Antihistamines and/or decongestants for otitis media with effusion (OME) in children
References
References to studies included in this review
Jump to:
References to studies excluded from this review
Jump to:
Additional references
Jump to:
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Quality score: 3/5 based on Jadad scoring (Randomization: 1+0; Blinding: 1+1; Withdrawals: 0). Follow up was 87% and the RCT was conducted over 3 years, from 1978‐81. Intention‐to‐treat analysis: yes | |
| Participants | 553 pediatric ENT patients were referred from outpatient clinics. Diagnosis was based on an algorithm involving otoscopy, tympanometry and middle ear muscle reflex testing. Exclusion criteria included: congenital craniofacial malformation, Down's Syndrome, history of tonsillectomy, adenoidectomy, or tympanostomy (myringotomy) tubes, structural middle ear abnormality, underlying hearing loss, severe upper airway obstruction, acute otitis media, purulent rhinitis, any sinusitis, or history of antihistamine or decongestant use in the preceding 30 days. | |
| Interventions | Antihistamine (chlorpheniramine) and decongestant (pseudoephedrine) combination versus placebo. | |
| Outcomes | The primary outcome was effusion or no effusion at 4 weeks. Other outcomes measured were hearing, medication side effects and the complication of recurrent OME | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 3/5 (Randomization: 1+0; Blinding: 1+1; Withdrawals: 0). Intention‐to‐treat analysis: no. Follow up was 89%. RCT was conducted over 3 years from 1981‐84. | |
| Participants | 318 children aged 7 months to 12 years were recruited from community practices and a hospital ambulatory care centre. Diagnosis was based on an algorithm using otoscopy and tympanometry. Exclusion criteria included: congenital craniofacial malformation, systemic illness, history of tonsillectomy, adenoidectomy, insertion of tubes (tympanostomy), structural middle ear abnormality, hearing loss ... | |
| Interventions | Antihistamine (chlorpheniramine) and decongestant (pseudoephedrine) combination versus placebo. | |
| Outcomes | The primary outcome was effusion or no effusion at 4 weeks with a secondary outcome measured at 12 weeks. Hearing improvement or no improvement was measured at 4 weeks and the complication of acute otitis media was assessed. Side effects of medications were counted. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 4/5 (Randomization: 1+0; Blinding: 1+1; Withdrawals: 1). Intention‐to‐treat analysis: no. Follow up was 89%. | |
| Participants | 66 children aged 6 months to 10 years were recruited from a pediatric outpatient clinic. All had completed antibiotic treatment before enrollment. Diagnosis was based on pneumatic otoscopy and tympanometry. Exclusion criteria included: history of cleft lip or palate, chronic disease, immunodeficiency disease, recent use of corticosteroids or known hearing loss > 25 dB bilaterally or > 35 dB unilaterally. | |
| Interventions | Antihistamine (chlorpheniramine), decongestant (pseudoephedrine) and placebo | |
| Outcomes | Our primary outcome was not measured (cure at or before 4 weeks), but a secondary outcome of effusion or no effusion at 12 weeks was measured as was the complication of Acute Otitis Media (AOM). | |
| Notes | Antihistamine arm of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | See Notes | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Same study as Dusdieker 1985 but different arm ‐ decongestant arm | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Quality score: 1/5 (Randomization: 0; Blinding: 1+0; Withdrawals: 0). Intention‐to‐treat analysis: no. Follow up was 78%. The trial took place in 1974. | |
| Participants | 94 children mainly less than 10 years were seen in an ENT clinic. Diagnosis was based on pneumatic otoscopy. Exclusion criteria: none reported. | |
| Interventions | Antihistamine (cinnarizine) and placebo. | |
| Outcomes | The only outcome measured was the secondary outcome of effusion or no effusion at less than 12 weeks (7 weeks here). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 3/5 (Randomization: 1; Blinding: 1+0; Withdrawals: 1). Intention‐to‐treat analysis: yes. Follow up 100% | |
| Participants | 172 children aged 6 months to 15 years were recruited from ENT clinics in Sweden. Diagnosis was based on pneumatic otoscopy and tympanometry. Exclusion criteria included: need for acute tympanocentesis, chronic illness, refusal to participate, difficult child to examine, previous side effects to one drug or the other. | |
| Interventions | Decongestant (oxymetazoline nasal drops or phenylpropanolamine orally) versus no treatment. | |
| Outcomes | The primary outcome of effusion or no effusion at 4 weeks was measured as was the secondary outcome of cure or no cure at 4‐8 weeks, all significant side effects and the complication of surgery (tympanostomy). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 1/5 (Randomization: 1+0; Blinding: 0; Withdrawals: 0). Intention‐to‐treat analysis: unable to determine. Follow up was 96%. | |
| Participants | 85 children aged 3‐12 years with bilateral OME were recruited. Diagnosis was based on an algorithm using otoscopy and tympanometry. Exclusion criteria: none given. | |
| Interventions | Decongestant (ephedrine nasal drops) or antihistamine/decongestant combination (brompheniramine/phenylpropanolamine) versus autoinflation (control). | |
| Outcomes | No individual patient data were given. The authors gave a simple statement that outcomes from all three interventions were the same. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | Quality score: 3/5 (Randomization: 1; Blinding: 1+1; Withdrawals: 0). Intention‐to‐treat analysis: no. Follow up was 94%. | |
| Participants | 61 children aged 1‐14 years were seen in an ENT Department in Norway. Diagnosis was based on pneumatic otoscopy, otomicroscopy, tympanometry and audiometry. Exclusion criterion: age less than one year. | |
| Interventions | Decongestant (phenylpropanolamine) or decongestant/antihistamine combination (phenylpropanolamine/brompheniramine) versus placebo. | |
| Outcomes | The primary outcome of effusion or not at 4 weeks was measured as was improvement or no improvement in hearing at 4 weeks. | |
| Notes | For 'Any Medication' comparison, this will be decongestant arm only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | See Notes | |
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | Same study as Haugeto 1981 but different arm, antihistamine/decongestant arm. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
| Methods | Quality score: 4/5 (Randomization: 1+0; Blinding: 1+1; Withdrawals: 1). Intention‐to‐treat analysis: no. Follow up was 50% and the duration of the study was 4 years from 1978‐82. | |
| Participants | 67 children aged 9 months to 10 years were recruited from private pediatrics offices approximately 2 weeks after treatment for AOM. Diagnosis was based on either clinical criteria (pneumatic otoscopy) or tympanometry. We used only tympanometry data. Exclusion criteria: none given. | |
| Interventions | Decongestant (phenylephrine) given intranasally versus intranasal placebo. | |
| Outcomes | The primary outcome of effusion or not at 4 weeks was measured. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 3/5 (Randomization: 1+0; Blinding: 1+1; Withdrawals: 0). Intention‐to‐treat analysis: no. Follow up was 88%. | |
| Participants | 42 children (no age range given) from GP practices were referred to a single ENT specialist. Diagnosis was based on clinical examination and tympanometry. Exclusion criteria: previous surgery to ears, nose or throat and abnormal palatal function. | |
| Interventions | Antihistamine/decongestant combination (triprolidine/pseudoephedrine) versus placebo | |
| Outcomes | Our primary outcome (effusion or not at 4 weeks or less) was not measured. Secondary (1‐3 months) and Late (greater than 3 months) were measured as was the outcome of surgery (tympanostomy). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 2/5 (Randomization: 1; Blinding: 1; Withdrawals: 0). Intention‐to‐treat analysis: unable to determine. Follow up was 97%. | |
| Participants | 58 children aged 5‐14 years were recruited from an ENT clinic. Diagnosis was based on a combination of history, otoscopy and audiology. Exclusion criterion: presence of AOM. | |
| Interventions | Antihistamine/decongestant combination (brompheniramine/phenylephrine and phenylpropanolamine) versus placebo. | |
| Outcomes | No individual patient data are available. The outcome measured was hearing loss at 4 weeks. The authors stated that no significant difference in hearing was found between the antihistamine/decongestant combination and the placebo group. All children got tympanostomies. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 2/5 (Randomization: 1; Blinding: 0; Withdrawals: 1). Intention‐to‐treat analysis: no. Follow up 95%. Study duration from Sept. 1984 to Jan. 1985. | |
| Participants | 39 children aged 3‐12 years with OME after tympanostomy and (adenoidectomy or tonsillectomy) were recruited from an ENT practice. Diagnosis was based on a thick effusion at tympanostomy at entry to the study and on, at outcome, an algorithm that included tympanometry, audiography and otoscopy. Exclusion criteria included: previous surgery for OME and use of mucolytics, antihistamines or decongestants in the preceding 72 days. | |
| Interventions | Antihistamine/decongestant combination (brompheniramine/phenylephrine and phenylpropanolamine) versus no treatment. | |
| Outcomes | Our primary outcome of effusion or not at 4 weeks was not measured. A secondary outcome of effusion or not at 6 weeks (by counting ears) was measured as was the side effect of nosebleed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | This is a report of the same study as Cantekin 1991 so the outcomes will not be reported separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 2/5 (Randomization: 0: Blinding: 1+1; Withdrawals: 0). Intention‐to‐treat analysis: no. Follow up was 91%. The study took place between March and December 1977. | |
| Participants | 83 children aged 3‐9 years with their first episode of OME were recruited from Pediatrics and ENT practices in Rhode Island. Diagnosis was based on a combination of otoscopy, audiometry and tympanometry. Exclusion criteria included: oral temperature greater than 37.8C and ear or nose deformity. | |
| Interventions | Antihistamine/decongestant combination (diphenhydrinate/pseudoephedrine) versus placebo. | |
| Outcomes | Our primary outcome of effusion or not at 4 weeks was not measured. A secondary outcome was measured by patient‐ear‐visit at 3 months. Hearing at 3 months was measured for improvement (by at least 20 dB) or no improvement. Adverse effects of sedation, hyperactivity or any of the above were measured after 1 month. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | This study was a report of late follow up of the previous study so the methods were the same. | |
| Participants | Participants were the same as above. | |
| Interventions | Interventions were the same as above. | |
| Outcomes | A late outcome of effusion or no effusion was measured by ears at 1 year. Improvement in hearing and school performance were also measured at 1 year. The complication of recurrence of OME at 1 year was measured. | |
| Notes | Late follow‐up of O'Shea 1980. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | Quality score: 2/5 (Randomization: 1; Blinding: 1; Withdrawals: 0). Intention‐to‐treat analysis: unable to determine. Follow up was 67%. | |
| Participants | 78 children over 6 months of age were recruited from a community based paediatric practice in upstate New York after a recent diagnosis of AOM treated with antibiotics and decongestant. Diagnosis was based on tympanometry. Exclusion criterion was presence of ear grommets (ventilation tubes). A history of previous OME and of allergies was recorded and used to generate outcomes. | |
| Interventions | Decongestant (pseudoephedrine) versus placebo. | |
| Outcomes | No individual patient data were given for our primary outcome. The authors stated that in all comparisons measured, patients who received oral decongestant consistently did worse than those on placebo although the difference did not always reach statistical significance. | |
| Notes | See outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | Quality score: 3/5 (Randomization: 1; Blinding 1+1; Withdrawals: 0). Intention‐to‐treat analysis: no Follow up was 68%. | |
| Participants | 21 children aged 1‐12 were recruited from an ENT clinic. A history of allergies was noted. Diagnosis was based on audiometry and pneumatic otoscopy. Exclusion criteria included: no AOM within 2 weeks of OME and normal hearing prior to OME. | |
| Interventions | Antihistamine/decongestant (brompheniramine/phenylpropanolamine) versus placebo. | |
| Outcomes | The primary outcome of effusion or not at 4 weeks was measured as was hearing. The surgical complication of tympanostomy was measured and side effects were assessed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| ALLOCATION: PARTICIPANTS: | |
| ALLOCATION: | |
| ALLOCATION: | |
| ALLOCATION: PARTICIPANTS: | |
| ALLOCATION: | |
| ALLOCATION: | |
| ALLOCATION: PARTICIPANTS: INTERVENTION: OUTCOME: | |
| ALLOCATION: | |
| ALLOCATION: | |
| ALLOCATION: | |
| ALLOCATION: | |
| ALLOCATION: | |
| ALLOCATION: PARTICIPANTS: | |
| ALLOCATION: | |
| ALLOCATION: | |
| ALLOCATION: | |
| ALLOCATION: PARTICIPANTS: | |
| ALLOCATION: PARTICIPANTS: | |
| ALLOCATION: | |
| ALLOCATION: PARTICIPANTS: INTERVENTION: | |
| ALLOCATION: | |
| ALLOCATION: PARTICIPANTS: | |
| ALLOCATION: PARTICIPANTS: INTERVENTION: | |
| ALLOCATION: PARTICIPANTS: INTERVENTION: OUTCOME: |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: cure or no cure at 1‐3 months Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.36] |
| Analysis 1.1 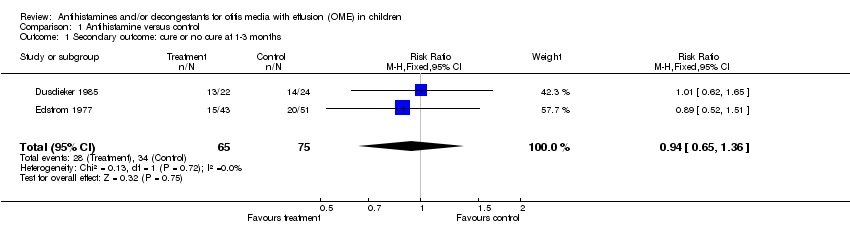 Comparison 1 Antihistamine versus control, Outcome 1 Secondary outcome: cure or no cure at 1‐3 months. | ||||
| 2 Complication: AOM Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.73] |
| Analysis 1.2  Comparison 1 Antihistamine versus control, Outcome 2 Complication: AOM. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: cure or no cure at or before 1 month Show forest plot | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.22] |
| Analysis 2.1 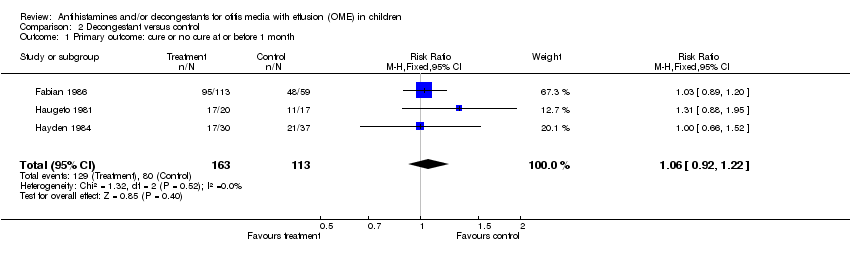 Comparison 2 Decongestant versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month. | ||||
| 2 Secondary outcome: cure or no cure at 1‐3 months Show forest plot | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.85, 1.30] |
| Analysis 2.2 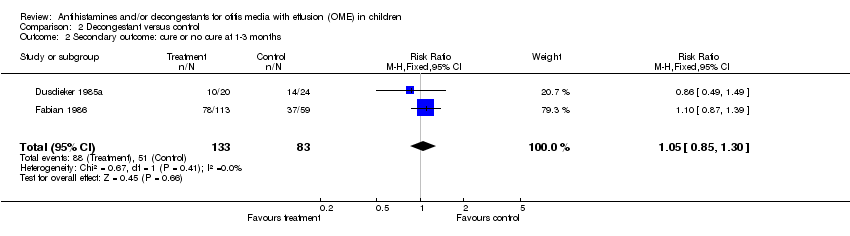 Comparison 2 Decongestant versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months. | ||||
| 3 Side effect: any significant side effects at or before one month Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.05 [0.66, 185.38] |
| Analysis 2.3  Comparison 2 Decongestant versus control, Outcome 3 Side effect: any significant side effects at or before one month. | ||||
| 4 Outcome: hearing on or about 1 month Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| Analysis 2.4  Comparison 2 Decongestant versus control, Outcome 4 Outcome: hearing on or about 1 month. | ||||
| 5 Outcome: surgery (tympanostomy (myringotomy)) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.62] |
| Analysis 2.5  Comparison 2 Decongestant versus control, Outcome 5 Outcome: surgery (tympanostomy (myringotomy)). | ||||
| 6 Complication: AOM Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.31] |
| Analysis 2.6  Comparison 2 Decongestant versus control, Outcome 6 Complication: AOM. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: cure or no cure at or before 1 month Show forest plot | 4 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.04] |
| Analysis 3.1 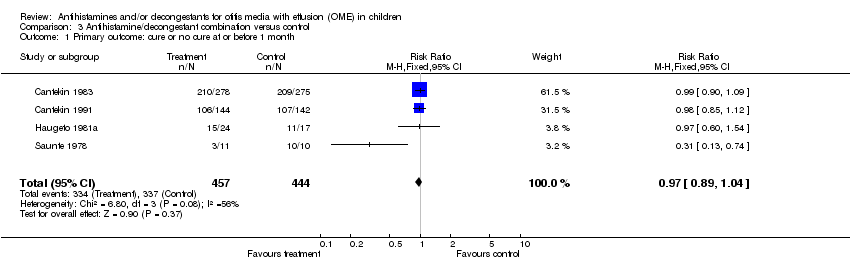 Comparison 3 Antihistamine/decongestant combination versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month. | ||||
| 2 Secondary outcome: cure or no cure at 1‐3 months Show forest plot | 3 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.40] |
| Analysis 3.2 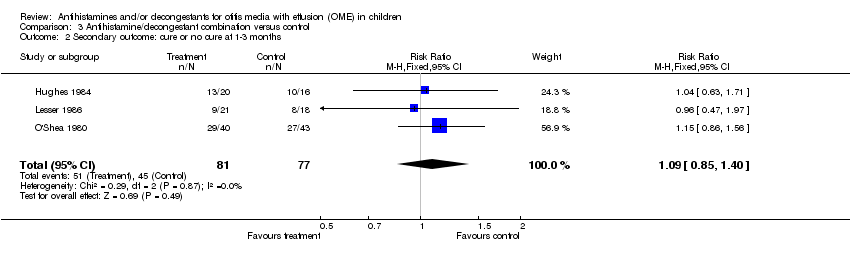 Comparison 3 Antihistamine/decongestant combination versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months. | ||||
| 3 Late outcome: cure or no cure after 3 months Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.72, 2.13] |
| Analysis 3.3  Comparison 3 Antihistamine/decongestant combination versus control, Outcome 3 Late outcome: cure or no cure after 3 months. | ||||
| 4 Side effect: any significant side effects at or before one month Show forest plot | 5 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.76, 3.67] |
| Analysis 3.4 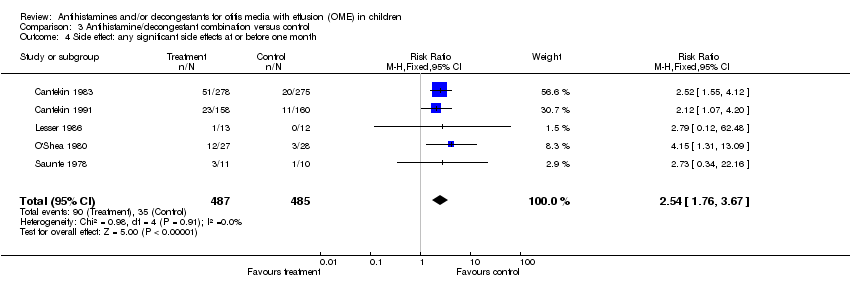 Comparison 3 Antihistamine/decongestant combination versus control, Outcome 4 Side effect: any significant side effects at or before one month. | ||||
| 5 Outcome: hearing at or less than 3 months Show forest plot | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.27] |
| Analysis 3.5 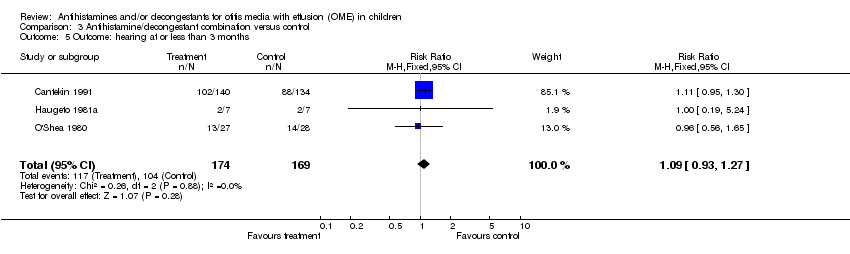 Comparison 3 Antihistamine/decongestant combination versus control, Outcome 5 Outcome: hearing at or less than 3 months. | ||||
| 6 Late outcome: hearing at 1 year Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.63, 3.56] |
| Analysis 3.6 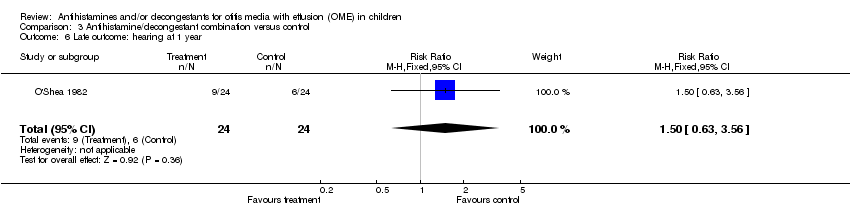 Comparison 3 Antihistamine/decongestant combination versus control, Outcome 6 Late outcome: hearing at 1 year. | ||||
| 7 Late outcome: school performance at 1 year Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.35, 1.86] |
| Analysis 3.7  Comparison 3 Antihistamine/decongestant combination versus control, Outcome 7 Late outcome: school performance at 1 year. | ||||
| 8 Outcome: surgery (tympanostomy (myringotomy)) Show forest plot | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.41] |
| Analysis 3.8  Comparison 3 Antihistamine/decongestant combination versus control, Outcome 8 Outcome: surgery (tympanostomy (myringotomy)). | ||||
| 9 Complication: recurrent OME Show forest plot | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.92, 1.83] |
| Analysis 3.9 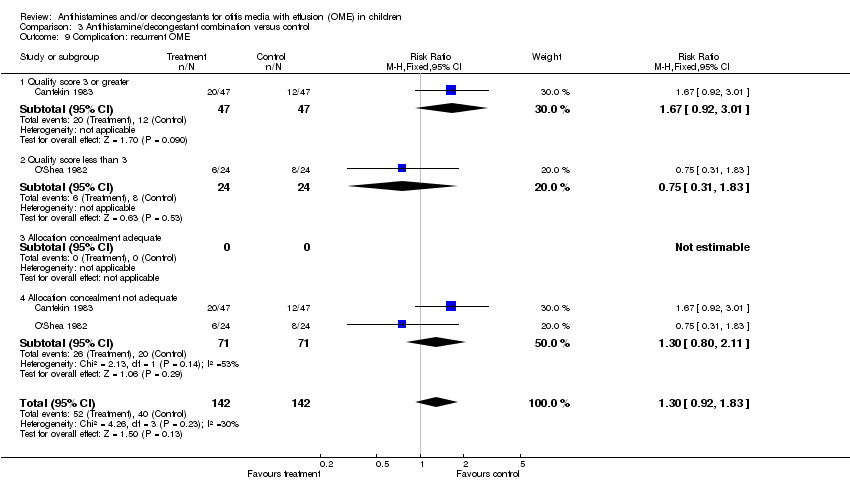 Comparison 3 Antihistamine/decongestant combination versus control, Outcome 9 Complication: recurrent OME. | ||||
| 9.1 Quality score 3 or greater | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.92, 3.01] |
| 9.2 Quality score less than 3 | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.31, 1.83] |
| 9.3 Allocation concealment adequate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Allocation concealment not adequate | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.80, 2.11] |
| 10 Complication: AOM Show forest plot | 1 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.46, 1.26] |
| Analysis 3.10 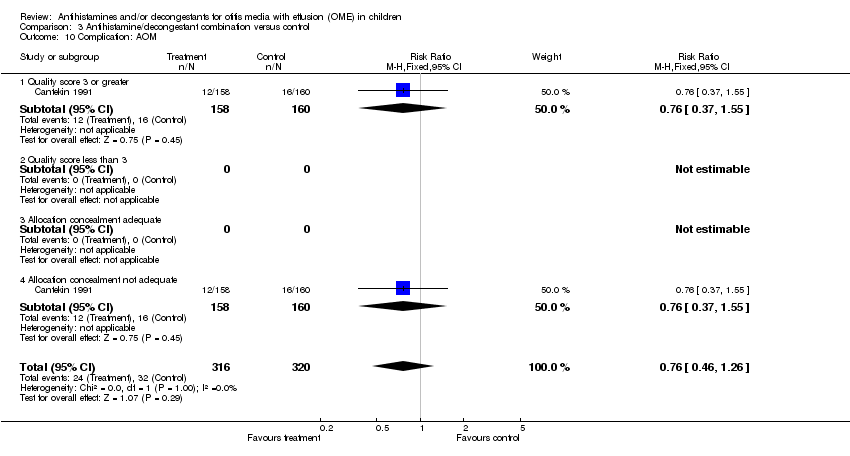 Comparison 3 Antihistamine/decongestant combination versus control, Outcome 10 Complication: AOM. | ||||
| 10.1 Quality score 3 or greater | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| 10.2 Quality score less than 3 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Allocation concealment adequate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Allocation concealment not adequate | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: cure or no cure at or before 1 month Show forest plot | 7 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.05] |
| Analysis 4.1  Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month. | ||||
| 2 Secondary outcome: cure or no cure at 1‐3 months Show forest plot | 7 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| Analysis 4.2  Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months. | ||||
| 3 Late outcome: cure or no cure after 3 months Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.72, 2.13] |
| Analysis 4.3 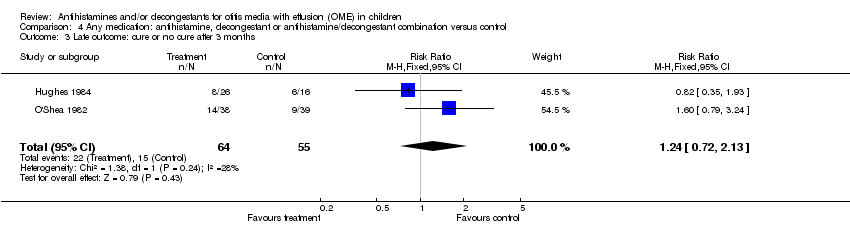 Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 3 Late outcome: cure or no cure after 3 months. | ||||
| 4 Side effect: any significant side effects at or before 1 month Show forest plot | 6 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.87, 3.88] |
| Analysis 4.4  Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 4 Side effect: any significant side effects at or before 1 month. | ||||
| 5 Outcome: hearing on or about 1 month Show forest plot | 4 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.27] |
| Analysis 4.5  Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 5 Outcome: hearing on or about 1 month. | ||||
| 6 Late outcome: hearing at 1 year Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.63, 3.56] |
| Analysis 4.6  Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 6 Late outcome: hearing at 1 year. | ||||
| 7 Late outcome: school performance at 1 year Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.35, 1.86] |
| Analysis 4.7  Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 7 Late outcome: school performance at 1 year. | ||||
| 8 Outcome: surgery (tympanostomy (myringotomy)) Show forest plot | 3 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.69, 1.32] |
| Analysis 4.8  Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 8 Outcome: surgery (tympanostomy (myringotomy)). | ||||
| 9 Complication: recurrent OME Show forest plot | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.80, 2.11] |
| Analysis 4.9 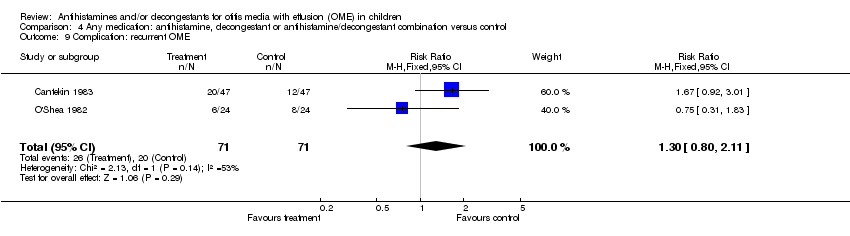 Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 9 Complication: recurrent OME. | ||||
| 10 Complication: AOM Show forest plot | 3 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.14] |
| Analysis 4.10 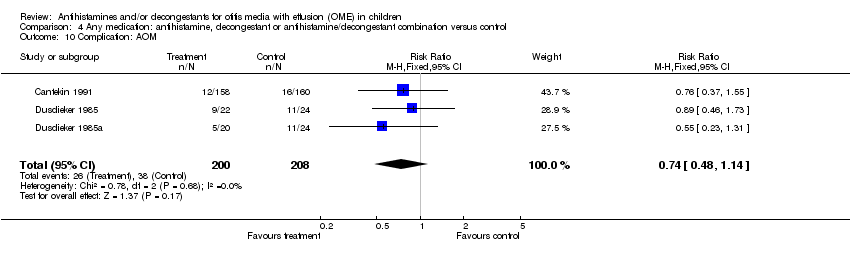 Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 10 Complication: AOM. | ||||

Comparison 1 Antihistamine versus control, Outcome 1 Secondary outcome: cure or no cure at 1‐3 months.

Comparison 1 Antihistamine versus control, Outcome 2 Complication: AOM.

Comparison 2 Decongestant versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month.

Comparison 2 Decongestant versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months.

Comparison 2 Decongestant versus control, Outcome 3 Side effect: any significant side effects at or before one month.

Comparison 2 Decongestant versus control, Outcome 4 Outcome: hearing on or about 1 month.

Comparison 2 Decongestant versus control, Outcome 5 Outcome: surgery (tympanostomy (myringotomy)).

Comparison 2 Decongestant versus control, Outcome 6 Complication: AOM.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 3 Late outcome: cure or no cure after 3 months.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 4 Side effect: any significant side effects at or before one month.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 5 Outcome: hearing at or less than 3 months.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 6 Late outcome: hearing at 1 year.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 7 Late outcome: school performance at 1 year.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 8 Outcome: surgery (tympanostomy (myringotomy)).

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 9 Complication: recurrent OME.

Comparison 3 Antihistamine/decongestant combination versus control, Outcome 10 Complication: AOM.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 1 Primary outcome: cure or no cure at or before 1 month.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 2 Secondary outcome: cure or no cure at 1‐3 months.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 3 Late outcome: cure or no cure after 3 months.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 4 Side effect: any significant side effects at or before 1 month.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 5 Outcome: hearing on or about 1 month.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 6 Late outcome: hearing at 1 year.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 7 Late outcome: school performance at 1 year.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 8 Outcome: surgery (tympanostomy (myringotomy)).

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 9 Complication: recurrent OME.

Comparison 4 Any medication: antihistamine, decongestant or antihistamine/decongestant combination versus control, Outcome 10 Complication: AOM.
| Filter |
| 40. limit 39 to (clinical trial or clinical trial, phase i or clinical trial, phase ii or clinical trial, phase iii or clinical trial, phase iv or controlled clinical trial or meta analysis or multicenter study or randomized controlled trial) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Secondary outcome: cure or no cure at 1‐3 months Show forest plot | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.65, 1.36] |
| 2 Complication: AOM Show forest plot | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.46, 1.73] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: cure or no cure at or before 1 month Show forest plot | 3 | 276 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.92, 1.22] |
| 2 Secondary outcome: cure or no cure at 1‐3 months Show forest plot | 2 | 216 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.85, 1.30] |
| 3 Side effect: any significant side effects at or before one month Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.05 [0.66, 185.38] |
| 4 Outcome: hearing on or about 1 month Show forest plot | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.16, 4.68] |
| 5 Outcome: surgery (tympanostomy (myringotomy)) Show forest plot | 1 | 172 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.71, 1.62] |
| 6 Complication: AOM Show forest plot | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.23, 1.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: cure or no cure at or before 1 month Show forest plot | 4 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.89, 1.04] |
| 2 Secondary outcome: cure or no cure at 1‐3 months Show forest plot | 3 | 158 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.85, 1.40] |
| 3 Late outcome: cure or no cure after 3 months Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.72, 2.13] |
| 4 Side effect: any significant side effects at or before one month Show forest plot | 5 | 972 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.54 [1.76, 3.67] |
| 5 Outcome: hearing at or less than 3 months Show forest plot | 3 | 343 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.93, 1.27] |
| 6 Late outcome: hearing at 1 year Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.63, 3.56] |
| 7 Late outcome: school performance at 1 year Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.35, 1.86] |
| 8 Outcome: surgery (tympanostomy (myringotomy)) Show forest plot | 2 | 57 | Risk Ratio (M‐H, Random, 95% CI) | 0.54 [0.09, 3.41] |
| 9 Complication: recurrent OME Show forest plot | 2 | 284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.92, 1.83] |
| 9.1 Quality score 3 or greater | 1 | 94 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.92, 3.01] |
| 9.2 Quality score less than 3 | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.31, 1.83] |
| 9.3 Allocation concealment adequate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.4 Allocation concealment not adequate | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.80, 2.11] |
| 10 Complication: AOM Show forest plot | 1 | 636 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.46, 1.26] |
| 10.1 Quality score 3 or greater | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| 10.2 Quality score less than 3 | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Allocation concealment adequate | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.4 Allocation concealment not adequate | 1 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.37, 1.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary outcome: cure or no cure at or before 1 month Show forest plot | 7 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.92, 1.05] |
| 2 Secondary outcome: cure or no cure at 1‐3 months Show forest plot | 7 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.89, 1.21] |
| 3 Late outcome: cure or no cure after 3 months Show forest plot | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.72, 2.13] |
| 4 Side effect: any significant side effects at or before 1 month Show forest plot | 6 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [1.87, 3.88] |
| 5 Outcome: hearing on or about 1 month Show forest plot | 4 | 358 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.93, 1.27] |
| 6 Late outcome: hearing at 1 year Show forest plot | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.63, 3.56] |
| 7 Late outcome: school performance at 1 year Show forest plot | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.35, 1.86] |
| 8 Outcome: surgery (tympanostomy (myringotomy)) Show forest plot | 3 | 229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.69, 1.32] |
| 9 Complication: recurrent OME Show forest plot | 2 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.3 [0.80, 2.11] |
| 10 Complication: AOM Show forest plot | 3 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.48, 1.14] |

