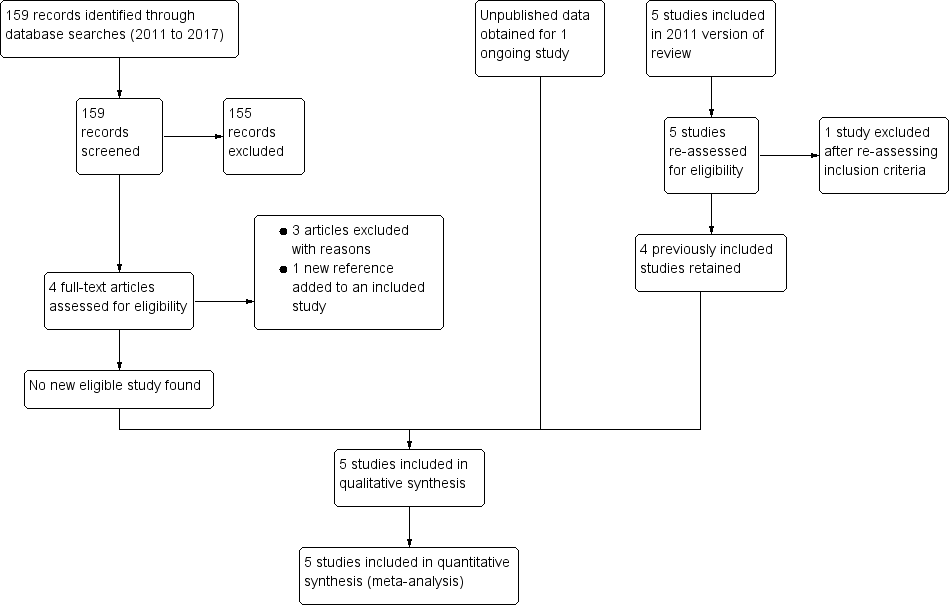
Administración de suplementos de vitamina A para reducir la transmisión del VIH de madre a hijo
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomized controlled trial (RCT) | |
| Participants | 303 HIV‐positive pregnant women from metropolitan Bloemfontein, South Africa. Most participants (56%) lived in informal settlements and all attended public health facilities. For the trial, women were asked to volunteer for HIV testing during their first antenatal visit. Pretest counselling was done in groups, and post‐test counselling was done individually. Women who were seropositive for HIV were asked to participate in the trial. | |
| Interventions | Vitamin A supplementation versus placebo | |
| Outcomes | Mother‐to‐child transmission (MTCT) of HIV | |
| Notes | All trial participants gave separate informed consent for the trial. All patients were recruited by one study physician and received verbal or written information (Sesotho, English, or Afrikaans information sheets). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not mention the method used to generate the randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method used to conceal the treatment allocation. |
| Blinding (performance bias and detection bias) | Low risk | The trial used an identical placebo; HIV diagnosis was done in the laboratory. |
| Incomplete outcome data (attrition bias) | High risk | More than 48% of women were lost to follow‐up and we do not know whether this was related to outcomes. |
| Selective reporting (reporting bias) | High risk | Not all prespecified outcomes were reported in various publications from this trial. |
| Other bias | Low risk | We do not believe that other biases were introduced, over and above the high loss to follow‐up and the selective reporting. |
| Methods | Described as randomized double‐blind. | |
| Participants | 728 HIV‐positive women enrolled at 17 to 39 weeks' gestation in KwaZulu‐Natal Province of South Africa; 30.6% of whom had serum retinol levels < 20 µg/dL. | |
| Interventions | Daily oral vitamin A (5000 IU retinyl palmitate and 30 mg beta‐carotene) or placebo. At delivery, women in the vitamin A group received a dose of 200,000 IU of retinyl palmitate while the placebo arm received an identical placebo. | |
| Outcomes | Stillbirths, HIV infection in the child, neonatal deaths, preterm birth, birthweight, low birthweight. | |
| Notes | No woman received any antiretroviral therapy (ART). It is not stated in the trial reports whether the women also received iron or folic acid, or both. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The trial authors did not describe the method used to generate the randomization sequence. |
| Allocation concealment (selection bias) | Unclear risk | The trial authors did not describe the method used to conceal the treatment allocation. |
| Blinding (performance bias and detection bias) | Low risk | Use of identical placebo; diagnosis of HIV was done in the laboratory. |
| Incomplete outcome data (attrition bias) | Low risk | Less than 10% of women were lost to follow‐up and we do not believe that this was related to the outcome. We do not believe this introduced bias. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes were reported in various publications from this trial. |
| Other bias | Low risk | We do not believe that other biases were introduced that could have affected study findings. |
| Methods | Randomized, placebo‐controlled, double‐blind. | |
| Participants | 1075 pregnant HIV‐positive women enrolled at 12 to 27 weeks' gestation in Dar es Salam, Tanzania. Of 1085 women initially randomized, 10 were eventually excluded for either being HIV‐negative (n = 7) or not pregnant (n = 3). The prevalence of vitamin A deficiency (< 0.70 µmol/L) was about 34% during the second trimester. | |
| Interventions | Daily oral dose of one of: vitamin A (30 mg beta carotene + 5000 IU retinyl palmitate) alone, multivitamins (20 mg vitamin B1, 20 mg vitamin B2, 25 mg vitamin B6, 100 mg niacin, 50 µg vitamin B12, 500 mg vitamin C, 30 mg vitamin E, and 0.8 mg folic) plus vitamin A, multivitamins without vitamin A, or placebo. At delivery, women receiving any vitamin A were given an additional 200,000 IU oral dose of vitamin A while the others received an extra dose of placebo. In this review, we used only data from the vitamin A only (intervention) and the placebo arms. | |
| Outcomes | Stillbirths, HIV infection in child, preterm delivery, low birthweight, postpartum CD4 levels. | |
| Notes | It is not mentioned in this trial whether any woman received ART. All women were given daily oral doses of iron and folic acid, and weekly doses of chloroquine. All children, regardless of which intervention group they were in, received 100,000 IU vitamin A at six months of age, and 200,000 IU of vitamin A every six months afterwards. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial used computer‐generated randomization sequence. |
| Allocation concealment (selection bias) | Low risk | Block randomization; blocks of 20. At enrolment, the investigators assigned each eligible woman the next numbered bottle of study drug. |
| Blinding (performance bias and detection bias) | Low risk | At enrolment, each eligible woman was assigned the next numbered bottle of study drug. Active tablets and placebo were indistinguishable, so that neither the participants nor the investigators could identify which participants were randomized to the which regimen. |
| Incomplete outcome data (attrition bias) | Low risk | Fifty‐four women (5.0%) were lost to follow‐up, and we do not believe that this was related to the outcome. We do not believe this introduced bias. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported on all outcomes specified in the goals of the study articles. |
| Other bias | Low risk | We do not believe that other biases were introduced that could have affected the study findings. |
| Methods | Randomized, placebo‐controlled trial. | |
| Participants | 4495 mother‐infants pairs who were part of the Zimbabwe Vitamin A for Mothers and Babies (ZVITAMBO) trial in Harare Zimbabwe. Mother‐infant pairs were enrolled at 96 hours (or less) after delivery. | |
| Interventions | A 2‐by‐2 factorial design with 4 treatment groups Aa, Ap, Pa, and Pp; where “A” was maternal vitamin A supplementation (400,000 IU), “P” was maternal placebo, “a” was infant vitamin A supplementation (50,000 IU), and “p” was infant placebo. In this review, we used only data from Ap (intervention) versus Pp (placebo). | |
| Outcomes | Primary outcome: breastfeeding–associated MTCT and HIV‐free survival. Secondary outcome: adverse effects in HIV‐positive women or their infants. | |
| Notes | All but 4 mothers initiated breastfeeding, no information on ART or cotrimoxazole prophylaxis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors performed randomization using computer‐generated blocks of 12. |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was concealed by pre‐packing study supplements in sequentially numbered series assigned to study identification numbers. Concealed envelopes with study number; link between number and treatment assignment kept at central location. |
| Blinding (performance bias and detection bias) | Low risk | Participants, care providers, and outcome assessors were blinded to the treatment allocation. Mothers were assigned an original study identification number at enrolment and were given the next sequentially numbered opaque bottle with supplements. |
| Incomplete outcome data (attrition bias) | Low risk | One hundred and forty‐three (3.2%) mother‐infant pairs were lost to follow‐up. We do not believe that the loss to follow‐up was related to the outcome. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported outcomes that were prespecified in the protocol (NCT00198718). |
| Other bias | Low risk | We do not believe that other biases were introduced that could have affected the study findings. |
| Methods | RCT. Participants were assigned to treatment using computer‐generated random numbers, and treatment was concealed by pre‐packing study supplements in sequentially numbered series assigned to study identification numbers. Sixty‐three women (9%) were lost to follow‐up before delivery and excluded from the analyses. The 14 pairs of twins in the study were excluded from the birth weight and mortality analyses because twins are known to have lower birth weights and higher mortality rates. | |
| Participants | 697 pregnant HIV‐positive women enrolled at 18 to 28 week's gestation in Blantyre, Malawi. The prevalence of vitamin A deficiency (< 0.70 µmol/L) was 51% during the second trimester. | |
| Interventions | All women received orally administered daily doses of iron (30 mg of elemental iron) and folate (400 µg) from enrolment until delivery. One‐half of the women were randomized to receive daily doses of orally administered vitamin A (10,000 IU). | |
| Outcomes | Stillbirths, HIV infection in child, preterm delivery, low birthweight, postpartum CD4 levels. | |
| Notes | All women received oral vitamin A (100,000 IU) at 6 weeks postpartum, as per policy of the Malawi Ministry of Health. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial authors determined treatment assignment by use of a computer random‐number generator. |
| Allocation concealment (selection bias) | Low risk | Treatment assignment was concealed by pre‐packing study supplements in sequentially numbered series assigned to study identification numbers. Mothers were assigned an original study identification number at enrolment and were given the next sequentially numbered opaque bottle with supplements. |
| Blinding (performance bias and detection bias) | Low risk | Supplements containing vitamin A, iron, and folate were identical in appearance to the supplements containing iron and folate. |
| Incomplete outcome data (attrition bias) | Low risk | Nine per cent of women were lost to follow‐up and we do not believe that this was related to the outcome. We do not believe this introduced bias. |
| Selective reporting (reporting bias) | Low risk | The trial authors reported on all outcomes specified in the goals of the study articles. |
| Other bias | Low risk | We do not believe that other biases were introduced that could have affected the study findings. |
Abbreviations: ART: antiretroviral therapy; MTCT: mother‐to‐child transmission; RCT: randomized controlled trial.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| The intervention consisted of multiple multivitamins (not vitamin A) and the participants were children born to HIV‐positive women (rather than the women themselves). | |
| The intervention consisted of multiple multivitamins and not vitamin A. | |
| The intervention consisted of multiple multivitamins (not vitamin A) and the participants were children born to HIV‐positive women (rather than the women themselves). | |
| The intervention consisted of multiple multivitamins (not vitamin A) and the participants were children born to HIV‐positive women (rather than the women themselves). |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HIV infection status of the child Show forest plot | 5 | 4428 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| Analysis 1.1  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 1 HIV infection status of the child. | ||||
| 1.1 Antepartum supplementation | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 1.2 Postpartum supplementation | 1 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.98, 1.27] |
| 1.3 Antepartum and postpartum supplementation | 2 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.59] |
| 2 Mean birthweight Show forest plot | 3 | 2181 | Mean Difference (IV, Random, 95% CI) | 34.12 [‐12.79, 81.02] |
| Analysis 1.2  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 2 Mean birthweight. | ||||
| 3 Low birthweight Show forest plot | 3 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.97] |
| Analysis 1.3 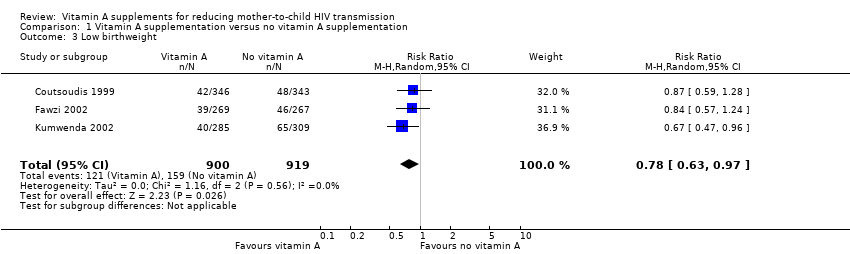 Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 3 Low birthweight. | ||||
| 4 Child death by two years of age Show forest plot | 3 | 3883 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.22] |
| Analysis 1.4  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 4 Child death by two years of age. | ||||
| 5 Preterm delivery Show forest plot | 2 | 1577 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.37] |
| Analysis 1.5  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 5 Preterm delivery. | ||||
| 6 Stillbirth Show forest plot | 3 | 2335 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.77] |
| Analysis 1.6  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 6 Stillbirth. | ||||
| 7 Maternal death Show forest plot | 2 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.43] |
| Analysis 1.7  Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 7 Maternal death. | ||||
| 8 Postpartum CD4 count Show forest plot | 1 | 511 | Mean Difference (IV, Random, 95% CI) | ‐13.0 [‐60.46, 34.46] |
| Analysis 1.8 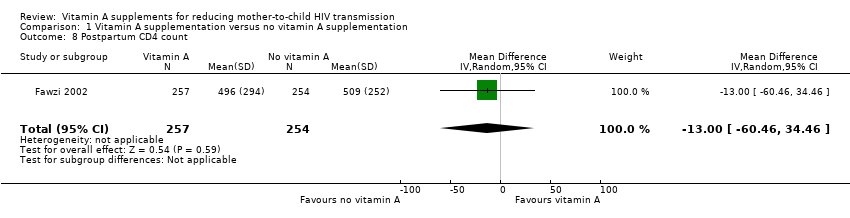 Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 8 Postpartum CD4 count. | ||||

ʽRisk of bias' graph: review authors' judgements about each ʽRisk of bias' item presented as percentages across all included trials
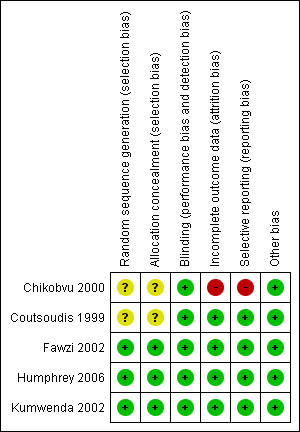
ʽRisk of bias' summary: review authors' judgements about each ʽRisk of bias' item for each included trial

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 1 HIV infection status of the child.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 2 Mean birthweight.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 3 Low birthweight.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 4 Child death by two years of age.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 5 Preterm delivery.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 6 Stillbirth.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 7 Maternal death.

Comparison 1 Vitamin A supplementation versus no vitamin A supplementation, Outcome 8 Postpartum CD4 count.
| Population: HIV‐positive women during pregnancy and immediate postpartum period Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
| Assumed risk with no vitamin A | Corresponding risk with vitamin A supplements | |||||
| HIV infection status of the child | 27 per 100 | 29 per 100 | RR 1.07 (0.91 to 1.26) | 4428 | ⊕⊕⊕⊝ due to imprecision | Vitamin A supplements probably have little or no effect on mother‐to‐child transmission of HIV. |
| Mean birthweight | 2964 g | 34 g higher | MD 34.12 (−12.79 to 81.02) | 2181 | ⊕⊕⊝⊝ due to imprecision | Vitamin A supplements may increase the mean birthweight |
| Low birthweight | 17 per 100 | 13 per 100 | RR 0.78 | 1819 | ⊕⊕⊕⊝ due to imprecision | Vitamin A supplements probably reduce the incidence of low birthweight babies. |
| Child death by two years of age | 14 per 100 | 15 per 100 | RR 1.06 | 3883 | ⊕⊕⊝⊝ due to imprecision | Vitamin A supplements may have little or no effect on child death by two years of age. |
| Preterm delivery | 20 per 100 | 17 per 100 | RR 0.84 | 1577 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on preterm deliveries. |
| Stillbirth | 3 per 100 | 3 per 100 | RR 1.13 | 2335 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on stillbirths. |
| Maternal death | 3 per 100 | 2 per 100 | RR 0.71 | 1267 | ⊕⊝⊝⊝ due to imprecision and selective reporting | It is uncertain whether or not vitamin A supplements have an effect on maternal deaths. |
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1We downgraded by 1 for imprecision, as the CI ranges from small benefits to a clinically important increase in harm. | ||||||
| Search set | CENTRAL | PubMed | Embase | WHO ICTRP | ClinicalTrials.gov |
| #1 | MeSH descriptor: [HIV Infections] explode all trees | Search ((HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tiab] OR hiv‐1*[tiab] OR hiv‐2*[tiab] OR hiv1[tiab] OR hiv2[tiab] OR hiv infect*[tiab] OR human immunodeficiency virus[tiab] OR human immunedeficiency virus[tiab] OR human immuno‐deficiency virus[tiab] OR human immune‐deficiency virus[tiab] OR ((human immun*[tiab]) AND (deficiency virus[tiab])) OR acquired immunodeficiency syndrome[tiab] OR acquired immunedeficiency syndrome[tiab] OR acquired immuno‐deficiency syndrome[tiab] OR acquired immune‐deficiency syndrome[tiab] OR ((acquired immun*[tiab]) AND (deficiency syndrome[tiab])) OR "sexually transmitted diseases, Viral"[MeSH:NoExp])) | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus' OR 'human immunodeficiency virus:ab,ti' OR hiv:ab,ti OR 'hiv‐1':ab,ti OR 'hiv‐2':ab,ti OR 'human immunodeficiency virus':ab,ti OR 'human immunedeficiency virus':ab,ti OR 'human immune‐deficiency virus':ab,ti OR 'human immuno‐deficiency virus':ab,ti OR 'acquired immunodeficiency syndrome':ab,ti OR 'acquired immuno‐deficiency syndrome':ab,ti OR 'acquired immune‐deficiency syndrome':ab,ti OR 'acquired immunedeficiency syndrome':ab,ti | hiv AND vitamin A OR hiv AND retinol OR hiv AND retinoic OR hiv AND micronutrients OR hiv AND carotene | HIV and "VITAMIN A" | Interventional Studies | Studies received from 05/20/2016 to 08/25/2017 |
| #2 | MeSH descriptor: [HIV] explode all trees | Search (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR "clinical trials as topic"[mesh: noexp] OR randomly [tiab] OR trial [tiab]) NOT (animals [mh] NOT humans [mh]) | 'randomized controlled trial'/de OR 'randomized controlled trial' OR random*:ab,ti OR trial:ti OR allocat*:ab,ti OR factorial*:ab,ti OR placebo*:ab,ti OR assign*:ab,ti OR volunteer*:ab,ti OR 'crossover procedure'/de OR 'crossover procedure' OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR ((doubl* NEAR/3 blind*):ab,ti) OR (singl*:ab,ti AND blind*:ab,ti) OR crossover*:ab,ti OR cross+over*:ab,ti OR ((cross NEXT/1 over*):ab,ti) | — | — |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or (hiv near infect*) or (human immunodeficiency virus) or (human immunedeficiency virus) or (human immune‐deficiency virus) or (human immuno‐deficiency virus) or (human immune deficiency virus) or (human immuno deficiency virus) or (acquired immunodeficiency syndrome) or (acquired immunedeficiency syndrome) or (acquired immuno‐deficiency syndrome) or (acquired immune‐deficiency syndrome) or (acquired immun* deficiency syndrome) (Word variations have been searched) | Search (infectious disease transmission, vertical[mh] OR vertical transmission[tiab] OR vertical infect*[tiab] OR infection transmission[tiab] OR mother‐to‐child transmission[tiab] OR MTCT[tiab]) | 'animal'/de OR 'animal experiment'/de OR 'invertebrate'/de OR 'animal tissue'/de OR 'animal cell'/de OR 'nonhuman'/de | — | — |
| #4 | MeSH descriptor: [Lymphoma, AIDS‐Related] this term only | Search (vitamin A[mh] OR vitamin*[tiab] OR caroten*[tiab] OR retinol[tiab] OR retinoic[tiab] OR micronutrient*[tiab]) | 'human'/de OR 'normal human'/de OR 'human cell'/de | — | — |
| #5 | MeSH descriptor: [Sexually Transmitted Diseases, Viral] this term only | Search (#1 AND #2 AND #3 AND #4) | #3 AND #4 | — | — |
| #6 | #1 or #2 or #3 or #4 or #5 | Search (((#1 AND #2 AND #3 AND #4))) AND ("2016/05/20"[Date ‐ Publication] : "2017/08/25"[Date ‐ Publication]) | #3 NOT #5 | — | — |
| #7 | [mh "infectious disease transmission, vertical"] or "vertical transmission":ti,ab,kw or vertical next infect*:ti,ab,kw or "infection transmission":ti,ab,kw or "mother‐to‐child transmission":ti,ab,kw or MTCT:ti,ab,kw (Word variations have been searched) | — | #2 NOT #6 | — | — |
| #8 | [mh "vitamin A"] or vitamin*:ti,ab,kw or caroten*:ti,ab,kw or retinol:ti,ab,kw or retinoic:ti,ab,kw or micronutrient*:ti,ab,kw (Word variations have been searched) | — | 'vertical transmission'/de OR 'vertical transmission':ab,ti OR 'infectious disease transmission':ab,ti OR 'mother+to+child transmission':ab,ti OR mtct:ab,ti | — | — |
| #9 | #6 and #7 and #8 | — | caroten*:ab,ti OR retinoic:ab,ti OR 'retinol'/de OR retinol:ab,ti OR vitamin*:ab,ti OR 'vitamin a'/de OR micronutrient*:ab,ti | — | — |
| #10 | #6 and #7 and #8 Publication Year from 2016 to 2017 | — | #1 AND #7 AND #8 AND #9 | — | — |
| #11 | — | — | #1 AND #7 AND #8 AND #9 AND [20‐5‐2016]/sd NOT [25‐8‐2017]/sd | — | — |
| Search set | CENTRAL | PubMed | Embase |
| #1 | HIV Infections | HIV Infections OR HIV OR hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR hiv infect* OR human immunodeficiency virus OR human immunedeficiency virus OR human immuno‐deficiency virus OR human immune‐deficiency virus OR (human immun* AND deficiency virus) OR acquired immunodeficiency syndrome OR acquired immunedeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR (acquired immun* AND deficiency syndrome) OR "sexually transmitted diseases, Viral" | human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus infection OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR human immunodeficiency virus OR hiv OR hiv‐1 OR hiv‐2 OR human immunodeficiency virus OR human immunedeficiency virus OR human immune‐deficiency virus OR human immuno‐deficiency virus OR acquired immunodeficiency syndrome OR acquired immuno‐deficiency syndrome OR acquired immune‐deficiency syndrome OR acquired immunedeficiency syndrome |
| #2 | HIV | randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR "clinical trials as topic" OR randomly OR trial) NOT (animals NOT humans) | randomized controlled trial OR randomized controlled trial OR random* OR trial OR allocat* OR factorial* OR placebo* OR assign* OR volunteer* OR crossover procedure OR crossover procedure OR double‐blind procedure OR double‐blind procedure OR single‐blind procedure OR single‐blind procedure OR doubl* NEAR/3 blind* OR singl* AND blind* OR crossover* OR cross+over* OR cross NEXT/1 over* |
| #3 | hiv or hiv‐1* or hiv‐2* or hiv1 or hiv2 or hiv near infect* or human immunodeficiency virus or human immunedeficiency virus or human immune‐deficiency virus or human immuno‐deficiency virus or human immune deficiency virus or human immuno deficiency virus or acquired immunodeficiency syndrome or acquired immunedeficiency syndrome or acquired immuno‐deficiency syndrome or acquired immune‐deficiency syndrome or acquired immun* deficiency syndrome | infectious disease transmission, vertical OR vertical transmission OR vertical infect* OR infection transmission OR mother‐to‐child transmission OR MTCT | animal OR animal experiment OR invertebrate OR animal tissue OR animal cell OR nonhuman |
| #4 | Lymphoma, AIDS‐Related | vitamin A OR vitamin* OR caroten* OR retinol OR retinoic OR micronutrient* | human OR normal human OR human cell |
| #5 | Sexually Transmitted Diseases, Viral | 1‐4/AND | #3 AND #4 |
| #6 | 1‐5/OR | 5 AND (2010/06/01 NOT 2016/05/20) | #3 NOT #5 |
| #7 | infectious disease transmission, vertical or vertical transmission or vertical next infect* or infection transmission or mother‐to‐child transmission or MTCT | — | #2 NOT #6 |
| #8 | vitamin A or vitamin* or caroten* or retinol or retinoic or micronutrient* | — | vertical transmission OR vertical transmission OR infectious disease transmission OR mother+to+child transmission OR mtct |
| #9 | 6‐8/AND | — | caroten* OR retinoic OR retinol OR retinol OR vitamin* OR vitamin a OR micronutrient* |
| #10 | Limit 9 to publication date 2010‐2016 | — | #1 AND #7 AND #8 AND #9 |
| #11 | — | — | #10 AND (2010/06/01 NOT 2016/05/20) |
| Search set | CENTRAL | PubMed | Embase |
| #1 | MeSH descriptor HIV Infections explode all trees | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MESH:NoExp] | 'human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection'/de OR 'human immunodeficiency virus infection' OR 'human immunodeficiency virus'/exp OR 'human immunodeficiency virus'/de OR 'human immunodeficiency virus'OR hiv:ti OR hiv:ab OR 'hiv‐1':ti OR 'hiv‐1':ab OR 'hiv‐2':ti OR 'hiv‐2':ab OR 'human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab OR 'human immuno‐deficiency virus':ti OR 'human immuno‐deficiency virus':ab OR 'human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab OR 'human immune‐deficiency virus':ti OR 'human immune‐deficiency virus':ab OR 'acquired immune‐deficiency syndrome':ti OR 'acquired immune‐deficiency syndrome':ab OR 'acquired immunedeficiency syndrome':ti OR 'acquired immunedeficiency syndrome':ab OR 'acquired immunodeficiency syndrome':ti OR 'acquired immunodeficiency syndrome':ab OR 'acquired immuno‐deficiency syndrome':ti OR 'acquired immuno‐deficiency syndrome':ab |
| #2 | MeSH descriptor HIV explode all trees | Search (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]) | random*:ti OR random*:ab OR factorial*:ti OR factorial*:ab OR cross?over*:ti OR cross?over*:ab OR crossover*:ti OR crossover*:ab OR placebo*:ti OR placebo*:ab OR (doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab) OR (singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab) OR assign*:ti OR assign*:ab OR allocat*:ti OR allocat*:ab OR volunteer*:ti OR volunteer*:ab OR 'crossover procedure'/exp OR 'crossover procedure'/de OR 'crossover procedure'OR 'double‐blind procedure'/exp OR 'double‐blind procedure'/de OR 'double‐blind procedure' OR 'single‐blind procedure'/exp OR 'single‐blind procedure'/de OR 'single‐blind procedure' OR 'randomised controlled trial'/exp OR 'randomised controlled trial'/de OR 'randomised controlled trial' |
| #3 | hiv OR hiv‐1* OR hiv‐2* OR hiv1 OR hiv2 OR HIV INFECT* OR HUMAN IMMUNODEFICIENCY VIRUS OR HUMAN IMMUNEDEFICIENCY VIRUS OR HUMAN IMMUNE‐DEFICIENCY VIRUS OR HUMAN IMMUNO‐DEFICIENCY VIRUS OR HUMAN IMMUN* DEFICIENCY VIRUS OR ACQUIRED IMMUNODEFICIENCY SYNDROME OR ACQUIRED IMMUNEDEFICIENCY SYNDROME OR ACQUIRED IMMUNO‐DEFICIENCY SYNDROME OR ACQUIRED IMMUNE‐DEFICIENCY SYNDROME OR ACQUIRED IMMUN* DEFICIENCY SYNDROME | Search mother‐to‐child‐transmission OR MTCT OR infectious disease transmission, vertical | 'mother‐to‐child transmission' OR 'mother to child transmission' OR mtct OR 'vertical transmission'/de OR 'vertical transmission' |
| #4 | MeSH descriptor Lymphoma, AIDS‐Related, this term only | Search caroten* OR retinoic OR retinol OR vitamin* OR vitamin A OR micronutrient* | caroten* OR retinoicOR 'retinol'/de OR retinolOR vitamin*OR 'vitamin a'/de OR 'vitamin a'OR micronutrient* |
| #5 | MeSH descriptor Sexually Transmitted Diseases, Viral, this term only | Search #1 AND #2 AND #3 AND #4 Limits: Publication Date from 2007/01/01 to 2010/06/08 | #1 AND #2AND #3AND #4 |
| #6 | (#1 OR #2 OR #3 OR #4 OR #5) | — | #1 AND #2AND #3AND #4AND [humans]/lim AND [embase]/lim AND [1‐1‐2007]/sd NOT [8‐6‐2010]/sd |
| #7 | mother‐to‐child‐transmission OR MTCT | — | — |
| #8 | MeSH descriptor Infectious Disease Transmission, Vertical, this term only | — | — |
| #9 | (#7 OR #8) | — | — |
| #10 | caroten* OR retinoic OR vitamin* OR vitamin A OR micronutrient* | — | — |
| #11 | (#6 AND #9 AND #10) | — | — |
| #12 | (#6 AND #9 AND #10), from 2007 to 2010 | — | — |
| Search set | PubMed | Embase | AIDSearch | GATEWAY |
| #1 | Search HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw] OR acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH] | (('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection') OR ('human immunodeficiency virus infection'/exp OR 'human immunodeficiency virus infection')) OR ((('human immunodeficiency virus'/exp OR 'human immunodeficiency virus') OR ('human immunodeficiency virus'/exp OR 'human immunodeficiency virus'))) OR ((('b cell lymphoma'/exp OR 'b cell lymphoma') OR ('b cell lymphoma'/exp OR 'b cell lymphoma'))) OR (hiv:ti OR hiv:ab) OR ('hiv‐1':ti OR 'hiv‐1':ab) OR ('hiv‐2':ti OR 'hiv‐2':ab) OR ('human immunodeficiency virus':ti OR 'human immunodeficiency virus':ab) OR ('human immunedeficiency virus':ti OR 'human immunedeficiency virus':ab) OR ('human | (HIV INFECTIONS) OR HIV OR HIV OR HIV‐1* OR HIV‐2* OR HIV1 OR HIV2 OR (HIV INFECT*) OR (HUMAN IMMUNODEFICIENCY VIRUS) OR (HUMAN IMMUNEDEFICIENCY VIRUS) OR (HUMAN IMMUNO‐DEFICIENCY VIRUS) OR (HUMAN IMMUNE‐DEFICIENCY VIRUS) OR ((HUMAN IMMUN*) AND (DEFICIENCY VIRUS)) OR (ACQUIRED IMMUNODEFICIENCY SYNDROME) OR (ACQUIRED IMMUNEDEFICIENCY SYNDROME) OR (ACQUIRED IMMUNO‐DEFICIENCY SYNDROME) OR (ACQUIRED IMMUNE‐DEFICIENCY SYNDROME) OR ((ACQUIRED IMMUN*) AND (DEFICIENCY SYNDROME)) OR (SEXUALLY TRANSMITTED DISEASES, VIRAL) | Search: (HIV Infections[MeSH] OR HIV[MeSH] OR hiv[tw] OR hiv‐1*[tw] OR hiv‐2*[tw] OR hiv1[tw] OR hiv2[tw] OR hiv infect*[tw] OR human immunodeficiency virus[tw] OR human immunedeficiency virus[tw] OR human immuno‐deficiency virus[tw] OR human immune‐deficiency virus[tw] OR ((human immun*) AND (deficiency virus[tw])) OR acquired immunodeficiency syndrome[tw]) AND (acquired immunedeficiency syndrome[tw] OR acquired immuno‐deficiency syndrome[tw] OR acquired immune‐deficiency syndrome[tw] OR ((acquired immun*) AND (deficiency syndrome[tw])) OR "sexually transmitted diseases, viral"[MH]) |
| #2 | Search randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR ( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh]) | (random*:ti OR random*:ab) OR (factorial*:ti OR factorial*:ab) OR (cross?over*:ti OR cross?over:ab OR crossover*:ti OR crossover*:ab) OR (placebo*:ti OR placebo*:ab) OR (((doubl*:ti AND blind*:ti) OR (doubl*:ab AND blind*:ab))) OR (((singl*:ti AND blind*:ti) OR (singl*:ab AND blind*:ab))) OR (assign*:ti OR assign*:ab) OR (volunteer*:ti OR volunteer*:ab) OR (((('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')) OR (('crossover procedure'/exp OR 'crossover procedure') OR ('crossover procedure'/exp OR 'crossover procedure')))) OR (((('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')) OR (('double‐blind procedure'/exp OR 'double‐blind procedure') OR ('double‐blind procedure'/exp OR 'double‐blind procedure')))) OR (((('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')) OR (('single‐blind procedure'/exp OR 'single‐blind procedure') OR ('single‐blind procedure'/exp OR 'single‐blind procedure')))) OR (((('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')) OR (('randomised controlled trial'/exp OR 'randomised controlled trial') OR ('randomised controlled trial'/exp OR 'randomised controlled trial')))) OR (allocat*:ti OR allocat*:ab) AND [2003‐2008]/py | ((RANDOMIZED CONTROLLED TRIAL) OR (CONTROLLED CLINICAL TRIAL) OR (RANDOMIZED CONTROLLED TRIALS) OR (RANDOM ALLOCATION) OR (DOUBLE‐BLIND METHOD) OR (SINGLE‐BLIND METHOD) OR (CLINICAL TRIAL) OR (CLINICAL TRIALS) OR ("CLINICAL TRIAL") OR ((SINGL* OR DOUBL* OR TREBL* OR TRIPL* AND (MASK* OR BLIND* )) OR PLACEBOS OR PLACEBO* OR RANDOM* OR (COMPARATIVE STUDY) OR (EVALUATION STUDIES) OR (FOLLOW‐UP STUDIES) OR (PROSPECTIVE STUDIES) OR CONTROL* OR PROSPECTIV* OR VOLUNTEER*)) NOT (ANIMALS NOT HUMAN ) | Search: (randomised controlled trial [pt] OR controlled clinical trial [pt] OR randomised controlled trials [mh] OR random allocation [mh] OR double‐blind method [mh] OR single‐blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR ("clinical trial" [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw]))) OR (( placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp] OR (comparative study) OR (comparative studies) OR (evaluation studies) OR follow‐up studies [mh] OR prospective studies [mh] OR control* [tw] OR prospectiv* [tw] OR volunteer* [tw]) NOT (animals [mh] NOT human [mh])) |
| #3 | Search (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) | 'mother‐to‐child transmission' OR mtct OR 'vertical disease transmission' AND [2003‐2008]/py | (MOTHER‐TO‐CHILD TRANSMISSION) OR MTCT OR (VERTICAL DISEASE TRANSMISSION) | Search: (DISEASE TRANSMISSION, VERTICAL) OR MTCT OR (MOTHER‐TO‐CHILD TRANSMISSION) |
| #4 | Search CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | caroten* OR retinoic OR ('retinol'/exp OR 'retinol') OR vitamin* OR micronutrient* AND [2003‐2008]/py | CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* | Search: CAROTEN* OR RETINOIC OR RETINOL OR VITAMIN* OR MICRONUTRIENT* |
| #5 | Search PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* | pregnant OR ('pregnancy'/exp OR 'pregnancy') OR antepartum OR ('ante partum') OR antenatal OR ('ante natal') OR prenatal OR ('pre natal') AND [2003‐2008]/py | PREGNANT OR PREGNANCY OR ANTEPARTUM OR (ANTE‐PARTUM) OR ANTENATAL OR (ANTE‐NATAL) OR PRENATAL OR (PRE‐NATAL) | Search: PREGNANT OR PREGNANCY OR ANTEPARTUM OR PRENATAL OR ANTE‐PARTUM OR PRE‐NATAL OR PREPART* |
| #6 | Search #1 AND #2 AND #3 AND #4 AND #5 Limits: Publication Date from 2003 to 2008 | #1 AND #2 AND #3 AND #4 AND #5 | #1 AND #2 AND #3 AND #4 AND #5 | #1 and #2 and #3 and #4 and #5 Limit: 2003:2008 |
| Outcome | Assumed risk | Source | Clinically important relative improvement | Sample size required |
| HIV infection in child | 27/100 | Analysis 1.1 | 25% | 1236 |
| Mean birthweight | 2964 | Analysis 1.2 | 25% | 6178 |
| Low birthweight | 17/100 | Analysis 1.3 | 25% | 2194 |
| Still birth | 3/100 | Analysis 1.4 | 25% | 14,264 |
| Preterm birth | 20/100 | Analysis 1.5 | 25% | 1806 |
| Child death | 14/100 | Analysis 1.6 | 25% | 2748 |
| Maternal death | 3/100 | Analysis 1.7 | 25% | 14,264 |
| We based the sample size calculations: 2‐sided tests, with ratio of 1:1, power of 0.8 and confidence level of 0.05. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 HIV infection status of the child Show forest plot | 5 | 4428 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 1.1 Antepartum supplementation | 2 | 650 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 1.2 Postpartum supplementation | 1 | 2248 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.98, 1.27] |
| 1.3 Antepartum and postpartum supplementation | 2 | 1530 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.86, 1.59] |
| 2 Mean birthweight Show forest plot | 3 | 2181 | Mean Difference (IV, Random, 95% CI) | 34.12 [‐12.79, 81.02] |
| 3 Low birthweight Show forest plot | 3 | 1819 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.97] |
| 4 Child death by two years of age Show forest plot | 3 | 3883 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.92, 1.22] |
| 5 Preterm delivery Show forest plot | 2 | 1577 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.52, 1.37] |
| 6 Stillbirth Show forest plot | 3 | 2335 | Risk Ratio (M‐H, Random, 95% CI) | 1.13 [0.72, 1.77] |
| 7 Maternal death Show forest plot | 2 | 1267 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.35, 1.43] |
| 8 Postpartum CD4 count Show forest plot | 1 | 511 | Mean Difference (IV, Random, 95% CI) | ‐13.0 [‐60.46, 34.46] |