Fluid therapy for acute bacterial meningitis
Appendices
Appendix 1. Search strategy for 2010 update
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (1966 to Week 4, July 2010); EMBASE (1980 to August 2010); and CINAHL (1982 to August 2010).
For the previous update in 2007 we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2007, Issue 1) which includes the Cochrane Acute Respiratory Infections Group's Specialised Register; MEDLINE (1966 to March 2007); EMBASE (1980 to March 2007); and CINAHL (1982 to February 2007). MEDLINE, EMBASE and CINAHL were searched using OVID software. The MEDLINE search strategy is in Appendix 2.
The following search strategy was used to search MEDLINE and CENTRAL. The MEDLINE search was combined with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). The search strategy was adapted to search Embase.com (see Appendix 3) and CINAHL (see Appendix 4).
MEDLINE (Ovid)
1 exp Meningitis/
2 meningit*.tw.
3 or/1‐2
4 exp Fluid Therapy/
5 fluid*.tw,nm.
6 Sodium Chloride/
7 saline*.tw,nm.
8 Rehydration Solutions/
9 (rehydrat* or hydrat* or dehydrat*).tw.
10 exp Water‐Electrolyte Balance/
11 electrolyt*.tw,nm.
12 (hyponatr* adj2 solution*).tw.
13 exp Albumins/
14 exp Plasma/
15 exp Plasma Substitutes/
16 albumin*.tw.
17 plasma*.tw.
18 (starch* or dextran* or gelofus* or haemacc* or hemacc*).tw.
19 or/4‐18
20 3 and 19
Appendix 2. Previous MEDLINE search strategy
The following search strategy was used to search MEDLINE and CENTRAL and adapted for EMBASE and CINAHL.
MEDLINE (OVID)
1 exp MENINGITIS/
2 meningit$.mp
3 or/1‐2
4 exp Fluid Therapy/
5 fluid resuscitation.mp.
6 fluid restriction.mp.
7 fluid maintenance.mp.
8 fluid management.mp.
9 intravenous fluid$.mp.
10 IV fluid$.mp.
11 hyponatr?emic solution$.mp.
12 exp Sodium Chloride/
13 saline.mp.
14 exp ALBUMINS/
15 exp PLASMA/
16 exp Plasma Substitutes/
17 (volume adj replac$).mp.
18 (human adj albumin$).mp.
19 ((frozen adj plasma) or (fresh adj plasma)).mp.
20 (plasma adj protein$).mp.
21 (hypoalbumin$ or (low adj albumin)).mp.
22 (starch or dextran$ or gelofus$ or haemacc$ or hemacc$).mp.
23 or/4‐22
24 2 and 23
Appendix 3. EMBASE (Elsevier) search strategy
#38 #36 AND #37
#37 819074
#37.8 #37.3 NOT #37.7
#37.7 #37.4 NOT #37.6
#37.6 #37.4 AND #37.5
#37.5 'human'/de AND [embase]/lim
#37.4 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de AND [embase]/lim
#37.3 #37.1 OR #37.2
#37.2 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti AND [embase]/lim
#37.1 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim
#36 #16 AND #35
#35 #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34
#34 albumin*:ab,ti OR plasma*:ab,ti OR starch*:ab,ti OR dextran*ti,ab OR gelofus*:ab,ti OR haemacc* OR hemacc*:ab,ti
#33 'albumin'/de
#32 'plasma'/de
#31 'plasma substitute'/exp
#30 electrolyt*:ab,ti
#29 'electrolyte balance'/exp
#28 'water deprivation'/de
#27 (parenteral NEAR/2 (solution* OR infusion*)):ab,ti
#26 'parenteral solution'/de
#25 hyponatr*:ab,ti
#24 saline*:ab,ti OR sodium*:ab,ti
#23 'sodium chloride'/de
#22 solution*:ab,ti OR rehydrat*:ab,ti OR dehydrat*:ab,ti OR hydrat*:ab,ti
#21 'oral rehydration solution'/de
#20 'solution and solubility'/exp
#19 fluid*:ab,ti
#18 'body fluid'/de OR 'cerebrospinal fluid'/de
#17 'fluid therapy'/exp
#16 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15
#15 meningit*:ab,ti OR meningococ*:ab,ti
#14 'streptococcus group b':ab,ti OR 'streptococcus agalactiae':ab,ti
#13 'streptococcus agalactiae'/de
#12 ((haemophilus OR hemophilus OR hib) NEAR/2 (mening* OR infect*)):ab,ti
#11 'haemophilus infection'/de OR 'haemophilus meningitis'/de
#10 'haemophilus influenzae type b'/de
#9 'listeria monocytogenes':ab,ti
#8 'listeria monocytogenes'/de OR 'listeriosis'/de
#7 'escherichia coli':ab,ti OR 'e coli':ab,ti
#6 'escherichia coli infection'/exp
#5 'neisseria meningitidis'/de
#4 'pneumococcal infection'/de OR 'pneumococcal meningitis'/de
#3 'meningococcosis'/exp
#2 'bacterial meningitis'/de OR 'group b streptococcal meningitis'/de OR 'haemophilus meningitis'/de OR 'pneumococcal meningitis'/de
#1 'meningitis'/de
Appendix 4. CINAHL (Ebsco) search strategy
S46 S45 AND EM 201006‐
S45 S34 AND S44
S44 S35 OR S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43
S43 (MH "Quantitative Studies")
S42 TI placebo* OR AB placebo*
S41 (MH "Placebos")
S40 TI random* OR AB random*
S39 (MH "Random Assignment")
S38 TI ((singl* or doubl* or tripl* or trebl*) N1 (blind* or mask*)) OR AB ((singl* or doubl* or tripl* or trebl*) N1 (blind* or mask*))
S37 TI clinical* trial* OR AB clinical* trial*
S36 PT clinical trial
S35 (MH "Clinical Trials+")
S34 S15 AND S33 S
S33 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32
S32 TI (plasma* or albumin* or starch* or dextran* or gelofus* or haemacc* or hemacc*) OR AB (plasma* or albumin* or starch* or dextran* or gelofus* or haemacc* or hemacc*)
S31 (MH "Plasma Substitutes+")
S30 (MH "Plasma+")
S29 (MH "Albumins+")
S28 TI electrolyt* OR AB electrolyt*
S27 (MH "Fluid‐Electrolyte Imbalance+")
S26 (MH "Fluid‐Electrolyte Balance+")
S25 (MH "Parenteral Nutrition Solutions")
S24 (MH "Infusions, Parenteral+")
S23 TI hyponatr* OR AB hyponatr*
S22 TI (sodium* or saline*) OR AB (sodium* or saline*)
S21 (MH "Sodium Chloride")
S20 TI (solution* or rehydrat* or dehydrat* or hydrat*) OR AB (solution* or rehydrat* or dehydrat* or hydrat*)
S19 (MH "Solutions+")
S18 TI fluid* OR AB fluid*
S17 (MH "Body Fluids") OR (MH "Body Water")
S16 (MH "Fluid Therapy+")
S15 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14
S14 TI (meningit* or meningococc*) OR AB (meningit* or meningococ*)
S13 TI ("streptococcus group b" or "streptococcus agalactiae") OR AB ("streptococcus group b" or "streptococcus agalactiae")
S12 TI hib OR AB hib
S11 TI ((haemophilus or hemophilus) N2 (mening* or infect*)) OR AB ((haemophilus or hemophilus) N2 (mening* or infect*))
S10 (MH "Haemophilus Influenzae")
S9 (MH "Haemophilus Infections")
S8 TI "listeria monocytogenes" OR AB "listeria monocytogenes"
S7 (MH "Listeria Infections")
S6 TI ("escherichia coli" or "e coli") OR AB ("escherichia coli" or "e coli")
S5 (MH "Escherichia Coli Infections")
S4 (MH "Neisseria Infections")
S3 (MH "Pneumococcal Infections+")
S2 (MH "Meningococcal Infections+")
S1 (MH "Meningitis") OR (MH "Meningitis, Bacterial+")
Appendix 5. LILACS (BIREME) search strategy
(mh:meningitis OR meningit* OR mh:c10.228.228.507* OR mh:c10.228.566* OR mh:"Meningococcal Infections" OR mh:c01.252.400.625.549* OR meningococ* OR mh:"Pneumococcal Infections" OR mh:c01.252.410.890.670* OR "Infecciones Neumocócicas" OR "Infecções Pneumocócicas" OR "Streptococcus pneumoniae Infections" OR mh:"Neisseria meningitidis" OR mh:b03.440.400.425.550.550.641* OR mh:b03.660.075.525.520.500* OR mh:"Escherichia coli Infections" OR "Escherichia coli" OR "e coli" OR mh:"Listeria monocytogenes" OR mh:"Meningitis, Listeria" OR "listeria monocytogenes" OR mh:"Haemophilus influenzae type b" OR "Haemophilus influenzae type b" OR hib OR mh:"Haemophilus Infections" OR "hemophilus infections" OR "haemophilus infections" OR "Infecciones por Haemophilus" OR "Infecções por Haemophilus" OR mh:"Streptococcus agalactiae" OR "Streptococcus Group B") AND (mh:"Fluid Therapy" OR mh:e02.319.360* OR fluidoterapia OR hidratação OR rehydrat* OR hydrat* OR dehydrat* OR "Terapia con Líquidos" OR rehidratación OR reidratação OR mh:"Body Fluids" OR fluid* OR "Líquidos Corporales" OR "Líquidos Corporais" OR mh:solutions OR solution* OR soluciones OR soluções OR mh:"Hypertonic Solutions" OR mh:"isotonic solutions" OR mh:"rehydration solutions" OR mh:"Sodium Chloride" OR saline* OR sodium* OR hyponatr* OR hiponatremia OR mh:"Infusions, Parenteral" OR mh:e02.319.267.510* OR "Infusiones Parenterales" OR "Infusões Parenterais" OR "Parenteral Infusions" OR mh:"Water Deprivation" OR "Privación de Agua" OR "Privação de Água" OR mh:"Water‐Electrolyte Balance" OR "Equilibrio Hidroelectrolítico" OR "Equilíbrio Hidroeletrolítico" OR mh:g02.111.917* OR mh:g03.960* OR mh:g07.700.360.888* OR "Balance Hidroelectrolítico" OR "Equilibrio Líquido" OR osmorregulación OR "Equilíbrio Hidreletrolítico" OR "Balanço Hidroeletrolítico" OR "Balanço Hidreletrolítico" OR "Balanço Líquido" OR "Regulação Osmótica" OR mh:"Water‐Electrolyte Imbalance" OR "Desequilibrio Hidroelectrolítico" OR "Desequilíbrio Hidroeletrolítico" OR electrolyt* OR mh:albumins OR albumin* OR mh:d12.776.034* OR mh:plasma OR plasma OR mh:a12.207.152.693* OR mh:a12.207.270.695* OR mh:a15.145.693* OR starch* OR dextran* OR gelofus* OR haemacc* OR hemacc*) AND db:("LILACS") AND type_of_study:("clinical_trials")
Appendix 6. Web of Science (Thomson Reuters) search strategy
| # 6 | #4 AND #3 Refined by: Publication Years=( 2010 OR 2011 OR 2012 OR 2013 ) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All years | |
|
| ||
| # 5 | #4 AND #3 Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All years | |
|
| ||
| # 4 | Topic=(random* or placebo* or crossover* or "cross over" or allocat* or ((singl* or doubl*) NEAR/1 blind*)) OR Title=(trial) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All years | |
|
| ||
| # 3 | #2 AND #1 Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All years | |
|
| ||
| # 2 | Topic=(fluid* or solution* or dehydrat* or hydrat* or rehydrat* or saline* or sodium* or hyponatr* or "parenteral infusion*" or "water deprivat*" or "water restrict*" or electrolyt* or albumin* or plasma* or starch* or dextran* or gelofus* or haemacc* or hemac*) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All years | |
|
| ||
| # 1 | Topic=(meningit* or meningococ*) Databases=SCI‐EXPANDED, CPCI‐S, CCR‐EXPANDED, IC Timespan=All years | |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
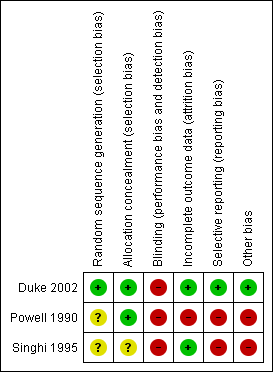
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Maintenance fluids versus restricted fluids, Outcome 1 Death.
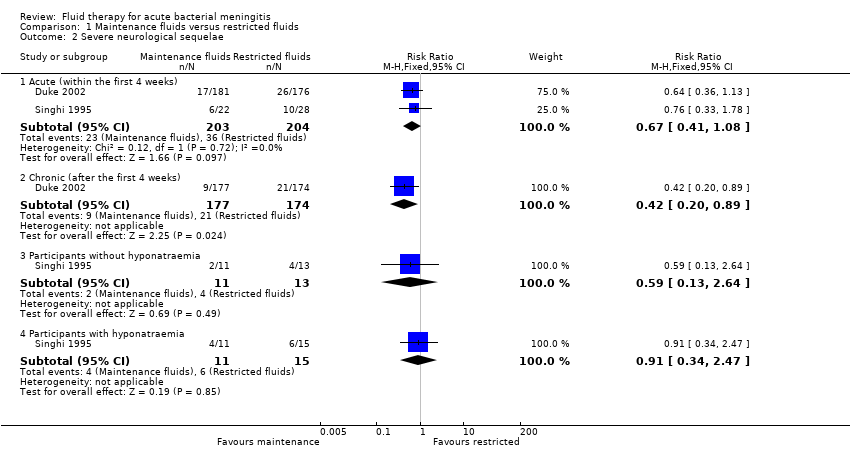
Comparison 1 Maintenance fluids versus restricted fluids, Outcome 2 Severe neurological sequelae.

Comparison 1 Maintenance fluids versus restricted fluids, Outcome 3 Mild to moderate neurological sequelae.

Comparison 1 Maintenance fluids versus restricted fluids, Outcome 4 Hemiparesis/hemiplegia.
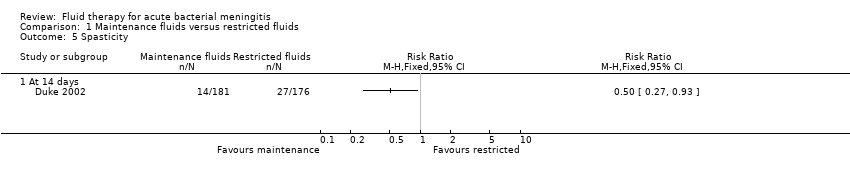
Comparison 1 Maintenance fluids versus restricted fluids, Outcome 5 Spasticity.

Comparison 1 Maintenance fluids versus restricted fluids, Outcome 6 Seizures.

Comparison 1 Maintenance fluids versus restricted fluids, Outcome 7 Visual impairment.

Comparison 1 Maintenance fluids versus restricted fluids, Outcome 8 No response to sound.
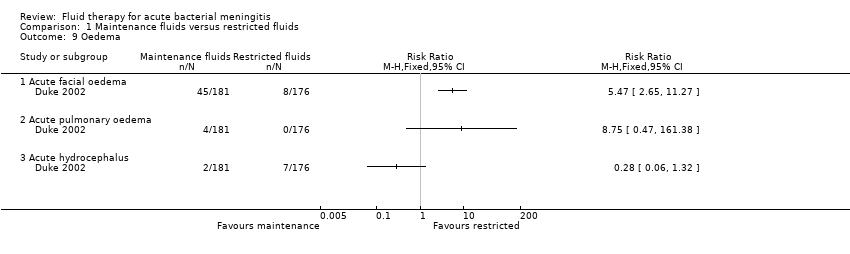
Comparison 1 Maintenance fluids versus restricted fluids, Outcome 9 Oedema.

Comparison 1 Maintenance fluids versus restricted fluids, Outcome 10 Total body water ‐ fall after 48 hours.
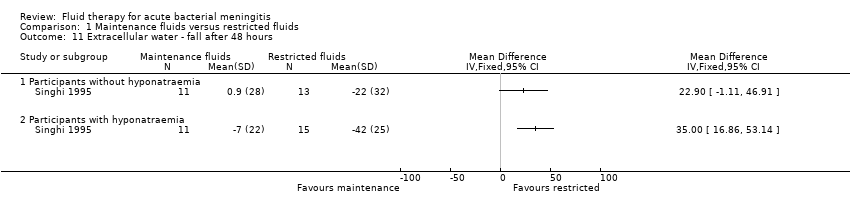
Comparison 1 Maintenance fluids versus restricted fluids, Outcome 11 Extracellular water ‐ fall after 48 hours.
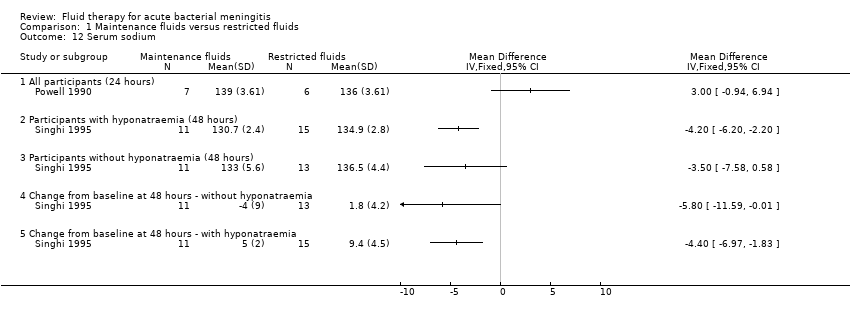
Comparison 1 Maintenance fluids versus restricted fluids, Outcome 12 Serum sodium.
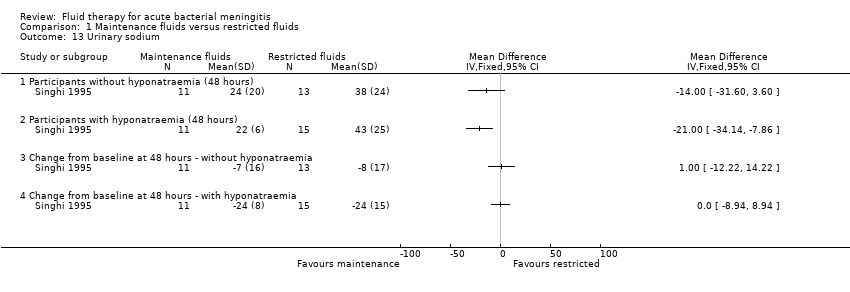
Comparison 1 Maintenance fluids versus restricted fluids, Outcome 13 Urinary sodium.
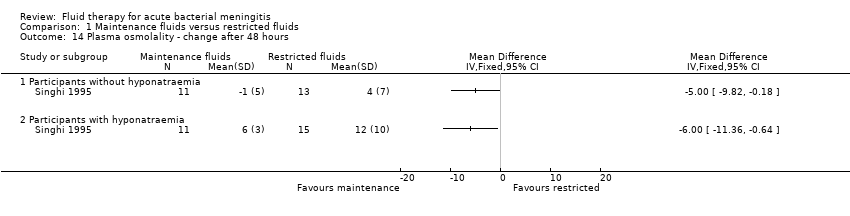
Comparison 1 Maintenance fluids versus restricted fluids, Outcome 14 Plasma osmolality ‐ change after 48 hours.
| Maintenance fluids versus restricted fluids for acute bacterial meningitis in paediatric populations | ||||||
| Patient or population: patients with acute bacterial meningitis in paediatric populations 1 | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Maintenance fluids versus restricted fluids | |||||
| Death ‐ all patients | Study population | RR 0.82 | 407 | ⊕⊕⊕⊝ | ||
| 186 per 1000 | 153 per 1000 | |||||
| Moderate | ||||||
| 213 per 1000 | 175 per 1000 | |||||
| Severe neurological sequelae ‐ acute (within the first 4 weeks) | Study population | RR 0.67 | 407 | ⊕⊝⊝⊝ | ||
| 176 per 1000 | 118 per 1000 | |||||
| Moderate | ||||||
| 252 per 1000 | 169 per 1000 | |||||
| Severe neurological sequelae ‐ chronic (after the first 4 weeks) | Study population | RR 0.42 | 351 | ⊕⊕⊕⊝ | ||
| 121 per 1000 | 51 per 1000 | |||||
| Moderate | ||||||
| 121 per 1000 | 51 per 1000 | |||||
| Mild to moderate neurological sequelae | Study population | RR 1.24 | 357 | ⊕⊕⊕⊝ | ||
| 62 per 1000 | 78 per 1000 | |||||
| Moderate | ||||||
| 63 per 1000 | 78 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% Confidence Interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 No studies were found comparing different intravenous fluid regimens in adult populations in the systematic review. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All participants | 2 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.53, 1.27] |
| 1.2 Participants with hyponatraemia | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.50] |
| 1.3 Participants without hyponatraemia | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.16, 3.90] |
| 2 Severe neurological sequelae Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Acute (within the first 4 weeks) | 2 | 407 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.41, 1.08] |
| 2.2 Chronic (after the first 4 weeks) | 1 | 351 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.20, 0.89] |
| 2.3 Participants without hyponatraemia | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.13, 2.64] |
| 2.4 Participants with hyponatraemia | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.34, 2.47] |
| 3 Mild to moderate neurological sequelae Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Hemiparesis/hemiplegia Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 At 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Spasticity Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 At 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Seizures Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.1 Within the first 72 hours | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Visual impairment Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 At 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 No response to sound Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 14 days | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Oedema Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Acute facial oedema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Acute pulmonary oedema | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 Acute hydrocephalus | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Total body water ‐ fall after 48 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 Participants without hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10.2 Participants with hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Extracellular water ‐ fall after 48 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11.1 Participants without hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Participants with hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Serum sodium Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 All participants (24 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.2 Participants with hyponatraemia (48 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.3 Participants without hyponatraemia (48 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.4 Change from baseline at 48 hours ‐ without hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12.5 Change from baseline at 48 hours ‐ with hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Urinary sodium Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Participants without hyponatraemia (48 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Participants with hyponatraemia (48 hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Change from baseline at 48 hours ‐ without hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 Change from baseline at 48 hours ‐ with hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Plasma osmolality ‐ change after 48 hours Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 Participants without hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 Participants with hyponatraemia | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

