Miel como tratamiento tópico para heridas
Information
- DOI:
- https://doi.org/10.1002/14651858.CD005083.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 06 March 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Wounds Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Andrew Jull: designed and co‐ordinated the review. Extracted data (first review), reviewed risk of bias assessments and data extraction (this update). Analysed or interpreted data and performed statistical analysis, wrote to study author/experts/companies, completed or reviewed the drafts, revisions, and the final review (first review and this update). He is guarantor of the review.
Nicky Cullum: checked others' data extraction and extracted data (this update); checked others' risk of bias assessment and conducted risk of bias assessments (this update). Analysed and interpreted data; constructed summary of findings tables (this update); completed drafts and revisions of the review and approved the final version of this update prior to publication.
Jo Dumville: checked others' data extraction and extracted data (this update); checked others' risk of bias assessment and conducted risk of bias assessments (this update). Analysed and interpreted data; constructed summary of findings tables (this update); completed drafts and revisions of the review and approved the final version of this update prior to publication.
Maggie Westby: checked others' data extraction and extracted data (this update); checked others' risk of bias assessment and conducted risk of bias assessments (this update). Analysed and interpreted data; constructed summary of findings tables (this update); completed drafts and revisions of the review and approved the final version of this update prior to publication.
Natalie Walker: designed the review and checked studies to be included, checked risk of bias assessment and the quality of statistical analysis (first review), performed part of writing or editing of the review (first review and first update). Approved final review prior to submission (first review and all updates).
Sohan Deshpande: checked studies to be included, extracted data, performed risk of bias assessments and contributed to writing (first update).
Contributions of editorial base:
Nicky Cullum: for the original review and first update ‐ edited the review, advised on methodology, interpretation and review content; approved the final review prior to submission.
Liz McInnes, Editor: approved the first review update prior to submission.
Sally Bell‐Syer: co‐ordinated the editorial process; advised on methodology, interpretation and content; edited the review and the updated versions of the review.
Ruth Foxlee: designed the search strategy and edited the search methods section.
David Tovey (Editor in Chief) approved the final version of this updated review (second update).
Toby Lasserson (Senior Editor) of the Cochrane Editorial Unit advised on the Summary of Findings Tables and approved the final version of this updated review (second update).
Sources of support
Internal sources
-
Senior Health Research Scholarship, University of Auckland, New Zealand.
-
School of Nursing, Midwifery and Social Work, University of Manchester, UK.
External sources
-
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane Wounds. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
-
The Douglas Senior Fellowship in Heart Health (Prevention), New Zealand.
Declarations of interest
Andrew Jull, Natalie Walker and Anthony Rodgers were investigators in the Honey as Adjuvant Leg ulcer Treatment (HALT) trial (ISRCTN 06161544), one of the trials included in this review. The Clinical Trials Research Unit, which employed Andrew Jull, Natalie Walker and Antony Rodgers received a small, unconditional cash contribution from a manufacturer of honey dressings for the conduct of the HALT trial.
Dr Walker is supported by a Heart Foundation Douglas Senior Fellowship in Heart Health (Prevention). She has provided consultancy to the manufacturers of smoking cessation medications, received honoraria for speaking at a research meeting and received benefits in kind and travel support from a pharmaceutical company that makes smoking cessation medications. She has also received product in kind from a pharmaceutical company that makes smoking cessation medications, for use in an investigator initiated phase III clinical trial.  She has been contracted by two companies to undertake clinical trials for them ‐ one company wanted her to evaluate a treatment for leg ulcers (but this was not honey) and the second was an asthma trial for a New Zealand Crown entity that decides, on behalf of District Health Boards, which medicines and pharmaceutical products are subsidised for use in the community and public hospitals.Â
Acknowledgements
The review authors would like to thank the following referees for their comments on the review, Wounds Group Editors: Mieke Flour, Andrea Nelson and Gill Worthy, referees: Margaret Harrison, Lois Orton, Consumer referee: Durhane Wong‐Rieger. Anthony Rodgers contributed to the original review but was not involved in the updating of the review, we would like to acknowledge his contribution. Elizabeth Royle copy edited the updated review.
The review authors would also like to thank David Tovey and Toby Lasserson of the Cochrane Editorial Unit for their advice and review of this updated review.
Dr Walker is supported by a Heart Foundation Douglas Senior Fellowship in Heart Health (Prevention).
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Mar 06 | Honey as a topical treatment for wounds | Review | Andrew B Jull, Nicky Cullum, Jo C Dumville, Maggie J Westby, Sohan Deshpande, Natalie Walker | |
| 2013 Feb 28 | Honey as a topical treatment for wounds | Review | Andrew B Jull, Natalie Walker, Sohan Deshpande | |
| 2008 Oct 08 | Honey as a topical treatment for wounds | Review | Andrew B Jull, Anthony Rodgers, Natalie Walker | |
| 2004 Apr 19 | Honey as a topical treatment for wounds | Protocol | Andrew B Jull, Anthony Rodgers, Natalie Walker | |
Differences between protocol and review
In the first version of the review, two trials that compared active interventions allocated wounds to the interventions rather than participants (Oluwatosin 2000; Okeniyi 2005). The participants had multiple wounds in many cases, and some participants would have received both interventions. In this update, a trial that required participants to have two burns (Malik 2010), and a trial that may have randomised participants but appears to have reported healing by pressure injury rather than by participant (Yapucu Gunes 2007), were excluded. The data in these trials were presented by wound and thus could not be combined (if possible) with trials where data were presented by participant in both the first version of the review and this update. Such methods were not foreseen in the protocol, where it was assumed that data would be presented by participant. Randomising by wound breaches the assumption of independence that underpins inferential testing, increases the weight of a study inappropriately if included in a meta‐analysis (by doubling the denominator) and thereby artificially improves the precision of the confidence interval for the summary statistic. Additionally, a trial requiring participants to have two wounds that randomises one wound to each treatment is not clinically generalisable as it has reduced between‐patient variability. Between‐patient variability in pragmatic trials drives external validity. The inclusion criteria have been adjusted in this update to reflect this change.
Notes
The authors have carefully considered and incorporated the observations and items of feedback submitted through the “Submit Comments” facility on the Cochrane Library for this review. These comments and the replies from the authors have been retained in this version of the review. This is to enable readers to follow the exchange and to form their own interpretation of the evidence that is now available.
During the updating of this review the review authors became aware that the publication Gethin G, Cowman S. Manuka honey vs. hydrogel ‐ a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Journal of Clinical Nursing 2009;18(3):466‐74 (http://onlinelibrary.wiley.com/doi/10.1111/jocn.12652/abstract) has been retracted by agreement between the journal Editor‐in‐Chief, the authors and John Wiley & Sons, Ltd. The retraction has been agreed due to errors in the data analysis which affect the article's findings. The review authors would like to confirm that the data in this updated review is taken from the source: Gethin G. Manuka honey versus hydrogel ‐ a prospective, open label, multicentre, randomised controlled trial to compare desloughing efficacy and healing outcomes in venous ulcers. Unpublished PhD thesis 2007.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Humans;
PICOs

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
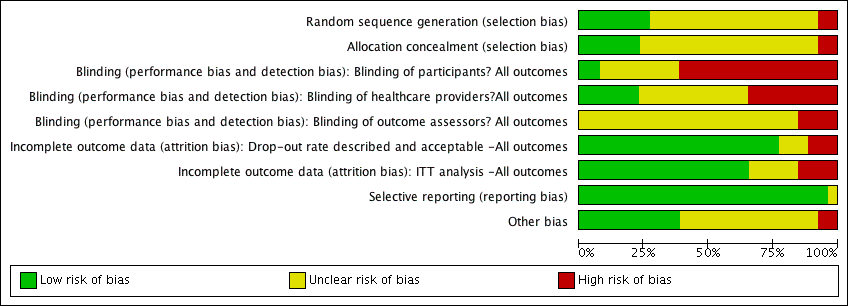
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 1 Time to healing.

Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 2 Adverse events.

Comparison 1 Minor acute wounds: honey vs. no honey (conventional dressings), Outcome 3 Infection.

Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 1 Time to healing (days).
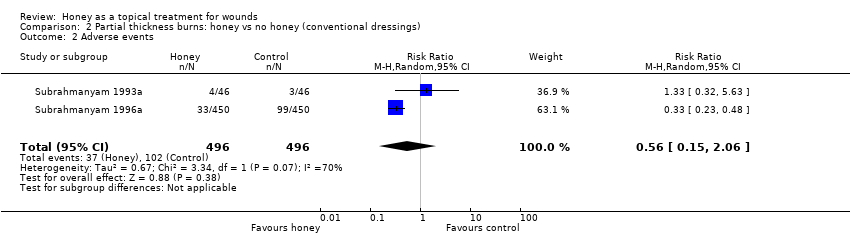
Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 2 Adverse events.
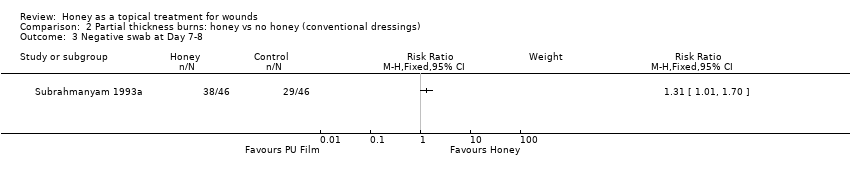
Comparison 2 Partial thickness burns: honey vs no honey (conventional dressings), Outcome 3 Negative swab at Day 7‐8.

Comparison 3 Burns: honey with delayed graft PRN vs early excision & grafting (no honey), Outcome 1 Time to healing (days).

Comparison 3 Burns: honey with delayed graft PRN vs early excision & grafting (no honey), Outcome 2 Mean duration of antibiotic therapy (days).
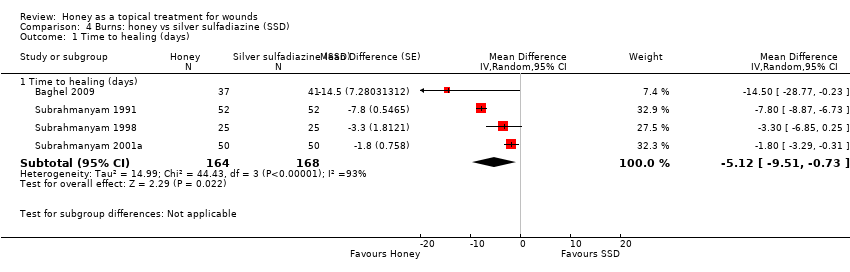
Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 1 Time to healing (days).
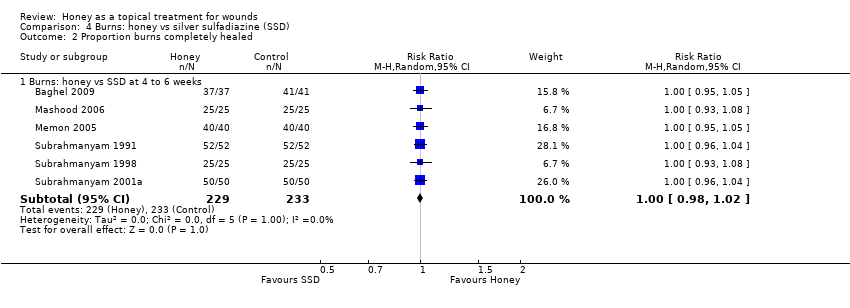
Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 2 Proportion burns completely healed.

Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 3 Adverse events.

Comparison 4 Burns: honey vs silver sulfadiazine (SSD), Outcome 4 Negative swab at Day 7.
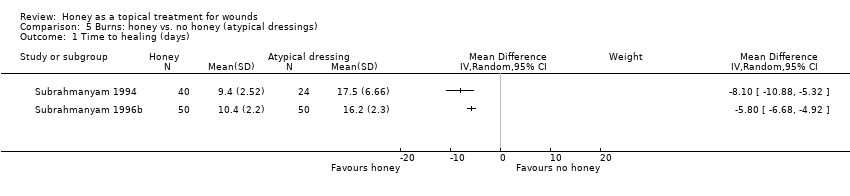
Comparison 5 Burns: honey vs. no honey (atypical dressings), Outcome 1 Time to healing (days).

Comparison 6 Mixed acute and chronic wounds, Outcome 1 Time to healing (days).

Comparison 6 Mixed acute and chronic wounds, Outcome 2 Negative swab at Day 7.

Comparison 7 Chronic wounds, Outcome 1 Proportion healed.

Comparison 7 Chronic wounds, Outcome 2 Time to healing (days).

Comparison 7 Chronic wounds, Outcome 3 Venous ulcers: proportion healed at 12 weeks.
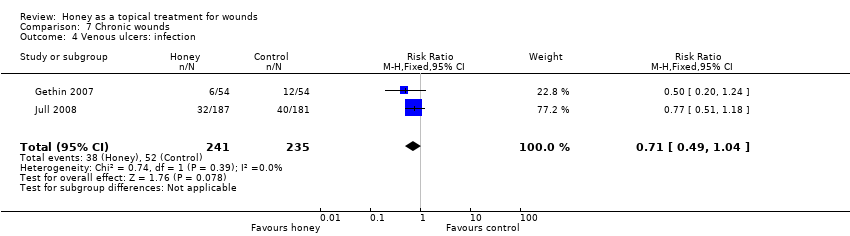
Comparison 7 Chronic wounds, Outcome 4 Venous ulcers: infection.

Comparison 7 Chronic wounds, Outcome 5 Mixed wounds: proportion healed.
| Honey compared with conventional dressings for minor acute wounds | ||||||
| Patient or population: patients with Minor acute wounds | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional dressings | Honey | |||||
| Complete healing (time to healing)(days) | The mean complete healing (time to healing) in the intervention groups was | 213 | ⊕⊝⊝⊝ | |||
| Adverse events | Study population | RR 1.19 | 82 | ⊕⊝⊝⊝ | ||
| 357 per 1000 | 425 per 1000 | |||||
| Infection | Study population | RR 0.91 | 151 | ⊕⊝⊝⊝ | ||
| 14 per 1000 | 13 per 1000 | |||||
| Costs | The mean cost of dressing materials per patient was 0.49 ZAR in the honey group and 12.06 ZAR in the control (hydrogel) group | 82 | ⊕⊝⊝⊝ | |||
| Quality of Life6 | Not reported | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias (one level): High risk of attrition bias in all three included studies | ||||||
| Honey compared with conventional dressings for burns | ||||||
| Patient or population: patients with Burns | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional dressings | Honey | |||||
| Complete healing (time to healing)(days) | The mean complete healing (time to healing) in the intervention groups was | 992 | ⊕⊕⊕⊕ | |||
| Adverse events | Study population | RR 0.56 | 992 | ⊕⊝⊝⊝ | ||
| 206 per 1000 | 115 per 1000 | |||||
| Negative wound swab | Study population | RR 1.31 | 92 | ⊕⊝⊝⊝ | ||
| 630 per 1000 | 826 per 1000 | |||||
| Costs5 | Not reported | Not estimable5 | N/A | N/A | ||
| Quality of life5 | Not reported | Not estimable5 | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to inconsistency (one level): High heterogeneity was detected with an I‐squared of 70% | ||||||
| Honey compared with silver sulfadiazine for burns | ||||||
| Patient or population: patients with Burns | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Silver sulfadiazine | Honey | |||||
| Complete healing | Study population | RR 1.00 | 462 | ⊕⊕⊕⊕ | ||
| 1000 per 1000 | 1000 per 1000 | |||||
| Mean time to complete healing (days) | The mean time to complete healing in the intervention groups was | 332 | ⊕⊝⊝⊝ | |||
| Adverse events | Study population | RR 0.29 | 412 | ⊕⊕⊕⊕ | ||
| 413 per 1000 | 120 per 1000 | |||||
| Negative wound swab | Study population | RR 3.92 | 412 | ⊕⊝⊝⊝ | ||
| 236 per 1000 | 923 per 1000 | |||||
| Costs | The cost of dressing treatment per % TBSA affected was 0.75 PKR for honey and 10 PKR for silver sulfadiazine. | 50 | ⊕⊕⊝⊝ | |||
| Quality of Life6 | Not reported | N/A | N/A | N/A | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to inconsistency (two levels): Very high level of statistical heterogeneity (I squared of 93%) | ||||||
| Honey for venous leg ulcers | ||||||
| Patient or population: patients with Venous leg ulcers | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Honey | |||||
| Complete healing (time to healing) | Study population | HR 1.1 | 368 | ⊕⊕⊝⊝ | ||
| 497 per 1000 | 531 per 1000 | |||||
| Complete healing (proportion wounds healed) | Study population | RR 1.15 | 476 | ⊕⊕⊝⊝ | ||
| 460 per 1000 | 529 per 1000 | |||||
| Adverse events | Study population | RR 1.28 | 368 | ⊕⊕⊝⊝ | ||
| 464 per 1000 | 594 per 1000 | |||||
| Infection | Study population | RR 0.71 | 476 | ⊕⊕⊝⊝ | ||
| 221 per 1000 | 157 per 1000 | |||||
| Costs | The mean cost in the intervention group was | 368 | ⊕⊝⊝⊝ | The ICER was sensitive to the inclusion of hospitalisation costs. Hospitalisation unlikely related to treatment and when these were excluded the ICER was in favour of control. | ||
| Quality of Life | The mean PCS in the intervention group was | 368 | ⊕⊕⊕⊝ | |||
| Quality of Life | The mean MCS in the intervention groups was | 368 | ⊕⊕⊕⊝ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias (one level): Unblinded outcome assessment | ||||||
| Adverse event | Honey treatment | Control treatment |
| Ulcer pain | 47/187 | 18/181 |
| Bleeding | 3/187 | 3/181 |
| Dermatitis | 8/187 | 8/181 |
| Deterioration of ulcer | 19/187 | 9/181 |
| Erythema | 6/187 | 4/181 |
| Oedema | 4/187 | 1/181 |
| Increased exudate | 5/187 | 1/181 |
| Deterioration of surrounding skin | 5/187 | 3/181 |
| New ulceration | 16/187 | 15/181 |
| Other | 6/187 | 3/181 |
| Cardiovascular | 4/187 | 3/181 |
| Cancer | 2/187 | 2/181 |
| Neurological | 4/187 | 1/181 |
| Gastrointestinal | 4/187 | 2/181 |
| Injury | 10/187 | 9/181 |
| Musculoskeletal | 13/187 | 9/181 |
| Respiratory | 6/187 | 3/181 |
| Other | 3/187 | 8/181 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing Show forest plot | 3 | 213 | Mean Difference (IV, Random, 95% CI) | 2.26 [‐3.09, 7.61] |
| 2 Adverse events Show forest plot | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.69, 2.05] |
| 3 Infection Show forest plot | 2 | 151 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.13, 6.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 2 | 992 | Mean Difference (IV, Fixed, 95% CI) | ‐4.68 [‐5.09, ‐4.28] |
| 2 Adverse events Show forest plot | 2 | 992 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.15, 2.06] |
| 3 Negative swab at Day 7‐8 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 13.60 [9.82, 17.38] |
| 2 Mean duration of antibiotic therapy (days) Show forest plot | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | 16.0 [8.85, 23.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 1.1 Time to healing (days) | 4 | 332 | Mean Difference (Random, 95% CI) | ‐5.12 [‐9.51, ‐0.73] |
| 2 Proportion burns completely healed Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Burns: honey vs SSD at 4 to 6 weeks | 6 | 462 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.98, 1.02] |
| 3 Adverse events Show forest plot | 5 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.20, 0.42] |
| 4 Negative swab at Day 7 Show forest plot | 5 | 412 | Risk Ratio (M‐H, Random, 95% CI) | 3.92 [1.32, 11.63] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to healing (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 Negative swab at Day 7 Show forest plot | 2 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion healed Show forest plot | 6 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.1 Infected post‐op wounds | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Pressure ulcers (grade I and II) at 10 days | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Leishmaniasis at 4 months (16 weeks) | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Venous leg ulcers at 12 weeks | 2 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Diabetic foot ulcers | 1 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Time to healing (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Fournier's gangrene | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Venous ulcers: proportion healed at 12 weeks Show forest plot | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.96, 1.38] |
| 4 Venous ulcers: infection Show forest plot | 2 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.49, 1.04] |
| 5 Mixed wounds: proportion healed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Mixed wounds healing 2' intention at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Mixed wounds healing 2' intention at 24 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

