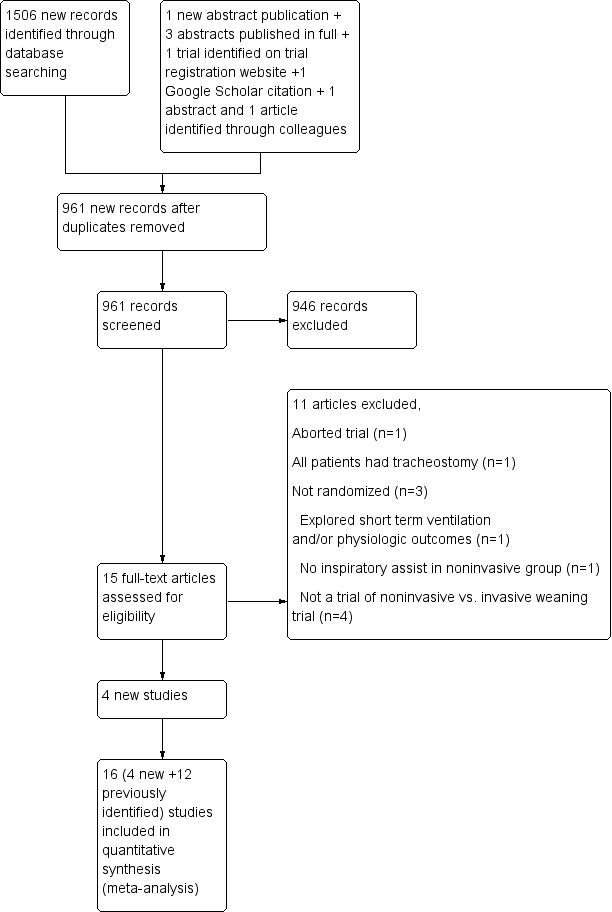
Noninvasive positive‐pressure ventilation as a weaning strategy for intubated adults with respiratory failure
Information
- DOI:
- https://doi.org/10.1002/14651858.CD004127.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 09 December 2013see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Emergency and Critical Care Group
- Copyright:
-
- Copyright © 2018 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Karen Burns (KB): proposed the research question and title; designed the protocol; reviewed and retrieved relevant articles; assessed methodologic quality of retrieved articles; abstracted data as the primary review author; entered data; conducted the statistical analysis and prepared the final review.
Neill Adhikari (NA): reviewed and retrieved relevant articles; assessed methodologic quality of retrieved articles; abstracted data as the second review author and revised the final review for important intellectual content.
Azra Premji (AP): assisted in retrieving articles and updating the text of the review. Also revised the final review for methodologic quality and scientific integrity.
Maureen Meade (MM): adjudicated disagreements; supervised the methodologic integrity of the review; reviewed the manuscript for methodologic and scientific integrity.
Sources of support
Internal sources
-
New source of support, Other.
External sources
-
Dr Burns is the recipient of a Canadian Institutes of Health Research Clinician Scientist Phase 2 Award, Canada.
Declarations of interest
Karen EA Burns: none known.
Maureen O Meade: Dr Meade has received in‐kind support from industry in the form of equipment loans for use in the context of a multicentre clinical trial.
Azra Premji: none known.
Neill KJ Adhikari: none known.
Acknowledgements
We would like to thank the the Cochrane editorial team and our peer reviewers (Dr Harald Herkner, Professor Nathan Pace, Dr Felix Ram, Dr Andrew Jones, Dr Bronagh Blackwood, Dr Eddy Fan and Dr Andrew MacDuff) for their contributions to the review. We would also like to thank the authors of the primary research, who provided additional information pertinent to the design and outcomes of their respective clinical trials, and Dr Sean Keenan, who, although not able to participate in the updated review, provided advice regarding study selection. We thank Haibo Zhang, Haibo Qiu, Wei Ehr Cheng, Zhuxian Feng and Hanpo Yu for their assistance with translating and contacting authors to clarify publication and study design in earlier issues of this review.
Version history
| Published | Title | Stage | Authors | Version |
| 2013 Dec 09 | Noninvasive positive‐pressure ventilation as a weaning strategy for intubated adults with respiratory failure | Review | Karen EA Burns, Maureen O Meade, Azra Premji, Neill KJ Adhikari | |
| 2010 Aug 04 | Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure | Review | Karen EA Burns, Neill KJ Adhikari, Sean P Keenan, Maureen O Meade | |
| 2003 Oct 20 | Noninvasive positive pressure ventilation as a weaning strategy for intubated adults with respiratory failure | Review | Karen E. A. Burns, Neill K. J. Adhikari, Maureen O. Meade | |
Notes
In the previously published protocol, as part of an a priori sensitivity analysis, we stated that we would assess the impact of the cause of respiratory failure (COPD vs non‐COPD) on (1) the proportion of weaning failures and (2) mortality. In the last version of this review, we identified two studies restricted to participants with COPD and three studies with mixed participant populations. In the absence of individual participant data, we compared studies restricted to COPD participants versus those with mixed participant populations. To explore for potential differences in response to NPPV, we compared studies enrolling at least 50% COPD participants versus those enrolling less than 50% COPD participants, in terms of mortality.
To search EMBASE, we used the following Emtree terms: respiratory failure (explode), positive end‐expiratory pressure (explode) and weaning (explode). In addition, we used the Emtag: artificial ventilation.
In the protocol, we stated that the MEDLINE search strategy would be limited to include the following publication types: clinical trials, controlled clinical trials, randomized controlled trials, multicenter studies and meta‐analyses. In the review, we did not limit the most recent literature search by publication type.
October 2013
Quality assessment
In this update, we evaluated and recorded the presence of true randomization and use of concealed allocation to minimize selection bias. Additionally, we evaluated reports of randomized trials for completeness of outcome data and selective outcomes reporting to assess for attrition and reporting biases, respectively.
Unlike in the previous review (Burns 2010), we did not include in our quality assessment the use of daily screening to identify participants capable of unassisted breathing; inclusion of predefined, permissive weaning criteria to identify weaning candidates (including but not limited to minute ventilation, tidal volume (VT), vital capacity, respiratory rate, rapid shallow breathing index, Glasgow Coma Scale, presence of spontaneous ventilatory efforts and a cough reflex, requirement for PEEP and ability to maintain arterial oxygen saturation above 90% on a fractional concentration of inspired oxygen (FiO2) of less than 0.50) and performance of spontaneous breathing trials (SBTs). We did not include assessment of the use of weaning protocols or guidelines (in both groups) and criteria for failure of prerandomization SBT, discontinuation of mechanical ventilation (in both groups) and extubation, reintubation due to poor reporting of these aspects of trial design and implementation and concerns over the reliability of efforts to acquire these details amidst language issues. We contacted study authors to ask them to describe specific features of their trials, including use of daily screening and a prerandomization SBT; however, we did not include them in the quality assessment in this update.
Summary of findings
We included in this update SoF tables for the outcomes of mortality, weaning failure, VAP and reintubation.
Exclusion criteria
We updated our exclusion criteria to exclude studies evaluating exclusively tracheostomized participants, as (1) tracheostomy was an outcome of this review, (2) these studies typically include a high proportion of participants undergoing prolonged mechanical ventilation and (3) application of the interventions could be different in the setting of a tracheostomy (e.g. participants randomly assigned to noninvasive weaning may meet criteria to return to invasive ventilation per tracheostomy and subsequently may be returned to noninvasive ventilation. Similarly, participants randomly assigned to invasive weaning may undergo a series of SBTs before extubation).
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Noninvasive Ventilation [*methods];
- Pneumonia, Ventilator-Associated [prevention & control];
- Positive-Pressure Respiration [*methods, mortality];
- Pulmonary Disease, Chronic Obstructive [mortality, *therapy];
- Quality of Life;
- Randomized Controlled Trials as Topic;
- Respiratory Insufficiency [mortality, *therapy];
- Ventilator Weaning [*methods];
Medical Subject Headings Check Words
Adult; Humans;
PICOs

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
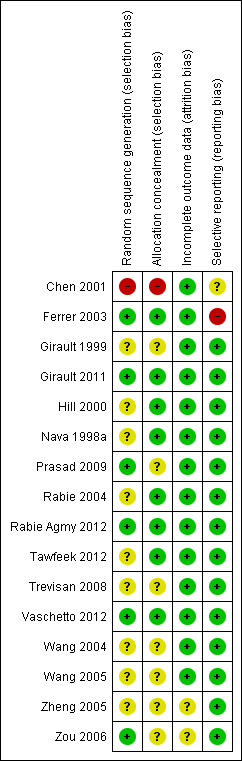
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.

Comparison 1 Noninvasive versus invasive weaning, Outcome 1 Mortality.

Comparison 2 Noninvasive versus invasive weaning, Outcome 1 Weaning failure.

Comparison 3 Noninvasive versus invasive weaning, Outcome 1 Nosocomial pneumonia.

Comparison 4 Noninvasive versus invasive weaning, Outcome 1 LOS ICU.

Comparison 5 Noninvasive versus invasive weaning, Outcome 1 LOS hospital.

Comparison 6 Noninvasive versus invasive weaning, Outcome 1 Average total duration of mechanical ventilatory support.

Comparison 7 Noninvasive versus invasive weaning, Outcome 1 Average duration of ventilation related to weaning.

Comparison 8 Noninvasive versus invasive weaning, Outcome 1 Duration of endotracheal mechanical ventilation.

Comparison 9 Noninvasive versus invasive weaning, Outcome 1 Reintubation.

Comparison 10 Noninvasive versus invasive weaning, Outcome 1 Arrhythmia.

Comparison 11 Noninvasive versus invasive weaning, Outcome 1 Tracheostomy.

Comparison 12 Sensitivity analysis: noninvasive versus invasive weaning, Outcome 1 Mortality excluding quasi‐randomized trial.

Comparison 12 Sensitivity analysis: noninvasive versus invasive weaning, Outcome 2 Nosocomial pneumonia excluding quasi‐randomized trial.

Comparison 13 Noninvasive versus invasive weaning, Outcome 1 Mortality greater than or equal to 50% COPD versus less than 50% COPD.

Comparison 14 Noninvasive versus invasive weaning, Outcome 1 Weaning failure greater than or equal to 50% COPD.

Comparison 15 Noninvasive versus invasive weaning, Outcome 1 Weaning failure.

Comparison 15 Noninvasive versus invasive weaning, Outcome 2 Nosocomial pneumonia.

Comparison 15 Noninvasive versus invasive weaning, Outcome 3 Average duration of ventilation related to weaning.

Comparison 15 Noninvasive versus invasive weaning, Outcome 4 Reintubation.
| Noninvasive versus invasive weaning for intubated adults with respiratory failure | ||||||
| Patient or population: intubated adults with respiratory failure | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Noninvasive versus invasive weaning | |||||
| Mortality—COPD | Study population | RR 0.36 | 632 | ⊕⊕⊕⊝ | ||
| 225 per 1000 | 81 per 1000 | |||||
| Moderate | ||||||
| 200 per 1000 | 72 per 1000 | |||||
| Mortality—mixed | Study population | RR 0.81 | 362 | ⊕⊕⊝⊝ | ||
| 239 per 1000 | 194 per 1000 | |||||
| Moderate | ||||||
| 270 per 1000 | 219 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| 1Fewer than 300 events. Test for subgroup differences (P = 0.02). | ||||||
| Noninvasive versus invasive weaning for intubated adults with respiratory failure | ||||||
| Patient or population: patients with intubated adults with respiratory failure | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Noninvasive versus invasive weaning | |||||
| Weaning failure | Study population | RR 0.63 | 605 | ⊕⊕⊕⊝ | ||
| 362 per 1000 | 228 per 1000 | |||||
| Moderate | ||||||
| 327 per 1000 | 206 per 1000 | |||||
| Nosocomial pneumonia | Study population | RR 0.25 | 953 | ⊕⊕⊝⊝ | ||
| 296 per 1000 | 74 per 1000 | |||||
| Moderate | ||||||
| 307 per 1000 | 77 per 1000 | |||||
| Average duration of ventilation related to weaning | The mean average duration of ventilation related to weaning in the intervention groups was | 645 | ⊕⊕⊝⊝ | |||
| Reintubation | Study population | RR 0.65 | 789 | ⊕⊕⊕⊝ | ||
| 310 per 1000 | 202 per 1000 | |||||
| Moderate | ||||||
| 286 per 1000 | 186 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Less than 300 events | ||||||
| Study | No of participants | Inclusion criteria (participants) | Inclusion criteria (weaning eligibility) | Experimental strategy | Control strategy |
| Nava 1998 | 50 | Exacerbation of COPD. Intubated for at least 36 to 48 hours | Simple weaning criteria, 1‐hour SBT failure | Noninvasive pressure support on conventional ventilator delivered with face mask | Invasive PS |
| Girault 1999 | 33 | Acute‐on‐chronic respiratory failure (COPD, restrictive, or mixed populations). Intubated for at least 48 hours | Simple weaning criteria, 2‐hour SBT failure | Flow or pressure mode with nasal or face mask | Flow or pressure mode (PS) |
| Hill 2000 | 21 | Acute respiratory failure | 30‐minute SBT failure | NPPV using VPAP in ST‐A mode | Invasive PS |
|
Chen 2001 |
24 |
Exacerbation of COPD. Intubated for at least 48 to 60 hours. Saturation > 88% on FiO2 of 40% |
Day 3+ weaning criteria |
Bilevel NPPV (pressure mode) |
Invasive PS |
| Ferrer 2003 | 43 | Acute respiratory failure and persistent weaning failure. Intubated for at least 72 hours | Two‐hour SBT failure on 3 consecutive days | Bilevel NPPV in ST mode delivered with face or nasal mask | AC or invasive PS |
| Rabie Agmy 2004 | 37 | Exacerbation of COPD | Two‐hour SBT failure | NPPV (proportional assist in timed mode) delivered by face or nasal mask | Invasive PS |
| Wang 2004 | 28 | COPD. Bronchopulmonary infection | PIC window | NPPV (pressure mode) delivered by mask (unspecified)
| SIMV + PS |
| Zheng 2005 | 33 | COPD. Severe pulmonary infection | PIC window | Bilevel NPPV (pressure mode) delivered by face or nasal mask | Invasive PS |
| Zou 2006 | 76 | COPD with severe respiratory failure. Pulmonary infection | PIC window | Bilevel NPPV (pressure, ST mode) delivered by nasal or oronasal mask | SIMV + PS |
| Wang 2005 | 90 | COPD with severe hypercapneic respiratory failure. Pneumonia or purulent bronchitis. Age < 85. Capable of self care in past year | PIC window | Bilevel NPPV (pressure mode) | SIMV + PS |
| Trevisan 2008 | 65 | Invasively ventilated > 48 hours | 30‐minute SBT failure | Bilevel NPPV (pressure mode) delivered by face mask | Invasive mechanical ventilation |
| Prasad 2009 | 30 | COPD. Hypercapneic respiratory failure | Two‐hour SBT failure | Bilevel NPPV (pressure mode) delivered by full face mask | Invasive PS |
| Girault 2011 | 138 | Chronic hypercapneic respiratory failure invasively ventilated for at least 48 hours | Two‐hour SBT failure | Noninvasive PS ± PEEP or bilevel NIV with face mask (initial choice) | Invasive PS with once‐daily SBT with T‐piece or PS ± PEEP |
| Rabie Agmy 2012 | 264 | Acute‐on‐chronic exacerbation of COPD
| Two‐hour SBT failure
| NPPV (pressure, ST mode) | Invasive PS |
| Tawfeek 2012 | 42 | Invasively ventilated for > 48 hours
| Two‐hour SBT failure
| Noninvasive PAV ventilation delivered by face mask | SIMV |
| Vaschetto 2012 | 20 | Hypoxemic respiratory failure invasively ventilated for at least 48 hours | PS with PEEP + inspiratory support, < 25 cm H2O
PEEP 8 to 13 cm H2O
PaO2/FiO2 200 to 300 mm Hg with FiO2< 0.6 | Helmet NPPV | Invasive PS with SBT when P/F ratio > 250 mm Hg |
| COPD = chronic obstructive pulmonary disease; NPPV = noninvasive positive‐pressure ventilation; PS = pressure support; PEEP = positive end‐expiratory pressure; PIC = pulmonary infection control window; ST = spontaneous timed; AC = assist control; SIMV = synchronized intermittent mandatory ventilation; P/F ratio = ratio of arterial concentration of oxygen to fractional concentration of oxygen administered; SBT = spontaneous breathing trial; PAV = proportional assist ventilation. | |||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 9 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 0.36 [0.24, 0.56] |
| 1.2 Mixed | 7 | 362 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.47, 1.40] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weaning failure Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 3 | 351 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.36, 0.74] |
| 1.2 Mixed | 5 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.35, 1.51] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Nosocomial pneumonia Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 9 | 632 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.13, 0.37] |
| 1.2 Mixed | 5 | 321 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.15, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LOS ICU Show forest plot | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 8 | 608 | Mean Difference (IV, Random, 95% CI) | ‐6.66 [‐9.41, ‐3.92] |
| 1.2 Mixed | 5 | 299 | Mean Difference (IV, Random, 95% CI) | ‐3.32 [‐6.78, 0.15] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LOS hospital Show forest plot | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 6 | 524 | Mean Difference (IV, Random, 95% CI) | ‐6.91 [‐10.83, ‐1.00] |
| 1.2 Mixed | 4 | 279 | Mean Difference (IV, Random, 95% CI) | ‐4.02 [‐9.41, 1.36] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Average total duration of mechanical ventilatory support Show forest plot | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 5 | 277 | Mean Difference (IV, Random, 95% CI) | ‐5.77 [‐10.64, ‐0.91] |
| 1.2 Mixed | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐5.20 [‐11.34, 0.93] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Average duration of ventilation related to weaning Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 4 | 355 | Mean Difference (IV, Random, 95% CI) | ‐1.43 [‐3.12, 0.26] |
| 1.2 Mixed | 5 | 290 | Mean Difference (IV, Random, 95% CI) | 0.17 [‐4.01, 4.35] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Duration of endotracheal mechanical ventilation Show forest plot | 12 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 7 | 558 | Mean Difference (IV, Random, 95% CI) | ‐7.53 [‐11.47, ‐3.60] |
| 1.2 Mixed | 5 | 159 | Mean Difference (IV, Random, 95% CI) | ‐6.85 [‐10.75, ‐2.95] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Reintubation Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 3 | 430 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.35, 0.70] |
| 1.2 Mixed | 7 | 359 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.47, 1.43] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Arrhythmia Show forest plot | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 2.0 [0.20, 19.78] |
| 1.2 Mixed | 2 | 171 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.26, 2.17] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Tracheostomy Show forest plot | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 COPD | 1 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 0.04 [0.00, 0.60] |
| 1.2 Mixed | 6 | 308 | Risk Ratio (M‐H, Random, 95% CI) | 0.23 [0.09, 0.57] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality excluding quasi‐randomized trial Show forest plot | 15 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Nosocomial pneumonia excluding quasi‐randomized trial Show forest plot | 13 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality greater than or equal to 50% COPD versus less than 50% COPD Show forest plot | 16 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Greater than or equal to 50% COPD | 12 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.29, 0.76] |
| 1.2 Less than 50% COPD | 4 | 148 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.47, 1.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weaning failure greater than or equal to 50% COPD Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Greater than or equal to 50% COPD | 5 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.46, 1.01] |
| 1.2 Less than 50% COPD | 3 | 83 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.12, 2.18] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Weaning failure Show forest plot | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2 Nosocomial pneumonia Show forest plot | 14 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Average duration of ventilation related to weaning Show forest plot | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4 Reintubation Show forest plot | 10 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |