition, comparison and outcome and then the different outcomes are brought together in summary of findings tables (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3; summary of findings Table 4), which are discussed in the final section (Discussion).
Results
Description of studies
Included studies
Twenty six trials met the inclusion criteria (please see the Characteristics of included studies) and were available for analysis; eight trials were added in updates (Baghel 2009; Gulati 2014; Kamaratos 2014; Mashood 2006; Memon 2005; Nilforoushzadeh 2007; Robson 2009; Shukrimi 2008), including one trial that was mistakenly excluded in the previous review, but was found in the first update to meet the inclusion criteria on re‐screening (Mashood 2006). Another trial was previously wrongly included and has now been excluded in this update (Subrahmanyam 1996c). Three trials are awaiting assessment while attempts are made to contact the authors to request further data (Askarpour 2009; Jan 2012; Maghsoudi 2011).
Ten separate trials were conducted by the same investigator (Subrahmanyam 1991; Subrahmanyam 1993a; Subrahmanyam 1993b; Subrahmanyam 1994; Subrahmanyam 1996a; Subrahmanyam 1996b; Subrahmanyam 1998; Subrahmanyam 1999; Subrahmanyam 2001a; Subrahmanyam 2004) based in India. Important information was missing from the published reports of these studies however some was provided on request.
Fourteen trials recruited participants with acute wounds: 11 with burns (Baghel 2009; Mashood 2006; Memon 2005; Subrahmanyam 1991; Subrahmanyam 1993b; Subrahmanyam 1994; Subrahmanyam 1996a; Subrahmanyam 1996b; Subrahmanyam 1998; Subrahmanyam 1999; Subrahmanyam 2001a), two with minor surgical excisions (Marshall 2005; McIntosh 2006), and one with minor trauma (Ingle 2006). Ten trials recruited participants with chronic wounds including venous leg ulcers (Jull 2008; Gethin 2007), infected surgical wounds (Al Waili 1999), pressure injuries (Weheida 1991), Fournier's gangrene (Subrahmanyam 2004), cutaneous Leishmaniasis (ulcers caused by protozoans injected by sandfly bite) (Nilforoushzadeh 2007), diabetic foot ulcers (Kamaratos 2014; Shukrimi 2008), and a variety of chronic wounds healing by secondary intention though mainly venous leg ulcers (Gulati 2014; Robson 2009). Two trials recruited participants with either chronic or acute wounds (Mphande 2007; Subrahmanyam 1993a).
Eight trials were conducted in community settings or outpatient clinics (Gulati 2014; Kamaratos 2014; Gethin 2007; Ingle 2006; Jull 2008; Marshall 2005; McIntosh 2006; Nilforoushzadeh 2007). The remaining trials were conducted in hospital settings, or a mixed inpatient and outpatient setting (Robson 2009). Eight trials reported recruiting only adults (Al Waili 1999; Gulati 2014; Gethin 2007; Ingle 2006; Jull 2008; Kamaratos 2014; Shukrimi 2008; Subrahmanyam 2004). The remaining trials did not specify an age range (Marshall 2005; McIntosh 2006; Robson 2009), or recruited both children and adults.
Monofloral honey (aloe, jarrah, jamun, jambhul or manuka) was used in ten trials (Gethin 2007; Gulati 2014; Ingle 2006; Jull 2008; Kamaratos 2014; Marshall 2005; McIntosh 2006; Robson 2009; Subrahmanyam 2001a; Subrahmanyam 2004); the type of honey used was not specified in the remaining trials. Honey was delivered as a honey impregnated gauze dressing in six trials (Kamaratos 2014; Mphande 2007; Nilforoushzadeh 2007; Subrahmanyam 1993a; Subrahmanyam 1994; Subrahmanyam 2004); as a honey impregnated alginate dressing in two (Jull 2008; McIntosh 2006); as honey spread between gauze in one trial (Memon 2005) and as topical honey covered with either a film dressing (Ingle 2006; Gulati 2014) or with gauze in the remaining trials. Six trials investigated honey as an adjunct treatment: four included people with venous leg ulcers, and, for these, honey was used as an adjunct to compression (Gethin 2007; Gulati 2014; Jull 2008; Robson 2009). One trial in people with Leishmaniasis gave honey alongside an injection of glucantamine (Nilforoushzadeh 2007). In a further trial, 50% patients receiving honey also received delayed autologous skin grafting, as necessary (Subrahmanyam 1999).
There was a range of comparison treatments in this review, which have been grouped under the broad categories of conventional dressings, silver sulfadiazine (SSD), antiseptics, early excision and atypical dressings. Seven trials compared honey with SSD, and of these, the comparator was a SSD impregnated dressing in four trials (Subrahmanyam 1991; Subrahmanyam 1993b; Subrahmanyam 1998; Subrahmanyam 2001a) and SSD cream in three (Baghel 2009; Mashood 2006; Memon 2005). Two studies compared honey with hydrogel (Gethin 2007; Ingle 2006). In the adjunct trials, the comparators were either other dressings plus compression (Gethin 2007; Gulati 2014; Jull 2008; Robson 2009) or glucantamine injection alone (Nilforoushzadeh 2007). The comparator for the trial giving honey plus delayed skin grafting was early tangential excision and skin grafting (Subrahmanyam 1999).
In view of the clinical diversity of the evidence, this review is organised by wound type, and then by comparison type.
There was a great deal of variation in the outcomes reported by the included studies which makes the drawing of overall conclusions very difficult. Many of the included studies report "mean time to healing" but it was frequently not clear whether every participant's wound had healed during follow up (in which case the mean time with associated SD or 95% confidence interval would be acceptable). However if all wounds in a study do not heal during the period of observation, simple calculation of the mean (or median) time to healing without accounting for censoring is inappropriate since, by definition, this approach excludes people who did not heal during follow up (as they cannot contribute to the numerator). Importantly excluding people who failed to heal from the data excludes treatment failures and will over‐estimate treatment success. Time to healing is a type of "time to event" outcome and should be analysed as such using a survival approach which allows people who did not heal to contribute data to the analysis for the period for which they were observed. Only three studies (Jull 2008; Nilforoushzadeh 2007; Robson 2009) analysed time to healing as time to event data. One study (Shukrimi 2008) reported time to readiness for wound closure surgery.
The multiplicity of time points at which healing was reported was a further problem which had not been anticipated in the protocol. We opted to analyse the outcomes for the longest point of follow up shared by several trials of the same comparison (since to extract and analyse all time points risks a Type I error).
Excluded studies
For details on the excluded studies please see Characteristics of excluded studies table. Of the 57 excluded studies, 24 were not RCTs or quasi‐RCTs (Abdelatif 2008; Ahmed 2003; Al Waili 2004c; Al Waili 2005; Bose 1982; Dunford 2004; Freeman 2010; Gethin 2005; Lusby 2002; Marshall 2002; Mayer 2014; Misirligou 2003; Moghazy 2010; Molan 2002; Molan 2006; Mwipatayi 2004; Nagane 2004; Robson 2002; Schumacher 2004; Subrahmanyam 1993; Thurnheer 1983; Tostes 1994; Vijaya 2012; Visscher 1996). Seven of the excluded studies were not in wounds (Al Waili 2003; Albietz 2006; Biswal 2003; Johnson 2005; Quadri 1998; Quadri 1999; Somaratne 2012). Three were studies in animal models (Al Waili 2004a; Al Waili 2004b; Subrahmanyam 2001b). Thirteen studies did not report sufficient information on healing (Bangroo 2005; Chokotho 2005; Gad 1988; Heidari 2013; Jeffery 2008; Lund‐Nielsen 2011; Mat Lazim 2013; Robson 2012; Rogers 2010; Rucigaj 2006; Saha 2012; Subrahmanyam 2003; Ur‐Rehman 2013). Three studies did not evaluate honey (Berchtold 1992; Muller 1985), one evaluated the effect of adding vitamins and polyethylene glycol to honey (Subrahmanyam 1996c) and a further two could not be obtained for assessment (Calderon Espina 1989; Rivero Varona 1999). Four studies had unit of analysis issues, they had randomised, or reported, by wound rather than by the participant. Such unit of randomisation or analysis issues were not considered in the protocol for the review, but we considered that such studies could not contribute usefully to this review. For further discussion on the rationale for this decision see "Differences between protocol and review" (Malik 2010; Okeniyi 2005; Oluwatosin 2000; Yapucu Gunes 2007).
Risk of bias in included studies
Risk of bias is summarised in the figures (Figure 1; Figure 2) with judgements explained in the Characteristics of included studies. Risk of bias was a key consideration in assessing the quality of the evidence and used (and justified) in the Summary of Findings Tables to downgrade the evidence where appropriate. Overall the quality of reporting was poor and it was frequently not possible to determine whether allocation was fully concealed. Two studies (Mphande 2007; Kamaratos 2014) used quasi‐random methods of allocation and these are at high risk of selection bias as a consequence. Most of the included studies were at risk of performance bias as neither participants nor health care providers were blinded to treatment allocation. The main outcomes of complete healing, adverse events and infection have, to varying extents, an element of subjectivity inherent in them and 14 out of 26 included studies reported having used blinded outcome assessment.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
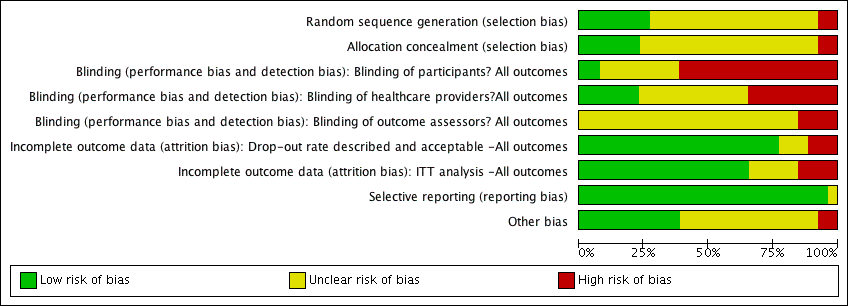
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Summary of findings for the main comparison Honey compared with conventional dressings for minor acute wounds; Summary of findings 2 Honey compared with conventional dressings for burns; Summary of findings 3 Honey compared with silver sulfadiazine for burns; Summary of findings 4 Honey for venous leg ulcers
The 26 trials included 3011 participants. The trials were generally small (median sample size 83.5, range 30 to 900), and there was very obvious clinical and methodological heterogeneity. It was not appropriate, therefore, to combine the trials in a single meta‐analysis to produce a summary statistic for honey overall, or even subgroup summary statistics for acute, chronic, or mixed wounds. Within the subgroups (acute, mixed acute and chronic, and chronic wounds) trials have been combined in meta‐analysis where appropriate. Otherwise the trials have been summarised narratively.
In common with wounds research in general, adverse event reporting was variable in nature and often poor in quality. Five trials did not report adverse events (Baghel 2009; Kamaratos 2014; Subrahmanyam 1996a; Subrahmanyam 1998; Weheida 1991), and five trials stated that there were no adverse events or no adverse events related to treatment (Gethin 2007; Gulati 2014; Marshall 2005; McIntosh 2006; Subrahmanyam 1996b). One trial reported the number of people with any adverse event (Jull 2008), as well as itemising specific types of events. The remaining trials appear to have limited reporting of events to specific types of events, rather than encouraging reports of any event. Adverse events are presented by wound type, and the comparators indicated in footnotes and any meta‐analysis uses a random‐effects model due to the heterogeneity. Although only one trial explicitly reported frequency of events by participant (Jull 2008), it is assumed one event equals one participant in all other trials (this may be an erroneous assumption).
The "infection" data in these studies are not well reported and impossible to analyse in a robust manner and interpret reliably across all wound types. One of the main problems is the lack of a definition of infection within most trial reports and an inconsistency between trials. Most of the burns trials reported "positive" swab cultures at baseline and then the proportion rendered "sterile" at subsequent time points (usually 7 days). However a positive swab culture is NOT the same as a clinical infection (the diagnosis of the latter being dependent on signs and symptoms as well as culture). Consequently we cannot draw conclusions about the treatment effects of honey dressings and comparators from these data. We have confined our analysis of the burns studies to the outcome of "proportion of burns with negative swabs at 7 days" however this is a less clinically relevant outcome than healing, or clinical infection.
1. Acute wounds
1.1 Minor acute wounds
1.1.1 Honey compared with conventional dressings
Three trials (213 participants) recruited participants with minor acute wounds (Ingle 2006; Marshall 2005; McIntosh 2006). In two trials, the wounds were surgical wounds created after partial or total toenail avulsions (Marshall 2005; McIntosh 2006), with the control group treated with paraffin gauze in one trial and an iodophor dressing in the other. The remaining trial recruited mine workers with lacerations or shallow abrasions and treated control participants with a hydrogel (Ingle 2006).
Outcome: healing
We combined the results of the three trials using a random effects model. It is unclear whether there is a difference in time to healing between honey and control (difference in mean days to healing was 2.26 days longer with honey, 95% CI ‐3.09 to 7.61 days (Analysis 1.1). Moderate heterogeneity (I² = 47%). Very low quality evidence (downgraded for risk of bias, inconsistency and imprecision) (summary of findings Table for the main comparison).
Outcome: adverse events
Ingle and colleagues reported the frequency of itching, burning and pain (Ingle 2006). There was no significant difference in the rates of these events between honey and hydrogel RR 1.19, 95% CI 0.69 to 2.05) (Analysis 1.2). No patient stopped treatment due to these sensations. It remains unclear as to whether there are more or fewer adverse events with honey dressings compared with non‐honey dressings in people with minor acute wounds. Very low quality evidence (downgraded for risk of bias, serious inconsistency and imprecision) (summary of findings Table for the main comparison).
Outcome: infection
Infection was not reported by Ingle 2006. There was only one instance of infection reported in each of the other two trials (Marshall 2005; McIntosh 2006): in 1/27 participants in the honey group compared with 0/24 in the iodine group of Marshall 2005, and in 1/48 participants of the iodine group compared with 0/52 in the honey group of McIntosh 2006 (pooled RR of infection 0.91, 95% CI 0.13 to 6.37, fixed effect, Analysis 1.3). It is therefore unclear if honey affects rates of wound infection in minor acute wounds. Very low quality evidence (downgraded for the reasons outlined above) (summary of findings Table for the main comparison).
Outcome: costs
Only Ingle 2006 reported on costs, and then only in terms of average costs of dressing materials per patient in each group. These were lower in the honey group (average cost per patient 0.49 Rand) than the hydrogel group (average cost per patient 12.06 Rand) however this does not appear to have been a commercial honey preparation (hence low cost).
Outcome: quality of life
Not reported.
1.2 Burns
There were six comparison treatments, which have been grouped under the four broad categories of conventional dressings, early excision, silver sulfadiazine (SSD) and atypical dressings for this review.
1.2.1 Honey compared with conventional dressings
Two trials (992 participants) compared honey with conventional dressings for the treatment of partial‐thickness burns (Subrahmanyam 1993a; Subrahmanyam 1996a). In one trial (Subrahmanyam 1993a) the comparison was a polyurethane film dressing and in the other trial (Subrahmanyam 1996a) the control participants were treated with a range of interventions: polyurethane film (OpSite, n = 90), paraffin gauze (n = 90), sterile linen dressings (n = 90), antimicrobial impregnated gauze (Soframycin, n = 90) or left exposed (n = 90).
Outcome: healing
Mean days to healing were reported but not the standard deviations, though this information was later provided by the author (personal communication: M Subrahmanyam). The two trials were pooled using a fixed effect model (I2 = 0%). Burns treated with honey healed more quickly (WMD ‐4.68 days, 95% CI ‐5.09 to ‐4.28 days, Analysis 2.1). High quality evidence (summary of findings Table 2).
Outcome: adverse events
In Subrahmanyam 1993a there were two cases of over‐granulation and two of contracture in the honey group compared with two of over‐granulation and one of contracture in the control group. In Subrahmanyam 1996a there were five cases of hypergranulation and 28 of scarring with honey and 12 of hypergranulation and 87 of scarring with conventional dressings. These data were pooled (random effects). Overall there was no clear difference in risk of adverse events (RR 0.56, 95% CI 0.15 to 2.06) (Analysis 2.2).Very low quality evidence (downgraded for inconsistency i.e., high heterogeneity, and imprecision) (summary of findings Table 2).
Outcome: infection
Subrahmanyam 1996a did not report infection rates. Subrahmanyam 1993a reported a greater proportion of honey participants having a negative swab at Day 8 (38/46 participants in the honey group cf. 29/46 in the polyurethane film group), (RR of a negative swab 1.31, 95%CI 1.01 to 1.70), Analysis 2.3. Low quality evidence (downgraded for imprecision and indirectness, in that a negative swab on a particular day is not a measure of infection per se)(summary of findings Table 2).
Outcomes: costs and quality of life
Not reported.
1.2.2 Honey plus delayed grafting compared with early excision and grafting (no honey)
One trial (50 participants) compared early tangential excision and skin grafting with honey dressings plus delayed skin grafting where needed, for the treatment of mixed partial‐ and full‐thickness burns (Subrahmanyam 1999).
Outcome: healing
Mean time to healing was not published, but was later provided by the author (personal communication: M Subrahmanyam). Burns healed more slowly when treated with honey (followed by delayed grafting where needed) than with early excision and grafting (no honey) (WMD 13.6 days, 95% CI 9.82 to 17.38 days, Analysis 3.1). The quality of this evidence was downgraded for imprecision on the basis that there is only one trial with a total of 50 participants and whilst the difference in time to healing was statistically significant and clinically important, this is a small single study. We also downgraded the quality of the evidence for indirectness, since honey is not the only systematic difference in the treatments for this comparison as the burn excision and grafting interventions were also different (therefore the trial does not directly address the question of whether honey dressings are effective for burns). Low quality evidence.
Outcome: adverse events
In Subrahmanyam 1999 there was a total of 6 adverse events in the honey group (3 deaths, 3 contractures), compared with one death in the early excision group. Low quality evidence (see above).
Outcome: infection
Infection rates per se were not reported for Subrahmanyam 1999 however days of antibiotic therapy (which is a proxy for infection), were. Participants in the honey group received a mean of 32 (SD 18) days of antibiotics compared with 16 (SD 3) in the early excision and graft group (difference in mean days of antibiotic therapy 16.00, 95% CI 8.85 to 23.15) (Analysis 3.2). Low quality evidence (downgraded two levels for indirectness since honey is not the only systematic difference between treatments and the outcome "days of antibiotic therapy" is only a proxy for infection).
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
1.2.3 Honey compared with silver sulfadiazine
Six trials (462 participants) compared honey with SSD (Baghel 2009; Mashood 2006; Memon 2005; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001a). The trials used different burn grading systems to report the depth of burns, which reflected the age of the trials and the lack of a clinical consensus on reporting burn depth. The burns, however, did tend towards the less severe end of the spectrum, although early staging systems (e.g. first‐, second‐ and third‐degree burns, or superficial, partial‐thickness and full‐thickness) do not have the sensitivity of more recent systems (i.e. epidermal, superficial dermal, mid‐dermal, deep dermal and full‐thickness), and participants with deep partial‐thickness and deep dermal burns are likely to require skin grafting (NZGG 2007).
Trials recruited participants with superficial and partial‐thickness burns (Baghel 2009), superficial burns (Subrahmanyam 1991; Subrahmanyam 1998), superficial, partial‐ and deep partial‐thickness burns (Mashood 2006), and one recruited participants with superficial, dermal, mid and deep dermal burns (Memon 2005). The remaining trial did not report their eligibility criteria but some of the participants had full thickness burns (Subrahmanyam 2001a).
The studies used different treatment regimens. All applied honey topically, covered with gauze. Three studies gave SSD as a topical cream covered by gauze (Baghel 2009; Mashood 2006; Memon 2005) and the rest as SSD impregnated gauze. Two studies changed both types of dressings daily (Mashood 2006; Subrahmanyam 1991); two changed both types of dressing on alternate days (Memon 2005; Subrahmanyam 2001a) and the other study did not report the frequency of dressing change (Baghel 2009). The study by Subrahmanyam 1998 changed the SSD dressings daily and the honey dressings on alternate days.
Outcome: healing
One trial reported mean time to healing and the risk of healing at different times (Subrahmanyam 2001a), and five trials reported either mean time to healing without standard deviations (Baghel 2009; Memon 2005), or risk of complete healing (Mashood 2006; Subrahmanyam 1991; Subrahmanyam 1998), but at different time points i.e., two, four, and six weeks (Mashood 2006), and seven, 10, 15, 21 and 30 days (Subrahmanyam 1998).
Additional information was sought from authors and provided by one author (personal communication: M Subrahmanyam). Baghel 2009 reported an exact p‐value for the comparison of time to healing and this was used to calculate the standard error for mean time to healing. Thus mean time to healing was used as the outcome, with the result that four trials out of six could be pooled for the outcome of mean time to healing using a random effects model (heterogeneity was extremely high, I² = 93%). In these four trials, people whose burns were treated with honey experienced an average reduction in healing time of 5 days (WMD ‐5.12 days, 95% CI ‐9.51 to ‐0.73) (Analysis 4.1). This was very low quality evidence (downgraded for inconsistency and imprecision) (summary of findings Table 3).
In the trial that did not report standard deviations with mean time to healing (Memon 2005), the honey‐treated group had a mean time to healing of 15.3 days and it was 20.0 days in the SSD group (Memon 2005). In the remaining trial (Mashood 2006), all 25 participants in the honey‐treated group were healed by four weeks, while all patients in the SSD group were healed by six weeks.
All six trials reported the risk of complete healing at either 4 or six weeks (or both) as well as several earlier time points. We wished to reduce the risk of Type I errors inherent in multiple endpoint analysis. We therefore report the pooled risk of complete healing for all six trials at 4 to 6 weeks (Mashood 2006; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001a; Baghel 2009; Memon 2005) using a random effects model because although the I2 was 0% these trials are clearly different in terms of frequency of dressing changes, types of burns and duration of follow up. There was no difference in the risk of burns healing by 4 to 6 weeks when treated with honey compared with SSD (RR 1.00 95% CI 0.98 to 1.02). High quality evidence (summary of findings Table 3).
Outcome: adverse events
Burns trials tended to report the frequencies of hypergranulation, contracture, hypertrophic scarring, minor scarring, itching and burning as adverse events however it was generally unclear if events were reported by individual without double counting, or (more likely) some individuals experienced more than one adverse event. Consequently we are not confident of the accuracy of the adverse events data. The adverse event data across the five trials that reported them (Baghel 2009; Mashood 2006; Memon 2005; Subrahmanyam 1991; Subrahmanyam 2001a) were pooled (random effects); importantly Mashood 2006 stated that there was "no difference" between groups in rates of contracture and hypertrophic scarring but only reported rates of itching and burning. Overall there were significantly fewer adverse events with honey than SSD (RR 0.29 95% CI 0.20 to 0.42) (Analysis 4.3). High quality evidence (summary of findings Table 3).
Outcome: infection
Infection related outcomes were reported in a variety of ways: most consistently five out of six trials reported the proportion of burns yielding negative swabs at Day 7 (Baghel 2009; Memon 2005; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001a). The report of Mashood 2006 merely stated the time to sterility (with no SD or other measure of variance) in each group. There was very high statistical heterogeneity when pooling the five studies (I2=94%). Overall wound swabs from honey treated burns were more likely to be negative at 7 days than were swabs from SSD treated burns (RR 3.92 95% CI 1.32 to 11.63) (Analysis 4.4) however this is very low quality evidence (downgraded for inconsistency, imprecision and indirectness) (summary of findings Table 3).
Outcomes: costs
Only Mashood 2006 reported any cost data and then only as standardised unit costs (per percentage of (total body surface area) TBSA) of dressing treatments. They reported that the honey cost 0.75 Rupees per percentage TBSA compared with SSD which costs 10 Rupees per percentage TBSA.
Outcomes: quality of life
Not reported.
1.2.4 Honey compared with atypical dressings
Two trials (164 participants) by the same investigator compared honey with atypical dressings or materials (Subrahmanyam 1994; Subrahmanyam 1996b). The first trial recruited 64 participants with partial‐thickness burns and compared honey‐impregnated gauze with treatment with amniotic membranes (Subrahmanyam 1994) and the second recruited 100 people with partial thickness burns and compared honey with boiled potato peel dressings (Subrahmanyam 1996b).
Outcome: healing
The mean time to healing was reported for both trials without SDs which were subsequently supplied by the author (personal communication: M Subrahmanyam). Burns treated with honey healed approximately 8 days more quickly than those treated with amniotic membranes (WMD ‐8.10 days, 95% CI ‐10.88 to ‐5.32). In the second trial, burns treated with honey healed approximately 6 days more quickly than those treated with boiled potato peel (WMD ‐5.80 days, 95% CI ‐6.68 to ‐4.92).
The comparator treatments for these two trials are too different to pool them. The evidence for both these comparisons should be downgraded for imprecision (due to the relative lack of evidence) and therefore this constitutes moderate quality evidence.
Outcome: adverse events
Subrahmanyam 1994 reported that 4/40 and 3/40 people in the honey group experienced scarring/contractures and severe pain respectively compared with 5/24 and 6/24 in the amniotic membrane group (we cannot assume that the people experiencing pain were separate from the people having scarring or contractures). It was stated that there were no allergies or side effects in either group in the other study (Subrahmanyam 1996b).
Outcome: infection
Both studies reported the outcome of a negative swab at Day 7. In Subrahmanyam 1994, 36/40 (90%) of honey treated burns had negative swabs at Day 7 compared with 18/24 (75%) of burns treated with amniotic membrane. In Subrahmanyam 1996b, 36/50 (72%) of burns treated with honey had negative swabs at Day 7 compared with 8/50 (16%) treated with potato peel.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
2. Mixed acute and chronic wounds
Two trials (140 participants) each recruited participants with a range of different acute and chronic wounds. Subrahmanyam 1993b recruited 100 participants with burns (50% of the study population), lower limb ulcers caused by trauma, pressure, diabetes, and venous disease, or trophic ulcers and compared honey with SSD. In a quasi‐randomised study, Mphande 2007 recruited 40 participants with ulcers, chronic osteomyelitis, abscesses, post‐surgical or traumatic wounds; the comparison treatment was sugar dressings.
Outcome: healing
For the Subrahmanyam 1993b study, information on overall mean time to healing was provided by the author (personal communication: M Subrahmanyam). Wounds treated with honey healed more quickly than those treated with SSD (difference in mean days to healing ‐13.0 days, 95% CI ‐10.76 to ‐15.24) (Analysis 6.1)
In the Mphande 2007 study, median time to complete healing was 31.5 days in the honey‐treated group and 56.0 days in the sugar‐treated group.
Outcome: adverse events
Subrahmanyam 1993b reported hypergranulation, hypertrophic scarring, contractures and irritation as adverse events: 2/50 (4%) in the honey group experienced these compared with 14/50 (28%) in the SSD group. Mphande 2007 did not refer to adverse events generally but did report on pain in terms of being pain free during dressing changes at 3 weeks and also pain during mobilisation at 3 weeks. 19/22 participants (86.4%) in the honey group were pain free during dressing changes at 3 weeks compared with 13/18 (72.2%) in the sugar dressing group. 20/22 participants (90.9%) in the honey group and 13/18 (72.2%) in the sugar group were pain free during mobilisation.
Outcome: infection
Both Mphande 2007 and Subrahmanyam 1993b reported the outcome of negative swabs at 7 days however these results cannot be pooled as the comparator treatments are so different. It is unclear whether honey is associated with more negative swabs at 7 days than either SSD or sugar dressings due to imprecision and risk of (performance) bias (Analysis 6.2).
Overall there is low quality evidence (downgraded for imprecision on account of the two small studies, and indirectness on account of the mixed patient population and difficulties of interpretation) that, on average, honey heals a heterogeneous population of acute and chronic wounds more quickly than SSD or sugar dressings though the comparative rates of adverse events and infection are unclear.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3. Chronic Wounds
Ten trials (819 participants) evaluated the effects of honey in chronic wounds. Two trials recruited people with venous leg ulcers (Gethin 2007; Jull 2008); one study (Robson 2009) recruited participants with any type of wound healing by secondary intention but most of these were leg ulcers (70%); one study recruited people with a range of chronic wounds though most were venous leg ulcers (Gulati 2014). Two studies recruited people with diabetic foot ulcers (Kamaratos 2014; Shukrimi 2008) and one study for each of the following: ulcers caused by Leishmaniasis (Nilforoushzadeh 2007); pressure injuries (Weheida 1991); infected post‐operative wounds (Al Waili 1999), and Fournier's gangrene (Subrahmanyam 2004). Five of these ten studies were added at the first or second update (Gulati 2014; Kamaratos 2014; Nilforoushzadeh 2007; Robson 2009; Shukrimi 2008),
Three trials reported either the mean or median time to healing (Al Waili 1999; Jull 2008), or the mean time to surgical closure (Shukrimi 2008); seven trials reported the proportion of participants with completely healed wounds (Gethin 2007; Gulati 2014; Jull 2008; Kamaratos 2014; Nilforoushzadeh 2007; Robson 2009; Weheida 1991). One trial only reported the outcome, "mean hospital stay", but data on mean time to healing were provided by the author (Subrahmanyam 2004). Given the clinical and methodological heterogeneity between the trials, it was not possible to combine the trials to produce an overall summary statistic for the effect of honey on chronic wounds and instead we consider the evidence by wound type below.
3.1 Infected post‐operative wounds
One trial (50 participants) randomly allocated participants with infected caesarean or hysterectomy wounds to twice daily applications of honey or antiseptic washes of 70% ethanol and povidone‐iodine, followed by gauze dressings (Al Waili 1999), in addition to systemic antibiotics. There was very limited information on baseline comparability and no real indication of the duration of treatment or length of follow‐up.
Outcome: healing
More people healed with honey (84.6%) than with antiseptic washes followed by gauze dressings (50.0%); RR 1.69 (95% CI 1.10 to 2.61) (Analysis 7.1) (moderate quality evidence; downgraded for imprecision). This equates to an increase in the absolute risk of healing of 35% (95%CI 8.7% to 55.4%).
Outcome: adverse events
People were less likely to be recorded as having experienced adverse events with honey: 4/26 honey treated wounds (15.3%) compared with 12/24 (50%) of control wounds dehisced; 6/24 (25%) of control wounds needed resuturing compared with none in the honey group (moderate quality evidence; downgraded for imprecision).
Outcome: infection
Al Waili 1999 reported the mean time (days) to a negative swab as a measure of infection; this was 6 days (SD 1.9) for honey compared with 14.8 days (SD 4.2) for antiseptics and gauze (moderate quality evidence, downgraded for imprecision).
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.2 Pressure injuries
One trial (40 participants) randomly allocated participants with uninfected grade I or grade II pressure injuries greater than 2 cm in diameter to daily applications of honey or saline‐soaked gauze dressings for 10 days treatment (Weheida 1991). There was limited information on baseline comparability.
Outcome: healing
More people treated with honey were healed at 10 days (100%) than those treated with saline soaks (70%); RR 1.41 (95% CI 1.05 to 1.90) Analysis 7.1 (very low quality evidence due to imprecision and potential selection bias as assessed by baseline imbalance).
Outcome: adverse events
Not reported.
Outcome: infection
Not reported.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.3 Fournier's gangrene
One trial in which 30 men with Fournier's gangrene (23 of whom were chronic alcoholics) were randomly allocated to be treated with monofloral (jamun) honey‐soaked gauze dressings or antiseptic EUSOL‐soaked gauze dressings (Subrahmanyam 2004). Fournier's gangrene is an infection of the scrotum that can also involve the perineum and abdominal wall.
Outcome: healing
Secondary suturing and skin grafting were required in 9/14 (64.3%) of the honey group and 9/16 (56.3%) of the EUSOL group. Only mean length of hospital stay was reported in the paper, but mean time to healing was supplied by the author (personal communication M Subrahmanyam). Mean time to healing was shorter in the honey‐treated group (MD ‐8.00 days, 95% CI ‐6.08 to ‐9.92 days, Analysis 7.2), but we note this was a very small sample size and the high rates of further surgical intervention (secondary suturing and skin grafting) required by participants in both groups is worth noting (very low quality evidence, on account of the very small sample size and single study).
Outcome: adverse events
One participant in the honey‐treated group and two in the EUSOL group died.
Outcome: infection
The primary condition (Fournier's gangrene) is itself the result of infection; rates of secondary infection were not reported.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.4 Cutaneous Leishmaniasis
One trial (100 participants) randomly allocated participants with ulcers caused by Leishmaniasis to treatment with intralesional injections of meglumine antimoniate (glucantamine) plus honey‐soaked gauze dressings or intralesional injections alone (Nilforoushzadeh 2007).
Outcome: healing
Thirteen participants withdrew from the honey dressings arm due to treatment failure (n=12) or contact dermatitis (n=1) (and therefore remain in this analysis in the denominator) whilst 10 withdrew from the meglumine antimoniate alone group due to treatment failure (similarly remaining in the denominator). Fewer people treated with injections plus honey had healed lesions compared with those not receiving honey at 4 months although this difference was not statistically significant (51.1% versus 71.1%) (RR 0.72; 95% CI 0.50 to 1.04) (Analysis 7.1) (low quality evidence due to imprecision and high risk of bias).
Outcome: adverse events
The study by Nilforoushzadeh 2007 reported one withdrawal due to sensitivity in the honey group and none in the control group.
Outcome: infection
Not reported.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.5 Venous leg ulcers
Two trials recruited only participants with venous leg ulcers. One trial (368 participants) recruited patients presenting to community‐based nursing services for assessment and treatment of their venous ulcers (Jull 2008). Participants were allocated to receive either manuka honey‐impregnated calcium alginate dressings or usual care. Participants allocated usual care could receive any dressing that was clinically indicated from the wide range normally available to community nurses (non‐adherent, alginate, hydrogel, hydrofibre, hydrocolloid, silver or iodophor dressings). Both groups received compression bandaging as a standard background treatment. Participants were treated for 12 weeks. The second trial recruited 108 participants with uninfected venous ulcers, the surfaces of which were at least 50% covered by slough (Gethin 2007). Participants were allocated to receive either manuka honey dressings or hydrogel dressings for four weeks and then standard care (the nature of which was individually determined by the clinician) for the remaining eight weeks of the 12 week follow‐up. Both groups received compression bandaging as a standard background treatment. A third trial (Robson 2009) compared honey with usual care in a population that included approximately 70% people with venous leg ulcers. Although it was not possible to separate out the results for people with venous leg ulcers that study also found no difference in risk of healing between honey and usual care (see section 3.6 below).
Outcome: healing
The Jull 2008 trial appropriately analysed healing as a time to event outcome using a survival approach. There was no difference in healing between groups treated with honey and usual (non‐honey) care (adjusted hazard ratio, HR, 1.1, 95% CI 0.8 to 1.5, P=0.451). There was no difference in the risk of healing at 12 weeks with or without honey (RR 1.12, 95% CI 0.92 to 1.37).
The Gethin 2007 study had change in area of slough at four weeks as the primary outcome and proportion of ulcers healed at 12 weeks as a secondary outcome. In this study 24/54 (44.4%) participants in the honey group had healed at 12 weeks compared with 18/54 (33.3%) in the control group (RR of healing 1.33, 95% CI 0.82 to 2.16).
Although the duration of treatment was dissimilar across the two trials, they were considered sufficiently alike to be able to provide meaningful information when combined (I² 0%). Overall it is not clear whether honey increases the healing of venous leg ulcers compared with no honey (RR 1.15, 95% CI 0.96 to 1.38) (Analysis 7.3) (low quality evidence; downgraded for risk of bias due to unblinded outcome assessment and imprecision) (summary of findings Table 4).
Outcome: adverse events
The Jull 2008 trial (368 participants) reported all adverse events, whether or not the event was believed to be related to the treatment, whereas the Gethin 2007 trial (108 participants) only referred to events that were attributable to the wound agent, of which there were none (and this approach is subject to ascertainment bias in an open label study). In the Jull 2008 trial there were significantly more adverse events (including deterioration of the ulcer) reported in the honey‐treated group than the control group (RR 1.28, 95% CI 1.05 to 1.56). The frequency of the different adverse events is presented in Table 1. It is notable that there were high frequencies of pain (47/187 versus 18/181) and of ulcer deterioration (19/187 versus 9/181) with honey (low quality evidence, downgraded for risk of bias and imprecision, summary of findings Table 4).
| Adverse event | Honey treatment | Control treatment |
| Ulcer pain | 47/187 | 18/181 |
| Bleeding | 3/187 | 3/181 |
| Dermatitis | 8/187 | 8/181 |
| Deterioration of ulcer | 19/187 | 9/181 |
| Erythema | 6/187 | 4/181 |
| Oedema | 4/187 | 1/181 |
| Increased exudate | 5/187 | 1/181 |
| Deterioration of surrounding skin | 5/187 | 3/181 |
| New ulceration | 16/187 | 15/181 |
| Other | 6/187 | 3/181 |
| Cardiovascular | 4/187 | 3/181 |
| Cancer | 2/187 | 2/181 |
| Neurological | 4/187 | 1/181 |
| Gastrointestinal | 4/187 | 2/181 |
| Injury | 10/187 | 9/181 |
| Musculoskeletal | 13/187 | 9/181 |
| Respiratory | 6/187 | 3/181 |
| Other | 3/187 | 8/181 |
Outcome: infection
The Jull 2008 trial reported risk of infection whilst the Gethin 2007 study reported withdrawals due to infection. Infection was operationally defined as clinical signs of infection or a positive swab result, and treatment with antibiotics in Jull 2008.These data were pooled (fixed effect) and it remains unclear if honey reduces leg ulcer infection rates relative to no honey (RR 0.71, 95% CI 0.49 to 1.04) (Analysis 7.4) (low quality evidence, downgraded for risk of bias, summary of findings Table 4).
Outcome: cost
Jull 2008 and colleagues conducted a full cost‐effectiveness analysis using a New Zealand health service perspective. Information was collected on dressings and related products, district nursing time, general practitioner and laboratory time, outpatient consultations, antibiotic use, and hospitalisation. In the base case analysis, the average cost of treatment with honey was NZD 917.00 per participant compared with NZD 972.68 per participant for usual care. This cost was driven by a small difference in hospitalisations that was considered likely to be due to chance variation (three participants in the honey group were hospitalised for ulcer‐related reasons for 10 days, compared to six participants hospitalised for 40 days). A sensitivity analysis excluding the hospitalisations found the average cost of treatment was reversed with usual care being cheaper (NZD 811.12 per participant) than treatment with honey (NZD 877.90 per participant).
Outcome: quality of life
The trial by Jull 2008 reported quality of life. Two generic instruments (SF‐36, EQ5D) and one disease‐specific instrument (Charing Cross Venous Ulcer Questionnaire) were used. There was little difference between the groups, with narrow confidence intervals, for both the physical summary component of SF‐36 (mean difference 1.1, 95% CI ‐0.8 to 3.0; scale 0 to 100, high is better, for a control group mean of 37.9) and the mental component summary score (mean difference 0.7, 95% CI ‐1.1 to 2.4, for a control group mean of 50.4). There was also little difference on EQ‐5D (mean difference 1.6, 95% CI ‐1.5 to 4.7; scale 0 to 100, high is better, for a control group mean of 73.5) or the Charing Cross Venous Ulcer Questionnaire.
3.5 Diabetic foot ulcers
Two trials (93 participants) recruited people with diabetes and foot ulcers of Wagner grade I or II (Kamaratos 2014) or Wagner grade II (Shukrimi 2008) and compared the effects of honey with either saline soaks (Kamaratos 2014) or povidone‐iodine gauze (Shukrimi 2008). In both studies participants also received initial debridement and antibiotics as necessary. Each trial measured and reported healing in a different way which precluded meta‐analysis.
Outcome: healing
There was no difference in healing between honey and saline gauze in the trial by Kamaratos 2014 (31/32 people completed healed by 16 weeks (97%) in the honey‐treated group compared with 28/31 (90%) in the saline‐gauze group). This equate to a RR for healing with honey of 1.07 (95% CI 0.94 to 1.22) (Analysis 7.1).
In Shukrimi 2008 the mean time to surgical closure was 14.4 days in the honey‐treated group and 15.4 days in the povidine‐iodine group, but it was unclear whether all wounds healed. The study did not report standard deviations or the numbers analysed, but stated the difference was not statistically significant.
Overall the evidence suggests there is little difference in the healing of diabetic foot ulcers between honey and saline‐soaked gauze or povidone iodine however this is low quality evidence (downgraded for high risk of bias and imprecision).
Outcome: adverse events
The study by Shukrimi 2008 reported subjective, impressions of pain and exudate only. Kamaratos 2014 did not mention adverse events or side effects.
Outcome: infection
Kamaratos 2014 reported negative wound swabs at 4 weeks: there was no difference between honey and saline dressings (100% of swabs in the honey group were negative at 4 weeks compared with 87% in the saline group; RR 1.07, 95%CI 0.94 to 1.22). Shukrimi 2008 did not report infection.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
3.6 Mixed chronic wounds
One trial (105 participants) recruited participants with different chronic wounds, of whom approximately 70% had venous leg ulcers (Robson 2009). Participants were allocated to receive either manuka honey or a usual care dressing. If slough was present, control participants were to be treated in the first instance with a hydrogel. Compression treatments were given as appropriate.
A second trial (45 participants) also recruited people with a range of chronic wounds, of whom 47% had venous leg ulcers (Gulati 2014). Participants received either sterilized honey followed by a film dressing or povidone iodine covered with a film dressing. People with venous ulcers also received elastic compression.
In Robson 2009 the reported healing rates at 12 weeks were 46.2% for honey compared with 34.0% for usual care (RR 1.36, 95% CI 0.84 to 2.19) and at 24 weeks, 72.7% versus 63.3% (RR 1.14, 95% CI 0.88 to 1.48) (Analysis 7.5). Robson 2009 also reported an unadjusted hazard ratio for healing of 1.30 (95% CI 0.77 to 2.19), with an adjusted analysis (for wound type, age, sex, wound area) of HR 1.51 (95% CI 0.88 to 2.58), Analysis 7.5.
In Gulati 2014 7/23 (30.4%) in the honey group completely healed at 6 weeks, compared with 0/22 in the povidone iodine group. We decided it was inappropriate to pool the healing data from Gulati 2014 and Robson 2009 due to the different patient populations, comparator interventions and durations of follow up.
Overall it is unclear whether honey speeds the healing of a mixed population of chronic wounds relative to usual care or povidone iodine; this evidence is of low quality (downgraded for risk of bias and imprecision).
Outcome: adverse events
In the Robson 2009 study there were 7 adverse events in the honey group (1 death, 1 pain, 2 cases of ulcer deterioration and 3 people discontinuing treatment due to other concomitant treatment) compared with 5 in the usual care group (1 death, 1 deterioration of the ulcer, 3 discontinuation due to other treatment). In Gulati 2014 there were no reported adverse events.
Outcome: infection
Not reported in either study.
Outcomes: costs
Not reported.
Outcomes: quality of life
Not reported.
Discussion
This is a complex review addressing a diverse range of wound types and with many trials at high or unclear risk of bias. Generally, the evidence, when assessing using GRADE, is of low or very low quality. This means that new, better quality research is highly likely to change the overall conclusions of this review. The findings are discussed below with respect to specific wound types and using a GRADE approach to addressing the quality of the evidence.
Summary of main results
1. Acute wounds
1.1 Minor acute wounds
It is unclear, on the basis of the very low quality evidence from three small trials (two in toenail bed avulsion and one in minor traumatic wounds) whether honey affects time to wound healing in minor acute wounds compared with conventional dressings. It is also unclear if honey and conventional dressings have different effects on adverse events or rates of infection in people with minor acute wounds (summary of findings Table for the main comparison).
1.2 Burns
The 11 trials of honey for burns used a wide range of comparator treatments, viz. various conventional dressings (Subrahmanyam 1993a; Subrahmanyam 1996a), early burn excision and grafting (Subrahmanyam 1999), silver sulfadiazine (SSD) (Baghel 2009; Mashood 2006; Memon 2005; Subrahmanyam 1991; Subrahmanyam 1998; Subrahmanyam 2001a), amniotic membrane (Subrahmanyam 1994) and potato peelings (Subrahmanyam 1996b).
The evidence for the effects of honey relative to these comparators is mixed, and is generally of low or very low quality. The strongest (high quality) evidence suggests that honey dressings heal partial thickness burns, on average, between 4 and 5 days more quickly than conventional dressings, however it is not clear if there is a difference in the rates of adverse events or infection (summary of findings Table 2).
One trial (low quality evidence) suggests that early excision and grafting heals burns on average 13.6 days more quickly (95% CI 9.82 days to 17.3 days) than honey followed by grafting as necessary, however the relative effects on adverse events and infection rates are unclear (Subrahmanyam 1999).
There is very low quality evidence that honey reduces the time for burns to heal by between 0.73 and 9.51 days (average difference was 5.12 days) compared with SSD dressings/cream and high quality evidence of a reduction in adverse events with honey and more negative wound swabs at day 7. There was no difference for the outcome of risk of complete healing by 4 to 6 weeks (high quality evidence) (summary of findings Table 3).
Perhaps unsurprisingly, the current evidence suggests that burns heal more quickly with honey than with amniotic membranes (by approximately 8 days) (Subrahmanyam 1994) and approximately 6 days more quickly than with potato peelings (Subrahmanyam 1996b) (single small studies, moderate quality evidence). The relative effects of these interventions on adverse events and infection are unclear.
2. Mixed acute and chronic wounds
The rationale for conducting trials in which the participants have either burns or a mix of chronic wounds is unclear. The aetiologies are so different that no matter what the results, the findings will be difficult to interpret and unlikely to influence clinical practice. Overall we found low quality evidence from two studies Mphande 2007; Subrahmanyam 1993b), that on average honey heals a heterogeneous population of acute and chronic wounds more quickly than sugar dressings or SSD. The comparative rates of adverse events and infection are unclear.
3. Chronic wounds
Most of the evidence for the effects of honey on chronic wounds is of low or very low quality. Most trials were small, with comparators that may not be relevant to current practice and at high or unclear risk of bias.
3.1 Infected post‐operative wounds
There is moderate quality evidence that honey increases the absolute risk of healing by 35% (95%CI 8.7% to 55.4%) relative to antiseptic (povidone iodine) washes followed by gauze. The single trial (Al Waili 1999) was small (50 participants) and the report lacked sufficient detail to permit the risk of bias to be determined accurately for most domains. The comparator was an antiseptic which has been proposed to impair wound healing although clinical evidence for such an effect is lacking (Leaper 1986). In the same study there were fewer adverse events and a shorter time to a negative wounds swab with honey.
3.2 Pressure ulcers
There is very low quality evidence that honey increases the relative risk of healing of pressure ulcers by 41% (95% CI 5% to 90%) relative to saline soaked gauze (Weheida 1991). This equates to an increase in the absolute risk of healing with honey of 30% (95%CI 7.7% to 51.9%). The quality of the evidence for the effect on healing from this trial was downgraded to very low quality for imprecision and possible selection bias. There were no data on adverse events or infection.
3.3 Fournier's gangrene
There is very low quality evidence that honey healed Fournier’s gangrene, on average, 8 days more quickly than Eusol soaks (1 trial, WMD ‐8.00 days, 95% CI ‐6.08 to ‐9.92 days). In this small study (30 participants) Subrahmanyam 2004), 64% (honey group) and 69% (EUSOL group) of participants also required secondary suturing and grafting. In addition, the comparator was EUSOL, an antiseptic that has been shown to impair wound healing in animal model studies (Brennan 1985).
3.4 Cutaneous Leishmaniasis
It is not clear whether honey influences the healing of lesions caused by Leishmaniasis when used as an adjuvant to meglumine antimoniate. The low quality evidence from a single small trial suggests that honey may impair healing compared with meglumine antimoniate alone (RR 0.72; 95% CI 0.50 to 1.04) (Nilforoushzadeh 2007) however the quality of this evidence for an effect on healing was downgraded for imprecision and high risk of bias. In terms of adverse events, there was one reported withdrawal from the honey group for sensitivity and none in the control group. Infection rates were not reported.
3.5 Venous leg ulcers
There is currently no clear evidence that honey improves the rate of healing of venous ulcers. Such evidence that there is, from two trials (Gethin 2007; Jull 2008) is low quality and finds no overall difference in healing (RR 1.15, 95% CI 0.96 to 1.38) (summary of findings Table 4). The larger of the two studies (Jull 2008) analysed healing (appropriately) as a time‐to‐event outcome which is more informative (and uses more of the information on healing) than crudely analysing the proportions of participants healed at a particular (arbitrary) time point. This study found no difference in the hazard of healing between honey and usual care (hazard ratio 1.1, 95%CI 0.8 to 1.5). These trials reported adverse events differently with Gethin 2007 only reporting those they "attributed" to the wound treatment (there were none). This approach has an inherently high risk of ascertainment bias in an open label study. By contrast Jull 2008 documented and reported all adverse events and there were more in the honey group than the control group, with pain and ulcer deterioration being frequent (absolute increase in risk of adverse events with honey was 12.9%, 95%CI 2.7% to 22.8%).
3.6 Diabetic foot ulcers
The effect of honey on diabetic foot ulcers cannot be determined. The two included studies (Kamaratos 2014; Shukrimi 2008) were small and the evidence for honey in this patient group was low quality.
Overall completeness and applicability of evidence
There are significant weaknesses in the completeness and applicability of the evidence overall. Most of the studies in burns have been conducted by one team in India (10 out of 26 included studies; Subrahmanyam 1991; Subrahmanyam 1993a; Subrahmanyam 1993b; Subrahmanyam 1994; Subrahmanyam 1996a; Subrahmanyam 1996b; Subrahmanyam 1998; Subrahmanyam 1999; Subrahmanyam 2001a; Subrahmanyam 2004) and we relied on further information supplied by the authors to supplement an absence of detail in the original published trial reports. This review is therefore disproportionately reliant on evidence from one single research team from one part of the world and this evidence may not be applicable elsewhere (particularly since the prevailing microbiological environment, health care facilities and climate are likely to have strong effects on burn outcomes and infection rates).
Only one study (Jull 2008) reported costs and quality of life. Clearly any impact of treatments on patients' quality of life is invaluable information for all decision makers but particularly patients. The relative cost‐effectiveness of competing treatments is essential information for health care funders and providers. We therefore urge that future research in this field uses contemporary methodologies to measure these outcomes.
There is relatively little replication of studies, with single, small studies for most comparisons. This weak evidence base makes it impossible to draw firm conclusions with confidence.
Some of the comparators chosen have little or no relevance to current clinical decision making (e.g., amniotic membrane, potato peelings). Other comparators will have relevance in some parts of the world but not others. For example antiseptics such as EUSOL and povidone iodine are used less frequently in open wounds than they used to be, in the developed world at least, because in vitro studies were interpreted as evidence that they may impair wound healing (Leaper 1986). Silver sulfadiazine, which is commonly used in burns and was the comparator in several of the studies included here, has been shown in a related review (Wasiak 2013) to impair the healing of burns relative to several comparator dressings.
Quality of the evidence
In common with most wounds‐related topic areas, the quality of the evidence in this review was generally low or very low ‐ mainly due to imprecision, risk of bias and inconsistency. Imprecision of effect estimates was usually low because there are only one or two small studies for each comparison. Risk of bias was generally high due to unblinded outcome assessment. Most studies did not guard against performance bias (by blinding participants and health care professionals) however the importance of performance bias in wound care studies is not clear. Blinded outcome assessment is however a substantial threat to validity (Hróbjartsson 2012). We downgraded the quality of the evidence for inconsistency where there was obvious clinical or statistical heterogeneity. Poor reporting is a major issue and the majority of studies were unclear for one or more important bias domains in the risk of bias assessment. We carefully considered downgrading the evidence for risk of bias where trial reports were unclear (most studies, see Figure 1). We decided against downgrading for unclear risk of bias (because it does not appear to be the norm) however this means that, if anything, the evidence is lower quality than we have rated it. The importance of following international standards for trial reporting (i.e., CONSORT, Schulz 2010), which most of the studies in this review did not adhere to, cannot be over‐emphasised .
The reporting of adverse events was poor in most trials, and non‐existent in a few trials. This makes accurate assessment of the risk of adverse events associated with honey dressings compared with comparators, difficult. The International Conference on Harmonization's Guideline for Good Clinical Practice (ICH GCP) defines an adverse event as any untoward medical occurrence in a trial subject who has been administered an intervention, whether related to the intervention or not. With the exception of Jull 2008, it was not clear if trials reported all adverse events as required by ICH GCP. Therefore the adverse event findings should be interpreted very cautiously, as the full adverse event profile of honey vs. comparators in different wounds is unknown.
The evidence regarding the effect of honey on wound infection rates was poor quality and difficult to interpret. The accurate identification of wound infection is problematic and since wound infection is dependent to some extent on the host response to the micro‐organism (e.g., manifesting as inflammation and pain), and the assessment of this is partly subjective, there is no "gold standard" diagnostic tool . Clinical presentation is an important indicator, but presentation may vary with wound type (Cutting 2005), therefore, trialists should strive for unambiguous definitions of infection to ensure that study results are interpretable and their meta‐analyses both feasible and sensible.
Infection is a significant and threatening consequence of burns. Wound sterility after a burn is maintained by careful attention to asepsis during wound care and the use of preventive agents. Honey dressings appear to increase the probability that a cultured swab from a burn will remain negative compared with a range of control treatments but the clinical importance of negative swabs at day 7 is unclear. Future trialists should identify and use reliable and valid measures of infection that can be applied in a range of settings.
Only one study conducted a full cost‐effectiveness analysis using a health services perspective (Jull 2008). As the effectiveness of honey was not established by the trial, honey cannot be considered the dominant strategy. In the same vein, this was also the only study that reported health related quality of life.
Potential biases in the review process
This review has a number of limitations, driven largely by the nature of the included studies. Firstly one of the included studies was led by one of the authors of this review (Jull 2008). This situation is not uncommon in Cochrane reviews and there are robust policies in place to reduce or eliminate any bias this may bring. Specifically data extraction, risk of bias assessment and analysis were checked by three further authors or editors (MW, NC, JD).
Secondly, several studies report and analyse mean time to healing as the main effect measure. Time to healing should be treated as a type of time‐to‐event outcome rather than a continuous measure, as this enables all participants to contribute data to the analysis irrespective of whether they experienced the outcome or remained in the study (only people who healed can contribute data to an analysis of mean time to healing as a continuous outcome). However, we were limited to using a common means of measurement wherever possible. Third, we attempted to contact authors where the original publication did not provide sufficient data, and then incorporate that data into the review. Where authors did not respond, we excluded the studies that did not report sufficient data even to be included in a narrative analysis. Finally it was not possible to evaluate the overall possibility of publication bias, as not all trials reported the same outcomes and overall the trials were too heterogeneous to combine.
Agreements and disagreements with other studies or reviews
Two relevant systematic reviews have been published since the last update of this review. In 2013 Vandamme 2013 concluded that honey stimulates wound healing in human burns, ulcers and other wounds, and that it debrides, removes odour, has anti‐inflammatory and pain reducing properties. There are key methodological differences between our review and the review by Vandamme 2013. They did not restrict inclusion to study designs at lowest risk of bias (but included published controlled trials, clinical trials and case reports as well as RCTs). Secondly there appears to have been no systematic assessment of study quality in the review by Vandamme 2013, but rather allusion to strengths and weaknesses of the included studies in an ad hoc way. Thirdly GRADE was not used to assess the quality of the evidence in the Vandamme 2013 review and conclusions were reached by vote counting rather than meta‐analysis. There is a great deal of overlap of study inclusion however (though we had excluded several studies included in their review e.g., Malik 2010, Yapucu Gunes 2007, Oluwatosin 2000, Lund‐Nielsen 2011, Misirligou 2003). Elsewhere the explanation for excluding studies from our review that were included in Vandamme 2013 was the different eligibility criteria for study population (they included studies of oral mucositis and radiation damage to skin). Notwithstanding these differences there was some agreement in the conclusions reached, viz. their conclusion that "Many of the included studies have methodological problems, and the quality of certain studies is low, making it difficult to formulate conclusive guidelines" (Vandamme 2013). Another systematic review was also published in 2013 (Rűttermann 2013). This review was prepared to support new German wound care guidelines and used the GRADE approach in summarising the quality of the evidence. Again the patient population differed slightly from ours in that they excluded studies in people with burns and other acute wounds. The review by Rűttermann 2013 concluded that honey does not accelerate wound healing and increases pain (a conclusion based solely on the studies of Gethin 2007 and Jull 2008 included here). There is much agreement therefore between our conclusions represented in this review update and those of other recent systematic reviews.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 1 Time to healing.

Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 2 Adverse events.

Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 3 Infection.

Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 1 Time to healing (days).
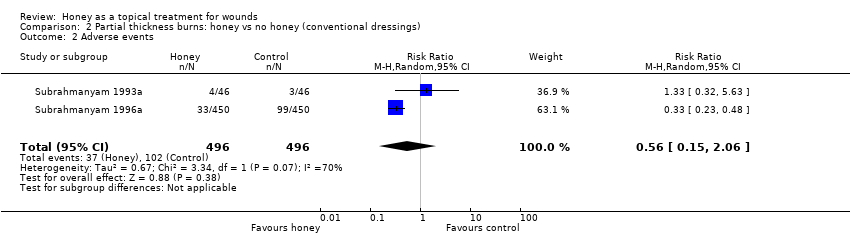
Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 2 Adverse events.
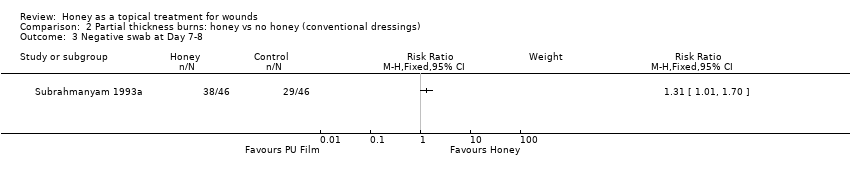
Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 3 Negative swab at Day 7‐8.

Comparison 3 Burns: honey with delayed graft PRN vs early excision & grafting (no honey), Outcome 1 Time to healing (days).

Comparison 3 Burns: honey with delayed graft PRN vs early excision & grafting (no honey), Outcome 2 Mean duration of antibiotic therapy (days).
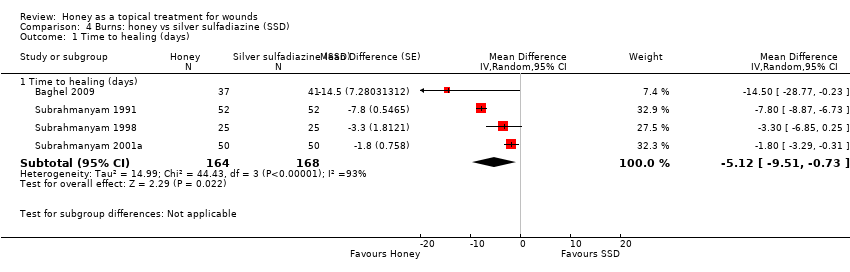
Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 1 Time to healing (days).
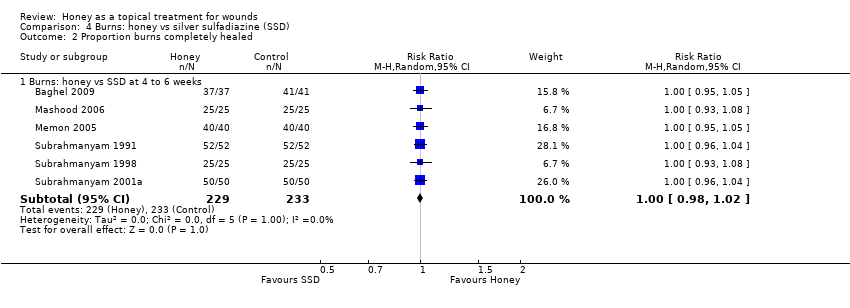
Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 2 Proportion burns completely healed.

Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 3 Adverse events.

Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 4 Negative swab at Day 7.
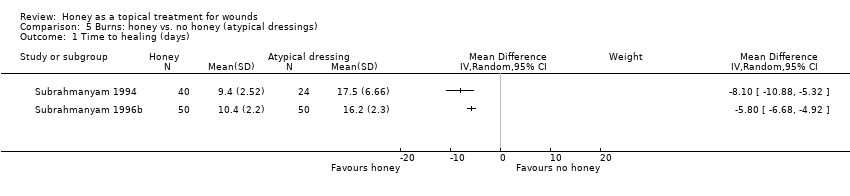
Comparison 5 Burns: honey vs. no honey (atypical dressings), Outcome 1 Time to healing (days).

Comparison 6 Mixed acute and chronic wounds, Outcome 1 Time to healing (days).

Comparison 6 Mixed acute and chronic wounds, Outcome 2 Negative swab at Day 7.

Comparison 7 Chronic wounds, Outcome 1 Proportion healed.

Comparison 7 Chronic wounds, Outcome 2 Time to healing (days).

Comparison 7 Chronic wounds, Outcome 3 Venous ulcers: proportion healed at 12 weeks.
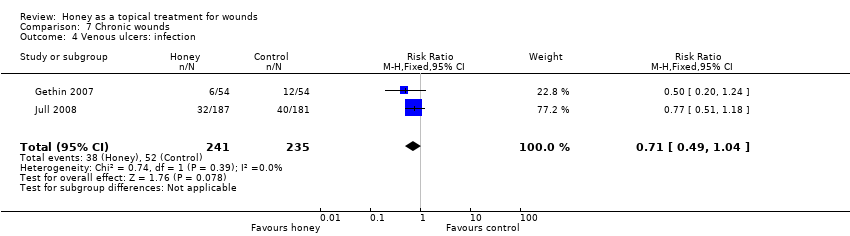
Comparison 7 Chronic wounds, Outcome 4 Venous ulcers: infection.

Comparison 7 Chronic wounds, Outcome 5 Mixed wounds: proportion healed.
| Honey compared with conventional dressings for minor acute wounds | ||||||
| Patient or population: patients with Minor acute wounds | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional dressings | Honey | |||||
| Complete healing (time to healing)(days) | The mean complete healing (time to healing) in the intervention groups was | 213 | ⊕⊝⊝⊝ | |||
| Adverse events | Study population | RR 1.19 | 82 | ⊕⊝⊝⊝ | ||
| 357 per 1000 | 425 per 1000 | |||||
| Infection | Study population | RR 0.91 | 151 | ⊕⊝⊝⊝ | ||
| 14 per 1000 | 13 per 1000 | |||||
| Costs | The mean cost of dressing materials per patient was 0.49 ZAR in the honey group and 12.06 ZAR in the control (hydrogel) group | 82 | ⊕⊝⊝⊝ | |||
| Quality of Life6 | Not reported | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias (one level): High risk of attrition bias in all three included studies | ||||||
| Honey compared with conventional dressings for burns | ||||||
| Patient or population: patients with Burns | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional dressings | Honey | |||||
| Complete healing (time to healing)(days) | The mean complete healing (time to healing) in the intervention groups was | 992 | ⊕⊕⊕⊕ | |||
| Adverse events | Study population | RR 0.56 | 992 | ⊕⊝⊝⊝ | ||
| 206 per 1000 | 115 per 1000 | |||||
| Negative wound swab | Study population | RR 1.31 | 92 | ⊕⊝⊝⊝ | ||
| 630 per 1000 | 826 per 1000 | |||||
| Costs5 | Not reported | Not estimable5 | N/A | N/A | ||
| Quality of life5 | Not reported | Not estimable5 | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to inconsistency (one level): High heterogeneity was detected with an I‐squared of 70% | ||||||
| Honey compared with silver sulfadiazine for burns | ||||||
| Patient or population: patients with Burns | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silver sulfadiazine | Honey | |||||
| Complete healing | Study population | RR 1.00 | 462 | ⊕⊕⊕⊕ | ||
| 1000 per 1000 | 1000 per 1000 | |||||
| Mean time to complete healing (days) | The mean time to complete healing in the intervention groups was | 332 | ⊕⊝⊝⊝ | |||
| Adverse events | Study population | RR 0.29 | 412 | ⊕⊕⊕⊕ | ||
| 413 per 1000 | 120 per 1000 | |||||
| Negative wound swab | Study population | RR 3.92 | 412 | ⊕⊝⊝⊝ | ||
| 236 per 1000 | 923 per 1000 | |||||
| Costs | The cost of dressing treatment per % TBSA affected was 0.75 PKR for honey and 10 PKR for silver sulfadiazine. | 50 | ⊕⊕⊝⊝ | |||
| Quality of Life6 | Not reported | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to inconsistency (two levels): Very high level of statistical heterogeneity (I squared of 93%) | ||||||
| Honey for venous leg ulcers | ||||||
| Patient or population: patients with Venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Honey | |||||
| Complete healing (time to healing) | Study population | HR 1.1 | 368 | ⊕⊕⊝⊝ | ||
| 497 per 1000 | 531 per 1000 | |||||
| Complete healing (proportion wounds healed) | Study population | RR 1.15 | 476 | ⊕⊕⊝⊝ | ||
| 460 per 1000 | 529 per 1000 | |||||
| Adverse events | Study population | RR 1.28 | 368 | ⊕⊕⊝⊝ | ||
| 464 per 1000 | 594 per 1000 | |||||
| Infection | Study population | RR 0.71 | 476 | ⊕⊕⊝⊝ | ||
| 221 per 1000 | 157 per 1000 | |||||
| Costs | The mean cost in the intervention group was | 368 | ⊕⊝⊝⊝ | The ICER was sensitive to the inclusion of hospitalisation costs. Hospitalisation unlikely related to treatment and when these were excluded the ICER was in favour of control. | ||
| Quality of Life | The mean PCS in the intervention group was | 368 | ⊕⊕⊕⊝ | |||
| Quality of Life | The mean MCS in the intervention groups was | 368 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias (one level): Unblinded outcome assessment | ||||||
| Adverse event | Honey treatment | Control treatment |
| Ulcer pain | 47/187 | 18/181 |
| Bleeding | 3/187 | 3/181 |
| Dermatitis | 8/187 | 8/181 |
| Deterioration of ulcer | 19/187 | 9/181 |
| Erythema | 6/187 | 4/181 |
| Oedema | 4/187 | 1/181 |
| Increased exudate | 5/187 | 1/181 |
| Deterioration of surrounding skin | 5/187 | 3/181 |
| New ulceration | 16/187 | 15/181 |
| Other | 6/187 | 3/181 |
| Cardiovascular | 4/187 | 3/181 |
| Cancer | 2/187 | 2/181 |
| Neurological | 4/187 | 1/181 |
| Gastrointestinal | 4/187 | 2/181 |
| Injury | 10/187 | 9/181 |
| Musculoskeletal | 13/187 | 9/181 |
| Respiratory | 6/187 | 3/181 |
| Other | 3/187 | 8/181 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing Show forest plot | 3 | 213 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐3.09, 7.61] |
| 2 Adverse events Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.69, 2.05] |
| 3 Infection Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 2 | 992 | Mean Difference (IV, Fixed, 95% CI) | ‐4.68 [‐5.09, ‐4.28] |
| 2 Adverse events Show forest plot | 2 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.06] |
| 3 Negative swab at Day 7‐8 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 13.60 [9.82, 17.38] |
| 2 Mean duration of antibiotic therapy (days) Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [8.85, 23.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Time to healing (days) | 4 | 332 | Mean Difference (Random, 95% CI) | ‐5.12 [‐9.51, ‐0.73] |
| 2 Proportion burns completely healed Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Burns: honey vs SSD at 4 to 6 weeks | 6 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.98, 1.02] |
| 3 Adverse events Show forest plot | 5 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.20, 0.42] |
| 4 Negative swab at Day 7 Show forest plot | 5 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 3.92 [1.32, 11.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Negative swab at Day 7 Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion healed Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Infected post‐op wounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pressure ulcers (grade I and II) at 10 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Leishmaniasis at 4 months (16 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Venous leg ulcers at 12 weeks | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Diabetic foot ulcers | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Time to healing (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fournier's gangrene | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Venous ulcers: proportion healed at 12 weeks Show forest plot | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 4 Venous ulcers: infection Show forest plot | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.49, 1.04] |
| 5 Mixed wounds: proportion healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Mixed wounds healing 2' intention at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mixed wounds healing 2' intention at 24 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

