Ultrazvučno temeljeni algoritmi za dijagnosticiranje tupih trauma abdomena u hitnoj medicini
Abstract
Background
Ultrasonography (performed by means of a four‐quadrant, focused assessment of sonography for trauma (FAST)) is regarded as a key instrument for the initial assessment of patients with suspected blunt abdominal and thoraco‐abdominal trauma in the emergency department setting. FAST has a high specificity but low sensitivity in detecting and excluding visceral injuries. Proponents of FAST argue that ultrasound‐based clinical pathways enhance the speed of primary trauma assessment, reduce the number of unnecessary multi‐detector computed tomography (MDCT) scans, and enable quicker triage to surgical and non‐surgical care. Given the proven accuracy, increasing availability of, and indication for, MDCT among patients with blunt abdominal and multiple injuries, we aimed to compile the best available evidence of the use of FAST‐based assessment compared with other primary trauma assessment protocols.
Objectives
To assess the effects of diagnostic algorithms using ultrasonography including in FAST examinations in the emergency department in relation to the early, late, and overall mortality of patients with suspected blunt abdominal trauma.
Search methods
The most recent search was run on 30th June 2015. We searched the Cochrane Injuries Group Specialised Register, The Cochrane Library, MEDLINE (OvidSP), EMBASE (OvidSP), ISI Web of Science (SCI‐EXPANDED, SSCI, CPCI‐S, and CPSI‐SSH), clinical trials registers, and screened reference lists. Trial authors were contacted for further information and individual patient data.
Selection criteria
We included randomised controlled trials (RCTs). Participants were patients with blunt torso, abdominal, or multiple trauma undergoing diagnostic investigations for abdominal organ injury. The intervention was diagnostic algorithms comprising emergency ultrasonography (US). The control was diagnostic algorithms without US examinations (for example, primary computed tomography (CT) or diagnostic peritoneal lavage (DPL)). Outcomes were mortality, use of CT or invasive procedures (DPL, laparoscopy, laparotomy), and cost‐effectiveness.
Data collection and analysis
Two authors (DS and CG) independently selected trials for inclusion, assessed methodological quality, and extracted data. Methodological quality was assessed using the Cochrane Collaboration risk of bias tool. Where possible, data were pooled and relative risks (RRs), risk differences (RDs), and weighted mean differences, each with 95% confidence intervals (CIs), were calculated by fixed‐effect or random‐effects models as appropriate.
Main results
We identified four studies meeting our inclusion criteria. Overall, trials were of poor to moderate methodological quality. Few trial authors responded to our written inquiries seeking to resolve controversial issues and to obtain individual patient data. Strong heterogeneity amongst the trials prompted discussion between the review authors as to whether the data should or should not be pooled; we decided in favour of a quantitative synthesis to provide a rough impression about the effect sizes achievable with US‐based triage algorithms. We pooled mortality data from three trials involving 1254 patients; the RR in favour of the FAST arm was 1.00 (95% CI 0.50 to 2.00). FAST‐based pathways reduced the number of CT scans (random‐effects model RD ‐0.52, 95% CI ‐0.83 to ‐0.21), but the meaning of this result was unclear.
Authors' conclusions
The experimental evidence justifying FAST‐based clinical pathways in diagnosing patients with suspected abdominal or multiple blunt trauma remains poor. Because of strong heterogeneity between the trial results, the quantitative information provided by this review may only be used in an exploratory fashion. It is unlikely that FAST will ever be investigated by means of a confirmatory, large‐scale RCT in the future. Thus, this Cochrane Review may be regarded as a review which provides the best available evidence for clinical practice guidelines and management recommendations. It can only be concluded from the few head‐to‐head studies that negative US scans are likely to reduce the incidence of MDCT scans which, given the low sensitivity of FAST (or reliability of negative results), may adversely affect the diagnostic yield of the trauma survey. At best, US has no negative impact on mortality or morbidity. Assuming that major blunt abdominal or multiple trauma is associated with 15% mortality and a CT‐based diagnostic work‐up is considered the current standard of care, 874, 3495, or 21,838 patients are needed per intervention group to demonstrate non‐inferiority of FAST to CT‐based algorithms with non‐inferiority margins of 5%, 2.5%, and 1%, power of 90%, and a type‐I error alpha of 5%.
PICOs
Laički sažetak
Korištenje ultrazvuka kao pomoć u dijagnostici pacijenata s tupom ozljedom trbuha
Brojni pacijenti koji su zaprimljeni u bolnicu nakon povrede imaju tupu (nepenetrantnu) ozljedu trbuha. Liječnici koji zbrinjavaju te pacijente moraju znati jesu li ozlijeđeni organi trbušne šupljine. Vjeruje se kako pregled ultrazvukom pomaže u dijagnosticiranju stanja pacijenta. U ovom Cochrane sustavnom pregledu autori su tražili studije koje uspoređuju stope smrtnosti u bolesnika s trbušnim ozljedama gdje se koristio ultrazvuk kao pomoć u dijagnostici sa stopama smrtnosti u kojima nije korišten ultrazvuk. Također su tražili dokaze može li uporaba ultrazvuka smanjiti potrebu za obavljanje drugih složenijih i skupljih dijagnostičkih postupaka. Međutim, učinjeno je vrlo malo istraživanja stoga su autori zaključili da nema dovoljno dokaza koji bi opravdali korištenje ultrazvuka kao dijela dijagnostike u pacijenata s ozljedom trbuha. Obzirom na stupanj nesigurnosti vjerojatno je opravdano pitati liječnika na dužnosti za potvrdni CT u pacijenata koji su zadobili ozljede s visokom mogućnošću teških posljedica (kao što su ozljede glave i mozga, prijelom vratne kralježnice, ozljede u području prsišta, trbuha i zdjelice, kao i ostalih ozljeda).
Authors' conclusions
Background
Description of the condition
Trauma is a global public concern. While penetrating injuries are mainly due to assaults and violence (especially stabbing and shooting), blunt injuries are typically caused by road traffic crashes or falls from a great height. According to the World Health Organization (WHO) Global Burden of Disease Study, road traffic crashes ranked eight (Lozano 2012) and 10th (Murray 2012) amongst all conditions contributing to global mortality and disability‐adjusted life years (DALY) lost in 2010. Consequently, the United Nations and WHO Decade of Action for Road Safety (http://www.who.int/roadsafety/decade_of_action/en) seeks not only to prevent crashes but to establish effective measures of post‐crash response to improve trauma outcomes worldwide (Peden 2010).
Closed abdominal injuries (for example, injuries to solid organs like the liver or spleen, mesenteric and hollow visceral tears) may lead to significant bleeding and haemodynamic instability after restoration of the circulation. Uncontrollable haemorrhage remains a leading cause of mortality from trauma (Brockamp 2012; Cohen 2012; Evans 2010; Pfeifer 2009). Point of care imaging aims at identifying life‐threatening injuries to enable priority‐oriented management ('treat first what kills first'). Interventions should be effective (in terms of diagnostic accuracy) and efficient (in terms of invasiveness, potential harms, time consumption, and resource use). False‐negative findings or delayed diagnoses bear the risk of severe complications. Physical signs and symptoms that indicate the presence of visceral lesions are unreliable (Nishijima 2012), especially in intubated or comatose patients.
Diagnostic problems with abdominal trauma must, however, be discussed in the light of the increasing trend towards non‐operative treatment of intra‐abdominal lesions (Demetriades 2006; Oyo‐Ita 2012; Raza 2013; van der Wilden 2012; Velmahos 2010). In addition, damage‐control haemostatic resuscitation protocols (Cirocchi 2013; Curry 2011; Gruen 2012; Ker 2012; Maegele 2012) as well as transvascular procedures (van der Vlies 2010) to stop bleeding from the liver, spleen, and mesenteric tears have significantly changed the management of abdominal trauma during the past decade.
Description of the intervention
Ultrasonography is a quick, non‐invasive, repeatable and inexpensive tool that has emerged as a key component of diagnostic algorithms and clinical pathways. In the trauma bay ultrasonography is mainly used in terms of focused assessment of sonography for trauma (FAST) to detect the presence of free fluid as an indicator of organ injury (Scalea 1999). However, the prevalence of organ injury without accompanying free fluid ranges from 5% to 37% (Yoshii 1998). With the evolution of ultrasound hardware and increasing resolution, FAST has been expanded to detect intrathoracic fluid and pneumothorax as well as solid organ injuries.
In a systematic review and meta‐analysis of the scientific literature we demonstrated that ultrasound has excellent specificity but rather low sensitivity (below 90%) in identifying both free fluid and organ lacerations (Stengel 2001). This means that a positive sonogram proves the presence of intraperitoneal injury, whereas a negative sonogram fails to confidently exclude traumatic organ lesions. In an update and meta‐regression analysis, we showed that sensitivity was overestimated by poorly designed studies due to partial verification bias (Stengel 2005). However, a major criticism of this study was that the findings considered false‐negative encompassed a broad range of minor and possibly trivial lesions that were unlikely to harm the patients.
Among the diagnostic tools available, diagnostic peritoneal lavage (DPL) has remained the standard initial diagnostic investigation for more than 20 years. Although regarded as a safe technique with high sensitivity (Griffin 2007), it has a significant false‐positive rate (Hoff 2002). This exposes the patients to the risk of an unnecessary laparotomy. In a retrospective analysis the incidence of short‐term complications caused by negative laparotomy was 43% (mainly pneumonia) in patients with associated extra‐abdominal injuries, and 20% in patients with no associated extra‐abdominal injuries (Morrison 1996). Still, DPL remains an optional diagnostic tool taught within the framework of Advanced Trauma Life Support (ATLS®) courses worldwide.
Without doubt, multi‐detector computed tomography (MDCT) represents the current diagnostic imaging standard in the trauma setting (Livingston 1998; Soto 2012). In most European trauma centres, the single‐pass whole‐body scan has emerged as the work‐up method of choice (Rademacher 2001; Stengel 2012). Data from the German Trauma Registry suggest a survival benefit with the routine use of the primary whole‐body scan (Huber‐Wagner 2009). The excess exposure to radiation, however, remains a matter of concern (Stengel 2009).
Why it is important to do this review
With the emerging role of single‐pass, whole‐body MDCT as the primary diagnostic imaging tool in trauma in the developed countries (DGU 2011), the utility and value of ultrasonography needs to be redefined. Additionally, the therapeutic consequences derived from the detection of intra‐abdominal bleeding have changed dramatically during the past few years. Damage‐control resuscitation strategies together with transvascular interventions (Bhullar 2012; Cirocchi 2013; Curry 2012; Holcomb 2007; Kirkpatrick 2008) that are currently available enable non‐operative management of most splenic and hepatic injuries (Malhotra 2000; Oyo‐Ita 2012).
On the other hand, MDCT might be neither an available nor an affordable tool for routine trauma investigation in low‐volume centres, rural areas, or developing countries. Thus, evidence is needed as to the effectiveness of the strategy of using ultrasound in diagnostic investigations of patients with suspected blunt abdominal injury or multiple trauma.
Objectives
The primary objective of this review is to study whether diagnostic algorithms using ultrasonography included in FAST examinations in the emergency department reduce early, late, and overall mortality in patients with suspected blunt abdominal trauma. Secondary objectives were to evaluate the impact of ultrasound‐based algorithms on morbidity (that is, subsequent development of inflammatory complications like systemic inflammatory response syndrome (SIRS) or acute respiratory distress syndrome (ARDS) (Keel 2005)), the number of additional diagnostic procedures, and functional and health‐related outcomes.
The following hypotheses were tested:
-
the use of ultrasonography in trauma algorithms is associated with reduced mortality compared with algorithms that do not involve a sonographic examination;
-
some patient subgroups (children, and hypotensive trauma victims) derive greater benefit from ultrasound diagnosis than others;
-
ultrasonography reduces the rate of non‐therapeutic laparotomies;
-
ultrasound decreases the frequency of invasive procedures, such as diagnostic peritoneal lavage (DPL);
-
ultrasound affects the frequency of CT scans;
-
ultrasound‐based clinical pathways are cost‐effective.
Methods
Criteria for considering studies for this review
Types of studies
We considered randomised controlled trials which compared trauma algorithms with ultrasonography, alone or in combination with other established diagnostic tests (computed tomography (CT), DPL, clinical monitoring), to algorithms without the use of ultrasound. Trials were included irrespective of blinding, number of patients allocated, and language of the article.
Types of participants
Haemodynamically stable or unstable patients with suspected blunt abdominal trauma as a single injury or an injury accompanying multiple trauma. Studies investigating patients with stab wounds and gunshot wounds were excluded.
Types of interventions
The experimental intervention consisted of diagnostic algorithms including ultrasonography either to detect free intra‐abdominal fluid (FAST) or organ injury, including follow‐up ultrasound examinations performed by radiologists, non‐radiologist clinicians, or ultrasound technicians, alone or in combination with subsequent confirmatory tests.
The control intervention was any algorithm that used only other established diagnostic tests (that is, CT, DPL, clinical monitoring).
Types of outcome measures
Primary outcomes
-
Overall mortality (as the proportion of patients)
Secondary outcomes
-
Mortality attributable to abdominal injury (i.e., rupture of solid and hollow organs, vascular injury)
-
Rates of missed injuries with and without surgical consequences (as defined by the results of subsequent laparotomy or laparoscopy, autopsy, follow‐up examinations during hospital stay, or necessity for re‐admission following discharge because of false‐negative findings)
-
Non‐therapeutic laparotomy rates (i.e., negative laparotomy performed for false‐positive findings of index tests, including misclassification of organ injury that, by intra‐operative judgement, would have been suitable for conservative treatment)
-
Short‐term (until discharge) and long‐term morbidity (i.e., SIRS, ARDS, sepsis, nosocomial pneumonia, wound infection, abdominal compartment syndrome)
-
Frequency of DPL procedures
-
Frequency of CT examinations
-
Time spent in the trauma bay (emergency department) until surgery, admission to the intensive care unit or peripheral wards, or ambulation
-
Duration of intensive care unit (ICU) stay (days)
-
Length of hospital stay (days)
Search methods for identification of studies
In order to reduce publication and retrieval bias we did not restrict our search by language, date, or publication status.
Electronic searches
The Cochrane Injuries Group Trials Search Co‐ordinator searched the following:
-
Cochrane Injuries Group Specialised Register (30 June 2015);
-
Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2015, Issue 6 of 12);
-
MEDLINE (OvidSP) (1946 to week 4 June 2015);
-
Embase (OvidSP) (1974 to 30 June 2015);
-
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED) (1970 to June 2015);
-
ISI Web of Science: Conference Proceedings Citation Index ‐ Science (CPCI‐S) (1990 to June 2015);
-
CINAHL Plus (EBSCO) (1939 to June 2015).
The authors additionally searched:
-
BIOSIS Previews (DIMDI) (1926 to 19 August 2015);
-
MEDLINE (DIMDI) (1946 to 19 August 2015);
-
PubMed (19 August 2015);
-
Embase (OvidSP) (DIMDI) (1980 to 19 August 2015).
The search strategies are reported in full in Appendix 1.
Searching other resources
Web‐based resources
These included the following:
-
narrative and systematic reviews, clinical practice guidelines, as well as health technology assessment reports;
-
the related articles option in PubMed;
-
Google Scholar.
Handsearching
We scanned reference lists of all relevant articles for further trials.
Author queries
Authors of potentially relevant abstracts were asked to provide full information. We also asked for individual patient data where possible. We contacted authors of relevant articles to enquire if they had information on any past, present, or future studies.
Data collection and analysis
Cochrane Injuries' Trials Search Co‐ordinator ran the searches and sent the search results to one of the authors (DS).
Selection of studies
Two authors (DS and CG) assessed titles or abstracts of all studies identified by the search and excluded clearly non‐relevant studies. Full text articles were obtained for potentially relevant studies and any studies with unclear methodology. All these studies were assessed for eligibility for this review by two authors examining their methods of randomisation and their adequacy of allocation concealment. Disagreements on inclusion were resolved by discussion and, if necessary, scrutiny by an independent third author (GR).
Data extraction and management
Two authors independently extracted the results of each included paper on a data extraction sheet. Disagreements were resolved by discussion.
Assessment of risk of bias in included studies
Each included trial was read independently by two authors for the following aspects of internal and external validity.
A. Was the assigned treatment adequately concealed prior to allocation?
2 = method did not allow disclosure of assignment;
1 = small but possible chance of disclosure of assignment or unclear;
0 = quasi‐randomised or open list or tables.
B. Were the outcomes of patients or participants who withdrew described and included in the analysis (intention to treat)?
2 = withdrawals well described and accounted for in analysis;
1 = withdrawals described and analysis not possible;
0 = no mention, inadequate mention, or obvious differences and no adjustment.
C. Were the outcome assessors blinded to the results of the index test (i.e., ultrasonography) or reference tests or patient outcome, or a combination?
2 = effective action taken to blind assessors;
1 = small or moderate chance of unblinding of assessors;
0 = not mentioned or not possible.
D. Were the treatment and control groups comparable at entry?
2 = good comparability of groups, or confounding adjusted for in analysis;
1 = confounding small or mentioned but not adjusted for;
0 = large potential for confounding, or not discussed.
E. Were care programmes, other than the trial options, identical?
2 = care programmes clearly identical;
1 = clear but trivial differences;
0 = not mentioned, or clear and important differences in care programmes.
F. Were the inclusion and exclusion criteria clearly defined?
2 = clearly defined;
1 = inadequately defined;
0 = not defined.
G. Were the interventions clearly defined?
2 = clearly defined interventions are applied with a standardised protocol;
1 = clearly defined interventions are applied but the application protocol is not standardised;
0 = intervention or application protocol, or both, are poor or not defined.
H. Were the outcome measures used clearly defined (by outcome)?
2 = clearly defined;
1 = inadequately defined;
0 = not defined.
I. Was the surveillance active, and of clinically appropriate duration?
2 = active surveillance and appropriate duration;
1 = active surveillance, but inadequate duration;
0 = surveillance not active or not defined.
Data synthesis
Mean differences and 95% confidence intervals were calculated for continuous variables. For dichotomous outcomes, relative risks (RRs) and risk differences (RDs) with 95% confidence intervals (CIs) were calculated. We used MetaView statistical software in RevMan 5.2 to pool the effect measures within a fixed‐effect or random‐effects model, where appropriate.
To evaluate the between‐study variability we tested for heterogeneity of results. We planned sensitivity and subgroup analyses (children, hypotensive patients, use of ultrasound as a primary versus subsequent work‐up modality, follow‐up examinations, operator experience). To control for possible publication bias, we aimed to test for funnel plot asymmetry.
Results
Description of studies
The study selection procedure is depicted in Figure 1. Most studies examined the diagnostic accuracy of ultrasonography to detect free intraperitoneal fluid or organ damage. Readers interested in the problem of efficacy (accuracy) will find a diagnostic meta‐analysis including a PRISMA flowchart depicting the study selection procedure in Stengel 2005. We identified nine studies that compared the effectiveness and efficiency of ultrasound‐based clinical pathways to algorithms that did not incorporate ultrasound examinations. Four of these (Branney 1997; Healey 1996; Hesse 1999; McKenney 2001) compared cohorts of patients admitted before and after introducing ultrasound as a screening tool and were excluded from further analysis.

Study selection process flow diagram for 2015 search update.
Of the five remaining trials, only two used a randomised format (Melniker 2006; Rose 2001). Another randomised trial (Navarrete‐Navarro 1996) sought to prove the superiority of early computed tomography over multimodal procedures (including bedside ultrasound) to clear suspected chest and abdominal trauma.
Two other studies enrolled patients in a quasi‐randomised fashion. The suitable algorithm was defined by ultrasound availability: ultrasound on weekdays from 8 am to 5 pm; no ultrasound on weekdays from 5 pm to 8 am and on weekends (Arrillaga 1999), or the presence of one of the investigators (Boulanger 1999). Since no patient had the opportunity to influence the date of injury, we considered these methods to fulfil proper allocation at random.
Since ultrasound findings prompted different forms of further investigation, care programmes varied between groups. We did not judge this difference to be a flaw but a desired observation indicating effectiveness (that is, a change in a doctor's decisions) and efficiency (a change in health‐related outcomes) of ultrasound‐based clinical pathways.
One study report (Kumar 2014a) from an updated search in June 2015 has been added to Studies awaiting classification and will be incorporated into the review at the next update.
Risk of bias in included studies
In general, details of the study populations were sparse or missing. Individual risk of bias items are illustrated in Figure 2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
One of the randomised trials (Rose 2001) met some of our design standards. Patients were assigned by a computer‐generated list, although it was not clear whether concealment was maintained (the trial author reply to clarify this issue is pending). Sample size considerations called for 50 patients in each group to detect a 20% difference in CT scan use between groups. A secondary outcome (30‐minute difference in time to laparotomy) mandating inclusion of 420 patients was mentioned in the methods section of the original paper. However, no data were provided on this endpoint. A flowchart sketched the study profile according to the CONSORT recommendations.
The Sonography Outcomes Assessment Program (SOAP)‐1 trial (Melniker 2006) was a randomised clinical trial (RCT) to assess the effect of point of care, limited ultrasonography (PLUS). At the time of the first review, economic data gathered from 115 participants had been published as an abstract (Melniker 2004). Mean hospital charges for the PLUS arm were USD 13,841 (95% CI 11,170 to 16,512), and USD 33,512 (95% CI 10,465 to 56,559) for the control group. A press release (http://www.diagnosticimaging.com/dinews/2003060301.shtml, June 2003) reported significantly decreased mortality in the experimental arm (6.3% versus 8.1%), a reduced ICU length of stay (2.1 days versus 3.2 days), and a reduced use of CT. We did not receive a response to our letter to the research team. In the meantime, some of the results have been published in full. Although the trial authors had laudable and honest goals, the original article is difficult to interpret. Of 525 patients screened, 262 were randomised and only 217 were included in the final analysis, which contradicted the intended intention‐to‐treat principle. All continuous measures were presented as means, medians, and interquartile ranges, and the lack of standard deviations did not allow for including the study in the pooled analysis. Composite complications including death were abstracted from the medical record, and were thus addressed in a retrospective fashion. Individual complication rates were neither tabulated nor indicated in the text. We will try again to contact the trial authors to ask for more details.
In contrast, the quasi‐RCTs thoroughly described patient selection criteria and interventions, but provided too few demographic data to estimate the degree and direction of bias. No attempts were made to control for effect modification by multivariate analysis. One of these trials (Boulanger 1999) addressed a large number of endpoints (the number of extra tests, laparotomy rates, mortality, accuracy, diagnostic time, and costs).
Effects of interventions
Owing to the small sample of studies eligible for this review, we did not explore publication bias. Results in each comparison category are shown in the MetaView summary analysis.
Mortality
Data were available from three studies (Arrillaga 1999; Boulanger 1999; Melniker 2006). There was no evidence of a difference in mortality: random‐effects RR 1.00 (95% CI 0.50 to 2.00). No data were provided on mortality attributable to abdominal injuries, missed abdominal injuries, or adverse events caused by any of the diagnostic tests or negative laparotomy.
The mortality outcome for Melniker 2006 also included the complication rate, however the data were included since events such as haemorrhagic shock, septic shock, and multisystem organ failure are potentially life‐threatening.
Use of computed tomography (CT) scans
Data were pooled from all four trials, showing significant heterogeneity (I2 = 98.4%). Ultrasound‐based algorithms reduced ordering of CT scans by 50%: random‐effects RD ‐0.52 (95% CI ‐0.83 to ‐0.21).
Use of diagnostic peritoneal lavage (DPL)
Two studies (Arrillaga 1999; Boulanger 1999) reported data on the use of DPL: ultrasound‐based algorithms reduced the number of DPL procedures by 6% (95% CI ‐0.09 to ‐0.02).
Cost‐effectiveness analysis
Two studies that aimed to estimate costs exhibited inconclusive results. We did not attempt to pool these results.
In Boulanger 1999 the ultrasound pathway proved superior to the control arm. Cost was calculated in Canadian Dollars in 1995 at the study site, Sunnybrook HSC, Toronto. The calculation included hospital costs and professional charges, but did not include the cost of the sonography or CT machines.
In Melniker 2006 mean hospital charges for the PLUS arm were USD 10,600 (interquartile range (IQR) 5700 to 19,000), and USD 16,400 (IQR 6700 to 43,600) for non‐PLUS patients.
Laparotomy
Data from three studies were combined for this endpoint (Boulanger 1999; Melniker 2006; Rose 2001). There was no evidence of a difference in laparotomy rates with ultrasound‐based algorithms (fixed‐effect RD 0.00, 95% CI ‐0.04 to 0.04).
Other secondary outcomes
We did not identify any RCTs that explored the impact of ultrasound‐based clinical pathways on other health‐related outcomes such as quality of life. In Boulanger 1999 ultrasound reduced the mean time from arrival to hospital to completion of the diagnostic algorithm from 151 minutes (95% CI 127 to 174) to 53 minutes (95% CI 48 to 58). In this study participants undergoing ultrasound had a 60% reduced RR of delayed recognition of intra‐abdominal trauma (mainly small bowel lacerations). Two non‐therapeutic laparotomies were performed in each group.
In Arrillaga 1999, mean length of stay and mean ICU days did not differ between groups. In this study, ultrasound significantly reduced the median disposition time from 80 minutes during weekdays, and 92 minutes during weekends, to 20 minutes in both cases.
In the SOAP‐1 trial (Melniker 2006), the time from emergency department arrival to operating room transfer was significantly shorter in the ultrasound group (median interval 60 (IQR 41 to 70) versus 157 (IQR 90 to 178) minutes).
Discussion
There is an increasing awareness of the limitations of the use of emergency ultrasound to disclose abdominal injury after blunt trauma. Because of its high specificity, a positive sonogram (either for free fluid or organ injury) proves the presence of intra‐abdominal damage. However, it is debatable whether identifying injured patients is a significant problem for trained emergency department teams. Given its poor overall sensitivity, ultrasound cannot be used to rule out abdominal injury (Miller 2003; Stengel 2005).
The first version of this review was published with the Cochrane Injuries Group (CIG) in 2005. At that time, the Cochrane Diagnostic Test Accuracy (DTA) group was working on methods and statistical software (that is, for computing summary receiver operating characteristics (SROC)) to compile accuracy data. The CIG mandated a classic effectiveness review, including data from RCTs. Algorithms to identify accuracy studies in MEDLINE and other databases are now established, and the RevMan software offers tools to aggregate DTA data across studies. Also, the reference standard has changed dramatically to 128‐row MDCT (including CT angiography) now available at the point of care. We recently applied for a Cochrane DTA review to update accuracy estimates of FAST and advanced abdominal ultrasound pathways for disclosing or verifying abdominal injuries.
This particular review, however, does not address false‐negative rates or any other indices of accuracy. We deliberately skipped decision nodes of the classic Fryback‐Thornbury cascade (Fryback 1991), investigating only patient‐centred outcomes associated with FAST‐based and non‐FAST based trauma surveys. Given the role and definition of FAST within the ATLS® concept, diagnostic efficacy and effectiveness of this modality may overlap in many ways. FAST is a point of care diagnostic tool, applied during resuscitation and even cardiopulmonary resuscitation (CPR), which may trigger therapeutic interventions like laparotomy and bypass confirmatory CT imaging.
The observed heterogeneity amongst the few eligible trials may have prevented pooling of data and computing summary estimates. All review authors agreed that, apart from substantial heterogeneity, point estimates derived from random‐effects model analysis may be more useful for clinicians and policy makers than simple tabulation of data. As noted, results must be used with caution and in an exploratory fashion .
Pre‐hospital or on‐site thoraco‐abdominal ultrasound has gained much interest during the past few years (Hoyer 2010). Yet, the contributors to this review were interested in the utility and value of point of care sonography in the trauma bay (that is, after hospital admission). Pre‐hospital and in‐hospital settings are too different to be compared in a single review.
It is troubling that an intervention regarded as a diagnostic standard has been so poorly evaluated. Since the first version of this review which was published in 2005, no further head‐to‐head studies have been performed, planned, or registered, and as things currently stand it seems unlikely that any RCT simply comparing ultrasonography‐based with non‐ultrasonography algorithms will be conducted in future. In fact, this kind of comparison will no longer make sense, given established principles of care of the injured in most countries. One may, however, speculate whether pre‐hospital ultrasonography used in land or air transport of trauma patients (Hasler 2012) or contrast‐enhanced ultrasound (Cagini 2013) will prompt or even demand experimental studies.
In suspected multiple trauma, reference data from the German Trauma Registry (Huber‐Wagner 2009; Huber‐Wagner 2013) imply that a primary CT scan is life‐saving. The observed reduction in CT scans (as a result of a negative FAST examination) might, in part, reflect a false sense of security. Emergency physicians and trauma surgeons are well advised to insist on CTs for admission and clinical monitoring, regardless of a negative sonogram. Trauma patients currently are exposed to substantial radiation for diagnostic purposes (Hui 2009). Any imaging algorithm must adhere to the 'As Low As Reasonably Achievable' (ALARA) principle of exposure to radiation, and future developments must seek to maintain image quality and diagnostic accuracy at much lower doses. Repeated ultrasound examinations may enhance the sensitivity of trauma ultrasound (Nunes 2001). Scheduled follow‐up examinations have established themselves in clinical practice because of their feasibility. However, if there is a high pre‐test probability of abdominal injuries, contrast‐enhanced CT still represents the diagnostic modality of choice.
Ultrasound‐based algorithms are often assumed to have merits in shortening the primary trauma assessment, triaging patients more precisely, avoiding unnecessary interventional procedures, and reducing costs. However, such assumptions are hardly supported by the available scientific data. Apart from a significant reduction in the frequency of ordering CT scans, we found no beneficial effect of ultrasound on patient‐centred endpoints. Divergent results prevented pooling of data for most endpoints of interest.
Of note, two studies of higher methodological quality (Boulanger 1999; Rose 2001) showed only a marginal reduction in CT frequency. Thus, it is open to debate whether abdominal ultrasound measurably affects the doctor's decision to order definitive diagnostic tests.
The meaning of the slightly increased RR of mortality in the ultrasound arm of two quasi‐RCTs (Arrillaga 1999; Boulanger 1999) is not straightforward and susceptible to residual confounding. Patients in this group might have been more severely injured, haemodynamically unstable, and considered unsuitable for CT imaging more frequently. Although similar Injury Severity Score (ISS) values were noted in both groups, no information was provided on the Abbreviated Injury Scale (AIS) for abdominal damage. Thus, imbalances between patient groups cannot be excluded.
We admit that our decision to pool data across studies despite extraordinarily strong heterogeneity may appear inconsistent. However, we considered it important to publish the best available qualitative and quantitative evidence about an established diagnostic instrument which had not been and will probably never be tested under the rigour of a multicentre RCT. We advise readers to take the heterogeneity of findings into account, and to regard pooled results as signposts rather than exact point estimates. The FAST examination always suffered from a paradox known as 'Buxton's Law' (Buxton 1987), that is, it is always too early to rigorously evaluate a new technology (that is, until the turning point of the learning curve has been reached), until it is suddenly too late (that is, after almost all practitioners have adopted the method because of convenience, preference, or other reasons).

Study selection process flow diagram for 2015 search update.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Mortality, Outcome 1 Relative risk of mortality.
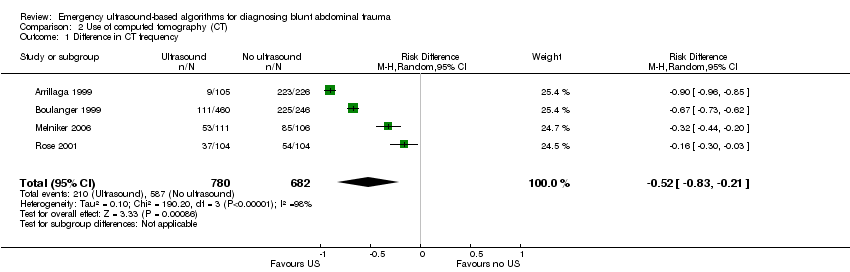
Comparison 2 Use of computed tomography (CT), Outcome 1 Difference in CT frequency.
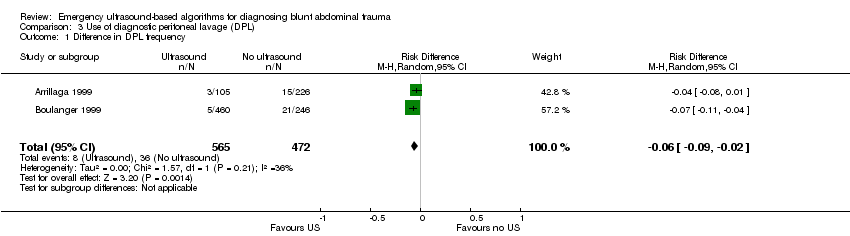
Comparison 3 Use of diagnostic peritoneal lavage (DPL), Outcome 1 Difference in DPL frequency.
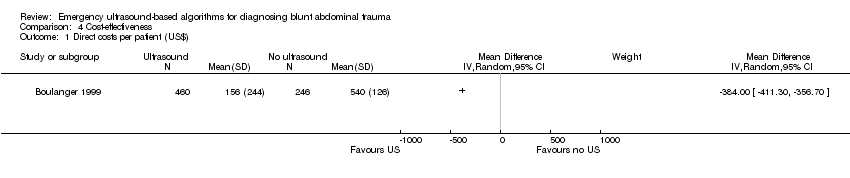
Comparison 4 Cost‐effectiveness, Outcome 1 Direct costs per patient (US$).

Comparison 5 Laparotomy, Outcome 1 Laparotomy rate.
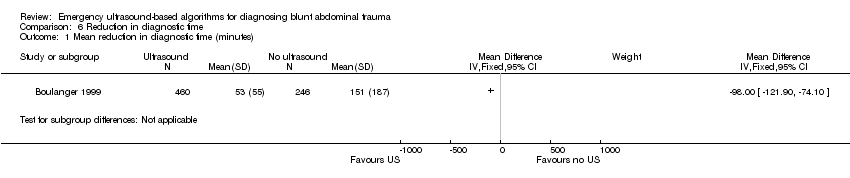
Comparison 6 Reduction in diagnostic time, Outcome 1 Mean reduction in diagnostic time (minutes).
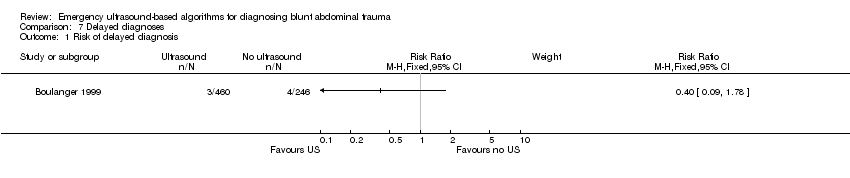
Comparison 7 Delayed diagnoses, Outcome 1 Risk of delayed diagnosis.
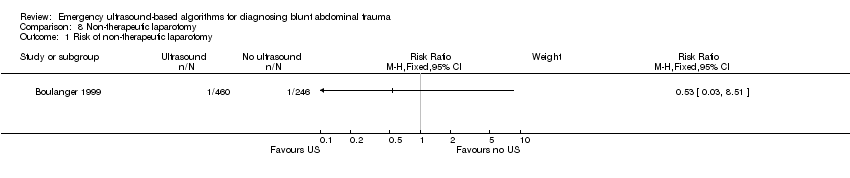
Comparison 8 Non‐therapeutic laparotomy, Outcome 1 Risk of non‐therapeutic laparotomy.
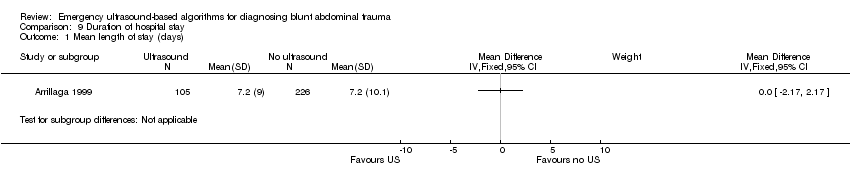
Comparison 9 Duration of hospital stay, Outcome 1 Mean length of stay (days).

Comparison 10 Intensive care, Outcome 1 Mean ICU days.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Relative risk of mortality Show forest plot | 3 | 1254 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.50, 2.00] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in CT frequency Show forest plot | 4 | 1462 | Risk Difference (M‐H, Random, 95% CI) | ‐0.52 [‐0.83, ‐0.21] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Difference in DPL frequency Show forest plot | 2 | 1037 | Risk Difference (M‐H, Random, 95% CI) | ‐0.06 [‐0.09, ‐0.02] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Direct costs per patient (US$) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Laparotomy rate Show forest plot | 3 | 1131 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.00 [‐0.04, 0.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean reduction in diagnostic time (minutes) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Risk of delayed diagnosis Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Risk of non‐therapeutic laparotomy Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean length of stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean ICU days Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |

