预防脑膜炎球菌感染的抗生素
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Cluster‐randomisation by households, quasi‐randomisation by order of admission to hospital, open, no loss to f/u | |
| Participants | Household contacts, Nigeria | |
| Interventions | Rifampin: 0 to 2 years: 4 x 75 mg; 2 to 4 years 4 x 150 mg; 5 to 14 years 4 x 300 mg; 15+ years 4 x 600 mg versus sulphadimidine: 0 to 4 years 4 x 250 mg; 5 to 14 years 4 x 500 mg; 15+ years 4 x 1 G | |
| Outcomes | Morbidity, eradication, resistance developed | |
| Notes | Data presented for carriers. Main serogroup: A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Low risk | No loss to f/u |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Double‐blind, no ITT | |
| Participants | Kindergarten and school children, Chile | |
| Interventions | Rifampin: 2 x 10 mg/kg versus placebo | |
| Outcomes | Eradication, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Cluster‐randomisation by households, open, no loss to f/u | |
| Participants | Household contacts, Malawi | |
| Interventions | Rifampicin: 2 to 18 years 4 x 20 mg/kg; > 18 years 4 x 600 mg | |
| Outcomes | Morbidity, eradication, re‐acquisition, adverse effects | |
| Notes | Data presented for carriers. Main serogroups: A W135. Ceftriaxone group not randomised and not included in analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Low risk | No loss to f/u |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, double‐blind, ITT | |
| Participants | Students, USA | |
| Interventions | Rifampin: 4 x 600 mg versus placebo | |
| Outcomes | Eradication, failure serogroup, adverse effects | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Students, USA | |
| Interventions | Cephalexin: 12 x 500 mg versus placebo | |
| Outcomes | Eradication, adverse effects, re‐acquisition | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Open, no ITT | |
| Participants | USSR | |
| Interventions | Rifampin: 4 x 300 mg versus none | |
| Outcomes | Eradication, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Coumermycin A1: 14 x 50 mg versus placebo | |
| Outcomes | Eradication, acquisition, failure serogroup | |
| Notes | Data extracted for 1) all (regardless of carrier status), 2) carriers only, 3) non‐carriers only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Rifampin: 4 x 600 mg versus placebo | |
| Outcomes | Eradication, failure serogroup of eradication failure | |
| Notes | Data extracted for 1) all (regardless of carrier status), 2) for carriers only, 3) for non‐carriers only. Main serogroup: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Minocycline 14 x 500 mg versus placebo | |
| Outcomes | Eradication | |
| Notes | Main serogroup: Y | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, open, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Minocycline: 14 x 500 mg versus none | |
| Outcomes | Eradication, adverse effects, resistance developed | |
| Notes | Data extracted for 1) all (regardless of carrier status) and 2) carriers only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Double‐blind, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Ampicillin: 30 x 500 mg versus oral penicillin G: 30 x 462 mg versus placebo | |
| Outcomes | Eradication, serogroup of eradication failure, resistance developed | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Double‐blind, ITT | |
| Participants | Volunteers | |
| Interventions | Ciprofloxacin 1 x 750 mg versus placebo | |
| Outcomes | Eradication, resistance developed, adverse effects | |
| Notes | Main serogroups: B, Z | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, open, no ITT | |
| Participants | Patients with extragenital gonorrhoea, USA | |
| Interventions | Bacampicillin: 12 x 400 mg versus amoxycillin: 6 x 500 mg | |
| Outcomes | Eradication, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, open, no ITT | |
| Participants | Nursing students, Cairo | |
| Interventions | Azythromycin: 1 x 500 mg versus rifampin: 4 x 600 mg | |
| Outcomes | Eradication, reacquisition, resistance developed, adverse effects | |
| Notes | Main serogroups: A, B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Cluster‐randomisation by companies, adequate generation of allocation, open, no ITT | |
| Participants | Army recruits, USA | |
| Interventions | Rifampin: 1 x 600 mg versus minocycline: 10 x 100 mg versus ampicillin: 10 x 500 mg versus placebo | |
| Outcomes | Morbidity, eradication, resistance developed | |
| Notes | Separate data provided for rifampin and minocycline treatment arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Outcome assessor blinded, ITT | |
| Participants | Patients with culture or smear positive for anogenital gonorrhoea or confirmed recent exposure to gonorrhoea | |
| Interventions | IM ceftriaxone 1 x 125 mg versus IM spectinomycin 1 x 2 g | |
| Outcomes | Eradication, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | No |
| Blinding of outcome assessment (detection bias) | Low risk | Yes |
| Incomplete outcome data (attrition bias) | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, open, no ITT, no drop‐outs | |
| Participants | Household contacts, USA | |
| Interventions | Rifampin: > 66 lb weight 4 x 600 mg/day; < 66 lb 4 x 300 mg/day versus none | |
| Outcomes | Morbidity, eradication, failure serogroup | |
| Notes | Data presented for carriers. Main serogroups: C, N | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Low risk | No drop‐outs |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Open, no ITT | |
| Participants | Volunteers, Turkey | |
| Interventions | Ciprofloxacin: 1 x 750 mg versus rifampin: 4 x 600 mg | |
| Outcomes | Eradication, adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Cluster‐randomisation by households, quasi‐randomisation assigned by order of arrival at study centre, open, no ITT | |
| Participants | Household contacts, Brazil | |
| Interventions | Sulphadiazine: 4 x 1 g versus minocycline: 1 x 200 mg + 5 x 100 mg versus rifampin: 4 x 600 mg versus minocycline/rifampin: as above | |
| Outcomes | Eradication, serogroup of eradication failure, resistance developed, adverse effects | |
| Notes | Data presented for carriers. Main serogroup: C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quasi‐randomisation |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | College students | |
| Interventions | Sch 29,482: 4 x 250 mg versus placebo | |
| Outcomes | Eradication | |
| Notes | Main serogroups: B, Z | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | High risk | Not used |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Volunteers | |
| Interventions | Ciprofloxacin: 10 x 500 mg versus placebo | |
| Outcomes | Eradication, resistance developed, failure serogroup adverse effects | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Adequate allocation generation, double‐blind, no ITT | |
| Participants | Army recruits | |
| Interventions | Ciprofloxacin: 4 x 250 mg versus placebo | |
| Outcomes | Eradication, serogroup of eradication failure, resistance developed, adverse effects | |
| Notes | Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate allocation generation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Cluster‐randomisation by households, open, no ITT | |
| Participants | Household contacts, Saudi Arabia | |
| Interventions | IM ceftriaxone 1 x 250 mg or 125 mg for children versus rifampin: 4 x 600 mg or 10 mg/kg versus none | |
| Outcomes | Eradication, acquisition in non‐carriers | |
| Notes | Data presented for carriers. Serogroup A | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
| Methods | Open, no ITT | |
| Participants | Household contacts, New Zealand | |
| Interventions | Rifampicin: 600 mg, children > 1 month, 10 mg/kg versus IM ceftriaxone: 250 mg < 12 years, 125 mg | |
| Outcomes | Morbidity, eradication, eradication of serogroup B, adverse effects | |
| Notes | Data presented for carriers. Main serogroup: B | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding of participants and personnel (performance bias) | High risk | Open |
| Blinding of outcome assessment (detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Unclear risk | No ITT |
| Selective reporting (reporting bias) | Low risk | No evidence |
| Other bias | Low risk | No evidence |
f/u: follow‐up
IM: intramuscular
ITT: intention‐to‐treat
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| No control group | |
| Trial of post‐exposure prophylaxis for H. influenzae and not N. meningitidis | |
| No randomisation | |
| Includes previously published data (Guttler 1971) included in this review. New data from a non‐randomised study also excluded | |
| No control group | |
| No randomisation | |
| No randomisation | |
| No randomisation | |
| No randomisation | |
| Not a controlled trial | |
| Not a RCT. The type of publication was a letter to the editor and did not contain any relevant data | |
| Not a controlled trial | |
| No randomisation | |
| No randomisation | |
| No control group | |
| No randomisation | |
| Patients with homozygous deficiency of the sixth component of complement (C6) with recurrent meningococcal meningitis | |
| No control group | |
| No control group | |
| No randomisation | |
| Not a RCT. The type of publication was a letter to the editor and did not contain any relevant data | |
| No randomisation |
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rifampin versus ceftriaxone Show forest plot | 1 | 856 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.10, 1.75] |
| Analysis 1.1 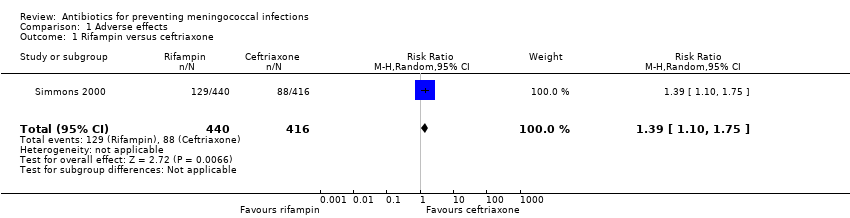 Comparison 1 Adverse effects, Outcome 1 Rifampin versus ceftriaxone. | ||||
| 2 Rifampin versus ciprofloxacin Show forest plot | 2 | 1598 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.56] |
| Analysis 1.2 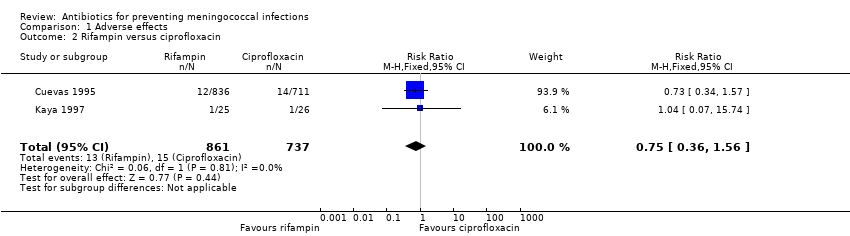 Comparison 1 Adverse effects, Outcome 2 Rifampin versus ciprofloxacin. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ciprofloxacin versus placebo Show forest plot | 3 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.12] |
| Analysis 2.1 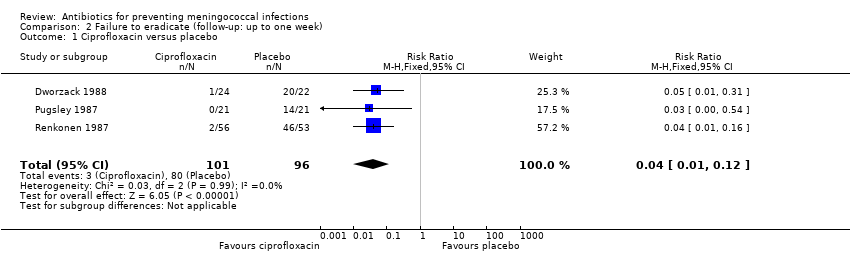 Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 1 Ciprofloxacin versus placebo. | ||||
| 2 Rifampin versus placebo Show forest plot | 6 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.13, 0.24] |
| Analysis 2.2  Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 2 Rifampin versus placebo. | ||||
| 3 Minocycline versus placebo Show forest plot | 3 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.21, 0.37] |
| Analysis 2.3  Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 3 Minocycline versus placebo. | ||||
| 4 Penicillin versus placebo Show forest plot | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.24, 0.94] |
| Analysis 2.4  Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 4 Penicillin versus placebo. | ||||
| 5 Other antibiotics versus placebo Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.5 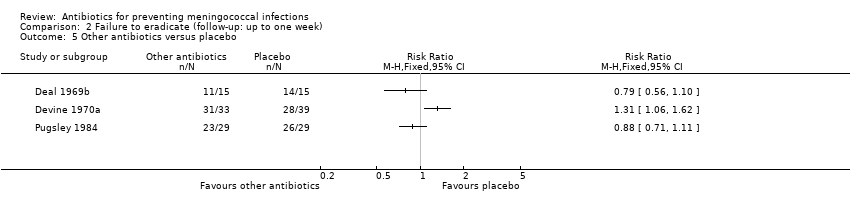 Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 5 Other antibiotics versus placebo. | ||||
| 6 Rifampin versus ciprofloxacin Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.11, 1.02] |
| Analysis 2.6  Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 6 Rifampin versus ciprofloxacin. | ||||
| 7 Rifampin versus ceftriaxone Show forest plot | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.73, 18.86] |
| Analysis 2.7  Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 7 Rifampin versus ceftriaxone. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ciprofloxacin versus placebo Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.42] |
| Analysis 3.1  Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 1 Ciprofloxacin versus placebo. | ||||
| 2 Rifampin versus placebo Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.14, 0.29] |
| Analysis 3.2  Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 2 Rifampin versus placebo. | ||||
| 3 Minocycline versus placebo Show forest plot | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.31] |
| Analysis 3.3  Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 3 Minocycline versus placebo. | ||||
| 4 Penicillin versus placebo Show forest plot | 2 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.51, 0.79] |
| Analysis 3.4  Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 4 Penicillin versus placebo. | ||||
| 5 Other antibiotics versus placebo Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.5 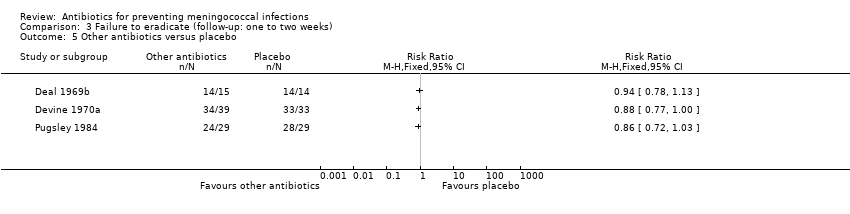 Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 5 Other antibiotics versus placebo. | ||||
| 6 Rifampin versus ciprofloxacin Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.09, 1.11] |
| Analysis 3.6  Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 6 Rifampin versus ciprofloxacin. | ||||
| 7 Rifampin versus ceftriaxone Show forest plot | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.93 [1.22, 28.68] |
| Analysis 3.7  Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 7 Rifampin versus ceftriaxone. | ||||
| 8 Rifampin versus minocycline Show forest plot | 2 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.57, 1.77] |
| Analysis 3.8  Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 8 Rifampin versus minocycline. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rifampin versus placebo Show forest plot | 3 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.16, 0.37] |
| Analysis 4.1  Comparison 4 Failure to eradicate (follow‐up: between two to three weeks), Outcome 1 Rifampin versus placebo. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rifampin versus placebo Show forest plot | 2 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.16, 0.38] |
| Analysis 5.1 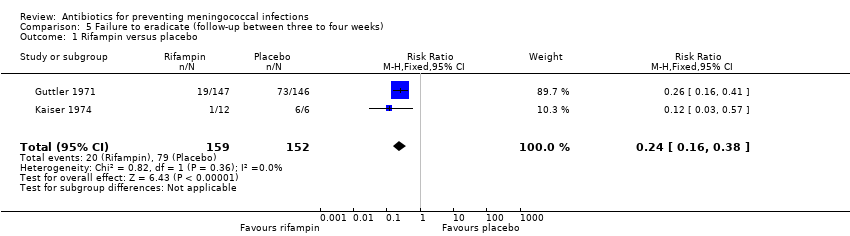 Comparison 5 Failure to eradicate (follow‐up between three to four weeks), Outcome 1 Rifampin versus placebo. | ||||
| 2 Penicillin versus placebo Show forest plot | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.53, 1.68] |
| Analysis 5.2 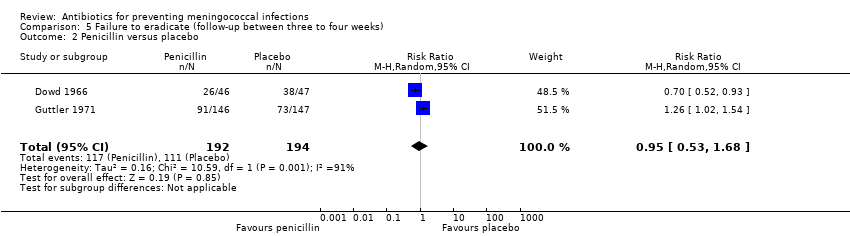 Comparison 5 Failure to eradicate (follow‐up between three to four weeks), Outcome 2 Penicillin versus placebo. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rifampin versus minocycline Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.48, 1.97] |
| Analysis 6.1  Comparison 6 Failure to eradicate (follow‐up: five weeks), Outcome 1 Rifampin versus minocycline. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Drop‐outs Show forest plot | 13 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| Analysis 7.1  Comparison 7 Exclusion after randomisation, Outcome 1 Drop‐outs. | ||||
| 1.1 Drop‐outs at around one week of follow‐up | 13 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
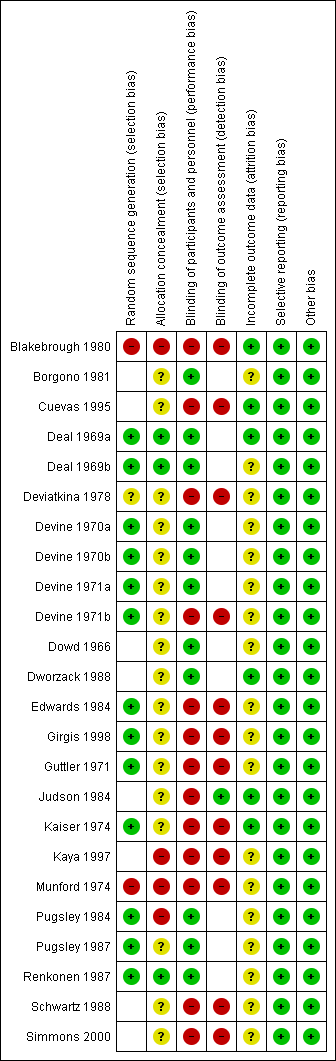
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Adverse effects, Outcome 1 Rifampin versus ceftriaxone.

Comparison 1 Adverse effects, Outcome 2 Rifampin versus ciprofloxacin.

Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 1 Ciprofloxacin versus placebo.

Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 2 Rifampin versus placebo.

Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 3 Minocycline versus placebo.

Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 4 Penicillin versus placebo.

Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 5 Other antibiotics versus placebo.

Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 6 Rifampin versus ciprofloxacin.

Comparison 2 Failure to eradicate (follow‐up: up to one week), Outcome 7 Rifampin versus ceftriaxone.

Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 1 Ciprofloxacin versus placebo.

Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 2 Rifampin versus placebo.

Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 3 Minocycline versus placebo.

Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 4 Penicillin versus placebo.

Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 5 Other antibiotics versus placebo.

Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 6 Rifampin versus ciprofloxacin.

Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 7 Rifampin versus ceftriaxone.

Comparison 3 Failure to eradicate (follow‐up: one to two weeks), Outcome 8 Rifampin versus minocycline.

Comparison 4 Failure to eradicate (follow‐up: between two to three weeks), Outcome 1 Rifampin versus placebo.

Comparison 5 Failure to eradicate (follow‐up between three to four weeks), Outcome 1 Rifampin versus placebo.

Comparison 5 Failure to eradicate (follow‐up between three to four weeks), Outcome 2 Penicillin versus placebo.

Comparison 6 Failure to eradicate (follow‐up: five weeks), Outcome 1 Rifampin versus minocycline.

Comparison 7 Exclusion after randomisation, Outcome 1 Drop‐outs.
| Study ID | Comparison | Study population | % carriers (N) |
| Rifampin versus sulphadimidine | Household contacts | 17 (479) | |
| Rifampin versus placebo | Children | 12 (2132) | |
| Rifampin versus ciprofloxacin versus ceftriaxone | Household contacts | 11 (1875) | |
| Rifampin versus placebo | Students | 14.4 (270) | |
| Cephalexin versus placebo | Students | 9.4 (352) | |
| Rifampin versus placebo | Military recruits | 64 (103) | |
| Minocycline versus placebo | Military recruits | 72 (121) | |
| Coumermycin A1 versus placebo | Military recruits | 55 (129) | |
| Ciprofloxacin versus placebo | Volunteers | 6.7 (620) | |
| Azithromycin versus placebo | Students | 24 (500) | |
| Rifampin versus minocycline | Military recruits | 21 (587) | |
| Rifampin versus placebo | Household contacts | 35 (54) | |
| Ciprofloxacin versus rifampin | Hospital staff | 18 (300) | |
| Rifampin versus minocycline versus minocycline/rifampin versus sulphadiazine | Household contacts | 25 (1187) | |
| Sch 29,482 versus placebo | Volunteers | 25 (555) | |
| Ciprofloxacin versus placebo | Students | 7 (461) | |
| Ciprofloxacin versus placebo | Military recruits | 38.6 (552) | |
| Rifampin versus ceftriaxone | Household contacts | 33 (347) | |
| Rifampin versus ceftriaxone | Household contacts | 21 (864) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rifampin versus ceftriaxone Show forest plot | 1 | 856 | Risk Ratio (M‐H, Random, 95% CI) | 1.39 [1.10, 1.75] |
| 2 Rifampin versus ciprofloxacin Show forest plot | 2 | 1598 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.36, 1.56] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ciprofloxacin versus placebo Show forest plot | 3 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.01, 0.12] |
| 2 Rifampin versus placebo Show forest plot | 6 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.17 [0.13, 0.24] |
| 3 Minocycline versus placebo Show forest plot | 3 | 464 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.21, 0.37] |
| 4 Penicillin versus placebo Show forest plot | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.24, 0.94] |
| 5 Other antibiotics versus placebo Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Rifampin versus ciprofloxacin Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.11, 1.02] |
| 7 Rifampin versus ceftriaxone Show forest plot | 2 | 286 | Risk Ratio (M‐H, Random, 95% CI) | 3.71 [0.73, 18.86] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Ciprofloxacin versus placebo Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.03 [0.00, 0.42] |
| 2 Rifampin versus placebo Show forest plot | 5 | 495 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.14, 0.29] |
| 3 Minocycline versus placebo Show forest plot | 2 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.37 [0.10, 1.31] |
| 4 Penicillin versus placebo Show forest plot | 2 | 386 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.51, 0.79] |
| 5 Other antibiotics versus placebo Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Rifampin versus ciprofloxacin Show forest plot | 2 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.09, 1.11] |
| 7 Rifampin versus ceftriaxone Show forest plot | 1 | 91 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.93 [1.22, 28.68] |
| 8 Rifampin versus minocycline Show forest plot | 2 | 419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.57, 1.77] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rifampin versus placebo Show forest plot | 3 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.16, 0.37] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rifampin versus placebo Show forest plot | 2 | 311 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.16, 0.38] |
| 2 Penicillin versus placebo Show forest plot | 2 | 386 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.53, 1.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Rifampin versus minocycline Show forest plot | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.48, 1.97] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Drop‐outs Show forest plot | 13 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |
| 1.1 Drop‐outs at around one week of follow‐up | 13 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.89, 1.15] |

