Laxatives for the management of constipation in people receiving palliative care
Information
- DOI:
- https://doi.org/10.1002/14651858.CD003448.pub4Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 13 May 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Pain, Palliative and Supportive Care Group
- Copyright:
-
- Copyright © 2020 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
MG, SW and CM developed the original protocol.
In the 2015 review update, BC, PS, PL and LJ independently assessed eligibility of studies in new searches. Data extraction undertaken by BC and checked by LJ. Statistical support provided by VV.
Updating of all review sections was drafted by BC and checked and critiqued by other members of the review update team (LJ, PL, PS , AT and VV).
Sources of support
Internal sources
-
Marie Curie Cancer Care, UK.
External sources
-
Janssen‐Cilag Ltd UK in original review (but not for the 2010 or 2015 review updates), UK.
Declarations of interest
BC has no relevant conflicts of interest to declare.
LJ has no relevant conflicts of interest to declare.
VV has no relevant conflicts of interest to declare.
AT has no relevant conflicts of interest to declare.
PS has no relevant conflicts of interest to declare.
PL has no relevant conflicts of interest to declare.
Janssen‐Cilag has funded a Marie Curie Care survey of the management of constipation in palliative care. Part of the remit of this study included a systematic review of the use of laxatives in the management of constipation for people receiving palliative care. Janssen‐Cilag do not manufacture or promote laxatives.
Acknowledgements
The researchers in the original review published in 2006 gratefully acknowledged the financial support provided by Janssen‐Cilag. Marie Curie Care funded both the 2010 and 2015 update of this review.
We acknowledge Claire Miles, Susie Wilkinson, Robyn Drake and Margaret Goodman, who were authors of earlier versions of this review and George Dowswell who assisted in the early stages of the updating of this review. We also acknowledge the support of Jessica Thomas, Caroline Struthers, Anna Hobson and Joanne Abbott of the Cochrane Pain, Palliative and Supportive Care Review Group.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pain, Palliative and Supportive Care Review Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 May 13 | Laxatives for the management of constipation in people receiving palliative care | Review | Bridget Candy, Louise Jones, Philip J Larkin, Victoria Vickerstaff, Adrian Tookman, Patrick Stone | |
| 2011 Jan 19 | Laxatives or methylnaltrexone for the management of constipation in palliative care patients | Review | Bridget Candy, Louise Jones, Margaret Lynn Goodman, Robyn Drake, Adrian Tookman | |
| 2006 Oct 18 | Laxatives for the management of constipation in palliative care patients | Review | Clare Miles, Deborah Fellowes, Margaret Lynn Goodman, Susie SM Wilkinson | |
| 2001 Oct 23 | Laxatives for the management of constipation in palliative care patients | Protocol | Margaret Lynn Goodman, Susie SM Wilkinson | |
Differences between protocol and review
A key difference between the earlier update and this 2015 update is that the review no longer includes trials on methylnaltrexone. Other differences by section are:
-
Background: re‐ordered, references updated.
-
Inclusion criteria: excludes opioid antagonists.
-
Methods: now includes further details on analysis and current methods of risk of bias assessment.
-
Results: includes analysis of one new study.
-
Discussion: conclusions changed following findings from the new study.
Notes
A restricted search in September 2019 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. The review will be re‐assessed for updating in two years. If appropriate, we will update the review before this date if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
- Analgesics, Opioid [adverse effects];
- Anthraquinones [therapeutic use];
- Cathartics [adverse effects, *therapeutic use];
- Constipation [chemically induced, *drug therapy];
- Lactulose [therapeutic use];
- Magnesium Hydroxide [therapeutic use];
- Naltrexone [adverse effects, *analogs & derivatives, therapeutic use];
- *Palliative Care;
- Paraffin [therapeutic use];
- Quaternary Ammonium Compounds [adverse effects, therapeutic use];
- Randomized Controlled Trials as Topic;
- Senna Extract [therapeutic use];
Medical Subject Headings Check Words
Humans;
PICOs

Study flow diagram for update search in 2014.
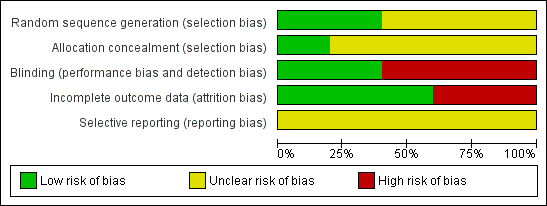
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Outcome or subgroup | Participants | Effect estimate* |
| Bowel movements in participants receiving strong opioid analgesia (taking ≥ 80 mg) | 17 | "Lactulose plus senna was associated with significantly higher frequency (regardless of which laxative taken first) (P value = < 0.01)" |
| Bowel movements in participants receiving opioid analgesia (< 80 mg) or no opioid analgesia | 21 | "No statistical difference between the trial arms" |
| No bowel movement in treatment week | Unclear | While participants were receiving co‐danthramer plus poloxamer, this occurred 11 times versus once in senna plus lactulose group (P value = 0.01) |
| Suspension of laxative therapy for 24 hours | Unclear | Occurred more frequently with lactulose plus senna (15 cases) than co‐danthramer plus poloxamer (5 cases) (P value = 0.05) |
| Rescue laxatives | Unclear | 14 participants received a rescue laxative only while taking co‐danthramer plus poloxamer but not with senna plus lactulose. 4 participants received rescue laxatives while taking senna plus lactulose but not with co‐danthramer plus poloxamer. 5 participants received rescue laxatives both while taking both trial treatments |
| Participant assessment of bowel function | Unclear | The reported mean change in participant assessment of their bowel function was not significant between drugs at the first week prior to cross‐over or in the week following cross‐over |
| Participant preference | 58 | "While favourable comments about agents effectiveness and flavour were evenly shared, twice as many patients disliked the flavour of co‐danthramer as that of lactulose with senna" |
| Diarrhoea | Unclear | "...diarrhoea resulted in the suspension of laxative therapy occurred more frequently with lactulose and senna compared to co‐danthramer (15 versus 5)" |
| Adverse effects | Unclear | 2 participants reported per‐anal soreness and burning on co‐danthramer plus poloxamer |
| Overall finding | ‐ | Outcomes were mixed on laxation response |
| * If data available and appropriate effect estimate was presented as an odds ratio (OR) or a mean difference (MD) with 95% confidence interval (CI). If not available or appropriate then effect was reported as stated in the trial. | ||
| Magnesium hydroxide plus liquid | Participants | Effect outcome* |
| Laxation response | 35 | "For all patients and for the subgroups who either were or were not receiving strong opioids there was no statistical difference in stool frequency between the two trial treatment groups". At the end of the trial, 19/35 (54%) participants had bowel function they accepted as normal |
| Treatment failure | 29 | 2 participants passed no spontaneous stool with either treatment |
| Loose stools | unclear | There was no significant difference between treatments in the proportion of participants reporting loose stools |
| Rescue laxatives | unclear | "...rectal measures were used on ten occasions during treatment with senna plus lactulose and 23 occasions while magnesium hydroxide plus liquid paraffin was being used" |
| Participant assessment of constipation | 35 | OR 1.10; 95% CI 0.28 to 4.26** |
| Participant assessment of diarrhoea | 35 | OR 0.67; 95% CI 0.10 to 4.58** |
| Participant assessment of normality of bowel function | 35 | OR 1.11; 95% CI 0.29 to 4.21** |
| Participant preference | 32 | 8/32 (magnesium hydroxide plus liquid paraffin) versus 19/32 (senna and lactulose group) |
| Adverse events | Unclear | In both groups, 1 participant found the treatment intolerably nauseating. 1 participant had gripping abdominal pain with lactulose and senna |
| Overall finding | ‐ | No difference in laxation response |
| * If data available and appropriate effect estimate was presented as an odds ratio (OR) or a mean difference (MD) with 95% confidence interval (CI). If not available or appropriate then effect was reported as stated in the trial. **Effect outcome used data prior to cross‐over. | ||
| Outcome or subgroup | Participants | Effect estimate* |
| Satisfactory bowel movements with no adverse effects | 28 | OR 7.67; 95% CI 0.37 to 158.01 |
| Overall finding | ‐ | No difference in laxation response |
| * If data available and appropriate effect estimate was presented as an odds ratio (OR) or a mean difference (MD) with 95% confidence interval (CI). If not available or appropriate then effect was reported as stated in the trial. | ||
| Outcome or subgroup | Participants | Effect estimate* |
| Mean number of defecation days | 75 | MD ‐0.10; 95% CI ‐0.60 to 0.40 |
| Defecation‐free days | 75 | MD 0.00; 95% CI ‐0.48 to 0.48 |
| General state of health | 75 | MD ‐0.10; 95% CI ‐0.31 to 0.11 |
| Overall finding | ‐ | No difference in laxation response |
| * If data available and appropriate effect estimate was presented as an odds ratio (OR) or a mean difference (MD) with 95% confidence interval (CI). If not available or appropriate then effect was reported as stated in the trial. | ||
| Outcome or subgroup | Participants | Effect estimate* |
| Stool frequency | 56 | No statistically significant difference in the overall mean number of bowel movements per day between the docusate plus senna (x statistic = 0.74 (SD 0.47) and placebo plus senna groups (x statistic = 0.69, SD 0.37) (P value = 0.58) |
| Bowel movement on ≥ 50% of days | 56 | OR 0.52; 95% CI 0.17 to 1.57 |
| Stool volume | 56 | Trialists reported no significant difference between trial arms in stool volume (P value = 0.06) |
| Stool consistency | 56 | Using the Bristol Stool Form Scale, more participants in the placebo plus senna group had Type 4 (smooth and soft) and Type 5 (soft blobs). In the docusate plus senna group, more participants had Type 3 (sausage, cracks in surface) and Type 6 (mushy stool) (P value = 0.01) |
| Participants' perceptions of the difficulty and completeness of defecation | 56 | No differences in reported difficulty in evacuation (13/40 in the docusate group versus 14/56 in the placebo group; OR 1.44; 95% CI 0.59 to 3.54). No difference in sense of completeness of evacuation (25/34 in the docustate plus senna group versus 44/56 in the placebo plus senna group ; OR 0.76; 95% CI 0.28 to 2.05) |
| Overall finding | ‐ | No difference in laxation response |
| * If data available and appropriate effect estimate is presented as an odds ratio (OR) or a mean difference (MD). If not available or appropriate then effect is reported as stated in the trial. SD: standard deviation. | ||

