تاثیر داروهای آنتیسایکوتیک در درمان درد حاد و مزمن در بزرگسالان
چکیده
پیشینه
این یک نسخه بهروز شده از مرور اصیل کاکرین است که در شماره 4، 2008 منتشر شد. نقش آنتیسایکوتیکها به عنوان مسکّنهای ادجوانت (کمکی) موضوع بحثهای طولانیمدت است. نورولپتانالژزی (neuroleptanalgesia) (یعنی حالت سکون، آگاهی تغییریافته، و بیدردی ناشی از ترکیبی از مصرف یک ضد درد اوپیوئیدی و یک داروی آنتیسایکوتیک)، که یک اصطلاح رایج برای مدیریت درد حاد است، تاثیر منفی بر روند بیماری و مورتالیتی کلی در بیماران مبتلا به آنژین ناپایدار (unstable angina) دارد. با این وجود، داروهای آنتیسایکوتیک برای درمان دردهای مزمن (به عنوان مثال سردرد مزمن، فیبرومیالژی و نوروپاتی ناشی از دیابت) استفاده میشوند. استفاده از آنتیسایکوتیکهای آتیپکال، یک کلاس جدید از داروهای آنتیسایکوتیک، ممکن است عوارض جانبی اکستراپیرامیدال کمتر شده و مزایای بیشتری از درمان در دسترس قرار گیرد.
اهداف
ارزیابی اثربخشی ضد دردی و عوارض جانبی آنتیسایکوتیکها در درمان درد حاد یا مزمن در بزرگسالان.
روشهای جستوجو
بانکهای اطلاعاتی زیر جستوجو شدند: CENTRAL، در کتابخانه کاکرین ، (شماره 12 از 12، 2012)؛ MEDLINE (1966 تا 11/1/2013)؛ EMBASE (1980 تا 2013 هفته 03) و PsycINFO (1806 تا هفته 3 ژانویه 2013). جستوجوها ابتدا در سال 2007، و سپس در سالهای 2011 و 2013 انجام شدند.
معیارهای انتخاب
کارآزماییهای تصادفیسازی و کنترل شده (randomised controlled trials; RCTs) با حضور بزرگسالان که هر دوزی را از یک داروی آنتیسایکوتیک خوراکی برای درمان درد حاد یا مزمن دریافت کرده، و در آن ارزیابی ذهنی درد به عنوان پیامد ثانویه توصیف شد.
گردآوری و تجزیهوتحلیل دادهها
دادهها توسط دو نویسنده مستقل استخراج شده، و نتایج برای یافتن تفاوتها مقایسه شدند. هرگونه اختلافنظر از طریق بحث، حلوفصل شد. کیفیت همه کارآزماییها بر اساس روشهای تعیین شده در بخش ششم کتابچه راهنمای کاکرین برای مرورهای سیستماتیک مداخلات (Cochrane Handbook for Systematic Reviews of Interventions) رتبهدهی شدند.
نتایج اصلی
در مجموع 770 شرکتکننده در 11 مطالعه وارد شده، شرکت کردند. دادههای پنج مطالعه تصادفیسازی شده دوسو کور (double‐blind)، تاثیرات مفید داروهای آنتیسایکوتیک را در درمان درد حاد و مزمن نشان دادند. تجزیهوتحلیل کمّی (quantitative) این مطالعات نشان داد که میانگین شدت درد پس از تجویز داروی آنتیسایکوتیک در مقایسه با دارونما (placebo) یا ترکیب فعال دیگر، کاهش معنیداری داشت، تفاوت میانگین وزندهی شده (WMD): 1.78‐؛ 95% CI؛ 2.71‐ تا 0.85‐، برای دادههای پیوسته (continuous data)؛ و نسبت خطر (relative risk): 0.43؛ 95% CI؛ 0.25 تا 0.73، تعداد افراد مورد نیاز برای درمان (numbers needed to treat; NNT): 2.6، برای دادههای دو حالتی (dichotomous data). با این وجود، تست ناهمگونی (heterogeneity) هم برای دادههای پیوسته (P = 0.0007) و هم برای دادههای دو حالتی (P = 0.04) معنیدار بود. بدیهی است که این امر باعث میشود NNT محاسبه شده کمتر قابل اعتماد باشد و هنگام تفسیر این نتایج، رعایت احتیاط لازم است.
بیشترین عوارض جانبی گزارش شده، تاثیرات خارج هرمی (یعنی حرکات غیرارادی، پارکینسونیسم و آکاتژی) و آرامبخش بودند.
نتیجهگیریهای نویسندگان
جستوجوی اخیر پنج مطالعه جدید را پیدا کرد که همگی کنار گذاشته شدند، بنابراین مرور مانند قبل باقی میماند.
داروهای آنتیسایکوتیک ممکن است به عنوان یک درمان کمکی در درمان شرایط دردناک استفاده شوند. با این وجود، پیش از استفاده از داروهای آنتیسایکوتیک برای درمان شرایط دردناک، باید عوارض جانبی اکستراپیرامیدال و آرامبخشی ناشی از آن در نظر گرفته شوند.
نتایج مربوط به داروهای آنتیسایکوتیک در درمان شرایط مختلف دردناک متفاوت بوده و اغلب حجم نمونه در RCTهای بررسی شده، کوچک هستند. انجام مطالعات بیشتر در مورد داروهای آنتیسایکوتیک آتیپکال در مطالعات بزرگتر دوسو کور و کنترل شده با دارونما که شامل ارزیابی استاندارد شده درد و مستند کردن آن باشد، ضروری است.
PICOs
خلاصه به زبان ساده
تاثیرات ضد دردی داروهای آنتیسایکوتیک در شرایط دردناک حاد و مزمن
داروهایی به نام «آنتیسایکوتیک»، که برای درمان برخی از سلامتهای روانی استفاده میشوند، گاهی اوقات برای درمان درد مزمن به کار میروند. نوع جدیدی از این داروها به نام «آنتیسایکوتیکهای آتیپکال» با عوارض جانبی کمتر و برخی مزایای بیشتر در دسترس قرار دارند. نویسندگان مرور تاثیر این داروها را بر درد و عوارض جانبی آنها ارزیابی کردند. بر اساس نتایج 5 مورد از 11 کارآزمایی وارد شده، برخی از تاثیرات مفید داروهای آنتیسایکوتیک در درمان درد حاد و مزمن وجود داشت. آنالیز این مطالعات کاهش قابلتوجهی را در درد پس از تجویز داروی آنتیسایکوتیک در مقایسه با دارونما یا داروی دیگر نشان داد، با این حال، این نتایج بر اساس مطالعات کوچک بوده و بنابراین ممکن است غیرقابل اعتماد باشند. همچنین مهم است که تاثیرات ناخواستهای را که این داروها ممکن است ایجاد کنند، در نظر بگیریم.
Authors' conclusions
Background
This review is an update of a previously published review in the Cochrane Database of Systematic Reviews (2008, Issue 4).
Antipsychotics (also called neuroleptics) can be classified according to their chemical structure into tricyclic antipsychotics (phenothiazines, thioxanthenes), butyrophenones, substituted benzamides and other chemical substances (dichlorphenyl‐piperazinyl‐chiloninones, diphenylbutylpiperidines, benzisoxazoles, benzisothiazylpiperazines, phenylpiperidines). Atypical antipsychotics differ from classical antipsychotics, or 'first generation antipsychotics', in the extrapyramidal side effects, effectiveness in negative symptomatology, and lower prolactin elevations with comparable antipsychotic efficacy. Classical antipsychotics have a predominant dopamine D2 antagonism, whereas the atypical antipsychotics also address other neurotransmitter systems, for example the serotonin system. In clinical practice cardiovascular side effects, especially a prolonged QTc, have to be kept in mind when treating patients with antipsychotics.
The therapeutic effects of antipsychotics make them a potential choice as drugs in the treatment of pain. To date the role of classic antipsychotics such as adjuvant analgesics has been a subject of longstanding controversy. Their clinical usefulness in the management of pain is questioned (Patt 1994). Neuroleptanalgesia (that is a state of quiescence, altered awareness, and analgesia produced by a combination of taking an opioid analgesic and an antipsychotic) as an established term for the management of acute pain was shown to negatively influence disease course and total mortality in unstable angina patients (Burduk 2000).
Antipsychotics are also used in a variety of different chronic pain states, from cancer pain (Bloomfield 1964; Breitbart 1998; Khojainova 2002) to chronic non‐cancer pain (Merskey 1997) as in chronic headache or chronic refractory headache (Hakkarainen 1977; Lu 2000; Silberstein 2002), fibromyalgia (Kiser 2001), musculoskeletal pain (Bloomfield 1964), low back pain (Bloomfield 1964; Jermyn 2001), chronic pain in older patients (Feinberg 2000), pain in AIDS (Breitbart 1998), post‐herpes zoster (Gobel 1997; Montilla 1963), chronic facial pain (Lechin 1989; Peschen‐Rosin 2002), and diabetic neuropathia (Gomez‐Perez 1985).
In a meta‐analysis on the analgesic potency of antipsychotics by Nix and colleagues (Nix 1998) only 10 out of 15 studies with a higher statistical power described a possible analgesic effect. None of the studies identified could differentiate between the effects of analgesia and sedation of the drugs used.
The way antipsychotics work to relieve pain is still under debate and may differ between different agents. For some pain syndromes (for example migraine) antidopaminergic properties may mediate the analgesic effects. Also, the serotonin antagonism of some antipsychotic agents is believed to mediate the analgesic effects (Schreiber 1999). Additionally, for some antipsychotics (for example olanzapine) their agonistic activity at alpha2‐adrenoceptors is believed to mediate analgesic effects (Silberstein 2002).
Besides discussions about the potential of antipsychotics to be used as analgesics, a potent antinociceptive effect has been shown for risperidone, an atypical antipsychotic, in an in vivo animal pain model (that is the tail‐flick assay). Further evaluation of risperidone with selective opioid antagonists revealed the involvement of µ1‐, µ2‐, and kappa1‐opioids and, to a lesser extent, delta‐opioid mechanisms. For olanzapine the alpha2‐adrenoceptors and to a lesser extent the opioid and serotonergic receptors are involved in the antinociception (Schreiber 1999).
With the arrival of atypical antipsychotics, a new class of antipsychotics, fewer extrapyramidal side effects and additional benefits are now available. These new treatments were not included in a meta‐analysis of reports on the analgesic effects of antipsychotics performed by Nix 1998. Therefore, a new meta‐analysis is needed to address the question of evidence based pain therapy with antipsychotics.
Objectives
To assess the analgesic efficacy and adverse effects of antipsychotics in acute or chronic pain in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) which were double blinded and which investigated the analgesic effects of antipsychotics as monotherapy or add‐on treatment in patients with acute or chronic pain, if pain assessment was either the primary or a secondary outcome. Reports were excluded if they were studies which were non‐randomised, studies of experimental pain, case reports, clinical observations (open studies) or studies of antipsychotics used to treat pain produced by other drugs.
Types of participants
Randomised controlled trials (RCTs) of adult patients of either gender who had acute or chronic pain, or both, of all degrees of severity, were included in this review.
Types of interventions
Any form of antipsychotic treatment (at any dose) listed below compared with no treatment, placebo, or other pain relieving treatment (for example non‐steroidal anti‐inflammatory drugs (NSAIDs), antidepressants, anticonvulsants, opiates).
Antipsychotic agents or neuroleptics:
-
amisulpride,
-
amoxapine,
-
chlormethiazole,
-
clopenthixol,
-
chlorpiprazine,
-
chlorpromazine,
-
chlorprothixene,
-
cloxazepine,
-
clozapine,
-
distraneurine,
-
dixyrazine,
-
droperidol
-
chlorpromazine,
-
flupentixol decanoate,
-
fluphenazine,
-
haloperidol,
-
levomepromazine,
-
loxapine,
-
melperone hydrochloride,
-
methotrimeprazine,
-
olanzapine,
-
oxilapine,
-
perphenazine,
-
pimozide,
-
prochlorperazine
-
prothipendyl hydrochloride,
-
quetiapine,
-
risperidone,
-
sulpiride,
-
thioridazine,
-
tiapride,
-
tisercin,
-
trifluoroperazine,
-
ziprsasidone,
-
zotepine,
-
zuclopenthixol.
For the update of this review in January 2013 we included two additional antipsychotics, namely droperidol and prochlorperazine, in our search strategy.
Types of outcome measures
Primary outcomes
The primary outcome measure for this review was the reduction in pain intensity as measured by a visual analogue scale (VAS), self reported global scale, verbal rating scale, numerical rating scale or categorical pain relief scale, and self reported pain relief. We used the effectiveness measures after the longest reported duration of treatment. We excluded studies which did not quantify pain using a scale. We included patient reported pain data, and excluded trials only reporting physician and carer pain assessment.
Secondary outcomes
An assessment of the frequency and severity of the commonly expected adverse effects was undertaken. Adverse effects were classified as minor if they were reported by a participant who continued with the medication and completed the trial. A major adverse effect was defined as one that caused the participant to withdraw from the study. Side effect data are recorded in Table 1.
| Author/Year | Substance | Type of side effect | Percentage |
| Ginsberg 1983 | Tiapride | Drowsiness (moderate to mild), mild gastric intolerance | 38.1% |
| Lechin 1989 | Pimozide | Physical and mental retardation, hand tremors, memory impairment, involuntary movements during sleep (jerkings) and slight Parkinson's disease manifestations | 83.3% |
| Langemark 1994 | Sulpiride | Sedation, depression, nausea, sleep disturbance, increased dreaming, uneasiness, weight gain, obstipation, amenorrhoea, galactorrhoea, impotence, restless legs, micturation difficulties, polyuria, accomodation difficulties, dry mouth, orthostatic hypotension | author only provided incidences, at least 34% |
| Roux 1983 | Tiapride | no side effects reported | ‐‐ |
| Johnston 1972 | Thioridazine | No untoward effects were observed or reported at any time during the study | ‐‐ |
| Davidsen 1979 | Levomepromazine | Dry mouth | 59% |
| Graff‐Radford 2000 | Fluphenazine | Sleepiness, dry mouth | ‐‐ |
| Zitman 1991 | Flupentixol | Dry mouth | ‐‐ |
| Judkins 1982 | Haloperidol | No serious side effects were observed, dry mouth | ‐‐ |
| Bussone 1980 | Tiapride | No extrapyramidal, neuroendocrine or neurovegetative side effects were observed | ‐‐ |
| Honkaniemi 2006 | Haloperidol | Motor agitation, sedation, hyperventilation and shortness of breath | 80% |
| Richman 2002 | Droperidol | Sedation, akathisia | Sedation: 6.7% (droperidol) vs. 13.4% (meperidine); akathisia 13.3% (only droperidol reported) |
Additional outcomes
-
Attrition, the numbers of participants withdrawing before completion of the study in an intervention versus placebo study and an intervention versus other treatment study due to non‐compliance, adverse effects or death
-
Measures of satisfaction or patient preference (if reported)
-
Assessment of quality of life (if reported)
Search methods for identification of studies
Electronic searches
This search was run for the original review in October 2007 and subsequent searches were run in 2011 and January 2013.
For the identification of studies to be included or considered for this review, the following databases were searched:
-
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2012, Issue 12);
-
MEDLINE (1966 to January 2013);
-
EMBASE (1989 to January 2013);
-
PsycINFO (1806 to January 2013).
Detailed search strategies were developed for each database searched and the MEDLINE search strategy from the original 2007 search is given in Appendix 1; for other search strategies please see Appendix 2 and the search strategies used for the 2013 updated searches can be found in Appendix 3. The searches attempted to identify all relevant studies irrespective of language. Non‐English papers would be assessed and translated, if necessary, with the assistance of a native speaker.
Searching other resources
We checked reference lists from retrieved trials for additional studies. We also sought relevant studies cited in reviews identified by searching the Cochrane Database of Systematic Reviews (CDSR) and the Database of Abstracts of Reviews and Effectiveness (DARE).
We contacted the corresponding authors of the identified articles and experts in the field for additional studies. Furthermore, we sent letters requesting information about published or unpublished trials to pharmaceutical companies which manufacture antipsychotics (Sanofi‐Synthelabo: amisulpride, fluphenazine, sulpiride, tiapride; Lundbeck: chlorprothixene, flupentixol decanoate, melperone, sertindole, zuclopenthixol; Novartis: clozapine, thioridazine; Eli Lilly: olanzapine; AstraZeneca: quetiapine; Janssen‐Cilag: haloperidol, pimozide, risperidone; Asta Medica: prothipendyl hydrochloride; Knoll Ltd: zotepine; Pfizer: ziprasidone; Gerot: levomepromazine; UCB‐Pharma: dixyrazine).
Data collection and analysis
Study selection
Two review authors (MA and MO) independently screened the titles and abstracts of all the references retrieved by the original search strategy. Two other authors (SS and TS) independently screened the titles and abstracts of all references retrieved by the search strategy for this update. The full text versions of relevant studies were retrieved by BW and were assessed independently by the authors (MA, MO, TS and SS) to determine whether they met the inclusion criteria. Disagreements were resolved by discussion among the four authors mentioned above.
Assessment of quality
Trials which met the inclusion criteria were graded independently for methodological quality and assessed for internal validity using the Oxford Quality Scale score (Jadad 1996):
-
randomisation (1 = yes; 0 = no);
-
method and description of randomisation (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate);
-
double blinding (1 = yes; 0 = no);
-
method and description of double blinding (0 = not described; 1 = described and adequate; ‐1 = described, but not adequate);
-
sufficient information about loss to follow‐up (1 = yes; 0 = no).
Each study was allocated a score of between one to five points. Because the inclusion criteria for this review required trials to be randomised, the minimum quality score was one. Higher scores indicated a higher quality of conducting or reporting, or both, of the trial. No trial that scored '0' met the inclusion criteria, the minimum score calculated was two.
Data extraction
The following data items were extracted from each of the included studies, where available:
-
trial characteristics (methods, duration, interventions);
-
patient characteristics (age, gender, type of pain condition);
-
trial results (patient reported pain intensity or pain relief);
-
adverse effects (major and minor);
-
study withdrawals (due to non‐compliance, adverse effects or death);
-
measures of satisfaction or patient preference (if reported); and
-
assessment of quality of life (if reported).
Data were extracted on to a standard form by two review authors working independently. Due to possible carry‐over effects, only the first phase of cross‐over studies was used.
Analysis
Statistical testing of heterogeneity between the trials was carried out by one author (EP) using RevMan Analyses 1.0.3 in Meta‐View 4.2.8 (RevMan 2012). Results from the trials were combined using a fixed‐effect model to calculate relative risks (RR) with 95% confidence intervals (CI) for dichotomous data and weighted mean differences (WMD) for continuous data.
If enough data were available, the number needed to treat to benefit (NNT) was calculated.
Subgroup analyses
The quality of the included trials was used in exploring any significant statistical heterogeneity between them. Cut‐off levels for the subgroup analysis values that were used were 'greater or equal to three' or 'less than or equal to two'.
Results
Description of studies
Study selection
The search for this update (from October 2007 to January 2013) resulted in 2083 hits. After screening of the titles and abstracts five potentially relevant studies were identified. Unfortunately, these studies had to be excluded because of the following reasons.
-
The study by Hill et al (Hill 2008) compared the efficacy of the antipsychotic to another antipsychotic (that is droperidol).
-
The study by Friedman et al (Friedman 2008) compared the efficacy of the antipsychotic to metoclopramide.
-
The study by Miller et al (Miller 2009) compared the efficacy of the antipsychotic to octreotide.
-
The study by Kostic et al (Kostic 2010) compared a combination of prochlorperazin with diphenhydramine to sumatriptan.
-
The study by Potvin et al (Potvin 2012) did not use pain as the primary outcome parameter.
The original search strategy, run in October 2007, resulted in 1908 hits. After screening of the titles and abstracts, 56 potentially relevant studies were identified. Forty‐one studies were excluded (see 'Characteristics of excluded studies' table). In short, 39 studies did not meet the quality criteria as assessed by the Oxford Quality Scale. Two high‐quality studies (Brousseau 2004; Weaver 2004;) were excluded because they reported the efficacy of an antipsychotic on headache in children and adolescents (Brousseau 2004) and compared two antipsychotics (droperidol vs. prochlorperazine) without the use of a placebo (Weaver 2004). Hence, 15 studies were considered for inclusion in this review. Two of these studies could not be assessed because a translation into English was not available (Govorin 1990; Lepola 1984). For one further study the authors could not be identified, and it was therefore excluded (Anon 1986).
Overall 12 RCTs of nine different antipsychotics were considered eligible (Bussone 1980; Davidsen 1979; Ginsberg 1983; Graff‐Radford 2000; Honkaniemi 2006; Johnston 1972; Judkins 1982; Langemark 1994; Lechin 1989; Richman 2002a; Roux 1983; Zitman 1991) (n = 772) for inclusion in the review, please see the 'Characteristics of included studies' table for full details of each included study. All included studies were clinic based and single centered, with one (Lechin 1989) explicitly stating the inclusion of outpatients. They were conducted at the departments for neurology (n = 4) (Bussone 1980; Honkaniemi 2006; Langemark 1994; Lechin 1989), anaesthesiology (n = 2) (Graff‐Radford 2000; Judkins 1982), psychiatry (n = 1) (Zitman 1992), neurosurgery (n = 1) (Roux 1983), oncology (n = 1) (Johnston 1972) and the emergency department (n = 2) (Davidsen 1979; Richman 2002a). The site of the remaining study (Ginsberg 1983) could not be specified. The sample size ranged from 29 to 316 participants. Most trials were limited by their small sample size. Only one trial included more than 200 participants (Davidsen 1979). Eight studies were placebo‐controlled and were considered for quantitative analysis. Data from six studies could not be included in the quantitative analysis because of the following reasons.
-
Two studies compared the efficacy of the antipsychotic to an opioid (Davidsen 1979) or a selective serotonin reuptake inhibitor (SSRI) (Langemark 1994).
-
One study only reported on the occurrence of headaches following an intervention (Roux 1983).
-
One study did not provide information about the duration of treatment (Bussone 1980).
-
The work of Johnston 1972 fulfilled the inclusion criteria, but due to missing data on variability, the study could not be included in the final analyses. Similarly, the study by Judkins 1982 had to be excluded due to missing data on the mean and variability of the selected outcome variables.
Risk of bias is shown in Figure 1 for each included study.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
For the meta‐analyses, the primary outcome variable was the difference in mean in the treatment and placebo groups (after ‐ baseline) and therefore reflected how much the VAS score could be reduced by the treatment. Additionally, the meta‐analysis was performed for the mean outcome VAS score following treatment in both groups. For the dichotomous event, feeling pain or no pain after treatment, the RR indicated the chance of having pain after receiving the intervention, an absolute risk reduction (ARR) < 1 means that the chance of having pain after the intervention is smaller in the treatment group than in the control group. The study authors were contacted to provide missing data, if necessary. Standard errors were converted into standard deviations. If the standard deviation of the difference in mean was not given, but the standard deviation from baseline and after treatment was given, imputation strategies were used (Higgins 2011). If values for the mean or standard deviation were not mentioned in the text, but were displayed in a figure, values were taken from the figure.
Study design
Nine of the included studies had a parallel design and three had a cross‐over design. Some trials had more than two arms and made more than one comparison.
Outcomes
Pain was patient reported in 12 trials. In one study information on pain was provided by the patients' weekly ratings (Johnston 1972). In six trials pain was reported using a pain diary (Bussone 1980; Ginsberg 1983; Graff‐Radford 2000; Langemark 1994; Lechin 1989; Zitman 1991). Three trials documented the analgesic requirements of the patients on a numeric VAS (Judkins 1982; Richman 2002a; Honkaniemi 2006). Two trials simply reported on the mere occurrence of pain (Davidsen 1979; Roux 1983).
Study methods
All 12 included studies were conducted in a double‐blind fashion. Eight trials compared an antipsychotic or a combination of analgesic and antipsychotic to placebo and three studies compared an antipsychotic or a combination of analgesic and antipsychotic to treatment with an active compound (that is antidepressants, antiepileptics or analgesics).
Antipsychotics
Trials using the following antipsychotics were found:
-
Five with tricyclic antipsychotics (flupentixol, fluphenazine, thioridazine, levomepromazine);
-
four with butyrophenones (droperidol, haloperidol);
-
three with benzamides (sulpiride, tiapride, pimozide).
Patient conditions
The underlying conditions studied were as follows:
-
somatoform pain disorder, one study;
-
post‐herpetic neuralgia, one study;
-
acute (migraine) headache, two studies;
-
pain in terminal cancer, one study;
-
postoperative pain, one study;
-
trigeminal neuralgia, one study;
-
acute rheumatic pain, one study;
-
chronic tension‐type headache, two studies;
-
post‐rachiocentesis headache, one study; and
-
acute myocardial infarction, one study.
Details of these eligible reports are provided in the 'Characteristics of included studies' table.
Risk of bias in included studies
Each study was scored independently for quality by two of the review authors (MA and MO) using the three‐item Oxford Quality Scale (Jadad 1996). The scores for individual trials are reported in the notes section of the 'Characteristics of included studies' table. The median quality score for the placebo‐controlled studies was three (all trials scored three), and for the active control studies it was also three (range three to four).
Effects of interventions
Overall 12 RCTs of nine different antipsychotics were considered eligible (Bussone 1980; Davidsen 1979; Ginsberg 1983; Graff‐Radford 2000; Honkaniemi 2006; Johnston 1972; Judkins 1982; Langemark 1994; Lechin 1989; Richman 2002a; Roux 1983; Zitman 1991) (total n = 743) for inclusion in the review. Forty studies were excluded and are listed in the 'Characteristics of excluded studies' table.
Tricyclic antipsychotics
Four trials (Davidsen 1979; Graff‐Radford 2000; Johnston 1972; Zitman 1991), with a total of 449 participants, studied the effects of tricyclic antipsychotics in different painful disorders. These trials studied the effect of tricyclic antipsychotics in:
-
pain following myocardial infarction (Davidsen 1979),
-
pain due to terminal cancer (Johnston 1972),
-
somatoform pain disorder (Zitman 1991), and
-
post‐herpetic neuralgias (Graff‐Radford 2000).
The description of the study results is given below.
Tricyclic antipsychotics versus placebo
One study indicated only a small positive effect of 75 mg thioridazine daily compared to placebo concerning global improvement and pain in terminal cancer patients (P < 0.1) (Johnston 1972). The study of Graff‐Radford et al had four arms (amitriptyline, amitriptyline + fluphenazine, fluphenazine, and placebo). For quantitative analysis we only included data from the fluphenazine and the placebo arms (Graff‐Radford 2000).
Tricyclic antipsychotics versus other active treatment
Three studies compared tricyclic antipsychotics to other active treatments, including amitriptyline (a tricyclic antidepressant) (Graff‐Radford 2000; Zitman 1991) and pethidine (an opioid) (Davidsen 1979).
Administration of levomepromazine proved to significantly reduce the recurrence of pain within the first 72 hours after an acute myocardial infarction compared to treatment with pethidine (P < 0.05) (Davidsen 1979). In the case of post‐herpetic neuralgia the decrease of pain in patients receiving fluphenazine did not reach statistical significance. Combination of the antipsychotic with a tricyclic antidepressant (that is amitriptyline) and comparison with treatment with amitriptyline alone failed to produce a significant advantage using fluphenazine: mean difference (MD) 0.54 (95% CI ‐1.49 to 2.57) (Graff‐Radford 2000). In another study, patients with a somatoform pain disorder receiving 75 mg amitriptyline or 75 mg amitriptyline plus 3 mg flupentixol experienced significantly less pain during the treatment. Yet, the comparison of pain reduction in both groups did not reveal a statistically significant MD (MD ‐0.60, 95% CI ‐2.10 to 0.90) (Zitman 1991).
Butyrophenones
Three placebo‐controlled RCTs (Honkaniemi 2006; Judkins 1982; Richman 2002a), with a total of 110 participants, studied the effects of butyrophenones on postoperative pain (Judkins 1982) and acute migraine headache (Honkaniemi 2006; Richman 2002a).
In the case of postoperative pain two different dosages of haloperidol (5 and 10 mg orally) were compared against placebo as premedication before major abdominal surgery (Judkins 1982). VAS scores for pain at 24 hours after surgery did not differ significantly between the three groups of participants. Only a significant reduction of postoperative emesis was found in both groups treated with haloperidol. In contrast, treatment of acute migraine headache with 5 mg intravenous haloperidol was shown to be significantly superior to placebo (MD ‐4.05, 95% CI ‐5.61 to ‐2.49) (Honkaniemi 2006). Another study on acute migraine compared 2.5mg intramuscular droperidol with 1.5mg/kg meperidine and failed to detect a significant difference regarding post‐treatment pain intensity (VAS) (47 vs. 37 mm, p=.033) (Richman 2002a).
Benzamides
Five double‐blind trials with a total of 240 participants, two of them placebo‐controlled (Bussone 1980; Roux 1983), studied the effects of benzamides on different types of headache (Bussone 1980; Langemark 1994; Roux 1983), trigeminal neuralgia (Lechin 1989) and acute rheumatic pain (Ginsberg 1983).
In the case of chronic tension‐type headache tiapride was compared with placebo (Bussone 1980) and sulpiride was compared with another active component, that is paroxetine an SSRI (Langemark 1994). It seemed noteworthy that five participants in the group receiving sulpiride dropped out during the study due to intolerable side effects (Langemark 1994), see Table 1 for further details.
Benzamides versus placebo
The effect of intravenous tiapride (dosage 200 mg) following rachiocentesis was studied in a small sample (n = 30) (Roux 1983). The authors only reported the percentage of patients who experienced pain within 48 hours after rachiocentesis, but did not provide any further details (for example pain severity or associated symptoms). When compared to the placebo, tiapride led to a reduction in the occurrence of headaches. In detail, 86.7% of patients who had received tiapride before the rachiocentesis and 46.6% of patients who had received placebo did not report headaches within 48 hours following the procedure. These results were statistically significant (P < 0.01).
An Italian group reported on the efficacy of 300 mg tiapride administered orally on chronic tension‐type headache, but failed to provide any information on the duration of the treatment conducted in the study (Bussone 1980). Forty per cent of the patients treated with tiapride were complete responders compared to no responders with placebo (Bussone 1980).
Benzamides versus other active treatment
Sulpiride (400 mg daily) and paroxetine (30 mg daily), an SSRI, were compared in a group of 50 patients suffering from chronic tension‐type headache in a cross‐over conditional‐response design (Langemark 1994). Each treatment period lasted eight weeks. Patients recorded their pain in headache diaries. Total pain scores differed significantly between groups (P = 0.03) favouring treatment with the antipsychotic.
Pimozide (12 mg daily) and carbamazepine (1200 mg daily), antiepileptic drugs established in the treatment of neuralgic pain, were tested in the treatment of pain due to trigeminal neuralgia in 48 participants, using a cross‐over design (Lechin 1989). During both treatment phases pimozide proved to be superior to carbamazepine regarding total trigeminal neuralgia pain scores (P < 0.01) (MD ‐4.11, 95% CI ‐8.08 to ‐0.14).
In the case of acute rheumatic pain tiapride (100 mg daily) and glafenine (200 mg daily), an anthranilic acid derivative with analgesic properties, were compared during a 14‐day double‐blind trial (Ginsberg 1983). Tiapride was significantly superior to glafenine regarding the time delay until the disappearance of the pain (P < 0.05). Concerning pain reduction there was a trend favouring the antipsychotic (RR 0.73, 95% CI 0.37 to 1.44), though it failed to show statistical significance. A separate analysis according to the sex of patients did not reveal any significant differences (Ginsberg 1983).
Five of these trials permitted a quantitative analysis of the study data according to our protocol. The results of these analyses are available in Analysis 1.1, Analysis 1.2 and Analysis 1.3.
Discussion
This systematic review revealed a small number (n = 12) of small‐sized clinical trials (total n = 772) that compared the analgesic effects of antipsychotics to placebo or active compounds in a randomised double‐blind fashion.
There are some preclinical studies in humans that link the dopaminergic system with pain. In one study an inverse correlation of pain threshold and the response criterion with the D2/D3 binding potential in the right putamen was found (Pertovaara 2004). This finding is supported by a number of animal studies which have suggested that dopamine is involved in the regulation of nociception. However, the data are contradictory as dopamine agonists have been shown to produce either antinociception (Shimizu 2004) or hyperalgesia (Paalzow 1992).
It appears reasonable to further investigate the analgesic effect of various antipsychotics in different pain syndromes. Thus, we describe the effects of different antipsychotics in the following painful conditions.
Headaches
The effects of antipsychotics have been studied in the treatment of acute migraine headache (Honkaniemi 2006; Richman 2002a), chronic tension‐type headache (Bussone 1980; Langemark 1994) and headaches following rachiocentesis (Roux 1983) using randomised double‐blind designs. All but one trial (Richman 2002a) demonstrated statistically significant positive results for antipsychotics, that is haloperidol, sulpirid and tiaprid. It seems noteworthy that administration of tiaprid did not lead to a greater reduction of headache intensity, but led to a faster amelioration of headache following rachiocentesis (Roux 1983).
Neuralgic pain
Treatment of patients with neuralgic pain (post‐herpetic neuralgia (Graff‐Radford 2000) and trigeminal neuralgia (Lechin 1989)) delivered both positive and negative results. Fluphenazine, used in the case of post‐herpetic neuralgia, did not prove to be superior to amitriptyline, a tricyclic antidepressant (Graff‐Radford 2000). On the other hand, a study with patients suffering from trigeminal neuralgia appeared to show that pimozide led to a significantly greater reduction of pain in a double‐blind cross‐over study (Lechin 1989).
Other painful conditions
The effects on several other painful conditions have been studied in double‐blind RCTs. We could only identify a single study for each condition, which met our inclusion criteria. This is one of the limitations of the data presented here. In summary, we can say that only one study reported a statistically significant positive effect on pain, that is pain following an acute myocardial infarction (Davidsen 1979). Intriguingly, that study turned out to hold the largest sample (n = 316) of all studies included. Trials on somatoform pain disorders (Zitman 1991), postoperative pain (Judkins 1982) and acute rheumatic pain (Ginsberg 1983) failed to deliver significant results favouring the treatment with antipsychotics.
Antipsychotics and acute pain
Four of six studies on acute painful conditions that were reviewed here (Ginsberg 1983; Judkins 1982; Richman 2002a; Roux 1983) did not find significant positive results for antipsychotics concerning reduction of pain intensity. One study (Davidsen 1979), using a large sample of patients, reported a statistically significant reduction of pain after an acute myocardial infarction. The data of Honkaniemi and colleagues demonstrated an excellent response of acute migraine headache to the administration of haloperidol (Honkaniemi 2006). In addition, after the administration of tiaprid post‐rachiocentesis headache disappeared more quickly when compared to placebo, although pain reduction was not greater than in patients who received placebo (Roux 1983).
Antipsychotics and chronic pain
Six studies included in this review focused on the management of chronic pain conditions. In the case of chronic tension‐type headaches two studies reported beneficial effects for patients treated with antipsychotics when compared to placebo or another active component (Bussone 1980; Langemark 1994). Results concerning neuralgic pain turned out to be both negative (Graff‐Radford 2000) and positive (Lechin 1989). In the latter study the antipsychotic was more efficient than carbamazepine, a well‐established drug in the treatment of neuralgias. Treatment of a somatoform (that is physical symptoms that mimic disease or injury for which there is no identifiable physical cause) pain disorder with flupentixol (Zitman 1991) and pain in terminal cancer patients with thioridazine (Johnston 1972) was not reported to be superior to placebo or another treatment (Zitman 1991).
Summarising the results of all 11 included RCTs we found a positive effect in painful conditions in six trials, whereas five trials failed to report any analgesic effect of the antipsychotics studied. Five trials proved eligible for a meta‐analysis. We next set out to perform quantitative analyses of studies on acute (n = 2) and chronic pain (n = 3) separately, but this does not seem reasonable to us due to the small number and tremendous clinical heterogeneity of the studies. In addition, the data reviewed here have further limitations as most trials studied small samples of patients and only one included more than 200 participants. Moreover, pain assessment varied among the different protocols. Some studies only reported on the (re‐)occurrence of pain, which is, from our point of view, not sufficient to assess the whole effect on painful states.
Thus, in the present review the evidence of analgesic properties of antipsychotics can only be described relying on each single study. From a clinical point of view further research on analgesic properties of antipsychotics is indicated in more RCTs. Since nowadays atypical antipsychotics, which are known to produce lesser extrapyramidal side effects, are available, this new group of antipsychotics clearly needs to be studied regarding their analgesic potency.
In this update we also added antipsychotics (that is prochlorperazine and droperidol) to our search strategy. Nevertheless we could not include any new studies. One of the excluded studies (Hill 2008) concluded that olanzapine, a newer atypical antipsychotic, was equally potent to droperidol, an older antipsychotic, to relieve headaches. This finding should encourage researchers to further study the efficacy of newer antipsychotic agents in the treatment of pain disorders.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Reduction in pain intensity, Outcome 1 Acute and chronic pain continuous data ‐ difference posttreatment minus baseline.
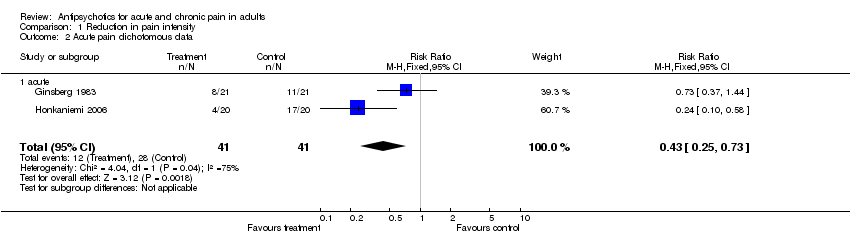
Comparison 1 Reduction in pain intensity, Outcome 2 Acute pain dichotomous data.

Comparison 1 Reduction in pain intensity, Outcome 3 Acute and chronic pain continuous data ‐ mean after treatment.
| Author/Year | Substance | Type of side effect | Percentage |
| Ginsberg 1983 | Tiapride | Drowsiness (moderate to mild), mild gastric intolerance | 38.1% |
| Lechin 1989 | Pimozide | Physical and mental retardation, hand tremors, memory impairment, involuntary movements during sleep (jerkings) and slight Parkinson's disease manifestations | 83.3% |
| Langemark 1994 | Sulpiride | Sedation, depression, nausea, sleep disturbance, increased dreaming, uneasiness, weight gain, obstipation, amenorrhoea, galactorrhoea, impotence, restless legs, micturation difficulties, polyuria, accomodation difficulties, dry mouth, orthostatic hypotension | author only provided incidences, at least 34% |
| Roux 1983 | Tiapride | no side effects reported | ‐‐ |
| Johnston 1972 | Thioridazine | No untoward effects were observed or reported at any time during the study | ‐‐ |
| Davidsen 1979 | Levomepromazine | Dry mouth | 59% |
| Graff‐Radford 2000 | Fluphenazine | Sleepiness, dry mouth | ‐‐ |
| Zitman 1991 | Flupentixol | Dry mouth | ‐‐ |
| Judkins 1982 | Haloperidol | No serious side effects were observed, dry mouth | ‐‐ |
| Bussone 1980 | Tiapride | No extrapyramidal, neuroendocrine or neurovegetative side effects were observed | ‐‐ |
| Honkaniemi 2006 | Haloperidol | Motor agitation, sedation, hyperventilation and shortness of breath | 80% |
| Richman 2002 | Droperidol | Sedation, akathisia | Sedation: 6.7% (droperidol) vs. 13.4% (meperidine); akathisia 13.3% (only droperidol reported) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Acute and chronic pain continuous data ‐ difference posttreatment minus baseline Show forest plot | 4 | 148 | Mean Difference (IV, Fixed, 95% CI) | ‐2.16 [‐1.00, ‐1.32] |
| 1.1 acute | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.52 [‐5.88, ‐3.16] |
| 1.2 chronic | 3 | 108 | Mean Difference (IV, Fixed, 95% CI) | ‐0.71 [‐1.78, 0.35] |
| 2 Acute pain dichotomous data Show forest plot | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.25, 0.73] |
| 2.1 acute | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.25, 0.73] |
| 3 Acute and chronic pain continuous data ‐ mean after treatment Show forest plot | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 acute | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 chronic | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

