Estrategias con antibióticos para erradicar la Pseudomonas aeruginosa en pacientes con fibrosis quística
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Double‐blind RCT. Placebo‐controlled. Parallel design. Duration: 28 days. Multicentre based in USA. | |
| Participants | 21 participants with a recent positive oropharyngeal culture and isolation of P aeruginosa from BAL at study entry. Age: 6 months ‐ 6 years. Gender: 11 males, 10 females. | |
| Interventions | Treatment: Tobramycin solution for inhalation (300 mg 2x daily for 28 days). Control: placebo inhalations. | |
| Outcomes | Eradication of P aeruginosa, nutritional status, modified Shwachman score, adverse effects. | |
| Notes | Oropharyngeal cultures performed at entry and on days 14, 28, 42 and 56 of the study. BAL from the same lobar segment on entry and day 28. Enrolement was discontinued due to an interim analysis, precipitated by poor accrual of participants, which showed a statistically significant microbiological effect of treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as a randomised controlled trial stratified by study centre and age (≦ 36 months; > 36 months), but the method of generation of allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Did not report how allocation was concealed. |
| Blinding (performance bias and detection bias) | Low risk | Reported as double blind, but paper did not provide any details regarding who was blinded or the method of blinding. We received the following helpful response from trial authors, regarding placebo: Active: Preservative free tobramycin sulfate, 60 mg/mL in 5 mL excipient (1/4 normal saline, pH 6.0) in low density polyethylene plastic ampoules inside a foil pouch (PathoGenesis Corporation). Placebo: 5 mL of vehicle with 1.25 mg of quinine sulfate added as a flavouring agent, packaged identically. PathoGenesis Corporation were responsible for the manufacture of the tobramycin and placebo for inhalation. |
| Incomplete outcome data (attrition bias) | Low risk | Analysed on an intention‐to‐treat basis. Reported data on all participants who were randomised. There were no dropouts reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found. |
| Other bias | High risk | Study was stopped early by the Data Monitoring Committee after recruitment of 21 from an anticipated 98 participants because of statistically significant treatment effect in favour of the tobramycin group. Study received sponsorship support from Chiron, manufacturer of tobramycin for inhalation as used in the study. |
| Methods | RCT. Parallel design. Duration: 3 months. Single centre based in Europe. | |
| Participants | 58 children with CF, all with new isolation of P aeruginosa (sputum or cough swabs). Age: median age 9 years, interquartile range (4.7 ‐ 13.1 years). Gender: 31 male, 27 female. Lung function: median FEV₁ at inclusion 98% predicted. | |
| Interventions | Treatment (n = 29): Inhaled TSI (300 mg 2x daily for 28 days). Control (n = 29): 3 months combination therapy with inhaled colistin (2 MU 2x daily) + oral ciprofloxacin (10 mg/kg 3x daily). | |
| Outcomes | Primary outcomes | |
| Notes | Participants were then switched to the other arm or treated with IV antibiotics if clinically indicated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised in blocks of 10. No description given of method of randomisation, nor of any stratification. |
| Allocation concealment (selection bias) | Unclear risk | Did not report how allocation was concealed. |
| Blinding (performance bias and detection bias) | High risk | Blinding not possible for participants and clinicians as treatments compared were inhaled versus inhaled and oral. No details regarding whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Intention‐to‐treat analysis on all 58 randomised participants. |
| Selective reporting (reporting bias) | High risk | Protocol published on ClinicalTrials.gov (identifier: NCT01400750). All pre‐specified outcomes reported. BMI z score, weight z score and frequency of exacerbations were reported not to have changed significantly for trial participants, but numerical data are not reported. |
| Other bias | Unclear risk | Primary outcome was assessed at end of treatment which was different for the 2 treatment groups 28 days for TSI participants versus 3 months for colistin/ciprofloxacin participants. |
| Methods | RCT. Parallel design. Duration: 27 months. Multicentre (21 centres) based in Europe (Germany, France, Spain, Austria, UK, Netherlands). | |
| Participants | 123 participants with CF free of P aeruginosa (88 randomised ‐ 31 participants not randomised because of high P aeruginosa antibody titres and 4 for other reasons). Age (mean (SD)): 28‐day TIS 8.7 (7.2) years, 56‐day TIS 8.7 (10.5) years. Gender: 28‐day TIS 26 (58%) males, 19 (42%) females; 56‐day TIS 22 (51%) males, 21 (49%) females. Lung function (mean (SD) FEV1 % predicted): 28‐day TIS 80.2 (18.9), 56‐day TIS 87.0 (19.2). | |
| Interventions | Group 1 (n = 45): 28 days of tobramycin solution for inhalation (TSI) (300 mg 2x daily), then stopped treatment. Group 2 (n = 43): 28 days of tobramycin solution for inhalation (TSI) (300 mg 2x daily), then randomised to a further 28 days (56 days in total). | |
| Outcomes | Primary outcome Secondary outcomes Proportion of patients free of P aeruginosa 1 month after the end of treatment Number and length of hospital admissions for respiratory indications Occurrence of other pathogens Changes in FEV₁, FVC & FEF25‐75 Weight, height and BMI. | |
| Notes | Also known as ELITE trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no description of randomisation techniques given. |
| Allocation concealment (selection bias) | Unclear risk | Did not report how allocation was concealed. |
| Blinding (performance bias and detection bias) | High risk | Open‐label study, no attempt at blinding. |
| Incomplete outcome data (attrition bias) | Low risk | 65 participants from 88 randomised achieved primary outcome. A total of 52 participants prematurely withdrawn from trial. 27 participants withdrew from the 28‐day treatment group with the following reasons: loss to follow up (n = 1); protocol deviation (n = 4); recurrence/non‐eradication (n = 21); other (n = 1). 25 participants withdrew from the 56‐day treatment group for the following reasons: withdrawn consent (n = 1); loss to follow up (n = 2); protocol deviation (n = 2); recurrence/no eradication (n = 19); abnormal audiology test (n = 1). |
| Selective reporting (reporting bias) | High risk | Study reports there were no major short‐ or long‐term (3 and 27 months) changes in spirometry, but does not record the figures for either of the 2 groups. Also, only summary statements and no numerical data are provided for weight, height or BMI. |
| Other bias | Unclear risk | Recruited fewer participants than planned; actually randomised 88 participants (primary outcome evaluable in 65) ‐ planned randomisation of 100 participants. Did not randomise 35 participants from the recruited cohort of 123 participants: 31 because of high P aeruginosa antibody levels, one for an adverse event, one where consent was withdrawn, one for a protocol deviation and one 'other' (unspecified) reason. Participants with raised antibody levels were not included because the investigators believed that they were chronically infected with P aeruginosa based on their antibody results. This trial was initially supported by Chiron and later Novartis Pharma, the manufacturer of TSI. |
| Methods | RCT. Parallel design. Duration: 28 days. Multicentre (13 centres) in Italy. | |
| Participants | 223 participants with first ever or new P aeruginosa infection. New infection defined as P aeruginosa isolation following bacterial clearance documented by 3 negative cultures within the previous 6 months. Age: over 1 year. Gender: 116 male, 107 female. | |
| Interventions | Group A (n = 105; 52 male and 53 female): 28 days 2x daily inhalation of 2 MU colistin with 2x daily doses of ciprofloxacin 15 mg/kg/dose. | |
| Outcomes | Primary outcome Lung function (FEV₁). Period of time free of P aeruginosa. Isolation of other pathogens including gram‐negative and aspergillus. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence generated by statistical software within permuted blocks of size 10, stratified according to age and FEV₁. |
| Allocation concealment (selection bias) | Low risk | Separation of individuals responsible for randomisation and treatment assignment. |
| Blinding (performance bias and detection bias) | High risk | Open‐label trial so no blinding of participants nor researchers. |
| Incomplete outcome data (attrition bias) | Low risk | 38 of 223 randomised participants (17%) dropped out of the trial. The biggest reason for dropping out was lack of compliance with follow up protocol (11 from Group A and 13 from Group B) and identification of a pulmonary exacerbation during early eradication therapy (4 from Group A and 5 from Group B). Analysis was by intention‐to‐treat. |
| Selective reporting (reporting bias) | Unclear risk | We have been unable to locate a published protocol for this trial. The details published on the EudraCT database (number 2008‐006502‐42) describe objectives but not outcomes. In the main paper, the methods section does not describe all the trial objectives. Only eradication, time free of P aeruginosa and spirometry are described in the methods section. These outcomes plus the additional outcomes of isolation of other organisms and adverse events are described in the results. |
| Other bias | Low risk | No evidence of other bias identified. |
| Methods | RCT. Multi‐centre (57 centres) in the USA. Inhaled tobramycin was provided in an open‐label fashion, while oral ciprofloxacin was provided in a double‐blinded fashion. | |
| Participants | 306 participants with CF, previously free of P aeruginosa or had not had positive isolates for 2 years or more. Age: 1 year or older and 12 years and younger. Gender: 150 male, 154 female. | |
| Interventions | All participants received eradication therapy with inhaled tobramycin (Novartis Pharmaceutical Corp) for 28 days with or without ciprofloxacin (Bayer Healthcare AG). The main randomised intervention of nebulised tobramycin, with or without oral ciprofloxacin, commenced after this initial 28 days of treatment: Group A: cycled therapy; Group B: culture‐based therapy. Furthermore, the time from isolation of P. aeruginosa to commencing trial therapy was up to 6 months and in this interval, some participants received antimicrobial therapy. | |
| Outcomes | Primary outcomes Secondary outcomes Clinical Frequency of pulmonary exacerbations, hospitalizations, and use of concomitant oral, inhaled, and IV antibiotics. Anthropometric measures (linear growth, weight gain). Pulmonary function tests including FVC, FEF25%–75%, and FEV₁ (participants 4 years of age and older, able to reproducibly perform spirometry). Total hospitalization days. Microbiological Changes in antibiotic susceptibility patterns (minimal inhibitory concentrations of 12 antibiotics). Colony morphology. Presence of mucoid isolates from baseline to the end of the trial. Emergence of intrinsically aminoglycoside and ciprofloxacin‐resistant non‐pseudomonal organisms (e.g. B cepacia, A xylosoxidans and S maltophilia). Adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was carried out by permuted blocks, and performed using a computer‐generated sequence. |
| Allocation concealment (selection bias) | Low risk | Randomization assignment was available at the sites via an interactive voice response system with e‐mail confirmation of the treatment assignment. |
| Blinding (performance bias and detection bias) | Unclear risk | Inhaled tobramycin was provided in an open‐label fashion, while oral ciprofloxacin was provided in a double‐blinded fashion. All trial personnel and participants were blinded to oral therapy assignment but not to cycled or culture‐based treatment allocation. The core trial investigators were blinded to all treatment allocation for the entire study. |
| Incomplete outcome data (attrition bias) | Low risk | Only 2 of 306 randomised participants excluded from the analysis (because they did not receive study treatment). |
| Selective reporting (reporting bias) | Low risk | Data on all primary and secondary outcomes reported. |
| Other bias | Low risk | No imbalance in baseline characteristics. Central trial team (not local investigators) blinded. |
| Methods | RCT. Parallel design. Duration: 27 months. Single‐centre trial based in Europe. | |
| Participants | 26 participants with a recent positive culture who have never received anti‐pseudomonal therapy. Age: 2 ‐ 9 years. Gender: 13 males, 13 females. | |
| Interventions | Treatment: oral ciprofloxacin (250 ‐ 750 mg) 2x daily and inhaled colistin (1 MU) for 3 weeks at entry and each time P aeruginosa isolated. Control: no anti‐pseudomonas chemotherapy. | |
| Outcomes | Time to chronic colonisation with P aeruginosa (defined as the presence of P aeruginosa in monthly routine sputum specimens for 6 consecutive months and/or the development of precipitating serum antibodies against P aeruginosa). | |
| Notes | Monthly sputum samples. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as a RCT without stratification, but the method of generation of allocation sequence was not stated. |
| Allocation concealment (selection bias) | Unclear risk | Did not report how allocation was concealed. |
| Blinding (performance bias and detection bias) | High risk | Did not use blinding, interventions different. |
| Incomplete outcome data (attrition bias) | Low risk | Analysed on an intention‐to‐treat basis. Reported data on all participants who were randomised. There were no dropouts reported. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting found. |
| Other bias | Low risk | No evidence of other bias identified. |
| Methods | RCT. Double‐blind, placebo‐controlled trial. Parallel design. Duration: 2 years. Multicentre trial based in Europe. | |
| Participants | 22 children with P aeruginosa‐negative throat swabs or sputum cultures for > 1 year and negative serum antibody titers were eligible. Age: 4 ‐ 18 years. Gender: 9 males, 13 females. | |
| Interventions | Treatment: nebulised tobramycin 80 mg inhaled 2x daily. Control: inhaled placebo. | |
| Outcomes | Time to clearance of P aeruginosa from the airway. | |
| Notes | Monthly sputum or oropharyngeal swabs during trial period. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation sequence was generated using a coin flip for pairs of participants. There is no information as to who was responsible for the coin flip or what controls were in place to ensure validity of the result of the coin flip. |
| Allocation concealment (selection bias) | Unclear risk | Did not report how allocation was concealed. |
| Blinding (performance bias and detection bias) | Low risk | Reported as double blind. Participants were blinded by providing a placebo inhalation with a similar taste to the treatment inhalation, but it is not clear whether the clinicians administering the treatment were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 out of 11 participants withdrew from treatment group; 5 out of 11 participants withdrew from placebo group. The trial was analysed on an available case basis. |
| Selective reporting (reporting bias) | High risk | Reported there was no change in spirometric pulmonary function during or after the treatment period, but no data were given |
| Other bias | Low risk | No evidence of other bias identified. |
A xylosoxidans: Achromobacter xylosoxidans
B cepacia: Burkholderia cepacia
BAL: bronchoalveolar lavage
BMI: body mass index
FEF25‐75: mid‐forced expiratory flow
FEV₁: forced expiratory volume at one second
FVC: forced vital capacity
IgG: immunoglobulin G
IV: intravenous
MU: million units
P aeruginosa: Pseudomonas aeruginosa
RCT: randomised controlled trial
S maltophilia: Stenotrophomonas maltophilia
TSI: tobramycin solution for inhalation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Drug tolerability trial, not eradication therapy. | |
| Drug tolerability trial, not eradication therapy. | |
| Eradication treatment not used. Observational study. | |
| Participants allocated to treatment by minimisation on the basis of IgG levels and clinical indications compared to therapy based on clinical indications alone. | |
| Symptomatic treatment not eradication. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| Historical control group. | |
| Pharmacokinetic and drug tolerability trial, not eradication therapy. | |
| Participants chronically infected with P aeruginosa. | |
| Not randomised and with no allocation concealment. | |
| Participants chronically infected with P aeruginosa. | |
| Case‐control study. | |
| No control group. | |
| Retrospective cohort study. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| The primary aim of this trial was not to evaluate eradication regimens for P aeruginosa and 112 of 118 participants were treated for an acute exacerbation or suppression of chronic infection with P aeruginosa. | |
| Trial not designed to look at eradication of P aeruginosa. At baseline, 47 of 59 participants had chronic infection with P aeruginosa. | |
| No control group. | |
| Sinonasal nebulisation of antibiotic aiming to eradicate from the sinuses only. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa, not an eradication trial, no randomisation. | |
| No control group and no randomisation. | |
| Not an eradication trial, participants chronically infected with P. aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| No control group. | |
| Participants chronically infected with P aeruginosa. | |
| Participants chronically infected with P aeruginosa. | |
| Pharmacokinetic trial of inhaled tobramycin, not eradication therapy. | |
| Pharmacokinetic trial. | |
| Symptomatic treatment not eradication. | |
| No randomisation or eradication therapy. | |
| Drug tolerability trial in chronic P aeruginosa infection, not eradication therapy. | |
| Pharmacokinetic trial. | |
| No control group. | |
| Participants chronically infected with P aeruginosa. | |
| Participants with chronic P aeruginosa. | |
| Primary outcome did not have a control group. Historical controls utilised for other outcomes. No randomisation. | |
| Trial of prophylaxis against future infection with P aeruginosa, not of eradication. | |
| Participants chronically infected with P aeruginosa. | |
| Historical control group. | |
| Randomised to therapy directed by the results of bronchoalveolar lavage compared to therapy based on clinical indications or upper respiratory samples. | |
| Participants chronically infected with P aeruginosa. |
P aeruginosa: Pseudomonas aeruginosa
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Single‐centre, randomised, prospective trial. |
| Participants | Stable children with CF and positive surveillance cultures for P aeruginosa. |
| Interventions | Nebulised tobramycin 300 mg 2x daily for 4 weeks or intravenous ceftazidime with tobramycin for 2 weeks at standard weight‐adjusted doses. |
| Outcomes | Primary efficacy endpoint was change in BAL fluid percentage neutrophils from the most affected lobe at bronchoscopy. Secondary outcomes included change in BAL fluid differential cell counts, cytokines and bacterial quantity. |
| Notes | 8 participants from a total of 15 had first ever isolate of P aeruginosa and can be included in this review. Outcome data for these 8 participants not published, author contacted for them. |
BAL: bronchoalveolar lavage
CF: cystic fibrosis
P. aeruginosa: Pseudomonas aeruginosa
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A Randomized, Double‐Blind, Placebo‐Controlled, Crossover Multi‐Center Study to Assess the Efficacy and Safety of Inhaled Tobramycin Nebuliser Solution (TOBI®) for the Treatment of Early Infections of P. Aeruginosa in Cystic Fibrosis Subjects Aged From 3 Months to Less Than 7 Years |
| Methods | Randomised, double‐blind trial. Multicentre in Europe and Canada. |
| Participants | Aim to enrol 50 participants aged 3 months to 6 years, both males and females. Inclusion criteria
Exclusion Criteria:
|
| Interventions | Experimental: TOBI (tobramycin inhaled solution)/placebo Participants randomized to TOBI received the investigational treatment for 28 days 2x daily in the 1st treatment cycle. At the end of 1st treatment cycle, participants who were positive for P aeruginosa entered the open‐label phase of the trial and received TOBI for 28 days 2x daily. Participants who were negative for P aeruginosa at the end of 1st treatment cycle and agreed to participate in the cross‐over treatment period received placebo for 28 days 2x daily (2nd treatment cycle). Comparator: placebo/TOBI Participants randomized to placebo group received 0.9 % saline (NaCl) for 28 days 2x daily in the 1st treatment cycle. At the end of 1st treatment cycle, participants who were positive for P aeruginosa entered the open‐label phase of the study and received TOBI for 28 days 2x daily. Participants who were negative for P aeruginosa at the end of 1st treatment cycle and agreed to participate in the cross‐over treatment period received TOBI for 28 days 2x daily (2nd treatment cycle). |
| Outcomes | Primary Outcome Percentage of participants P aeruginosa‐free after completion of the first treatment cycle (Day 29) as assessed by sputum/throat swab cultures Percentage of participants free from P aeruginosa 28 days after termination of the second treatment cycle (Day 91) as assessed by sputum/throat swab cultures |
| Starting date | April 2010 |
| Contact information | Prof Felix Ratjen, The Hospital for Sick Children, Toronto, Canada. |
| Notes | Completion date: June 2015 (final data collection date for primary outcome measure). Sponsors: Novartis Pharmaceuticals |
| Trial name or title | TORPEDO‐CF (Trial of Optimal Therapy for Pseudomonas Eradication in Cystic Fibrosis) |
| Methods | Multi‐centre, parallel group, RCT. |
| Participants | Inclusion criteria |
| Interventions | Objective: this trial will assess whether 10 days IV ceftazidime with tobramycin is superior to 3 months oral ciprofloxacin. Both treatment regimens will be in conjunction with 3 months nebulised colistin. Arm A: 14 days IV ceftazidime 50 mg/kg/dose, to a maximum of 3 g 3x daily and IV tobramycin 10 mg/kg/day either 1x daily or in divided doses (maximum 660 mg/day). |
| Outcomes | Primary outcome 1. Successful eradication of P aeruginosa infection 3 months after allocated treatment has started, remaining infection‐free through to 15 months after the start of allocated treatment Secondary outcomes 1. Time to reoccurrence of original P aeruginosa infection |
| Starting date | 24/05/2010. |
| Contact information | Dr Simon Langton Hewer Bristol Royal Hospital for Children Bristol BS2 8BJ |
| Notes | Anticipated end date: 01/11/2014 HTA 07/51/01 |
A xylosoxidans: Alcaligenes xylosoxidans
B cepacia: Burkholderia cepacia
BMI: body mass index
CF: cystic fibrosis
FEF25‐75: mid‐forced expiratory flow
FEV₁: forced expiratory volume at 1 second
FVC: forced vital capacity
IV: intravenous
MRSA: Methicillin‐resistant Staphylococcus aureus
NHS: National Health Service
P aeruginosa: Pseudomonas aeruginosa
QALY: quality‐adjusted life year
RCT: randomised controlled trial
S maltophilia: Stenotrophomonous maltophilia
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P aeruginosa (300 mg 2x daily) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.1  Comparison 1 Inhaled tobramycin versus placebo, Outcome 1 Positive respiratory culture for P aeruginosa (300 mg 2x daily). | ||||
| 1.1 At 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 2 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Positive respiratory culture for P aeruginosa (80 mg 2x daily) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.2 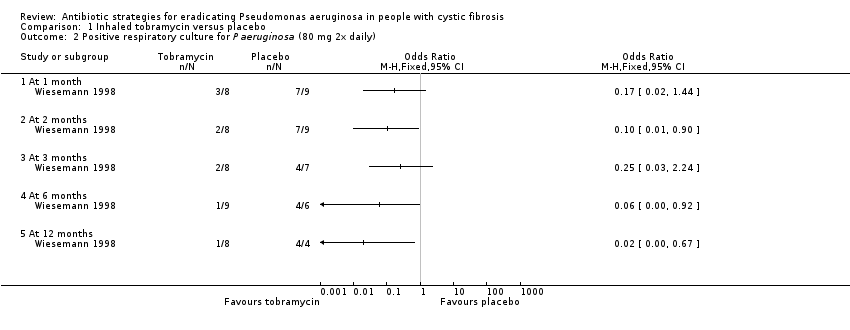 Comparison 1 Inhaled tobramycin versus placebo, Outcome 2 Positive respiratory culture for P aeruginosa (80 mg 2x daily). | ||||
| 2.1 At 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 2 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 At 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Positive respiratory culture for P aeruginosa (combined available case analysis) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 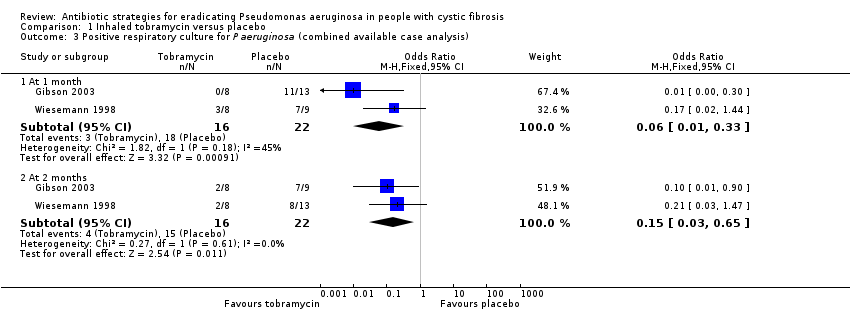 Comparison 1 Inhaled tobramycin versus placebo, Outcome 3 Positive respiratory culture for P aeruginosa (combined available case analysis). | ||||
| 3.1 At 1 month | 2 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.33] |
| 3.2 At 2 months | 2 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.65] |
| 4 Positive respiratory culture for P aeruginosa (combined) ‐ best case Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 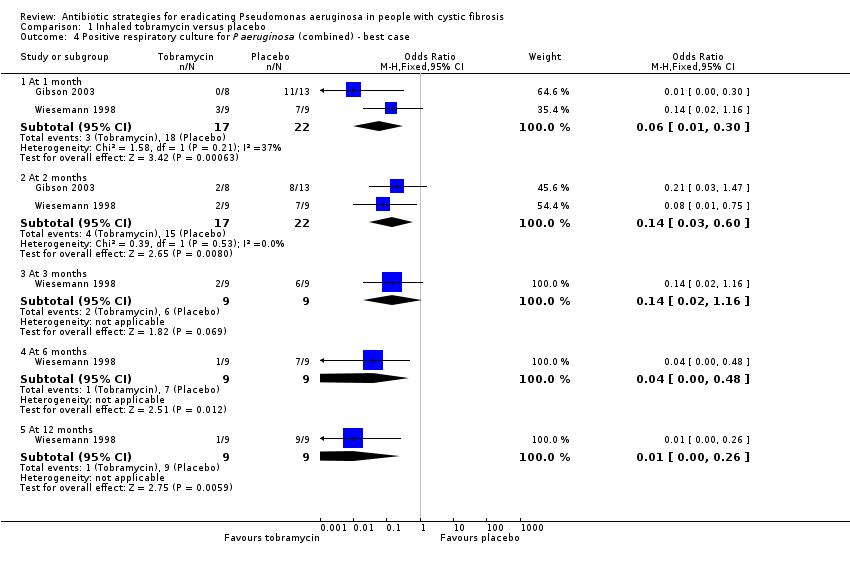 Comparison 1 Inhaled tobramycin versus placebo, Outcome 4 Positive respiratory culture for P aeruginosa (combined) ‐ best case. | ||||
| 4.1 At 1 month | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.30] |
| 4.2 At 2 months | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| 4.3 At 3 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.16] |
| 4.4 At 6 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.48] |
| 4.5 At 12 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.26] |
| 5 Positive respiratory culture for P aeruginosa (combined) ‐ worst case Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5  Comparison 1 Inhaled tobramycin versus placebo, Outcome 5 Positive respiratory culture for P aeruginosa (combined) ‐ worst case. | ||||
| 5.1 At 1 month | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.38] |
| 5.2 At 2 months | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.73] |
| 5.3 At 3 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.05, 2.77] |
| 5.4 At 6 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 1.83] |
| 5.5 At 12 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.05, 2.77] |
| 6 Weight (kg) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Inhaled tobramycin versus placebo, Outcome 6 Weight (kg) ‐ change from baseline. | ||||
| 6.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 2 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.7  Comparison 1 Inhaled tobramycin versus placebo, Outcome 7 Adverse events. | ||||
| 7.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Modified Shwachmann score ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8  Comparison 1 Inhaled tobramycin versus placebo, Outcome 8 Modified Shwachmann score ‐ change from baseline. | ||||
| 8.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 At 2 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion colonised with P aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1 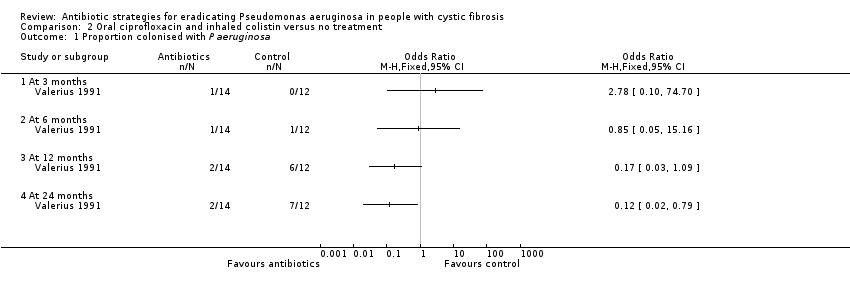 Comparison 2 Oral ciprofloxacin and inhaled colistin versus no treatment, Outcome 1 Proportion colonised with P aeruginosa. | ||||
| 1.1 At 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 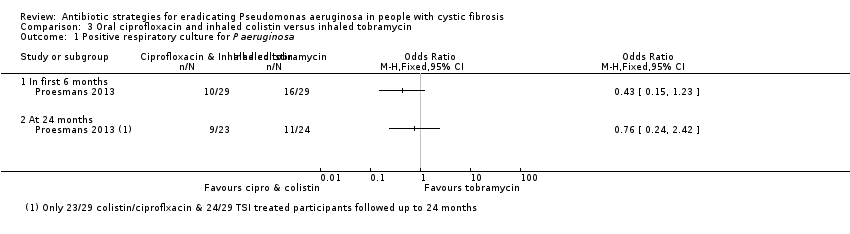 Comparison 3 Oral ciprofloxacin and inhaled colistin versus inhaled tobramycin, Outcome 1 Positive respiratory culture for P aeruginosa. | ||||
| 1.1 In first 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.2  Comparison 3 Oral ciprofloxacin and inhaled colistin versus inhaled tobramycin, Outcome 2 Adverse events. | ||||
| 2.1 Severe cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to next isolation of P aeruginosa from BAL, sputum or oropharyngeal cultures Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 1 Time to next isolation of P aeruginosa from BAL, sputum or oropharyngeal cultures. | ||||
| 2 Number of respiratory exacerbations Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.2 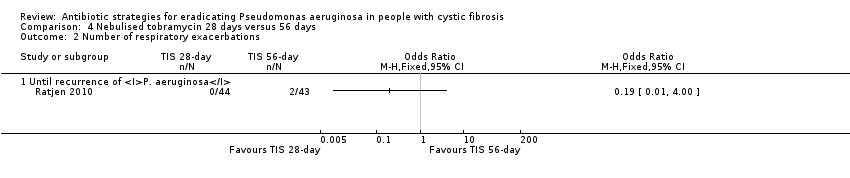 Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 2 Number of respiratory exacerbations. | ||||
| 2.1 Until recurrence of P. aeruginosa | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events (up to 3 months) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.3  Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 3 Adverse events (up to 3 months). | ||||
| 3.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Productive cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Haemoptysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Rhinitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Sinusitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Nasopharyngitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Tonsilitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Oropharyngeal pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Dysphonia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 URTI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Lung disorder | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Bronchitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 P. aeruginosa infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 Influenza | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Otitis media | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 Deafness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.18 Drug level increased | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.19 Pyrexia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.20 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.21 Varicella | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events (over 3 months) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4 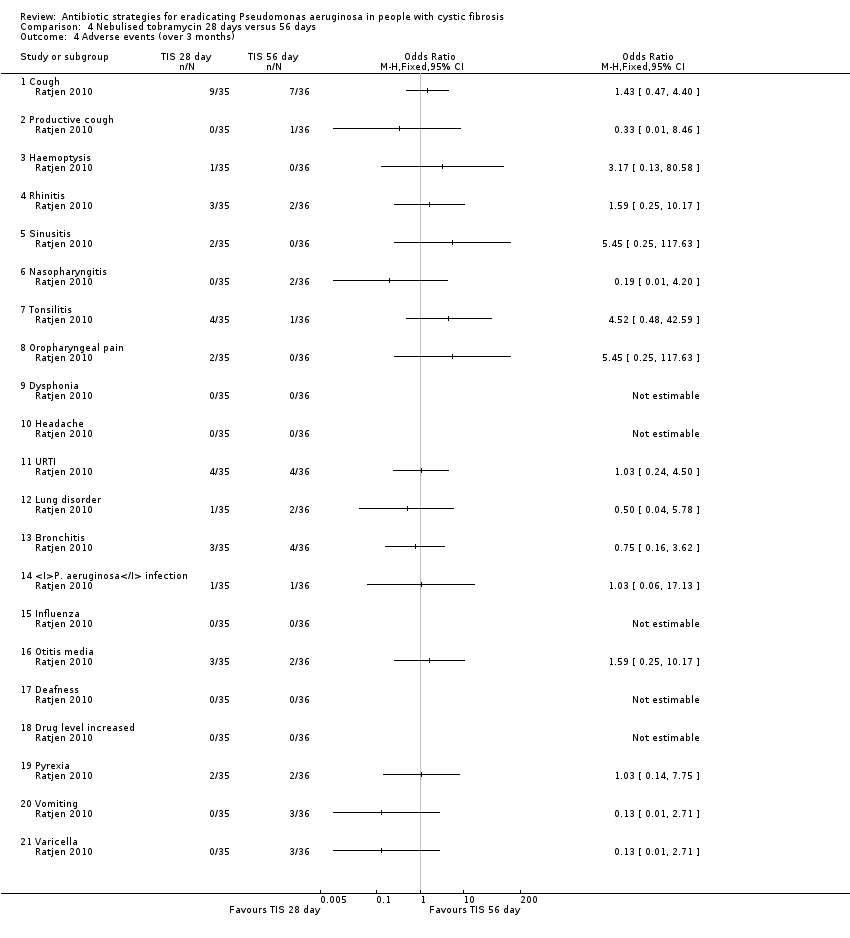 Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 4 Adverse events (over 3 months). | ||||
| 4.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Productive cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Haemoptysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Rhinitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Sinusitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Nasopharyngitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Tonsilitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Oropharyngeal pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Dysphonia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 URTI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Lung disorder | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.13 Bronchitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.14 P. aeruginosa infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.15 Influenza | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.16 Otitis media | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.17 Deafness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.18 Drug level increased | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.19 Pyrexia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.20 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.21 Varicella | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 1 Positive respiratory culture for P aeruginosa. | ||||
| 1.1 In first 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At end of follow up (median 16 months) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV₁ % predicted (relative change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 2 FEV₁ % predicted (relative change from baseline). | ||||
| 2.1 At mean 54 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Microbiology status (post‐trial) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 3 Microbiology status (post‐trial). | ||||
| 3.1 Stenotrophomonas maltophilia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Achromobacter xylosoxidans | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Aspergillus species | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events leading to trial discontinuation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 4 Adverse events leading to trial discontinuation. | ||||
| 4.1 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Photosensitivity | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Wheeze | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Pulmonary exacerbation during early eradication treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Lack of compliance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with one or more isolates of P aeruginosa from respiratory tract Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.1 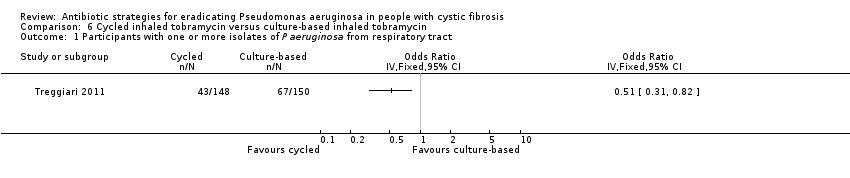 Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 1 Participants with one or more isolates of P aeruginosa from respiratory tract. | ||||
| 2 FEV₁ % predicted ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.2  Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 2 FEV₁ % predicted ‐ change from baseline. | ||||
| 2.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Weight (kg) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.3  Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 3 Weight (kg) ‐ change from baseline. | ||||
| 3.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Height (cm) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.4  Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 4 Height (cm) ‐ change from baseline. | ||||
| 4.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time to severe pulmonary exacerbation Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 6.5 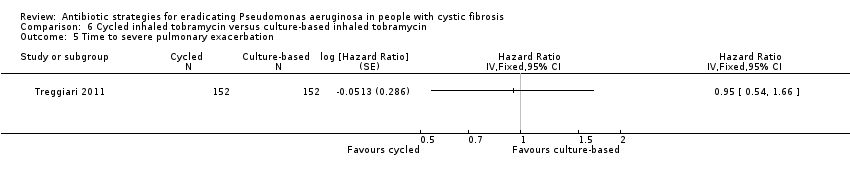 Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 5 Time to severe pulmonary exacerbation. | ||||
| 6 Participants with one or more severe pulmonary exacerbations Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.6  Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 6 Participants with one or more severe pulmonary exacerbations. | ||||
| 7 Time to pulmonary exacerbation (any severity) Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 6.7 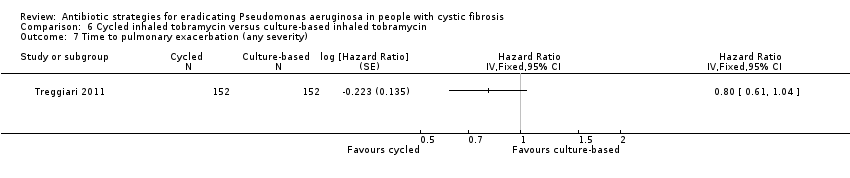 Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 7 Time to pulmonary exacerbation (any severity). | ||||
| 8 Participants with one or more pulmonary exacerbations (any severity) Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.8  Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 8 Participants with one or more pulmonary exacerbations (any severity). | ||||
| 9 Participants with new isolates of Stenotrophomonas maltophilia Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.9  Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 9 Participants with new isolates of Stenotrophomonas maltophilia. | ||||
| 10 Participants with one or more serious adverse event Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.10  Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 10 Participants with one or more serious adverse event. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with one or more isolates of P aeruginosa from respiratory tract Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 1 Participants with one or more isolates of P aeruginosa from respiratory tract. | ||||
| 2 FEV₁ % predicted ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 2 FEV₁ % predicted ‐ change from baseline. | ||||
| 2.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Weight (kg) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 3 Weight (kg) ‐ change from baseline. | ||||
| 3.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Height (cm) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.4 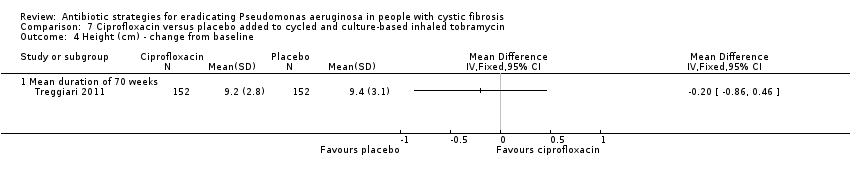 Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 4 Height (cm) ‐ change from baseline. | ||||
| 4.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time to severe pulmonary exacerbation Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 7.5 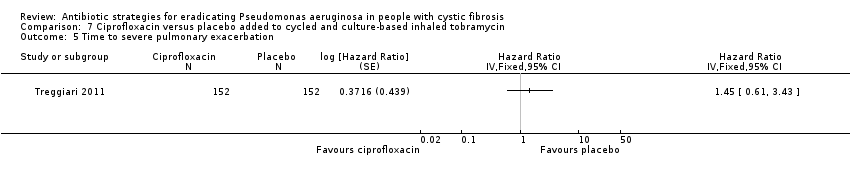 Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 5 Time to severe pulmonary exacerbation. | ||||
| 6 Participants with one or more severe pulmonary exacerbations Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.6  Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 6 Participants with one or more severe pulmonary exacerbations. | ||||
| 7 Time to pulmonary exacerbation (any severity) Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| Analysis 7.7  Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 7 Time to pulmonary exacerbation (any severity). | ||||
| 8 Participants with one of more pulmonary exacerbation (any severity) Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.8 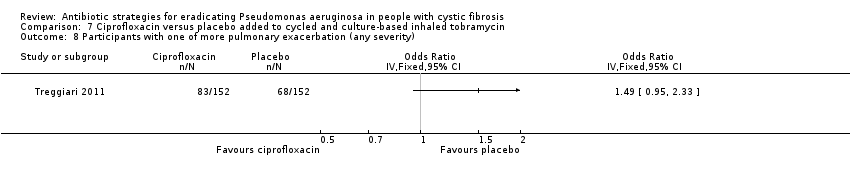 Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 8 Participants with one of more pulmonary exacerbation (any severity). | ||||
| 9 Participants with new isolates of Stenotrophomonas maltophilia Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.9  Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 9 Participants with new isolates of Stenotrophomonas maltophilia. | ||||
| 10 Participants with one or more serious adverse event Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.10  Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 10 Participants with one or more serious adverse event. | ||||

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 1 Positive respiratory culture for P aeruginosa (300 mg 2x daily).

Comparison 1 Inhaled tobramycin versus placebo, Outcome 2 Positive respiratory culture for P aeruginosa (80 mg 2x daily).

Comparison 1 Inhaled tobramycin versus placebo, Outcome 3 Positive respiratory culture for P aeruginosa (combined available case analysis).

Comparison 1 Inhaled tobramycin versus placebo, Outcome 4 Positive respiratory culture for P aeruginosa (combined) ‐ best case.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 5 Positive respiratory culture for P aeruginosa (combined) ‐ worst case.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 6 Weight (kg) ‐ change from baseline.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 7 Adverse events.

Comparison 1 Inhaled tobramycin versus placebo, Outcome 8 Modified Shwachmann score ‐ change from baseline.

Comparison 2 Oral ciprofloxacin and inhaled colistin versus no treatment, Outcome 1 Proportion colonised with P aeruginosa.

Comparison 3 Oral ciprofloxacin and inhaled colistin versus inhaled tobramycin, Outcome 1 Positive respiratory culture for P aeruginosa.

Comparison 3 Oral ciprofloxacin and inhaled colistin versus inhaled tobramycin, Outcome 2 Adverse events.

Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 1 Time to next isolation of P aeruginosa from BAL, sputum or oropharyngeal cultures.

Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 2 Number of respiratory exacerbations.

Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 3 Adverse events (up to 3 months).

Comparison 4 Nebulised tobramycin 28 days versus 56 days, Outcome 4 Adverse events (over 3 months).

Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 1 Positive respiratory culture for P aeruginosa.

Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 2 FEV₁ % predicted (relative change from baseline).

Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 3 Microbiology status (post‐trial).

Comparison 5 Inhaled colistin/oral ciprofloxacin versus inhaled tobramycin/oral ciprofloxacin, Outcome 4 Adverse events leading to trial discontinuation.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 1 Participants with one or more isolates of P aeruginosa from respiratory tract.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 2 FEV₁ % predicted ‐ change from baseline.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 3 Weight (kg) ‐ change from baseline.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 4 Height (cm) ‐ change from baseline.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 5 Time to severe pulmonary exacerbation.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 6 Participants with one or more severe pulmonary exacerbations.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 7 Time to pulmonary exacerbation (any severity).

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 8 Participants with one or more pulmonary exacerbations (any severity).

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 9 Participants with new isolates of Stenotrophomonas maltophilia.

Comparison 6 Cycled inhaled tobramycin versus culture‐based inhaled tobramycin, Outcome 10 Participants with one or more serious adverse event.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 1 Participants with one or more isolates of P aeruginosa from respiratory tract.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 2 FEV₁ % predicted ‐ change from baseline.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 3 Weight (kg) ‐ change from baseline.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 4 Height (cm) ‐ change from baseline.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 5 Time to severe pulmonary exacerbation.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 6 Participants with one or more severe pulmonary exacerbations.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 7 Time to pulmonary exacerbation (any severity).

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 8 Participants with one of more pulmonary exacerbation (any severity).

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 9 Participants with new isolates of Stenotrophomonas maltophilia.

Comparison 7 Ciprofloxacin versus placebo added to cycled and culture‐based inhaled tobramycin, Outcome 10 Participants with one or more serious adverse event.
| Inhaled tobramycin compared with placebo for eradicating Pseudomonas aeruginosa in people with cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis and a positive microbiological isolate of Pseudomonas aeruginosa from a respiratory tract specimen Settings: outpatients Intervention: inhaled tobramycin Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Inhaled tobramycin | |||||
| Eradication of P aeruginosa from the respiratory tract: Proportion with positive respiratory culture for P aeruginosa Follow‐up: 2 months (further results reported up to 2 years) | 682 per 1000 | 102 per 1000 (20 to 443 per 1000) | OR 0.15 (95% CI 0.03 to 0.65) | 38 | ⊕⊝⊝⊝ | The two studies gave very different doses of inhaled tobramycin (80 mg or 300 mg 2x daily). Results across different time points and sensitivity analyses to account for missing data in one trial were variable, showing no consistently significant advantage to inhaled tobramycin over placebo. |
| FEV₁ Follow‐up: up to 2 years | There were no changes in spirometric pulmonary function during or after the treatment period. | NR | up to 224 | ⊕⊝⊝⊝ | No numerical data were reported. | |
| FVC Follow‐up: up to 2 years | There were no changes in spirometric pulmonary function during or after the treatment period. | NR | up to 224 | ⊕⊝⊝⊝ | No numerical data were reported. | |
| Growth and nutritional status: change in weight (kg) from baseline Follow‐up: up to 2 months | The mean change in weight from baseline was 0.3 kg in the placebo group. | The mean change in weight from baseline was 0.1 kg higher (0.38 kg lower to 0.58 kg higher) in the inhaled tobramycin group. | NA | 21 | ⊕⊕⊝⊝ | There was also no difference in the mean change in weight from baseline between groups at 1 month MD 0.20 kg (95% CI ‐0.28 to 0.68). |
| Frequency of infective pulmonary exacerbations: number of exacerbations per patient year Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Isolation of other micro‐organisms from the respiratory tract: number of positive cultures per patient year Follow‐up: up to 2 months | There were no changes in the prevalence of other micro‐organisms, including multi‐resistant organisms, cultured from respiratory secretions. | NR | 21 | ⊕⊝⊝⊝ | No numerical data were reported. | |
| Adverse effects to antibiotics: cough Follow‐up: up to 2 months | 923 per 1000 | 535 per 1000 (28 to 1000 per 1000) | OR 0.58 (95% CI 0.03 to 10.86) | 21 | ⊕⊝⊝⊝ | No other specific adverse events were reported. The other included study in this comparison stated that there was no evidence of a difference in serum creatinine levels or auditory threshold between the groups. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to risk of bias; methodological information was limited and unclear in the included studies and there were concerns regarding incomplete outcome data, selective reporting and other biases due to the early termination of one study. 2. Downgraded once due to imprecision: wide confidence intervals around the pooled effect and variable results shown at different time points. 3. Downgraded once due to applicability: the included studies recruited only children; results are not applicable to adults. 4. In the included trial, 22 participants were randomised but it is not clear if all participants contributed to this outcome. 5. Downgraded once due to imprecision: no numerical results available. 6. Downgraded once due to imprecision: very wide confidence intervals around the effect size. | ||||||
| Oral ciprofloxacin and inhaled colistin compared with no treatment for eradicating Pseudomonas aeruginosa in people with cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis and a positive microbiological isolate of P. aeruginosa from a respiratory tract specimen Settings: outpatients Intervention: oral ciprofloxacin and inhaled colistin Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Oral ciprofloxacin and inhaled colistin | |||||
| Eradication of P aeruginosa from the respiratory tract Follow‐up: NA | Outcome not reported | NA | NA | NA | ||
| FEV₁ Follow‐up: NA | Outcome not reported | NA | NA | NA | ||
| FVC Follow‐up: NA | Outcome not reported | NA | NA | NA | ||
| Growth and nutritional status Follow‐up: NA | Outcome not reported | NA | NA | NA | ||
| Frequency of infective pulmonary exacerbations: number of exacerbations per patient year Follow‐up: NA | Outcome not reported | NA | NA | NA | ||
| Isolation of other micro‐organisms from the respiratory tract: number of positive cultures per patient year Follow‐up: NA | Outcome not reported | NA | NA | NA | ||
| Adverse effects to antibiotics Follow‐up: 27 months | No adverse effects were reported in either group | NR | 26 (1 RCT) | ⊕⊝⊝⊝ | No numerical data were reported. | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. Downgraded once due to risk of bias; methodological information was limited and unclear in the included study and there was a high risk of bias due to lack of blinding. 2. Downgraded once due to applicability: the included study recruited only children; results are not applicable to adults. 3. Downgraded once due to imprecision: no numerical results available. | ||||||
| Oral ciprofloxacin and inhaled colistin compared to inhaled tobramycin for eradicating Pseudomonas aeruginosa in people with cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis and a positive microbiological isolate of P aeruginosa from a respiratory tract specimen Settings: outpatients Intervention: oral ciprofloxacin and inhaled colistin Comparison: inhaled tobramycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inhaled tobramycin | Oral ciprofloxacin and inhaled colistin | |||||
| Eradication of P aeruginosa from the respiratory tract: Proportion with positive respiratory culture for P aeruginosa Follow‐up: up to 24 months | 458 per 1000 | 348 per 1000 (110 to 1000 per 1000) | OR 0.76 (95% CI 0.24 to 2.42) | up to 581 | ⊕⊝⊝⊝ | There was also no significant difference between treatment groups within the first 6 months, OR 0.43 (95% CI 0.15 to 1.23). |
| FEV₁: change from baseline (% predicted) Follow‐up: up to 24 months | Median change from baseline in FEV₁ (% predicted) for all the participants was ‐1%. | NR | up to 581 | ⊕⊝⊝⊝ | Changes in FEV₁ are not reported separately for each treatment arm. | |
| FVC Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Growth and nutritional status: BMI and weight z score Follow‐up: up to 24 months | Both BMI z score and weight z score were reported not to have changed significantly for trial participants as a whole. | NR | up to 581 | ⊕⊝⊝⊝ | Numerical data were not reported for comparative results across the treatment groups. | |
| Frequency of infective pulmonary exacerbations: number of exacerbations per patient year Follow‐up: up to 24 months | During the first six months of follow up, there was no difference between the two treatment arms in number of oral antibiotic treatment days. | NR | up to 581 | ⊕⊝⊝⊝ | These oral antibiotics were given for symptoms and not because of failed eradication. No numerical data were reported | |
| Isolation of other micro‐organisms from the respiratory tract: number of positive cultures per patient year Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Adverse effects to antibiotics: severe cough Follow‐up: up to 24 months | 34 per 1000 | 11 per 1000 (0 to 280 per 1000) | OR 0.32 (95% CI 0.01 to 8.24) | up to 581 | ⊕⊝⊝⊝ | No other specific adverse events were reported. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. In the included trial, 58 participants were randomised but not all participants contributed to all outcomes (unclear how many participants contributed to some outcomes). 2. Downgraded once due to risk of bias; methodological information was limited and unclear in the included study and there were concerns of bias due to selective reporting of results. 3. Downgraded once due to applicability: the included studies recruited only children; results are not applicable to adults. 4. Downgraded once due to imprecision: very wide confidence intervals around the effect size. 5. Downgraded once due to imprecision: no numerical comparative results available. | ||||||
| Inhaled tobramycin (28 days) compared with inhaled tobramycin (56 days) for eradicating Pseudomonas aeruginosa in people with cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis and a positive microbiological isolate of P aeruginosa from a respiratory tract specimen Settings: outpatients Intervention: inhaled tobramycin (28 days) Comparison: inhaled tobramycin (56 days) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inhaled tobramycin (56 days) | Inhaled tobramycin (28 days) | |||||
| Eradication of P aeruginosa from the respiratory tract: time to next isolation of P aeruginosa from BAL, sputum or oropharyngeal cultures Follow‐up: 27 months | By 26.12 months, 50% of people in the 56 day group can expect to have experienced a recurrence of P aeruginosa. | By 25.18 months, 50% of people in the 28 day group can expect to have experienced a recurrence of P aeruginosa. | HR 0.81 (95% CI 0.37 to 1.76) | 651 (1 RCT) | ⊕⊕⊝⊝ | |
| FEV₁: % predicted Follow‐up: 27 months | There were no major short‐ or long‐term changes in spirometric parameters were observed during the study period. | NR | up to 881 | ⊕⊝⊝⊝ | Changes in lung function were not reported separately for each treatment arm. | |
| FVC: % predicted Follow‐up: 27 months | There were no major short‐ or long‐term changes in spirometric parameters were observed during the study period. | NR | up to 881 | ⊕⊝⊝⊝ | Changes in lung function were not reported separately for each treatment arm. | |
| Growth and nutritional status: weight, height and BMI Follow‐up: 27 months | No significant differences in weight, height or body mass index were reported. | NR | up to 881 | ⊕⊝⊝⊝ | Numerical data were not reported or comparative results across the treatment groups. | |
| Frequency of infective pulmonary exacerbations: number of exacerbations per patient year Follow‐up: 27 months | 47 per 1000 | 9 per 1000 (0 to 188 per 1000) | OR 0.19 (95% CI 0.01 to 4.00) | 771 | ⊕⊝⊝⊝ | |
| Isolation of other micro‐organisms from the respiratory tract: number of positive cultures per patient year Follow‐up: 27 months | There were no consistent trends reported in the isolation of non‐P aeruginosa organisms (one isolate only of Stenotrophomonas maltophilia which was seen in the 28‐day arm). | NR | up to 881 | ⊕⊝⊝⊝ | Numerical data were not reported or comparative results across the treatment groups. | |
| Adverse effects to antibiotics Follow‐up: up to 27 months | There were no significant differences between treatment groups in terms of any reported adverse events at any time point. | NA | up to 771 | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. In the included trial, 88 participants were randomised but not all participants contributed to all outcomes (unclear how many participants contributed to some outcomes). 2. Downgraded once due to risk of bias; methodological information was limited and unclear in the included study and there were concerns of bias due to selective reporting of results and lack of blinding. 3. Downgraded once due to applicability: the included studies recruited only children; results are not applicable to adults. 4. Downgraded once due to imprecision: no numerical comparative results available. 5. Downgraded once due to imprecision: very wide confidence intervals around the effect size 6. Downgraded once due to imprecision: some wide confidence intervals around effects sizes (small event rates) and a lot of adverse events analysed increasing the statistical chance of a spurious finding. | ||||||
| Inhaled colistin plus oral ciprofloxacin compared to inhaled tobramycin plus oral ciprofloxacin for eradicating Pseudomonas aeruginosa in people with cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis and a positive microbiological isolate of P aeruginosa from a respiratory tract specimen Settings: outpatients Intervention: inhaled colistin plus oral ciprofloxacin Comparison: inhaled tobramycin plus oral ciprofloxacin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inhaled tobramycin plus oral ciprofloxacin | Inhaled colistin plus oral ciprofloxacin | |||||
| Eradication of P aeruginosa from the respiratory tract: proportion with positive respiratory culture for P aeruginosa Follow‐up: median 16 months | 315 per 1000 | 403 per 1000 (227 to 721 per 1000) | OR 1.28 (95% CI 0.72 to 2.29) | up to 2231 | ⊕⊕⊝⊝ | There was also no significant difference between treatment groups within the first 6 months, OR 1.11 (95% CI 0.64 to 1.92). |
| FEV₁: relative change in % predicted FEV1 from baseline Follow‐up: mean 54 days | The mean relative change in % predicted FEV₁ from baseline was 4.55% in the inhaled tobramycin plus oral ciprofloxacin group. | The mean relative change in % predicted FEV₁ from baseline was 2.4% lower (5.89% lower to 1.09% higher) in the inhaled colistin plus oral ciprofloxacin group. | NA | 1281 (1 RCT) | ⊕⊕⊝⊝ | |
| FVC Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Growth and nutritional status Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Frequency of infective pulmonary exacerbations: number of exacerbations per patient year Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Isolation of other micro‐organisms from the respiratory tract: number of positive cultures per patient year Follow‐up: median 16 months | There were no differences during follow up between the two groups for isolation of: Stenotrophomonas maltophilia, Achromobacter xylosoxidans or Aspergillus species. | NA | 2051 | ⊕⊕⊕⊝ | ||
| Adverse effects to antibiotics: leading to trial discontinuation Follow‐up: median 16 months | 21 out of 118 (18%) participants discontinued the trial early due to adverse events in the inhaled tobramycin plus oral ciprofloxacin group. | 17 out of 105 (16%) participants discontinued the trial early due to adverse events in the inhaled colistin plus oral ciprofloxacin group. | NA | 223 | ⊕⊕⊕⊝ | Reasons for discontinuations included vomiting, photosensitivity, wheeze and pulmonary exacerbation. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. In the included trial, 223 participants were randomised but not all participants contributed to all outcomes (unclear how many participants contributed to some outcomes, spirometry not performed in very young children). 2. Downgraded once due to risk of bias; methodological information was limited and unclear in the included study and there were potential concerns of bias due to selective reporting of results and lack of blinding. 3. Downgraded once due to imprecision: wide confidence intervals around the effect size. 4. Downgraded once due to applicability: a large proportion of the randomised participants (95 out of 223, 42%) did not contribute to this outcome. | ||||||
| Cycled inhaled tobramycin compared to culture‐based inhaled tobramycin for eradicating Pseudomonas aeruginosa in people with cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis and a positive microbiological isolate of P aeruginosa from a respiratory tract specimen Settings: outpatients Intervention: cycled inhaled tobramycin Comparison: culture‐based inhaled tobramycin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Culture‐based inhaled tobramycin | Cycled inhaled tobramycin | |||||
| Eradication of P aeruginosa from the respiratory tract: proportion of participants with one or more isolates of P aeruginosa from the respiratory tract Follow‐up: 18 months | 467 per 1000 | 228 per 1000 (145 to 383 per 1000) | OR 0.51 (95% CI 0.31 to 0.82) | 2981 | ⊕⊕⊕⊝ | The original trial report published age group–adjusted ORs which are slightly different to the results of this review. |
| FEV₁: mean 70‐week % change in FEV₁ (% predicted) Follow‐up: 70 weeks | The mean 70‐week % change in FEV₁ (% predicted) was ‐1.61% in the culture‐based inhaled tobramycin group. | The mean 70‐week % change in FEV₁ (% predicted) was 2.38% higher (2% lower to 6.76% higher) in the cycle‐based inhaled tobramycin group. | NA | 1431 | ⊕⊕⊝⊝ | |
| FVC Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Growth and nutritional status: mean 70‐week change from baseline in weight (kg) and height (cm) Follow‐up: 70 weeks | There were no significant differences between treatment groups in mean 70‐week change from baseline in weight (kg) or height (cm). | NA | 3041 | ⊕⊕⊕⊝ | ||
| Frequency of infective pulmonary exacerbations: proportion of participants with one or more pulmonary exacerbations (any severity) Follow‐up: 18 months | 533 per 1000 | 400 per 1000 (256 to 624 per 1000) | OR 0.75 (95% 0.48 to 1.17) | 3041 | ⊕⊕⊕⊝ | There was also no significant difference between groups in terms of proportion of participants with one or more severe pulmonary exacerbation or in terms of time to pulmonary exacerbation (severe or any severity). |
| Isolation of other micro‐organisms from the respiratory tract: proportion of participants with new isolates of Stenotrophomonas maltophilia Follow‐up: 18 months | 184 per 1000 | 217 per 1000 (118 to 390 per 1000) | OR 1.18 (95% CI 0.65 to 2.12) | 2791 | ⊕⊕⊕⊝ | |
| Adverse effects to antibiotics: proportion of participants with one or more serious adverse events Follow‐up: 18 months | 289 per 1000 | 246 per 1000 (147 to 405 per 1000) | OR 0.85 (95% 0.51 to 1.40) | 3041 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. In the included trial, 306 participants were randomised, 304 received treatment but not all participants contributed to all outcomes (unclear how many participants contributed to some outcomes, spirometry not performed in very young children (less than 4 years of age)). 2. Downgraded once due to applicability: the included studies recruited only children; results are not applicable to adults. Also the included trial required patients to have been free of P aeruginosa for at least two years so results may not be applicable to a wider population. 3. Downgraded once due to applicability: a large proportion of the randomised and treated participants (161 out of 304, 53%) did not contribute to this outcome. | ||||||
| Ciprofloxacin compared to placebo added to cycled and culture‐based inhaled tobramycin therapy for eradicating Pseudomonas aeruginosa in people with cystic fibrosis | ||||||
| Patient or population: adults and children with cystic fibrosis and a positive microbiological isolate of P aeruginosa from a respiratory tract specimen Settings: outpatients Intervention: ciprofloxacin added to cycled and culture‐based inhaled tobramycin therapy Comparison: placebo added to cycled and culture‐based inhaled tobramycin therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo added to cycled and culture‐based inhaled tobramycin therapy | Ciprofloxacin added to cycled and culture‐based inhaled tobramycin therapy | |||||
| Eradication of P aeruginosa from the respiratory tract: proportion of participants with one or more isolates of P aeruginosa from the respiratory tract Follow‐up: 18 months | 362 per 1000 | 322 per 1000 (199 to 521 per 1000) | OR 0.89 (95% CI 0.55 to 1.44) | 2981 | ⊕⊕⊕⊝ | The original trial report published age group–adjusted ORs which are slightly different to the results of this review. |
| FEV₁: mean 70‐week % change in FEV₁ (% predicted) Follow‐up: 70 weeks | The mean 70‐week % change in FEV₁ (% predicted) was ‐1.85% in the placebo added to cycled and culture‐based inhaled tobramycin therapy group. | The mean 70‐week % change in FEV₁ (% predicted) was 3.02% higher (1.33% lower to 7.37% higher) in the ciprofloxacin added to cycled and culture‐based inhaled tobramycin therapy group. | NA | 1431 | ⊕⊕⊝⊝ | |
| FVC Follow‐up: NA | Outcome not reported. | NA | NA | NA | ||
| Growth and nutritional status: mean 70‐week change from baseline in weight (kg) and height (cm) Follow‐up: 70 weeks | There were no significant differences between treatment groups in mean 70‐week change from baseline in weight (kg) or height (cm). | NA | 3041 | ⊕⊕⊕⊝ | ||
| Frequency of infective pulmonary exacerbations: proportion of participants with one or more pulmonary exacerbations (any severity) Follow‐up: 18 months | 447 per 1000 | 666 per 1000 (425 to 1000) | OR 1.49 (95% CI 0.95 to 2.33) | 3041 | ⊕⊕⊕⊝ | There was also no significant difference between groups in terms of proportion of participants with one or more severe pulmonary exacerbation or in terms of time to pulmonary exacerbation (severe or any severity). |
| Isolation of other micro‐organisms from the respiratory tract: proportion of participants with new isolates of Stenotrophomonas maltophilia Follow‐up: 18 months | 183 per 1000 | 220 per 1000 (121 to 395 per 1000) | OR 1.20 (95% CI 0.66 to 2.16) | 2791 | ⊕⊕⊕⊝ | |
| Adverse effects to antibiotics: proportion of participants with one or more serious adverse event Follow‐up: 18 months | 230 per 1000 | 354 per 1000 (214 to 591 per 1000) | OR 1.54 (95% CI 0.93 to 2.57) | 3041 | ⊕⊕⊕⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1. In the included trial, 306 participants were randomised, 304 received treatment but not all participants contributed to all outcomes (unclear how many participants contributed to some outcomes, spirometry not performed in very young children (less than 4 years of age)). 2. Downgraded once due to applicability: the included studies recruited only children; results are not applicable to adults. Also the included trial required patients to have been free of P. aeruginosa for at least two years so results may not be applicable to a wider population. 3. Downgraded once due to applicability: a large proportion of the randomised and treated participants (161 out of 304, 53%) did not contribute to this outcome. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P aeruginosa (300 mg 2x daily) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 2 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Positive respiratory culture for P aeruginosa (80 mg 2x daily) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 At 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 At 2 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 At 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.4 At 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.5 At 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Positive respiratory culture for P aeruginosa (combined available case analysis) Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 At 1 month | 2 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.33] |
| 3.2 At 2 months | 2 | 38 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.03, 0.65] |
| 4 Positive respiratory culture for P aeruginosa (combined) ‐ best case Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 At 1 month | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.06 [0.01, 0.30] |
| 4.2 At 2 months | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.03, 0.60] |
| 4.3 At 3 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.02, 1.16] |
| 4.4 At 6 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.04 [0.00, 0.48] |
| 4.5 At 12 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.01 [0.00, 0.26] |
| 5 Positive respiratory culture for P aeruginosa (combined) ‐ worst case Show forest plot | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 At 1 month | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.02, 0.38] |
| 5.2 At 2 months | 2 | 39 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.04, 0.73] |
| 5.3 At 3 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.05, 2.77] |
| 5.4 At 6 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.01, 1.83] |
| 5.5 At 12 months | 1 | 18 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.05, 2.77] |
| 6 Weight (kg) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 At 2 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Modified Shwachmann score ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 At 1 month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8.2 At 2 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Proportion colonised with P aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 At 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 At 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 At 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 In first 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At 24 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Adverse events Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Severe cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to next isolation of P aeruginosa from BAL, sputum or oropharyngeal cultures Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 2 Number of respiratory exacerbations Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Until recurrence of P. aeruginosa | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Adverse events (up to 3 months) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Productive cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Haemoptysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Rhinitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.5 Sinusitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.6 Nasopharyngitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.7 Tonsilitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.8 Oropharyngeal pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.9 Dysphonia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.10 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.11 URTI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.12 Lung disorder | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.13 Bronchitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.14 P. aeruginosa infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.15 Influenza | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.16 Otitis media | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.17 Deafness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.18 Drug level increased | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.19 Pyrexia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.20 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.21 Varicella | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events (over 3 months) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Productive cough | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Haemoptysis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Rhinitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Sinusitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.6 Nasopharyngitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.7 Tonsilitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.8 Oropharyngeal pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.9 Dysphonia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.10 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.11 URTI | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.12 Lung disorder | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.13 Bronchitis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.14 P. aeruginosa infection | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.15 Influenza | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.16 Otitis media | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.17 Deafness | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.18 Drug level increased | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.19 Pyrexia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.20 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.21 Varicella | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Positive respiratory culture for P aeruginosa Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 In first 6 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 At end of follow up (median 16 months) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEV₁ % predicted (relative change from baseline) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 At mean 54 days | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Microbiology status (post‐trial) Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Stenotrophomonas maltophilia | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Achromobacter xylosoxidans | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Aspergillus species | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Adverse events leading to trial discontinuation Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 Vomiting | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Photosensitivity | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Wheeze | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Pulmonary exacerbation during early eradication treatment | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Lack of compliance | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with one or more isolates of P aeruginosa from respiratory tract Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 FEV₁ % predicted ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Weight (kg) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Height (cm) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time to severe pulmonary exacerbation Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 6 Participants with one or more severe pulmonary exacerbations Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Time to pulmonary exacerbation (any severity) Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 8 Participants with one or more pulmonary exacerbations (any severity) Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Participants with new isolates of Stenotrophomonas maltophilia Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Participants with one or more serious adverse event Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants with one or more isolates of P aeruginosa from respiratory tract Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 2 FEV₁ % predicted ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Weight (kg) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Height (cm) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Mean duration of 70 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Time to severe pulmonary exacerbation Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 6 Participants with one or more severe pulmonary exacerbations Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Time to pulmonary exacerbation (any severity) Show forest plot | 1 | Hazard Ratio (Fixed, 95% CI) | Totals not selected | |
| 8 Participants with one of more pulmonary exacerbation (any severity) Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Participants with new isolates of Stenotrophomonas maltophilia Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 10 Participants with one or more serious adverse event Show forest plot | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |

