Antisepsia manual quirúrgica para reducir la infección del sitio quirúrgico
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised controlled trial (described as an equivalence study) Generation of random number sequence: no details given | |
| Participants | 1 hospital General Surgery division; surgeons (no further information on personnel) 600 patients initially randomised from general surgery; data reported on 500 General Surgery: abdominal (e.g. cholecystectomy) and other; mixture of clean and clean‐contaminated operations | |
| Interventions | Group 1 ‐ traditional surgeons' handscrub for 3‐5 min using 7.5% povidone iodine (Betadine) or 4% chlorhexidine gluconate (Hibiscrub) (228 patients) Group 2 ‐ As with group 1 for first case; subsequent antisepsis with alcohol handrub with 62% ethanol (Purrel) 10 ml, allowed to dry (272 patients) | |
| Outcomes | Surgical site infection defined as any one or more of the following: symptoms and signs (pain, swelling, redness, hotness, tenderness, indurations, purulent discharge, opened wound) occurring within 30 d from surgery (examinations before discharge, at 1 week, at 1 month, and C/S results); no further detail supplied. | |
| Notes | All patients had standardised skin preparation. 76% of patients had prophylactic antibiotics (indicated for specified surgeries); no difference between arms. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on sequence generation Quote: "Participants were randomised to either a routine hand scrub or an alcohol hand‐rub upon selecting a sealed envelope for each case." No further information. |
| Allocation concealment (selection bias) | Unclear risk | No further information; not clear if envelopes were opaque or sequentially numbered |
| Blinding (performance bias and detection bias) | High risk | Clear differences between the procedures employed mean blinding of participants was not possible |
| Blinding (performance bias and detection bias) | Unclear risk | No information reported |
| Blinding (performance bias and detection bias) | Low risk | Assessment of wound appearance and swab by personnel unaware of allocation. Quote: "Surgeons who examined surgical sites were unaware of the groups' allocation"; "A swab was sent for C/S from any suspected SSI. Health care personnel taking swabs and interpreting results of C/S were also unaware of how hand disinfection for each group had been allocated." |
| Incomplete outcome data (attrition bias) | High risk | 100 (1 in 6) participants originally randomised were excluded from analysis. The reasons for this are not fully explored. Quote: "Initially an equal number of cases (300 patients in each group) were randomised to each method. However, more cases were further excluded from each group as they turned out to be non‐eligible for inclusion after the original randomization (e.g. acute or chronic cholecystitis on histopathological examination), incomplete forms, failed follow‐ups, etc." |
| Selective reporting (reporting bias) | Low risk | Authors reported all specified objectives. Quote: "The objective of this study is to determine the equal efficacy of alcohol‐based hand‐rub as compared to traditional surgical scrub in the prevention of SSI as the primary outcome measure; the compliance of surgical staff and skin tolerance as the secondary outcome measure; also keeping in mind cost effectiveness and the potential change in surgical practice at least in our institution" |
| Other bias | Low risk | No other sources of bias detected |
| Methods | Randomised controlled trial | |
| Participants | 22 operating room nurses | |
| Interventions | Group 1 ‐ 3 min scrub using aqueous chlorhexidine gluconate | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | Participants did not take part in any surgical procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Twenty‐two operating room nurses were randomly divided into two groups as follows: the PVI group (n = 11) and the CHG group (n = 11). All the nurses were examined for bacterial contamination of their hands before and after surgical handwashing".
Comment: Evidence of randomisation, however not enough evidence to suggest truly randomised sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Twenty‐two operating room nurses were randomly divided into two groups as follows: the PVI group (n = 11) and the CHG group (n = 11). All the nurses were examined for bacterial contamination of their hands before and after surgical handwashing".
Comment: No evidence that appropriate allocation concealment took place |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "All the nurses were examined for bacterial contamination of their hands before and after surgical handwashing"
Comment: No evidence of blinding of participants or personnel to blinding to intervention |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "All the nurses were examined for bacterial contamination of their hands before and after surgical handwashing"
Comment: No evidence of blinding of participants or personnel to blinding to intervention |
| Blinding (performance bias and detection bias) | Unclear risk | Tap water outcome Quote: "We took water samples from 4 faucets just before hand washing and 1 ml of each sample was injected onto Brain‐Heart infusion bouillon plats (Eiken K. K) and cultured at 37⁰Celsius for 48 hours for bacterial detection." Comment: Samples seemingly taken by personnel, however unclear as to the role bias would play in the quantitative study of bacterial colonies in water faucets. Hands and fingers outcome Quote: "The samples were collected and pre‐treated according to the Glove Juice method. In detail, the sample liquid was taken from the right glove just before hand washing and from the left glove after hand washing". This was then "cultured at 37⁰Celcius for 48 hours, thereafter the number of bacterial colonies was counted". Comment: Unclear as to whether those obtaining the samples were blinded to the intervention. It is likely that they were not blinded; however the overall judgement is unclear. |
| Incomplete outcome data (attrition bias) | Low risk | No direct quotes, however no losses to follow‐up encountered
Comment: Low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | Quote: no direct quotes. Comment: Both outcome assessments of bacterial contamination of tap water and hands and fingers before and after surgical handwashing were accounted for in the results. |
| Other bias | Low risk | No other sources of bias detected |
| Methods | Randomised cross‐over controlled trial | |
| Participants | 18 operating room staff working in ophthalmic, podiatric and general surgery | |
| Interventions | Group 1 ‐ brush application of 7.5% povidone iodine aqueous scrub | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: Participants "were assigned to at random to one of three groups. Each group used one of the three solutions for five consecutive days. The following week, each group used a different scrub solution, such that all participants used each product over the study duration" Comment: Unclear as to whether a random sequence generator was used. |
| Allocation concealment (selection bias) | Unclear risk | Quote: Participants "were assigned to at random to one of three groups. Each group used one of the three solutions for five consecutive days. The following week, each group used a different scrub solution, such that all participants used each product over the study duration"
Comment: Unclear as to whether allocation was concealed. |
| Blinding (performance bias and detection bias) | High risk | Quote: "Participants could not be blinded to the three solutions due to differences in their nature and method of application". Comment: Study describes that blinding of participants was not possible.There is no information given as to whether the investigators were blinded, but it is likely there were similarly unblinded to the intervention for the reasons given for the participants above. The judgement for participant blinding is therefore high risk. |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "Participants could not be blinded to the three solutions due to differences in their nature and method of application". Comment: Study describes that blinding of participants was not possible.There is no information given as to whether the investigators were blinded, but it is likely there were similarly unblinded to the intervention for the reasons given for the participants above. The judgement for blinding of caregivers here is unclear. |
| Blinding (performance bias and detection bias) | Unclear risk | Bacterial sampling Quote: "The subject introduced their hands into this bag and the investigator massaged their hands externally with emphasis on web spaces and subungual areas" Comment: no attempt made at blinding method of obtaining bacterial sample, which would advise a high risk of bias decision Microbial assay Quote: "Samples were sent to the microbiology laboratory immediately after collection in a blinded manner" Comment: There was adequate evidence that approach microbial testing was blinded. Therefore, overall assessment of the risk of bias for outcome assessment is unclear. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Eighteen surgical staff members participated in this study. All members used each of the three scrub solutions over the duration or the study . . . Two volunteers developed a skin rash and a burning sensation on their hands within and a few minutes after their first scrub with the ABWA product. They also reported a metallic taste in their mouth and palpitations. Although none of their symptoms were severe and resolved shortly thereafter without any medical intervention, they were removed from the study. All the remaining staff volunteers completed the study". Comment: small loss to follow‐up and full explanations given as to the reasons for dropout |
| Selective reporting (reporting bias) | Low risk | No direct quotes, although the efficacy of the product in terms of reduction of log reduction in bacterial counts and product preference by participants are both included in the Results in full and comprehensive manner, as outlined in the Methods. |
| Other bias | Unclear risk | Cross‐over design, unclear if accounted for in analysis |
| Methods | Cluster randomised controlled trial | |
| Participants | 4 surgeons working in a trauma surgery | |
| Interventions | Group 1 ‐ 3 min scrub using aqueous chlorhexidine gluconate All surgeons washed with chlorhexidine (no further detail) for 5 min for first procedure with thorough cleaning under fingernails. | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | The 4 surgeons, who were not blinded, were randomised once and tested 53 times | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Orthopaedic surgeons were allocated to one of two different hand‐washing protocols using a randomisation table"
Comment: evidence of random sequence generation, therefore judged as low risk |
| Allocation concealment (selection bias) | Unclear risk | Quote: "[T]he surgeon was randomised to wash for 5 min with either chlorhexidine or alcohol gel"
Comment: no evidence that there was an attempt at allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The hand‐washing protocol dictated that all surgeons should wash for 5 min with chlorhexidine for their first case with thorough cleaning under the fingernails. Thereafter, the surgeon was randomised to wash for 5 min with either the chlorhexidine or alcohol gel. Alcohol was allowed to dry on the hands prior to double gloving".
Comment: no evidence that participants or personnel were blinded to intervention |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "The hand‐washing protocol dictated that all surgeons should wash for 5 min with chlorhexidine for their first case with thorough cleaning under the fingernails. Thereafter, the surgeon was randomised to wash for 5 min with either the chlorhexidine or alcohol gel. Alcohol was allowed to dry on the hands prior to double gloving".
Comment: no evidence that participants or personnel were blinded to intervention |
| Blinding (performance bias and detection bias) | Low risk | Quote: "The number of bacterial colonies present after 24 h and 48 h of incubation were recorded for each agar plate by a microbiologist blinded to the washing protocol used"
Comment: adequate blinding of assessment outcome |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Overall, 41 procedures and 82 episodes of handwashings were included in the study. Two episodes were discarded due to contamination at the time of glove removal. There was no incidence of outer glove perforation during this study"
Comment: good evidence to suggest losses to follow‐up were accounted for and there was minimal effect of attrition bias |
| Selective reporting (reporting bias) | Low risk | No direct quotes, but the assessment variable of bacterial colonisation after different methods of handwashing (which was outlined in the methodology) is accounted for in the results. |
| Other bias | Unclear risk | This appears to be a clustered randomised trial; it does not seem that clustering was taken into account in the analysis. |
| Methods | Randomised cross‐over controlled trial | |
| Participants | 154 members of the surgical teams working in plastic surgery and traumatology | |
| Interventions | Group 1 ‐ 3 min scrub using aqueous chlorhexidine gluconate, n = 50 | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Healthy volunteers washed with one of the three products for 1 week for 3 consecutive weeks. The order of the washings was randomised" Comment: indication that a randomisation process was undertaken, however not clear how the sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Healthy volunteers washed with one of the three products for 1 week for 3 consecutive weeks. The order of the washings was randomised" Comment: no evidence of allocation concealment given |
| Blinding (performance bias and detection bias) | High risk | Quote: "The effect of standard surgical washings with 7.5% iodine povidone or 4% chlorhexidine (both with scrubbing for 3 min following a standard technique) was compared with the effect of washing (without scrubbing with 2.3% N‐duopropenide in 60⁰ ispopropranol with dermoprotective substance). The latter solution was poured over the hands, which were then rubbed together, and when it began to dry, it was reapplied over 3 min." Comment: The study design here is a cross‐over design, reducing potential effects of bias; however, there is no evidence that participants or personnel were blinded to the intervention given, and the conditions differed sufficiently that blinding would not have been possible. |
| Blinding (performance bias and detection bias) | High risk | Quote: "The effect of standard surgical washings with 7.5% iodine povidone or 4% chlorhexidine (both with scrubbing for 3 min following a standard technique) was compared with the effect of washing (without scrubbing with 2.3% N‐duopropenide in 60⁰ isopropanol with dermoprotective substance. The latter solution was poured over the hands, which were then rubbed together, and when it began to dry, it was reapplied over 3 min." Comment: The study design here is a cross‐over design, reducing potential effects of bias; however, there is no evidence that participants or personnel were blinded to the intervention given, and the conditions differed sufficiently that blinding would not have been possible. |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "We used fingertips to sample hand bacteria as the standard European Norm (pr EN 1500) except we had not artificially contaminated the hands: five fingertips are rubbed for 1 min on a Petri dish containing 10 ml of TBS plus an antiseptic neutralize ". These were cultured for "48 h at 37⁰C, and the then the CFU/hand (the five fingertips), were counted and transformed into a decimal logarithm". Comment: Despite the assessment being quantitative, it is not clear whether those who obtained the bacterial samples were independent of the study or blinded to the intervention. |
| Incomplete outcome data (attrition bias) | Low risk | No direct quotes, although no losses to follow‐up recorded in Results Comment: no obvious source of attrition bias |
| Selective reporting (reporting bias) | Low risk | No direct quotes Comment: tabular format of results incorporates the assessment outcomes outlined (CFUs) between the groups, suggesting a low risk |
| Other bias | Unclear risk | Although the trial had a cross‐over design, it did not appear that this was reflected in the analysis. |
| Methods | Randomised cross‐over trial (participants took part in each of 3 groups) | |
| Participants | 24 surgeons | |
| Interventions | Group 1 ‐ 1 min wash with soap and water followed by 5 min rub with an alcoholic disinfectant | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just states "random order" with no further information |
| Allocation concealment (selection bias) | Unclear risk | As with sequence generation; no further information |
| Blinding (performance bias and detection bias) | Unclear risk | Standard technique compared with shorter techniques precludes blinding of personnel but no further information |
| Blinding (performance bias and detection bias) | Unclear risk | Standard technique compared with shorter techniques precludes blinding of personnel but no further information |
| Blinding (performance bias and detection bias) | Unclear risk | No information on blinding of assessment reported |
| Incomplete outcome data (attrition bias) | Low risk | No evidence of dropouts/loss of data |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
| Other bias | Unclear risk | Although the trial had a cross‐over design, it did not appear that this was reflected in the analysis. |
| Methods | Cluster‐randomised controlled cross‐over trial Generation of random number sequence: appropriate | |
| Participants | 66 surgeons and trainees; 3317 patients | |
| Interventions | Group 1 ‐ plain soap and water: 4‐5 min clean running water and plain soap; sterile cotton hand towel dry. 5 clusters (n = 1682 patients) Group 2 ‐ As group 1 before first procedure of day and subsequently in case of visible soiling, then alcohol‐based handrub (75% isopropyl alcohol, 1.45% glycerol, 0.125% hydrogen peroxide) for 3 min and kept wet (7‐10 ml per preparation) | |
| Outcomes | SSI (defined using modified US Centers for Disease Control and Prevention definitions for nosocomial infection) detected by tours of hospital wards; reviews in outpatient clinic; telephone contact: diagnosis established jointly by study collaborators | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Surgical hand preparation procedures were assigned randomly to the 6 participating operating theatres by tossing a coin, with a cross‐over every 2 months. There was no indication of baseline imbalance for important variables such as type of surgery, contamination level of the surgery or use of antibiotic prophylaxis. |
| Allocation concealment (selection bias) | Low risk | Allocation was decided by toss of a coin. Not clear who undertook this process and if it was concealed from the sites. Given that this was a cluster trial with cross‐over, the potential for bias stemming from allocation concealment was limited. As the Cochrane Handbook for Systematic Reviews of Interventions notes, "Cluster‐randomised trials often randomise all clusters at once, so lack of concealment of an allocation sequence should not usually be an issue." |
| Blinding (performance bias and detection bias) | High risk | Personnel were aware of allocation as procedures differed in obvious ways. Compliance by surgical teams was determined by observation of practices. A trained observer who did not belong to the surgical team checked whether each sink had the correct hand preparation and whether all surgeons (including visiting staff) followed the recommended hand preparation procedures. |
| Blinding (performance bias and detection bias) | Unclear risk | No information reported |
| Blinding (performance bias and detection bias) | Low risk | Diagnosis, documentation and determination of SSI by personnel without knowledge of allocation. "SSI was diagnosed . . . and documented by a trained nurse who visited the surgical wards three to four times each week during the 30 days after surgery. Patients discharged before this were reviewed in the outpatient clinic or contacted by telephone . . . The nurse was blinded to the method of surgical hand preparation. The diagnosis of SSI was established jointly by the study collaborators; differences in SSI ascertainment were resolved by consensus without knowledge of the study allocation of the patient." |
| Incomplete outcome data (attrition bias) | Low risk | 3722 patients underwent a surgical procedure in the operating theatre, and 3317 were included in the 10 study clusters. Postdischarge surveillance data could not be obtained for 184 patients (5.5%). |
| Selective reporting (reporting bias) | Low risk | The primary objective of this cluster‐randomised, cross‐over trial was to compare the efficacy of plain soap and water with alcohol‐based handrub, using SSI rates as the main outcome measure. The feasibility and affordability of the local production of an alcohol‐based handrub was also investigated, together with an assessment of its acceptability among healthcare workers. All specified outcomes were reported. |
| Other bias | Low risk | There was no evidence of other sources of bias. Clustered nature of the data was taken into account in the trial. |
| Methods | Cluster‐randomised controlled equivalence trial | |
| Participants | Surgical teams within 6 hospitals were randomised. 4387 patients undergoing clean and clean‐contaminated surgery were included in the study. | |
| Interventions | Group 1 ‐ 5 min scrub using either 4% povidone iodine or 4% chlorhexidine gluconate | |
| Outcomes | Outcome measure: SSIs in patients at 30 d using CDC definition | |
| Notes | Unclear if clustering is adjusted for in the analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each participating surgical service was assigned a 2‐digit random number by using a random number table. Surgical services corresponding to the 3 higher numbers were assigned to hand‐rubbing with AAS and the remaining 3 services were assigned to traditional hand‐scrubbing". Comment: adequate evidence of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Each participating surgical service was assigned a 2‐digit random number by using a random number table. Surgical services corresponding to the 3 higher numbers were assigned to handrubbing with AAS and the remaining 3 services were assigned to traditional hand‐scrubbing" Comment: no evidence of allocation concealment |
| Blinding (performance bias and detection bias) | High risk | Quote: "[O]bservers of the clinical outcome could not be blinded to the hand antisepsis protocol." The study also mentions that "compliance observers did not belong to the operating department team but were usually present in the surgical suite. To avoid a Hawthorne effect the surgical teams were not informed of the timing of the evaluations". Comment: Although the effect of blinding has been considered, in the comparison of different scrubbing protocols it would be difficult to blind the participant or personnel. The risk of bias is still high in this instance, however. |
| Blinding (performance bias and detection bias) | High risk | Quote: "[O]bservers of the clinical outcome could not be blinded to the hand antisepsis protocol." The study also mentions that "compliance observers did not belong to the operating department team but were usually present in the surgical suite. To avoid a Hawthorne effect the surgical teams were not informed of the timing of the evaluations". Comment: Although the effect of blinding has been considered, in the comparison of different scrubbing protocols it would be difficult to blind the participant or personnel. The risk of bias is still high in this instance, however. |
| Blinding (performance bias and detection bias) | High risk | Surgical site infection outcome Quote: "According to CDC guidelines, all SSIs had to be confirmed by the surgeon or the physician in charge on the patient. Thus, observers of the clinical outcome could not be blinded to the hand antisepsis protocol" Comment: As the surgeon was the participant in this case. it is clear that this could constitute a high risk of bias. Tolerance and compliance outcome Quote: "[T]he surgical personnel (77 subjects) were asked to estimate the effect of the 2 protocols on their skin. We used 2 10 cm visual analogue scales, at month 0 and after 3 crossovers; 0 cm representing absence of an tolerance problem and 10 cm representing maximal dryness with chapped hands and desquamation or maximal irritation with erythema, burning sensation, and abrasion." Comment: The surgeon was the (unblinded) participant who reported the variables, so the results may be affected by performance bias. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "During the study period . . . 4823 consecutive patients underwent surgery. Among these, 385 patients underwent contaminated or dirty‐contaminated surgery, and 51 were lost to follow up at 30 days (17 in the hand‐rubbing group). The remaining 4387 patients (68.5% of whom underwent clean surgery) were considered for analysis". Comment: As the sample size is large, the numbers lost to follow‐up are not significant enough to warrant a risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | Quote: "Thirty‐day surgical site infections rates were the primary end point; operating department teams' tolerance of and compliance with hand antisepsis were secondary end points". Comment: adequate evidence in the results that these endpoints were accounted for comprehensively |
| Other bias | Unclear risk | This appears to be a clustered cross‐over study; it does not seem that clustering was taken into account in the analysis. |
| Methods | Randomised controlled cross‐over trial (Latin square design ‐ participants took part in each of 4 interventions) | |
| Participants | 34 anaesthetic, recovery and ward nurses | |
| Interventions | Group 1 ‐ 5 min initial scrub and 3 min subsequent scrub using chlorhexidine | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | Participants did not take part in any surgical procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to one of four groups, and each group was assigned to one of the four scrub regimens each week. Control on the treatment order was achieved through a Latin square design, as described by Winder." Comment: adequate evidence of an appropriate study design, but on balance not enough evidence of truly random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects were randomly assigned to one of four groups, and each group was assigned to one of the four scrub regimens each week. Control on the treatment order was achieved through a Latin square design, as described by Winder."
Comment: no indication that allocation to each group was concealed to the personnel |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "Subjects were randomly assigned to one of four groups, and each group was assigned to one of the four scrub regimens each week . . . Subjects were supervised by the investigator while they scrubbed on all test occasions."
Comment: no evidence to suggest that there was appropriate blinding of participants or personnel during the study |
| Blinding (performance bias and detection bias) | Unclear risk | Quote: "Subjects were randomly assigned to one of four groups, and each group was assigned to one of the four scrub regimens each week . . . Subjects were supervised by the investigator while they scrubbed on all test occasions."
Comment: no evidence to suggest that there was appropriate blinding of participants or personnel during the study |
| Blinding (performance bias and detection bias) | Unclear risk | Bacterial contamination Quote: "While the glove was still on the hand, a sample of the fluid was taken . . . [S]amples were collected on four occasions for each condition: (1) immediately before scrubbing (both hands), (2) immediately after the initial surgical scrub (non‐dominant hand only) (3) 2 hours after the initial surgical scrub, immediately before the consecutive scrub (dominant hand) and (4) 2 hours after one consecutive surgical scrub (dominant hand)."
Comment: no indication that those collecting the samples, administering the fluid or those performing the microbial assays were in any way blinded to the intervention or protocol |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Thirty‐six subjects were recruited, but two subjects withdrew from the experiment before completing all four treatments (scrubs) because of skin reactions, including erythema, burning sensations and local swelling"
Comment: adequate evidence that losses to follow‐up were small and fully accounted for |
| Selective reporting (reporting bias) | Low risk | No direct quotes, but the results of each 'scrub' are displayed fully at baseline and subsequent time intervals in the Results as laid out in the Methodology. |
| Other bias | Unclear risk | Although the trial had a cross‐over design, it did not appear that this was reflected in the analysis. |
| Methods | Randomised controlled trial cross‐over (Latin square design ‐ participants took part in each of 5 interventions) | |
| Participants | 23 operating room nurses | |
| Interventions | Group 1 ‐ 5 min initial and 3.5 min subsequent scrub using 4% chlorhexidine | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | Participants did not take part in any surgical procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Subjects who agreed to participate in the study followed each of the scrub protocols in turn, the order controlled by the use of a Latin square design. That is, every nurse was required to complete every protocol but not in the same sequence."
Comment: clear that the study design does reduce selection bias; however not clear whether there is enough evidence to suggest random sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Subjects who agreed to participate in the study followed each of the scrub protocols in turn, the order controlled by the use of a Latin square design. That is, every nurse was required to complete every protocol but not in the same sequence." Comment: no evidence to suggest that allocation was concealed to personnel |
| Blinding (performance bias and detection bias) | High risk | Quote: "Subjects who agreed to participate in the study . . . followed each scrub protocol each day for one week (referred to as the 'test week') with a week of normal activities between each test week. They were issued with a supply of the specific antiseptic to be used in excess to their requirements for scrubbing so that the appropriate antiseptic could be used exclusively during the test week. Subjects were assessed before commencing each scrub protocol and at the end of the test week to determine changes in the number of colony forming units (cfu) after scrubbing and changes in the condition of the hands."
Comment: Although study design allows for repeated testing, there is no evidence that there was effective blinding of participants and personnel to the different interventions, and differences between the conditions would have been clearly apparent to those taking part. |
| Blinding (performance bias and detection bias) | High risk | Quote: "Subjects who agreed to participate in the study . . . followed each scrub protocol each day for one week (referred to as the 'test week)' with a week of normal activities between each test week. They were issued with a supply of the specific antiseptic to be used in excess to their requirements for scrubbing so that the appropriate antiseptic could be used exclusively during the test week. Subjects were assessed before commencing each scrub protocol and at the end of the test week to determine changes in the number of colony forming units (cfu) after scrubbing and changes in the condition of the hands."
Comment: Although study design allows for repeated testing, there is no evidence that there was effective blinding of participants and personnel to the different interventions, and differences between the conditions would have been clearly apparent to those taking part. |
| Blinding (performance bias and detection bias) | Unclear risk | Sampling method Quote: "A glove juice sampling method to estimate the numbers of CFU present on the hands" Comment: no indication that those performing the sampling or performing the microbial assays were independent to the study Skin condition Quote: "Larson's Weekly Skin Assessment Rating Scale was used to rate the condition of the hands with respect to appearance, integrity, moisture and sensation. Participants rated themselves on a weekly basis. An independent rater, blind to the protocol being followed by the subject, also rated the subject's dominant hand each week." Comment: evidence that reasonable measures were undertaken to blind outcome assessment. Overall assessment unclear for outcome assessment blinding. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Of the 32 participants recruited, 23 completed all five scrub protocols (scrubs) because it was discovered [that several participants] were allergic or sensitive to one of the more antiseptics. One felt that her skin was already too dry to be able to participate further. One person could not complete the study because she was unexpectedly off work for an extended period and the other two gave no reason for withdrawing." Comment: fairly high rates of losses to follow‐up and no information given as to why some participants did not complete the study. Although it seems that the researchers acted appropriately here, it is difficult to give a judgement other than high risk |
| Selective reporting (reporting bias) | Low risk | No direct quotes, but reasonable evidence to suggest that both bacterial contamination and skin condition have been appropriately and fully tested and reported upon effectively. |
| Other bias | Unclear risk | Although the trial had a cross‐over design, it did not appear that this was reflected in the analysis. |
| Methods | Randomised cross‐over trial | |
| Participants | 75 surgeons | |
| Interventions | Group 1 ‐ surgical scrub using 4% chlorhexidine (details of the duration are not given) | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Dermal tolerance study Quote: "The first study was performed with a panel of 60 volunteers divided in two subgroups of 30 persons each." Comment: no indication of how the subgroups were divided Surgical hand disinfection study Quote: "In a randomised cross‐over clinical trial the antimicrobial efficacy of Sterillium for the surgical hand‐rub was tested against Hibiscrub in the Kantonsspital Basel over a period of 11 weeks . . . two weeks were needed for recruitment, four weeks to conduct the Sterillium arm of the study, one week's interval and then four weeks for the Hibiscrub part." Comment: no further information given as to how participants were recruited and how they were assigned and allocated to which intervention Hygienic hand disinfection study Quote: "The antimicrobial efficacy of each product was compared with that of 60% (v/v) 2‐propanol on artificially contaminated hands (E. coli K 12, NCTC 10538) using a cross‐over design with 15 volunteers." Comment: no indication of if a randomised sequence was used and what method was implemented |
| Allocation concealment (selection bias) | Unclear risk | Dermal tolerance Quote: "The first study was performed with a panel of 60 volunteers divided in two subgroups of 30 persons each" Comment: No indication of how allocation was concealed Surgical hand disinfection Quote: "In a randomised cross‐over clinical trial the antimicrobial efficacy of Sterillium for the surgical hand‐rub was tested against Hibiscrub in the Kantonsspital Basel over a period of 11 weeks . . . two weeks were needed for recruitment, four weeks to conduct the Sterilium arm of the study, one week's interval and then four weeks for the Hibiscrub part." Comment: no indication if allocation was concealed Hygienic hand disinfection Quote: "The antimicrobial efficacy of each product was compared with that of 60% (v/v) 2‐propanol on artificially contaminated hands (E. coli K 12, NCTC 10538) using a cross‐over design with 15 volunteers." Comment: no Indication if allocation was concealed |
| Blinding (performance bias and detection bias) | Unclear risk | Dermal tolerance Quote: "The first panel started with Hibiscrub, the second with Sterilium. Both products were used over seven weeks after one week of preconditioning. After an interval of four weeks the second run started with a single crossover of products." Comment: although cross‐over trial used, no indication that the participants or personnel were blinded to the intervention Surgical hand disinfection Quote: "Organisms were recovered by the glove juice method. Plastic bags with the sampling fluid were placed on the subjects hands. The bag on each hand was secured and massaged for 1 min in a uniform manner by a laboratory technician." Comment: no indication whether participants were blinded to intervention and unclear whether technician was blinded to which intervention had been used Hygienic hand disinfection Quote: "The antimicrobial efficacy of each product was compared with that of 60% (v/v) 2‐propanol on artificially contaminated hands (E. coli K 12, NCTC 10 538) using a cross‐over design with 15 volunteers. The hands were first washed for 1 min with soft soap, dried with paper towels immersed in the contamination fluid up to the mid‐metacarpals for 5 s with fingers spread and then allowed to dry for 3 min" Comment: unclear as to whether participants or personnel were blinded to the interventions |
| Blinding (performance bias and detection bias) | Unclear risk | Dermal tolerance Quote: "The first panel started with Hibiscrub, the second with Sterilium. Both products were used over seven weeks after one week of preconditioning. After an interval of four weeks the second run started with a single crossover of products". Comment: although cross‐over design used, no indication that the participants or personnel were blinded to the intervention Surgical hand disinfection Quote: "Organisms were recovered by the glove juice method. Plastic bags with the sampling fluid were placed on the subjects hands. The bag on each hand was secured and massaged for 1 min in a uniform manner by a laboratory technician" Comment: no indication whether participants were blinded to intervention and unclear whether technician was blinded to which intervention had been used Hygienic hand disinfection Quote: "The antimicrobial efficacy of each product was compared with that of 60% (v/v) 2‐propanol on artificially contaminated hands (E. coli K 12, NCTC 10 538) using a cross‐over design with 15 volunteers. The hands were first washed for 1 min with soft soap, dried with paper towels immersed in the contamination fluid up to the mid‐metacarpals for 5 s with fingers spread and then allowed to dry for 3 min." Comment: unclear as to whether participants or personnel were blinded to the interventions |
| Blinding (performance bias and detection bias) | Unclear risk | Dermal tolerance Quote: "The following parameters were measured before and after application: Clinical assessment by observation of the hands and the forearms of the volunteers by a dermatologist. The number of volunteers which dropped out of the study because of skin damage as decided by a dermatologist" Comment: no indication that the dermatologist was independent to the study Surgical hand disinfection Quote: "The bag on each hand was secured and massaged for 1 min in a uniform manner by a laboratory technician" Comment: no indication that the laboratory technician was blinded to the intervention Hygienic hand disinfection Quote: "Control microbial counts were obtained by rubbing the fingertips for 1 minute in a Petri dish containing a liquid broth using a separate dish for each hand. Either 3 ml of the hand gel or two aliquots of 3 ml of the reference alcohol were applied to the hands. The rub‐in period was 30 s for the hand gels and 60 s for the reference alcohol as prescribed by EN 1500." Comment: no mention of whether those supervising the process or the overseeing the microbial assays were independent of the study |
| Incomplete outcome data (attrition bias) | Unclear risk | Dermal tolerance Quote: "A dramatic finding is the very high number of subjects dropping out of the Hibiscrub group. Altogether 15 persons gave up using the Hibiscrub for reasons related to the use of the product, but there was only person who discontinued Sterillium." Comment: This total represented a large proportion of the total participants used. Despite attributing the high rates of dropout to reasons related to the product, the risk of attrition bias is fairly high in this case. Surgical hand disinfection No direct quotes, but no indication given as to the total number of participants used or whether there was any observed loss to follow‐up. Therefore the judgement remains unclear. Hygienic hand disinfection Quote:"The antimicrobial efficacy of each product was compared with that of 60% (v/v) 2‐propanol on artificially contaminated hands (E. coli K 12, NCTC 10538) using a cross‐over design with 15 volunteers." Comment: No losses to follow‐up were discussed within the results; however, there is no evidence to suggest no losses to follow‐up occurred. Overall judgement therefore remains unclear for outcome assessment. |
| Selective reporting (reporting bias) | Low risk | Dermal tolerance No direct quotes; however, the form used to tabulate the results gives no quantitative figures for variables that were predominantly quantitative in nature. For example "D‐squames", "Electrical capacity", "Transepidermal water loss" are expressed as either "−− very poor", "− poor", "+ good" or "++ very good". It is unclear why the outcomes have been reported in this way. Surgical hand disinfection No direct quotes, although the results indicate the microbial concentration in the sampling fluid before and after treatment, outlined in the methodology and expressed as logarithm. Hygienic hand disinfection No direct quotes, although all the hand disinfectants that were discussed in the methodology as appropriate for testing are discussed and tabulated in an appropriate manner. Overall judgement of low risk. |
| Other bias | Low risk | No other sources of bias detected; cross‐over design taken into account in analysis |
| Methods | Parallel group randomised controlled trial. Unit of randomisation and analysis is individual staff member. Generation of random number sequence: computerised generation | |
| Participants | 164 nurses, operating department practitioners and healthcare assistants | |
| Interventions | Group 1 ‐ chlorhexidine (aqueous chlorhexidine gluconate 4% (Hibiscrub)) alone. Total application time of 2 min, n = 54 Group 2 ‐ chlorhexidine as group 1 plus cleaning nails with disposable nail pick (before scrub under running water), n = 54 Group 3 ‐ chlorhexidine as above plus cleaning nails with disposable nail brush (before scrub under running water), n = 54 All groups were observed and timed when scrubbing. Total antiseptic application time in each group was 2 min (measured dose of 2 x 2 ml for 1 min/dose). | |
| Outcomes | Primary outcome: number of CFUs on dominant hand Method of testing: modified glove juice method (sterile Gammex Powder Free, Ansell glove) | |
| Notes | No surgical procedures were performed; all staff performed circulating duties within the operating theatre for 1 h. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An appropriate method of generating the randomisation sequence was reported: "Randomisation was in random size blocks in multiples of three and was generated by a statistician using a computer software package." |
| Allocation concealment (selection bias) | Low risk | A recognised method for ensuring allocation concealment was reported: "Group allocation details were placed inside sequentially numbered sealed opaque envelopes by an individual independent from the study. The envelopes were opened by participants after baseline bacterial counts had been taken and immediately before the scrub intervention was performed. The researcher conducting the baseline sample was unaware of each participant's group allocation." |
| Blinding (performance bias and detection bias) | High risk | Scrub protocols differed such that all participants were aware of their allocation |
| Blinding (performance bias and detection bias) | High risk | For personnel (not caregivers): "The researcher conducting the baseline sample was unaware of each participant's group allocation. As the researcher observed the participants' scrubbing they were therefore aware of each participant's group allocation when conducting the post‐scrub sample.” |
| Blinding (performance bias and detection bias) | Low risk | CFU assessment was performed in a blinded manner: "Laboratory staff estimating the bacterial counts were unaware of group allocation status.” |
| Incomplete outcome data (attrition bias) | Low risk | Data missing for only 2 participants; reasons given: "164 operating department staff took part in the study. No participants dropped out, but the laboratory results for two people were spoiled. The findings on 162 participants are presented.” |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported: "The primary purpose of the trial was to compare any two of the trial groups by measuring the difference in post‐intervention CFU per hand. A secondary purpose was to identify any relationships between participants and baseline CFU counts." |
| Other bias | Low risk | No evidence of other sources of bias |
| Methods | Parallel group randomised controlled trial. Unit of randomisation and analysis is patient Generation of random number sequence: not reported | |
| Participants | 400 staff classified as surgeons, "instrumentalists" and helpers 100 patients undergoing clean or clean‐contaminated surgery | |
| Interventions | Group 1 ‐ aqueous scrub with 4% chlorhexidine gluconate with brush and sterile water. Mean duration of scrub 3.9 (SD 1.07) min. Group 2 ‐ alcohol rub with 61% ethanol, 1% chlorhexidine gluconate. Mean duration 2.0 (SD 0.47) min. | |
| Outcomes | SSI after 1 month (CDC criteria; method of diagnosis not further reported) CFUs on hands (20% of personnel only): reports number of personnel with positive cultures (no further detail) | |
| Notes | Only 20% of the 400 enrolled staff were assessed for bacteria on hands; these were classified as having or not having a positive culture. No data on number of CFUs on hands were reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just says "used closed envelopes" |
| Allocation concealment (selection bias) | Unclear risk | Just says "used closed envelopes" |
| Blinding (performance bias and detection bias) | Unclear risk | No information given but interventions clearly differed |
| Blinding (performance bias and detection bias) | Unclear risk | No information given but interventions clearly differed |
| Blinding (performance bias and detection bias) | Unclear risk | No information on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Low risk | Data on all patients reported for SSI Data on 20% of personnel collected for CFUs; results reported for all those collected |
| Selective reporting (reporting bias) | Low risk | All specified outcomes reported |
| Other bias | Low risk | No other sources of bias detected |
| Methods | Randomised cross‐over trial | |
| Participants | 25 operating theatre nurses and surgical technologists | |
| Interventions | Group 1 ‐ 3 min surgical scrub using either 4% chlorhexidine, 2% chlorhexidine or parachlorometaxylenol | |
| Outcomes | Outcome measure: CFUs on participants' hands | |
| Notes | Participants did not take part in any surgical procedures. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote "We randomly assigned subjects to one of two study groups (i.e., two‐minute, three‐minute surgical hand scrub times)." Comment: no indication as whether a truly randomised sequence was generated |
| Allocation concealment (selection bias) | Unclear risk | Quote "We randomly assigned subjects to one of two study groups (i.e., two‐minute, three‐minute surgical hand scrub times)." Comment: no indication as to whether allocation was concealed to the participants or personnel. The study was a cross‐over trial, therefore all the participants were likely to have undertaken the same interventions; however, the role of bias is unclear in this case. |
| Blinding (performance bias and detection bias) | High risk | No direct quotes given, but no mention as to whether the subjects or the personnel were blinded to the intervention. It is very likely that the personnel were not blinded as they would be able to calculate the time spent handwashing. |
| Blinding (performance bias and detection bias) | High risk | No direct quotes given, but no mention as to whether the subjects or the personnel were blinded to the intervention. It is very likely that the personnel were not blinded as they would be able to calculate the time spent handwashing. |
| Blinding (performance bias and detection bias) | High risk | No direct quotes given, but investigators were responsible for measurement of time and for preparation of solution for bacterial culture, as well as the sampling and measurement of log CFU counts. Although this is an objective measure, it is reasonable to suggest that as the investigators were not blinded to the intervention then the risk of bias here could be considered as high. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Each subject was able to complete both surgical hand scrub trials, which resulted in 300 agar plates for incubation and enumeration." Comment: adequate evidence of no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | The main outcome variable was the log counts of bacterial colonies found on participants' hands after washing their hands for a specified time. This was fully represented in table format in the results section. |
| Other bias | Unclear risk | Although the trial had a cross‐over design. it did not appear that this was reflected in the analysis |
AAS: aqueous alcohol solution;ABWA: alcohol‐based water‐aided; ASA: American Society of Anesthesiologists; CDC: Centers for Disease Control; CFU: colony forming units; CHG: chlorhexidine gluconate; C/S: culture and sensitivity PVI: povidone iodine; SSI: surgical site infection; v/v: volume/volume per cent.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Not a randomised controlled trial | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| Evaluated patient skin preparations | |
| Evaluated patient skin preparations | |
| A discussion paper | |
| A laboratory and ward study ‐ not hand antisepsis | |
| Hand hygiene study | |
| Study carried out on volunteers, not scrub staff in an operating theatre | |
| Wound irrigation study | |
| Infection in ITU | |
| A laboratory‐based study | |
| Gloving study | |
| Participants were not randomised | |
| A laboratory‐based study | |
| Not a randomised controlled trial | |
| Evaluated patient skin preparations | |
| Body exhaust suit study | |
| Infection surveillance study | |
| An editorial | |
| Evaluated skin condition rather than SSIs or CFUs | |
| Study of dressings | |
| Discussion paper | |
| Evaluated patient skin preparations | |
| Study of dressings | |
| Not a randomised controlled trial. Participants used product A for 2 weeks then swapped to product B for the following 2 weeks. | |
| Study of MRSA decolonisation | |
| Not a randomised controlled trial | |
| Literature review | |
| Not relevant to this review | |
| A laboratory‐based study | |
| Study was not randomised. Participants performed antisepsis using their usual solution. There were no comparison groups | |
| Randomised controlled trial comparing antiseptic solutions on patients' skin | |
| Chemical agents | |
| Not a randomised controlled trial | |
| Chlorhexidine scrub which was left on the surgeons arm was compared with a surgeons arm where the chlorhexidine scrub was rinsed off. Did not meet the objectives of this review | |
| Study on hygienic hand washing | |
| Study of gloves in surgery | |
| Staff survey | |
| Participants were randomised to 2 groups. Group 1 participants were tested after 1 hour and group 2 participants were tested after 2 hours. Participants in both groups used product A 1 day and product B the next day | |
| Participants' hands were covered with bacterial inoculum. A laboratory‐based study | |
| A laboratory‐based study | |
| Antibiotic prophylaxic study | |
| Non‐systematic literature review | |
| Dog bite study | |
| Evaluated patient skin preparations | |
| A laboratory style study using non clinical hospital staff | |
| Antimicrobial suture study | |
| Not randomised | |
| A retrospective study | |
| Discussion paper | |
| MRSA decontamination study | |
| Evaluated patient skin preparations | |
| Study carried out on volunteers, not scrub staff in an operating theatre | |
| Experimental and clinical conditions | |
| Measured the impact of hand care products on alcohol rubs | |
| Testing methods not suitable; not randomised | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| Skin preparation of patient | |
| A laboratory‐based study | |
| A study of skin antiseptics used on patients skin | |
| A laboratory‐based study and participants were not randomised | |
| No comparison group was used in the first part of the trial. Comparison groups were used in the second part of the trial, but solutions were applied to the forearm rather than as surgical scrubs | |
| A laboratory‐based study | |
| Anaesthetic agents study | |
| Not a randomised controlled trial | |
| Measured condition of skin on hands of participants; did not compare CFUs or SSIs | |
| Not randomised | |
| Not relevant topic. | |
| Not randomised | |
| Descriptive paper of scrubbing methods | |
| Study focused on handwashing rather than hand antisepsis | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| Study of handwashing in intensive care | |
| Not randomised to appropriate groups. 5 participants were randomised to a reference group at the beginning of the study. The participants randomised to the intervention group used an alcohol rub for 3 weeks and then a surgical scrub for 3 weeks | |
| Analgesics study | |
| Patient skin prep study | |
| A laboratory‐based study | |
| Study on topical agents | |
| Wound management | |
| Study carried out on volunteers, not scrub staff in an operating theatre | |
| Not relevant to this review | |
| A laboratory‐based study | |
| A literature review | |
| Evaluated patient skin preparations | |
| Patient skin preparation study | |
| Laboratory‐based study | |
| Hand hygiene study | |
| A discussion paper | |
| A discussion paper | |
| A laboratory‐based study | |
| Not relevant topic | |
| A study of handwashing rather than hand antisepsis. | |
| Study of dressings | |
| Not a randomised controlled trial; summary of previously published studies | |
| A laboratory‐based study | |
| Cross‐over trial but without any randomisation | |
| Wound irrigation study | |
| Bowel prep study | |
| All participants carried out intervention 1 on day 1, intervention 2 on day 2 and intervention 3 on day 3. No randomisation | |
| Study carried out on volunteers, not scrub staff in an operating theatre | |
| Assessed intra‐operative rescrubbing | |
| Central venous catheter study | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| Not randomised | |
| Not randomised | |
| Study carried out on volunteers, not scrub staff in an operating theatre | |
| Study of wound management | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| Study of handwashing rather than hand antisepsis | |
| A laboratory‐based study | |
| A laboratory‐based study | |
| Explores hand hygiene rather than hand antisepsis | |
| Laboratory‐based study | |
| A laboratory‐based study | |
| Study of clean air systems | |
| Evaluated user satisfaction | |
| No evidence that the study was randomised | |
| Study of dressings | |
| Evaluated patient skin preparations | |
| MRSA decolonisation | |
| Discussion paper | |
| Lab based study of effect of saline in antimicrobial skin preparations | |
| Evaluated patient skin preparations | |
| Study of antibiotics | |
| Patient skin preparation study | |
| Earlier systematic review | |
| Literature review | |
| Not a randomised controlled trial; no control group | |
| Wound irrigation study | |
| Evaluated iodine based wound dressings | |
| Looked at compliance with various handwashing methods | |
| Study of local anaesthetic | |
| Each intervention group contained a scrub solution, a patient prep solution and a follow‐up wound cleansing product. It was not possible to look at the effect of the scrub solution on its own | |
| Study of glove juice and rings | |
| Study of handwashing in a neo‐natal unit | |
| Anaesthetics study | |
| Study of antibiotics in hand injuries | |
| Hand hygiene literature review | |
| Not a randomised controlled trial | |
| A study of handwashing, not hand antisepsis. |
MRSA: methicillin‐resistant Staphylococcus aureus
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | Awaiting information from author |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SSI Show forest plot | 1 | 3133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| Analysis 1.1 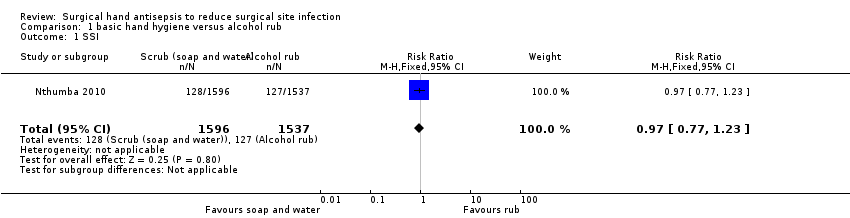 Comparison 1 basic hand hygiene versus alcohol rub, Outcome 1 SSI. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 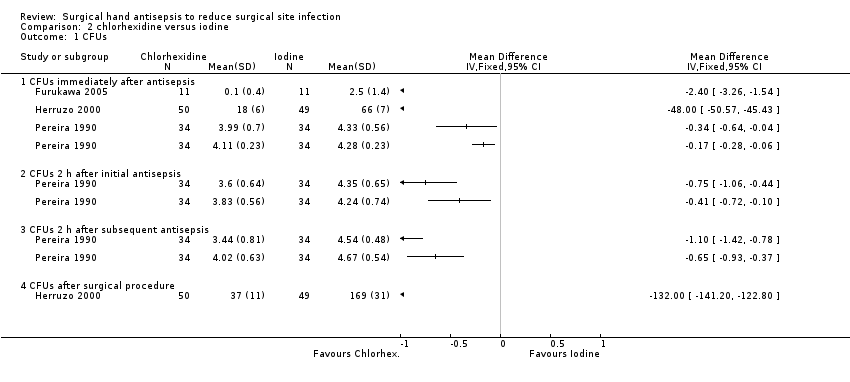 Comparison 2 chlorhexidine versus iodine, Outcome 1 CFUs. | ||||
| 1.1 CFUs immediately after antisepsis | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 CFUs 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 CFUs 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 CFUs after surgical procedure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1  Comparison 3 chlorhexidine versus iodine plus triclosan, Outcome 1 CFUs. | ||||
| 1.1 CFUs immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 CFUs 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 CFUs 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 alcohol rub versus other alcohol rub, Outcome 1 CFUs. | ||||
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SSI Show forest plot | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.23, 1.34] |
| Analysis 5.1  Comparison 5 scrub versus alcohol‐only rub, Outcome 1 SSI. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SSI Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 6.1 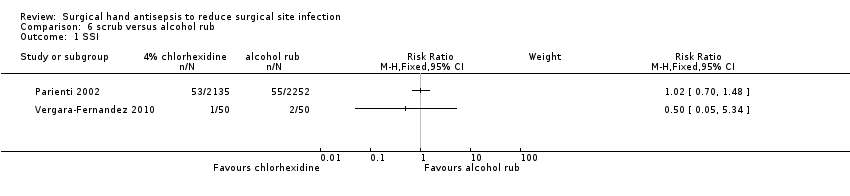 Comparison 6 scrub versus alcohol rub, Outcome 1 SSI. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 scrub (chlorhexidine) versus alcohol rub + additional ingredient, Outcome 1 CFUs. | ||||
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After surgical procedure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 8.1 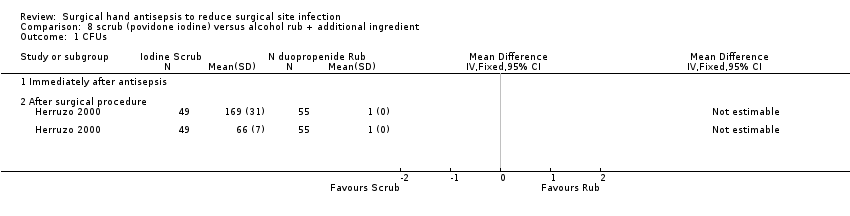 Comparison 8 scrub (povidone iodine) versus alcohol rub + additional ingredient, Outcome 1 CFUs. | ||||
| 1.1 Immediately after antisepsis | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After surgical procedure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 9.1  Comparison 9 scrub (chlorhexidine) versus rub + additional ingredient, Outcome 1 CFUs. | ||||
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After surgical procedure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐135.6 [‐153.39, ‐117.81] |
| Analysis 10.1  Comparison 10 scrub (chlorhexidine) versus alcohol rub + additional ingredient, Outcome 1 CFUs. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs immediately after antisepsis Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.14, 0.38] |
| Analysis 11.1 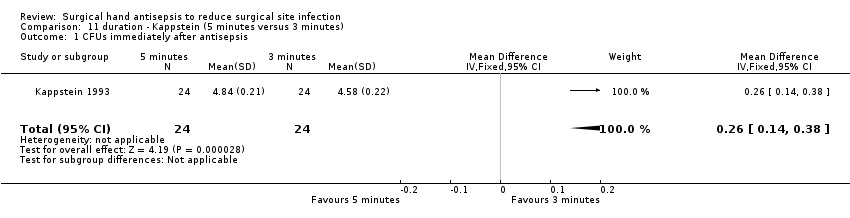 Comparison 11 duration ‐ Kappstein (5 minutes versus 3 minutes), Outcome 1 CFUs immediately after antisepsis. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 12.1  Comparison 12 duration ‐ 5 + 3 min versus 3 + 0.5 min with chlorhexidine), Outcome 1 CFUs. | ||||
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 13.1  Comparison 13 duration ‐ 5 + 3 min versus 3 + 0.5 minutes with iodine), Outcome 1 CFUs. | ||||
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 14.1  Comparison 14 duration ‐ 5 + 3.5 min versus 3 + 2.5 min chlorhexidine), Outcome 1 CFUs. | ||||
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUS Show forest plot | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.03, 0.51] |
| Analysis 15.1 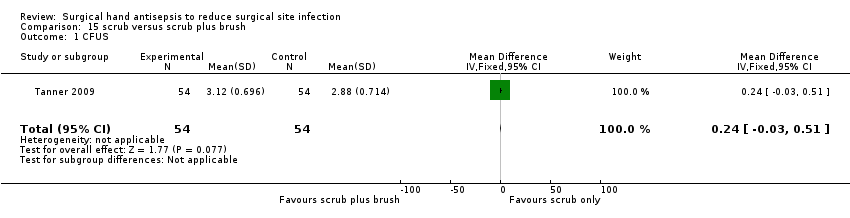 Comparison 15 scrub versus scrub plus brush, Outcome 1 CFUS. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.14, 0.40] |
| Analysis 16.1 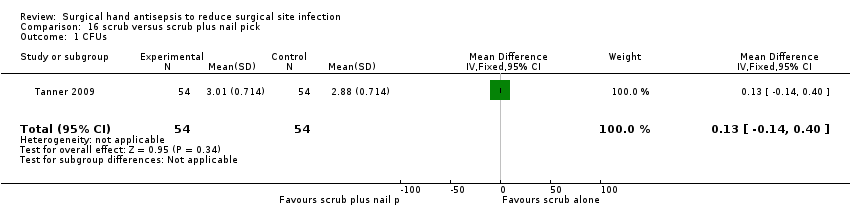 Comparison 16 scrub versus scrub plus nail pick, Outcome 1 CFUs. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.16, 0.38] |
| Analysis 17.1  Comparison 17 scrub plus brush versus scrub plus nail pick, Outcome 1 CFUs. | ||||
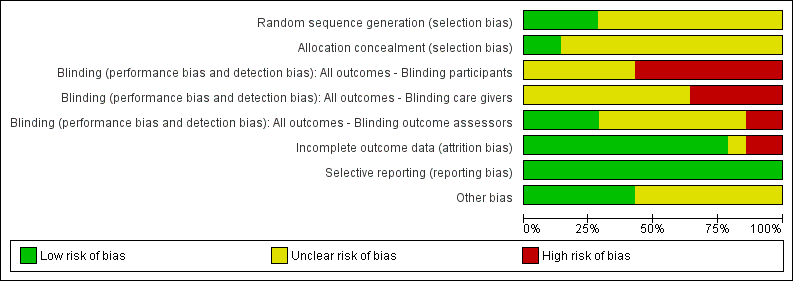
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 basic hand hygiene versus alcohol rub, Outcome 1 SSI.

Comparison 2 chlorhexidine versus iodine, Outcome 1 CFUs.

Comparison 3 chlorhexidine versus iodine plus triclosan, Outcome 1 CFUs.

Comparison 4 alcohol rub versus other alcohol rub, Outcome 1 CFUs.

Comparison 5 scrub versus alcohol‐only rub, Outcome 1 SSI.

Comparison 6 scrub versus alcohol rub, Outcome 1 SSI.

Comparison 7 scrub (chlorhexidine) versus alcohol rub + additional ingredient, Outcome 1 CFUs.

Comparison 8 scrub (povidone iodine) versus alcohol rub + additional ingredient, Outcome 1 CFUs.

Comparison 9 scrub (chlorhexidine) versus rub + additional ingredient, Outcome 1 CFUs.

Comparison 10 scrub (chlorhexidine) versus alcohol rub + additional ingredient, Outcome 1 CFUs.

Comparison 11 duration ‐ Kappstein (5 minutes versus 3 minutes), Outcome 1 CFUs immediately after antisepsis.

Comparison 12 duration ‐ 5 + 3 min versus 3 + 0.5 min with chlorhexidine), Outcome 1 CFUs.

Comparison 13 duration ‐ 5 + 3 min versus 3 + 0.5 minutes with iodine), Outcome 1 CFUs.

Comparison 14 duration ‐ 5 + 3.5 min versus 3 + 2.5 min chlorhexidine), Outcome 1 CFUs.

Comparison 15 scrub versus scrub plus brush, Outcome 1 CFUS.

Comparison 16 scrub versus scrub plus nail pick, Outcome 1 CFUs.

Comparison 17 scrub plus brush versus scrub plus nail pick, Outcome 1 CFUs.
| Trial arms | |||||||||
| Study | 1 | 2 | 3 | 4 | 5 | Country | Trial involved Surgery | SSI | CFU |
| n = 600 patients (data on 500) | Aqueous scrub | Alcohol rub | NA | NA | NA | Saudi Arabia | ✓ Clean and clean‐contaminated operations. Mainly abdominal. | ✓ CDC guidelines | ✕ |
| n = 22 operating nurses | Aqueous scrub | Aqueous scrub | NA | NA | NA | Japan | ✕ | ✕ | ✓ Iimmediately after antisepsis; glove juice method |
| n = 22 operating staff | Aqueous scrub | Alcohol rub + active ingredient | NA | NA | NA | USA | ✓ Ophthalmic, podiatric and general surgery | ✕ | ✓ Before antisepsis and immediately after antisepsis on day 1, after 6 hours on days 2 and 5; glove juice method |
| n = 4 surgeons (randomised and tested 53 times) | Aqueous scrub | Alcohol rub + active ingredient | alcohol rub + active ingredient | NA | NA | UK | ✕ Trauma | ✕ | ✓ At the end of the surgical procedure; glove juice method |
| n = 154 surgical staff | Aqueous scrub | Aqueous scrub | NA | NA | NA | Spain | ✓ Plastic surgery and traumatology | ✕ | ✓ Before antisepsis, immediately after antisepsis and at the end of the surgical procedure; finger press testing with agar plates |
| n = 24 surgeons | Aqueous scrub 1 (duration1) | Aqueous scrub 2 (duration 2) | NA | NA | NA | Germany | ✕ | ✕ | ✓ Before antisepsis and immediately after antisepsis; glove juice method |
| n = 66 surgical staff and 3317 patients | Alcohol rub + active ingredient | Standard hand hygiene | NA | NA | NA | Kenya | ✓ Clean and clean‐contaminated operations. Mixed surgery types. | ✓ Modified CDC guidelines | ✕ |
| n = 4387 patients | Aqueous scrub | Alcohol rub + active ingredient | NA | NA | NA | France | ✓ Mix of procedures | ✓ CDC guidelines | ✕ |
| n = 34 nurses | Aqueous scrub 1 Duration 1 | Aqueous scrub 2 Duration 1 | Aqueous scrub 1 Duration 2 | Aqueous scrub 2 Duration 2 | NA | Australia | ✕ | ✕ | ✓ Immediately after antisepsis, 2 hours after initial antisepsis, 2 hours after subsequent antisepsis; glove juice method |
| n = 34 operating room nurses | Aqueous scrub 1 (duration1) | Aqueous scrub 2 (duration 2) | Aqueous scrub 3 (duration 2) | Alcohol rub + active ingredient 1 | Alcohol rub + active ingredient 2 | Australia | ✕ | ✕ | ✓ Immediately after antisepsis, 2 hours after initial antisepsis, 2 hours after subsequent antisepsis; glove juice method |
| n= 75 surgeons | Aqueous scrub | Alcohol rub + active ingredient | NA | NA | NA | Germany | ✓ No detail | ✕ | ✓ Immediately after antisepsis and after surgical procedure completed; glove juice method |
| n= 164 staff | Aqueous scrub | Aqueous scrub +nail pick | Aqueous scrub +nail brush | NA | NA | UK | ✕ | ✕ | ✓ 1 hour after antisepsis; modified glove juice method |
| n = 100 patients | Aqueous scrub | Alcohol rub + active ingredient | NA | NA | NA | Mexico | ✓ Clean and clean‐contaminated operations. Mixed surgery types. | ✓ CDC guidelines | ✕ Only 20% of the 400 enrolled staff were assessed for bacteria on hands; data not included |
| n = 25 operating theatre nurses and surgical technologists | Aqueous scrub 1 (duration 1) | Aqueous scrub 2 (duration 2) | NA | NA | NA | USA | ✕ | ✕ | ✓ 1 hour after antisepsis; glove juice method |
| NA: not applicable | |||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SSI Show forest plot | 1 | 3133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.77, 1.23] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 CFUs immediately after antisepsis | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 CFUs 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 CFUs 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 CFUs after surgical procedure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 CFUs immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 CFUs 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 CFUs 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SSI Show forest plot | 1 | 500 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.23, 1.34] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 SSI Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After surgical procedure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediately after antisepsis | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After surgical procedure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After surgical procedure | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐135.6 [‐153.39, ‐117.81] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs immediately after antisepsis Show forest plot | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [0.14, 0.38] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 After initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Immediately after antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 2 h after initial antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 2 h after subsequent antisepsis | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUS Show forest plot | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.24 [‐0.03, 0.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.14, 0.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CFUs Show forest plot | 1 | 108 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.16, 0.38] |

