Campos electromagnéticos para el tratamiento de la osteoartritis
Appendices
Appendix 1. MEDLINE search strategy
MEDLINE updated to 3 October 2013
1. exp osteoarthritis/
2. osteoarthr$.tw.
3. (degenerative adj2 arthritis).tw.
4. arthrosis.tw.
5. or/1‐4
6. Electromagnetic Fields/
7. electromagnetic$.tw.
8. exp Electric Stimulation Therapy/
9. pulsed.tw.
10. (electric$ adj3 stimulat$).tw.
11. (alternat$ adj3 electric$).tw.
12. physical therapy modalities/
13. (physical adj therap$).tw.
14. physiotherap$.tw.
15. or/6‐14
16. 5 and 15
17. randomized controlled trial.pt.
18. controlled clinical trial.pt.
19. randomized.ab.
20. placebo.ab.
21. drug therapy.fs.
22. randomly.ab.
23. trial.ab.
24. groups.ab.
25. or/17‐24
26. (animals not (humans and animals)).sh.
27. 25 not 26
28. 16 and 27
Appendix 2. CINAHL search strategy
CINAHL updated to 3 October 2013
S30 S16 and S29
S29 S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or S26 or S27 or S28
S28 TI Allocat* random* or AB Allocat* random*
S27 (MH "Quantitative Studies")
S26 (MH "Placebos")
S25 TI Placebo* or AB Placebo*
S24 TI Random* allocat* or AB Random* allocat* Search S23 (MH "Random Assignment")
S22 TI Randomi?ed control* trial* or AB Randomi?ed control* trial*
S21 AB singl* blind* or AB singl* mask* or AB doub* blind* or AB doubl* mask* or AB trebl* blind* or AB trebl* mask* or AB
tripl* blind* or AB tripl* mask*
S20 TI singl* blind* or TI singl* mask* or TI doub* blind* or TI doubl* mask* or TI trebl* blind* or TI trebl* mask* or TI tripl*
blind* or TI tripl* mask*
S19 TI clinical* trial* or AB clinical* trial*
S18 PT clinical trial
S17 (MH "Clinical Trials+")
S16 S5 and S15
S15 S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14
S14 ti physiotherap* or ab physiotherap*
S13 ti physical therap* or ab physical therap* Search
S12 (MH "Physical Therapy+")
S11 ti alternat* N3 electric* or ab alternat* N3 electric*
S10 ti electric$* N3 stimulat* or ab electric$* N3 stimulat*
S9 ti pulsed or ab pulsed
S8 (MH "Electric Stimulation+")
S7 ti electromagnetic* or ab electromagnetic*
S6 (MH "Electromagnetic Fields")
S5 S1 or S2 or S3 or S4
S4 ti arthrosis or ab arthrosis
S3 ti degenerative N2 arthritis or ab degenerative N2 arthritis
S2 ti osteoarthr* or ab osteoarthr*
S1 (MH "Osteoarthritis+")
Appendix 3. EMBASE search strategy
EMBASE updated to 3 October 2013
1. exp osteoarthritis/
2. osteoarthr$.tw.
3. (degenerative adj2 arthritis).tw.
4. arthrosis.tw.
5. or/1‐4
6. electromagnetic field/
7. electromagnetic$.tw.
8. exp electrostimulation therapy/
9. pulsed.tw.
10. (electric$ adj3 stimulat$).tw.
11. (alternat$ adj3 electric$).tw.
12. exp physiotherapy/
13. (physical adj therap$).tw.
14. physiotherap$.tw.
15. or/6‐14
16. 5 and 15
17. (random$ or placebo$).ti,ab.
18. ((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
19. controlled clinical trial$.ti,ab.
20. RETRACTED ARTICLE/
21. or/17‐20
22. (animal$ not human$).sh,hw.
23. 21 not 22
24. 16 and 23
Appendix 4. CENTRAL search strategy
CENTRAL updated to Issue 9, 2013
#1 MeSH descriptor Osteoarthritis explode all trees
#2 degenerative near/2 arthritis
#3 osteoarthr*
#4 arthrosis:ti,ab
#5 (#1 OR #2 OR #3 OR #4)
#6 MeSH descriptor Electromagnetic Fields explode all trees
#7 electromagnetic*:ti,ab
#8 MeSH descriptor Electric Stimulation Therapy explode all trees
#9 pulsed:ti,ab
#10 electric* near/3 stimulat*:ti,ab
#11 alternat* near/3 electric*:ti,ab
#12 MeSH descriptor Physical Therapy Modalities explode all trees
#13 physical next therap*:ti,ab
#14 physiotherap*:ti,ab
#15 (#6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14)
#16 (#5 AND #15)
Appendix 5. PEDro search strategy
PEDro updated to 3 October 2013
Abstract and title: electro
Subdiscipline: musculoskeletal
Abstract and title: osteoarthritis
Therapy: electrotherapies, heat and cold
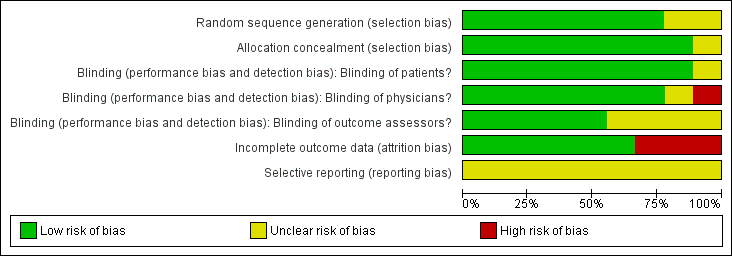
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
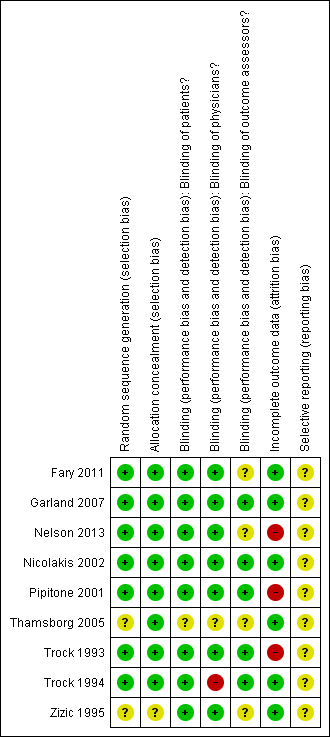
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
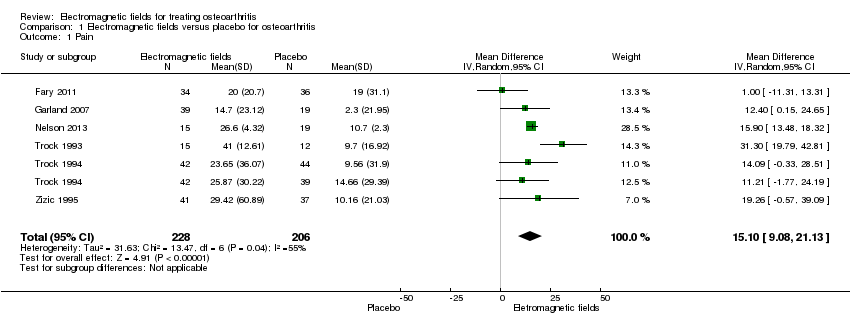
Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 1 Pain.
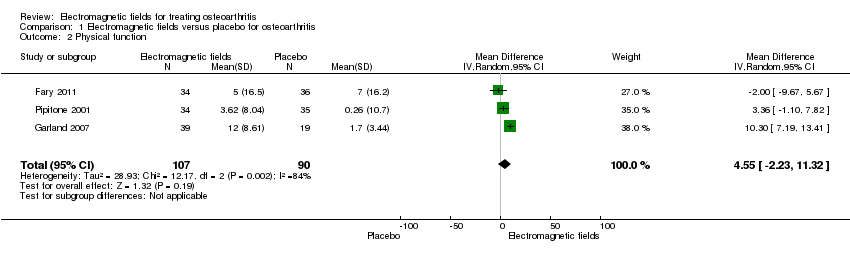
Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 2 Physical function.

Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 3 Quality of life.

Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 4 Number of patients experiencing any adverse event.

Comparison 1 Electromagnetic fields versus placebo for osteoarthritis, Outcome 5 Number of patients who withdrew because of adverse events.
| Electromagnetic field treatment compared to placebo for the treatment of osteoarthritis | ||||||
| Patient or population: patients with osteoarthritis | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Electromagnetic field treatment | |||||
| Pain Scale from: 0 to 100 (Higher scores mean worse pain) | The mean change in pain in the control groups was 10.7 | The mean change in pain in the intervention groups was | 434 | ⊕⊕⊕⊝ | MD 15.10 (95% CI 9.08 to 21.13) Absolute risk difference: 15% (95% CI 9.08% to 21.13%) Relative per cent change: 21.03% (95% CI 12.65% to 29.43%) NNT: 2 (95% CI 1 to 6) | |
| Physical function WOMAC function Scale from: 0 to 100 (Higher scores mean more severe limitation) | The mean change in physical function in the control groups was | The mean change in physical function in the intervention groups was | 197 | ⊕⊕⊝⊝ | MD 4.55 (95% CI ‐2.23 to 11.32) Absolute risk difference: 4.55% (95% CI ‐2.23% to 11.32%) Relative per cent change: 268% (95% CI ‐131% to 666%) NNT: not statistically significant | |
| Quality of life SF‐36 item Scale from: 0 to 100 (Lower scores mean worse quality) Follow‐up: mean 16 weeks | The mean change in quality of life in the control groups was | The mean change in quality of life in the intervention groups was | 145 | ⊕⊕⊕⊝ | SMD 0.09 (95% CI ‐0.36 to 0.54) Absolute risk difference: 1% (95% CI ‐2.92% to 4.37%) Relative per cent change: 30.38% (95% CI ‐121.5% to 182.25%) NNT: not statistically significant | |
| Radiographic progression Bone scintigraphic examinations Follow‐up: mean 2.5 months | See comment | See comment | Not estimable | 78 | See comment | No related data were available |
| Number of patients experiencing any adverse event Follow‐up: mean 1 month | 167 per 1000 | 195 per 1000 | RR 1.17 | 288 | ⊕⊕⊕⊝ | Absolute risk difference: 3% (95% CI ‐6% to 12%) Relative per cent change: 17% (95% CI ‐28% to 92%) NNT: not statistically significant |
| Number of patients who withdrew because of adverse events Follow‐up: mean 6 months | 27 per 1000 | 24 per 1000 (2 to 376) | RR 0.90 (0.06 to 13.92) | 78 | ⊕⊕⊝⊝ | Only 1 study: 1 participant withdrew from each group because of adverse skin reactions unrelated to the therapy |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded for moderate heterogeneity (I2 = 55%); unclear risk for random sequence generation (Zizic 1995), allocation concealment (Zizic 1995), blinding of outcome assessors (Fary 2011; Nelson 2013; Zizic 1995), selective reporting (all six studies) and high risk for incomplete outcome data (Zizic 1995). | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pain Show forest plot | 6 | 434 | Mean Difference (IV, Random, 95% CI) | 15.10 [9.08, 21.13] |
| 2 Physical function Show forest plot | 3 | 197 | Mean Difference (IV, Random, 95% CI) | 4.55 [‐2.23, 11.32] |
| 3 Quality of life Show forest plot | 2 | 139 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.36, 0.54] |
| 4 Number of patients experiencing any adverse event Show forest plot | 4 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.72, 1.92] |
| 5 Number of patients who withdrew because of adverse events Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |


