Regímenes cíclicos para la transferencia de embriones congelados‐descongelados
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register search strategy
Gynaecology and Fertility database search from inception to 13 December 2016
PROCITE platform
Keywords CONTAINS "cryopreservation" or "frozen embryo transfer" or "frozen embryos" or "frozen‐thawed cycle" or "frozen‐thawed embryo transfer" or "frozen‐thawed embryos" or "FET" or "cryopreserved embryos" or "cryopreserved‐thawed embryos" or "embryo vitrification" or "vitrification" or "vitrified" or "vitrified‐warmed embryos" or "frozen‐thawed" or "embryo vitrification" or Title CONTAINS "cryopreservation" or "frozen embryo transfer" or "frozen embryos" or "frozen‐thawed cycle" or "frozen‐thawed embryo transfer" or "frozen‐thawed embryos" or "FET" or "cryopreserved embryos" or "cryopreserved‐thawed embryos" or "embryo vitrification" or "vitrification" or "vitrified" or "vitrified‐warmed embryos" or "embryo vilification"
AND
Keywords CONTAINS "ovulation induction" or "endometrial preparation" or "*Clomiphene" or "clomiphene citrate" or "menotrophin" or "menotropin" or "HMG" or "human menopausal gonadotrophin" or "gonadotropin‐releasing hormone" or "gonadotropin releasing hormone agonist" or "gonadotrophin stimulation" or "Gonadotrophin releasing hormones" or "Gonadorelin" or "GnRh" or "GnRHa" or "GnRH a" or "GnRH agonist"or "GnRH agonists" or "GnRHa‐gonadotropin" or "rFSH" or "Fsh" or "FSH HMG" or "follicle stimulating hormone" or "follitropin" or "natural cycle" or "natural cycles" or "artificial cycle" or" modified natural cycle" or" estrogen" or "Estrogens" or "Progesterone" or "Estradiol" or "hormone therapy" or "hormone therapy estrogen" or "hormone replacement therapy" or "letrozole" or "tamoxifen" or "stimulated cycle" or "stimulation of endometrium embryo transfer" or "stimulation protocol" or Title CONTAINS "natural cycle"or "natural cycles" or "artificial cycle" or "endometrial preparation" or "*Clomiphene" or "modified natural cycle" (155 hits)
Appendix 2. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 13th December 2016
Web platform
1 Cryopreservation/ (253)
2 (Cryopreserv$ adj7 embryo$).tw. (176)
3 (Cryopreserv$ adj7 blastocyst$).tw. (32)
4 freezing/ or vitrification/ (95)
5 (vitrification adj7 embryo$).tw. (45)
6 (vitrification adj7 blastocyst$).tw. (34)
7 (frozen adj5 embryo$).tw. (196)
8 (freez$ adj5 embryo$).tw. (55)
9 (freez$ adj5 blastocyst$).tw. (7)
10 (frozen adj5 blastocyst$).tw. (25)
11 FET.tw. (94)
12 (Cryo‐preserv$ adj7 embryo$).tw. (0)
13 (Cryo‐preserv$ adj7 blastocyst$).tw. (0)
14 or/1‐13 (728)
15 exp Ovulation Induction/ (1030)
16 ((ovar$ adj5 stimula$) or (ovulat$ adj5 induct$)).tw. (1683)
17 (endometri$ adj2 prepar$).tw. (89)
18 Clomiphene.tw. or Clomiphene/ (875)
19 clomid.tw. (26)
20 exp Menotropins/ (358)
21 (Menotropin$ or menopausal gonadotrop$ or HMG).tw. (1421)
22 exp Follicle Stimulating Hormone/ (1639)
23 (Follicle Stimulating Hormone or FSH).tw. (2873)
24 Gonadotropin‐Releasing Hormone/ad, ag, aa, de, pd, tu [Administration & Dosage, Agonists, Analogs & Derivatives, Drug Effects, Pharmacology, Therapeutic Use] (137)
25 Gonadotrop?in‐Releasing Hormone$.tw. (1194)
26 GnRH$.tw. (2051)
27 exp Estrogens/ (5949)
28 (?estrogen$ or ?estradiol).tw. (8709)
29 exp Progesterone/ or progesterone.tw. (4154)
30 (natural$ adj4 cycle$).tw. (157)
31 (artificial$ adj2 cycle$).tw. (34)
32 (cycle$ adj2 regimen$).tw. (266)
33 pituitary suppression.tw. (103)
34 human menopausal.tw. (401)
35 spontaneous ovulation.tw. (24)
36 HCG trigger$.tw. (52)
37 (stimulat$ adj3 cycle$).tw. (438)
38 (hormone$ adj2 replacement).tw. (2088)
39 (endometri$ adj2 stimulat$).tw. (87)
40 HRT.tw. (1234)
41 or/15‐40 (18545)
42 14 and 41 (208)
Appendix 3. MEDLINE search strategy
From inception to 13th December 2016
Ovid platform
1 Cryopreservation/ (21360)
2 (Cryopreserv$ adj7 embryo$).tw. (3130)
3 (Cryopreserv$ adj7 blastocyst$).tw. (445)
4 freezing/ or vitrification/ (23937)
5 (vitrifi$ adj5 embryo$).tw. (957)
6 (vitrifi$ adj5 blastocyst$).tw. (641)
7 (frozen adj5 embryo$).tw. (2517)
8 (freez$ adj5 embryo$).tw. (1110)
9 (freez$ adj5 blastocyst$).tw. (175)
10 (frozen adj5 blastocyst$).tw. (335)
11 FET.tw. (2142)
12 (Cryo‐preserv$ adj7 embryo$).tw. (10)
13 or/1‐12 (47595)
14 exp Ovulation Induction/ (11949)
15 ((ovar$ adj5 stimula$) or (ovulat$ adj5 induc$)).tw. (16099)
16 (endometri$ adj2 prepar$).tw. (460)
17 hormon$ regimen$.tw. (279)
18 Clomiphene.tw. or Clomiphene/ (6564)
19 clomid.tw. (175)
20 (Tamoxifen or Letrozole).tw. (23668)
21 aromatase inhibitor$.tw. (6705)
22 anti‐?estrogen$.tw. (2427)
23 exp Menotropins/ (3127)
24 (Menotropin$ or menopausal gonadotrop$ or HMG).tw. (15378)
25 exp Follicle Stimulating Hormone/ (37398)
26 (Follicle Stimulating Hormone or FSH or rFSH or rhFSH).tw. (39284)
27 Gonadotropin‐Releasing Hormone/ad, ag, aa, de, pd, tu [Administration & Dosage, Agonists, Analogs & Derivatives, Drug Effects, Pharmacology, Therapeutic Use] (15972)
28 Gonadotrop?in‐Releasing Hormone$.tw. (16126)
29 GnRH$.tw. (21800)
30 exp Estrogens/ (162202)
31 (?estrogen$ or ?estradiol).tw. (177832)
32 exp Progesterone/ or progesterone.tw. (108635)
33 exogenous steroid$.tw. (506)
34 (natural$ adj4 cycle$).tw. (2642)
35 (artificial$ adj3 cycle$).tw. (482)
36 (cycle$ adj2 regimen$).tw. (278)
37 pituitary suppression.tw. (320)
38 human menopausal.tw. (2109)
39 spontaneous ovulation.tw. (408)
40 (HCG adj3 trigger$).tw. (263)
41 hormone therapy.tw. (12827)
42 (stimulat$ adj3 cycle$).tw. (3805)
43 (hormone$ adj2 replacement).tw. (15866)
44 (endometri$ adj2 stimulat$).tw. (547)
45 (HRT or HT).tw. (70350)
46 or/14‐45 (464462)
47 randomized controlled trial.pt. (469833)
48 controlled clinical trial.pt. (95075)
49 randomized.ab. (405868)
50 randomised.ab. (81587)
51 placebo.tw. (197475)
52 clinical trials as topic.sh. (189503)
53 randomly.ab. (286433)
54 trial.ti. (179694)
55 (crossover or cross‐over or cross over).tw. (76212)
56 or/47‐55 (1211177)
57 exp animals/ not humans.sh. (4669484)
58 56 not 57 (1117076)
59 13 and 46 and 58 (262)
Appendix 4. Embase search strategy
From inception to 13th December 2016
Ovid platform
1 cryopreservation/ (33757)
2 (Cryopreserv$ adj7 embryo$).tw. (4445)
3 (Cryo‐preserv$ adj7 embryo$).tw. (33)
4 (Cryopreserv$ adj7 blastocyst$).tw. (826)
5 freezing/ or vitrification/ (35415)
6 (vitrifi$ adj5 embryo$).tw. (1716)
7 (vitrifi$ adj5 blastocyst$).tw. (1242)
8 (frozen adj5 embryo$).tw. (3976)
9 (freez$ adj5 embryo$).tw. (1588)
10 (freez$ adj5 blastocyst$).tw. (283)
11 (frozen adj5 blastocyst$).tw. (688)
12 FET.tw. (2754)
13 freeze thawing/ or freezing/ (36470)
14 vitrification/ (4402)
15 or/1‐14 (71558)
16 exp ovulation induction/ (13019)
17 ((ovar$ adj5 stimula$) or (ovulat$ adj5 induc$)).tw. (20432)
18 (endometri$ adj2 prepar$).tw. (701)
19 hormon$ regimen$.tw. (316)
20 Clomiphene.tw. or Clomiphene/ (9591)
21 clomid.tw. (1068)
22 (Tamoxifen or Letrozole).tw. (30044)
23 aromatase inhibitor$.tw. (9118)
24 exp human menopausal gonadotropin/ (9848)
25 (Menotropin$ or menopausal gonadotrop$ or HMG).tw. (17974)
26 exp follitropin/ (55231)
27 (Follicle Stimulating Hormone or FSH or rFSH or rhFSH).tw. (46559)
28 gonadorelin/ (35623)
29 Gonadotrop?in‐Releasing Hormone$.tw. (16598)
30 GnRH$.tw. (25145)
31 exp estrogen/ (255742)
32 (?estrogen$ or ?estradiol).tw. (194821)
33 exp progesterone/ (85120)
34 exp Progesterone/ or progesterone.tw. (117583)
35 (natural$ adj2 cycle$).tw. (2548)
36 (artificial$ adj2 cycle$).tw. (425)
37 (cycle$ adj2 regimen$).tw. (523)
38 pituitary suppression.tw. (409)
39 human menopausal.tw. (2323)
40 spontaneous ovulation.tw. (478)
41 (HCG adj3 trigger$).tw. (693)
42 (stimulat$ adj3 cycle$).tw. (4793)
43 exogenous steroid$.tw. (560)
44 exogenous steroid$.tw. (560)
45 (hormone adj2 therap$).tw. (33347)
46 (endometri$ adj2 stimulat$).tw. (682)
47 or/16‐46 (491845)
48 15 and 47 (5220)
49 Clinical Trial/ (1004097)
50 Randomized Controlled Trial/ (465768)
51 exp randomization/ (83937)
52 Single Blind Procedure/ (27791)
53 Double Blind Procedure/ (137638)
54 Crossover Procedure/ (54096)
55 Placebo/ (323380)
56 Randomi?ed controlled trial$.tw. (150645)
57 Rct.tw. (22599)
58 random allocation.tw. (1637)
59 randomly allocated.tw. (26733)
60 allocated randomly.tw. (2210)
61 (allocated adj2 random).tw. (844)
62 Single blind$.tw. (18743)
63 Double blind$.tw. (173480)
64 ((treble or triple) adj blind$).tw. (657)
65 placebo$.tw. (248420)
66 prospective study/ (389403)
67 or/49‐66 (1790257)
68 case study/ (93504)
69 case report.tw. (324719)
70 abstract report/ or letter/ (989413)
71 or/68‐70 (1398438)
72 67 not 71 (1739458)
73 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5736041)
74 72 not 73 (1678022)
75 48 and 74 (720)
Appendix 5. PsycINFO search strategy
From inception to 13th December 2016
Ovid platform
1 exp reproductive technology/ (1610)
2 (frozen adj5 embryo$).tw. (27)
3 ((frozen‐thawed or cryopreserv$) adj5 embryo$).tw. (28)
4 exp Embryo/ (1632)
5 FET.tw. (56)
6 ((embry$ adj5 transf$) or embryo replacement or embryo deposition).tw. (272)
7 or/1‐6 (3386)
8 exp Ovulation/ (346)
9 ((ovar$ adj5 stimula$) or (ovulat$ adj5 induct$)).tw. (172)
10 (endometri$ adj2 prepar$).tw. (1)
11 Clomiphene.tw. (46)
12 clomid.tw. (1)
13 (Menotropin$ or menopausal gonadotrop$ or HMG).tw. (205)
14 (Follicle Stimulating Hormone or FSH).tw. (659)
15 exp Gonadotropic Hormones/ (4043)
16 Gonadotropin‐Releasing Hormone$.tw. (689)
17 GnRH$.tw. (887)
18 exp Estrogens/ (6065)
19 Estrogen$.tw. (7196)
20 exp Progesterone/ (2010)
21 Progesterone.tw. (3831)
22 oestrogen$.tw. (690)
23 (natural$ adj2 cycle$).tw. (127)
24 (artificial$ adj2 cycle$).tw. (28)
25 (cycle$ adj2 regimen$).tw. (5)
26 or/8‐25 (15793)
27 7 and 26 (80)
28 random.tw. (48495)
29 control.tw. (375857)
30 double‐blind.tw. (20313)
31 clinical trials/ (10039)
32 placebo/ (4746)
33 exp Treatment/ (669890)
34 or/28‐33 (1034673)
35 27 and 34 (36)
Appendix 6. CINAHL search strategy
From inception to 13th December 2016
EBSCO platform
| # | Query | Results |
| S48 | S35 AND S47 | 30 |
| S47 | S36 OR S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 | 1,097,934 |
| S46 | TX allocat* random* | 5,898 |
| S45 | (MH "Quantitative Studies") | 15,110 |
| S44 | (MH "Placebos") | 9,934 |
| S43 | TX placebo* | 42,609 |
| S42 | TX random* allocat* | 5,898 |
| S41 | (MH "Random Assignment") | 42,016 |
| S40 | TX randomi* control* trial* | 115,809 |
| S39 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 866,076 |
| S38 | TX clinic* n1 trial* | 196,374 |
| S37 | PT Clinical trial | 79,958 |
| S36 | (MH "Clinical Trials+") | 207,314 |
| S35 | S12 AND S34 | 107 |
| S34 | S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 | 20,839 |
| S33 | TX(stimulat* N3 cycle*) | 229 |
| S32 | TX spontaneous* ovulat* | 23 |
| S31 | TX pituitary suppression | 79 |
| S30 | TX(cycle* N2 regimen*) | 91 |
| S29 | TX(artificial* N2 cycle*) | 8 |
| S28 | TX(natural* N2 cycle*) | 120 |
| S27 | TX Progesterone | 4,593 |
| S26 | (MM "Progesterone") | 1,105 |
| S25 | TX estrogen* or TX oestrogen* | 14,913 |
| S24 | (MM "Estrogens") | 2,993 |
| S23 | TX GnRH* | 499 |
| S22 | TX Gonadotrop?in‐Releasing Hormone* | 90 |
| S21 | (MM "Gonadorelin") OR (MM "Pituitary Hormone Release Inhibiting Hormones") | 479 |
| S20 | TX(Follicle Stimulating Hormone or FSH) | 1,763 |
| S19 | (MM "Follicle‐Stimulating Hormone") | 262 |
| S18 | TX(Menotropin* or menopausal gonadotrop* or HMG) | 722 |
| S17 | TX Clomiphene or TX clomid | 360 |
| S16 | (MM "Clomiphene") | 121 |
| S15 | TX(endometri* N2 prepar*) | 18 |
| S14 | TX((ovar* N5 stimula*) or (ovulat* N5 induct*)) | 877 |
| S13 | (MM "Ovulation Induction") | 260 |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 | 1,100 |
| S11 | TX FET | 101 |
| S10 | TX(frozen N5 blastocyst*) | 5 |
| S9 | TX(freez* N5 blastocyst*) | 0 |
| S8 | TX(freez* N5 embryo*) | 51 |
| S7 | TX(frozen N5 embryo*) | 129 |
| S6 | TX(vitrification N7 blastocyst*) | 8 |
| S5 | TX(vitrification N7 embryo*) | 18 |
| S4 | (MM "Freezing") | 136 |
| S3 | TX(Cryopreserv* N7 blastocyst*) | 12 |
| S2 | TX(Cryopreserv* N7 embryo*) | 170 |
| S1 | (MM "Cryopreservation+") | 668 |
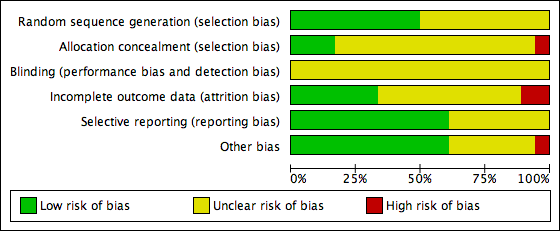
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Comparison 1 Natural cycle FET versus HT FET, Outcome 1 Clinical pregnancy rate per woman.
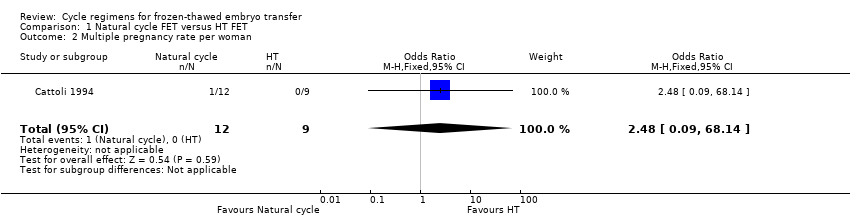
Comparison 1 Natural cycle FET versus HT FET, Outcome 2 Multiple pregnancy rate per woman.
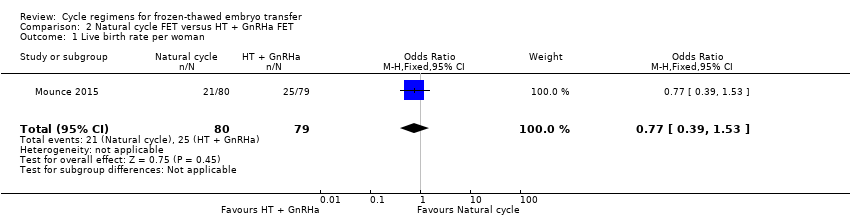
Comparison 2 Natural cycle FET versus HT + GnRHa FET, Outcome 1 Live birth rate per woman.

Comparison 2 Natural cycle FET versus HT + GnRHa FET, Outcome 2 Clinical pregnancy rate per woman.
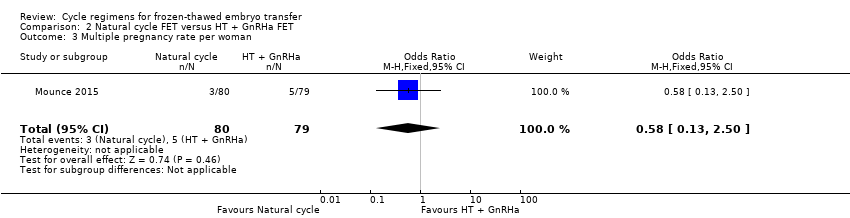
Comparison 2 Natural cycle FET versus HT + GnRHa FET, Outcome 3 Multiple pregnancy rate per woman.

Comparison 3 Natural cycle FET versus modified natural cycle FET (HCG trigger), Outcome 1 Live birth rate per woman.

Comparison 3 Natural cycle FET versus modified natural cycle FET (HCG trigger), Outcome 2 Miscarriage rate per woman.

Comparison 3 Natural cycle FET versus modified natural cycle FET (HCG trigger), Outcome 3 Ongoing pregnancy rate per woman.

Comparison 3 Natural cycle FET versus modified natural cycle FET (HCG trigger), Outcome 4 Clinical pregnancy rate per woman.

Comparison 4 Modified natural cycle FET (HCG trigger) versus HT FET, Outcome 1 Live birth rate per woman.

Comparison 4 Modified natural cycle FET (HCG trigger) versus HT FET, Outcome 2 Ongoing pregnancy rate per woman.
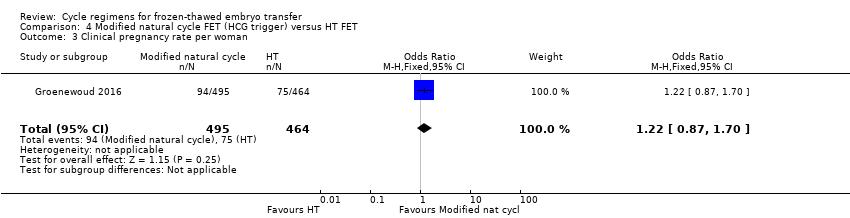
Comparison 4 Modified natural cycle FET (HCG trigger) versus HT FET, Outcome 3 Clinical pregnancy rate per woman.

Comparison 4 Modified natural cycle FET (HCG trigger) versus HT FET, Outcome 4 Cycle cancellation rate per woman.

Comparison 4 Modified natural cycle FET (HCG trigger) versus HT FET, Outcome 5 Endometrial thickness.
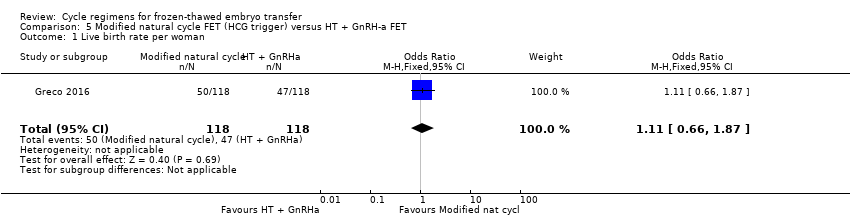
Comparison 5 Modified natural cycle FET (HCG trigger) versus HT + GnRH‐a FET, Outcome 1 Live birth rate per woman.

Comparison 5 Modified natural cycle FET (HCG trigger) versus HT + GnRH‐a FET, Outcome 2 Miscarriage rate per woman.
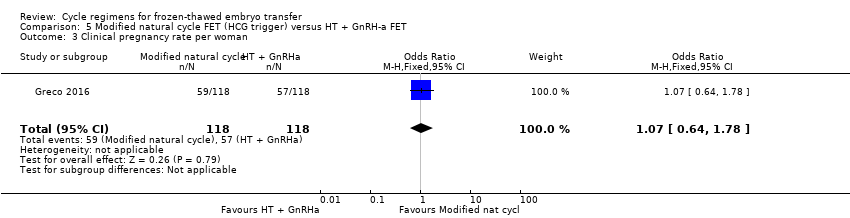
Comparison 5 Modified natural cycle FET (HCG trigger) versus HT + GnRH‐a FET, Outcome 3 Clinical pregnancy rate per woman.
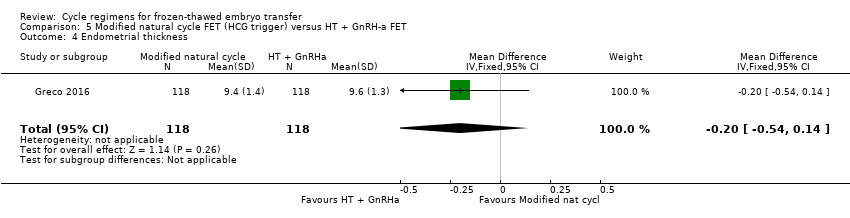
Comparison 5 Modified natural cycle FET (HCG trigger) versus HT + GnRH‐a FET, Outcome 4 Endometrial thickness.

Comparison 6 HT FET versus HT + GnRH‐a, Outcome 1 Live birth rate per woman.
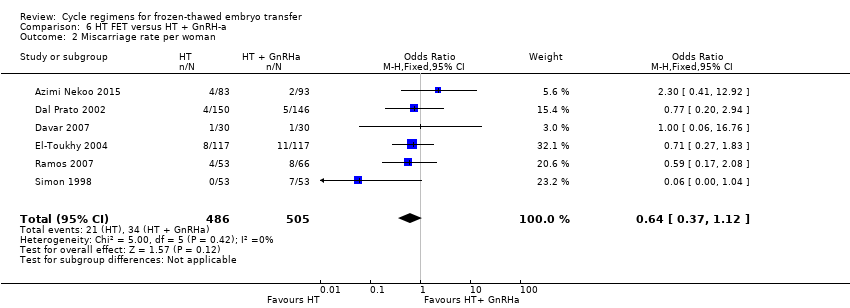
Comparison 6 HT FET versus HT + GnRH‐a, Outcome 2 Miscarriage rate per woman.
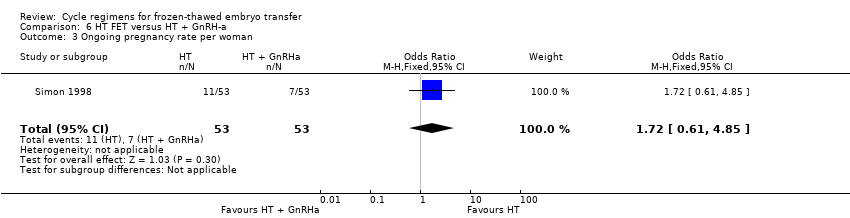
Comparison 6 HT FET versus HT + GnRH‐a, Outcome 3 Ongoing pregnancy rate per woman.
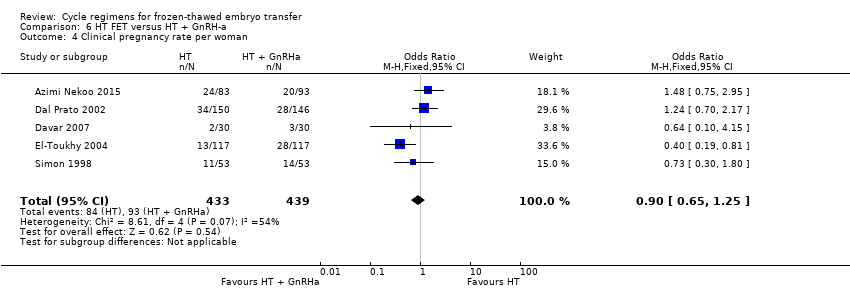
Comparison 6 HT FET versus HT + GnRH‐a, Outcome 4 Clinical pregnancy rate per woman.

Comparison 6 HT FET versus HT + GnRH‐a, Outcome 5 Cycle cancellation rate per woman.

Comparison 6 HT FET versus HT + GnRH‐a, Outcome 6 Endometrial thickness.

Comparison 7 HT FET versus FSH FET, Outcome 1 Clinical pregnancy rate per woman.
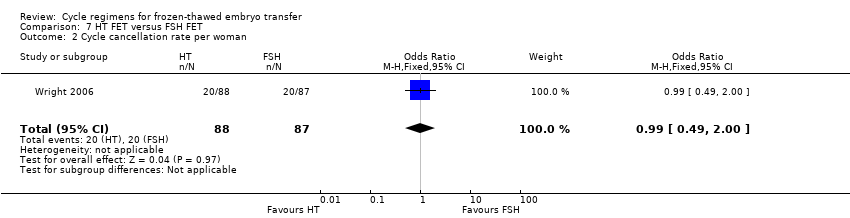
Comparison 7 HT FET versus FSH FET, Outcome 2 Cycle cancellation rate per woman.
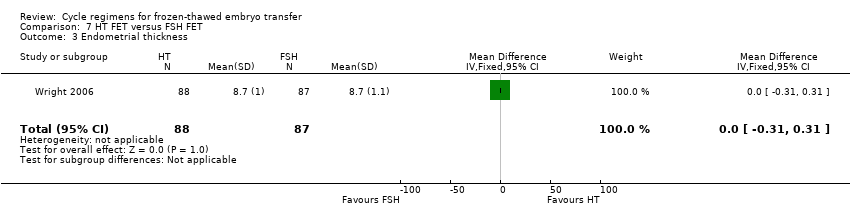
Comparison 7 HT FET versus FSH FET, Outcome 3 Endometrial thickness.
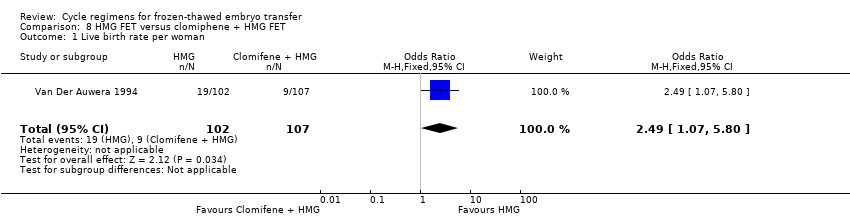
Comparison 8 HMG FET versus clomiphene + HMG FET, Outcome 1 Live birth rate per woman.
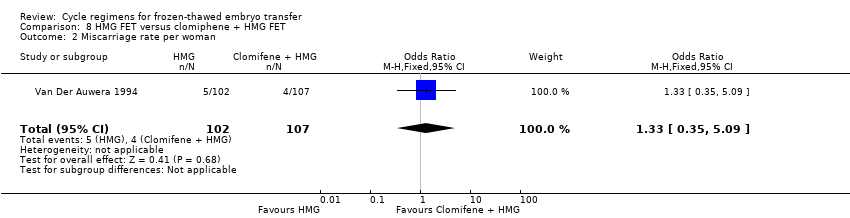
Comparison 8 HMG FET versus clomiphene + HMG FET, Outcome 2 Miscarriage rate per woman.

Comparison 8 HMG FET versus clomiphene + HMG FET, Outcome 3 Multiple pregnancy rate per woman.
| Natural cycle FET versus HT FET | ||||||
| Population: subfertile women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| HT FET | Natural cycle FET | |||||
| Live birth rate per woman | No data available | Not estimable | ‐ | |||
| Miscarriage rate per woman | No data available | Not estimable | ‐ | |||
| Ongoing pregnancy rate per woman | No data available | Not estimable | ‐ | |||
| Multiple pregnancy rate per woman | See comment | OR 2.48 | 21 | ⊕⊝⊝⊝ | No events in the control group | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for serious risk of bias: study at unclear risk of bias in all domains. | ||||||
| Natural cycle FET versus HT + GnRHa suppression FET | ||||||
| Population: subfertile women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| HT + GnRHa FET | Natural cycle FET | |||||
| Live birth rate per woman | 316 per 1000 | 262 per 1000 | OR 0.77 | 159 | ⊕⊕⊝⊝ | Only 46 events |
| Miscarriage rate per woman | No data available | Not estimable | ‐ | |||
| Ongoing pregnancy rate per woman | No data available | Not estimable | ‐ | |||
| Multiple pregnancy rate per woman | 63 per 1000 | 38 per 1000 | OR 0.58 | 159 | ⊕⊕⊝⊝ | Only 8 events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded two levels due to very serious imprecision: single study, few events, confidence interval compatible with benefit in either group or with no effect. | ||||||
| Natural cycle FET versus other regimens for primary or secondary subfertility | ||||||
| Population: subfertile women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Modified natural cycle FET (HCG trigger) | Natural cycle FET | |||||
| Live birth rate per woman | 267 per 1000 | 167 per 1000 | OR 0.55 | 60 | ⊕⊝⊝⊝ | Only 13 events |
| Miscarriage rate per woman | 24 per 1000 | 5 per 1000 | OR 0.20 | 168 | ⊕⊝⊝⊝ | Only 2 events |
| Ongoing pregnancy rate per woman | 107 per 1000 | 226 per 1000 | OR 2.44 | 168 | ⊕⊝⊝⊝ | Only 28 events |
| Multiple pregnancy per woman | No data available | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1One other study compared natural cycle FET versus natural cycle plus human menopausal gonadotrophin, but did not report any per‐woman data. | ||||||
| Modified natural cycle FET (HCG trigger) versus HT FET | ||||||
| Population: subfertile women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| HT FET | Modified natural cycle FET (HCG trigger) | |||||
| Live birth rate per woman | 88 per 1000 | 114 per 1000 | OR 1.34 | 959 | ⊕⊕⊝⊝ | |
| Miscarriage rate per woman | No data available | Not estimable | ‐ | |||
| Ongoing pregnancy rate per woman | 97 per 1000 | 115 per 1000 | OR 1.21 | 959 | ⊕⊕⊝⊝ | |
| Multiple pregnancy rate per woman | No data available | Not estimable | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to serious risk of bias: high attrition rate, unclear risk of allocation concealment | ||||||
| Modified natural cycle FET (HCG trigger) versus HT + GnRHa FET | ||||||
| Population: subfertile women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| HT + GnRHa FET | Modified natural cycle FET (HCG trigger) | |||||
| Live birth rate per woman | 398 per 1000 | 423 per 1000 | OR 1.11 | 236 | ⊕⊕⊝⊝ | |
| Miscarriage rate per woman | 68 per 1000 | 51 per 1000 | OR 0.74 | 236 | ⊕⊕⊝⊝ | |
| Ongoing pregnancy rate | No data available | Not estimable | ‐ | |||
| Multiple pregnancy rate per woman | No data available | Not estimable | ‐ | |||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to serious risk of bias: study at unclear risk of in most domains of bias (allocation concealment, blinding, selective reporting and other sources of bias). | ||||||
| HT FET versus other regimens for primary or secondary subfertility | ||||||
| Population: women with primary or secondary subfertility | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| HT + GnRHa FET | HT FET | |||||
| Live birth rate per woman | 742 per 1000 | 223 per 1000 | OR 0.10 | 75 | ⊕⊕⊝⊝ | Only 33 events |
| Miscarriage rate per woman | 48 per 1000 | 31 per 1000 | OR 0.64 | 991 | ⊕⊕⊝⊝ | ‐ |
| Ongoing pregnancy rate per woman | 132 per 1000 | 207 per 1000 | OR 1.72 | 106 | ⊕⊝⊝⊝ | Only 18 events |
| Multiple pregnancy rate per woman | No data available | |||||
| *The basis for the assumed risk is the mean control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level due to serious imprecision: single study, few events. | ||||||
| HMG FET versus clomiphene + HMG FET | ||||||
| Population: subfertile women | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Clomiphene+ HMG FET | HMG FET | |||||
| Live birth rate per woman | 84 per 1000 | 186 per 1000 | OR 2.49 | 209 | ⊕⊝⊝⊝ | Only 26 events |
| Miscarriage rate per woman | 37 per 1000 | 49 per 1000 | OR 1.33 | 209 | ⊕⊝⊝⊝ | Only 9 events |
| Ongoing pregnancy rate per woman | No data available | Not estimable | ‐ | |||
| Multiple pregnancy rate per woman | 28 per 1000 | 39 per 1000 | OR 1.41 | 209 | ⊕⊝⊝⊝ | Only 7 events |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded one level for serious risk of bias: study at unclear risk of bias in all domains. | ||||||
| Study | Intervention (number of embryo transfer) | Control (number of embryo transfer) | Live birth rate | P value |
| Natural cycle FET (n = 332) | HMG FET (n = 340) | 32/332 vs 45/340 | n/s | |
| FET: frozen‐thawed embryo transfer; HMG: human menopausal gonadotrophin; n/s: not significant. | ||||
| Study | Intervention (number of embryo transfer) | Control (number of embryo transfer) | Miscarriage rate | P value |
| Natural cycle FET | HT FET | 41.7% vs 22.2% | n/s | |
| FET: frozen‐thawed embryo transfer; HT: hormone therapy; n/s: not significant. | ||||
| Study | Intervention (number of embryo transfer) | Control (number of embryo transfer) | Ongoing pregnancy rate | P value |
| Natural cycle FET | HT FET | 24.1% vs 21.9% | n/s | |
| FET: frozen‐thawed embryo transfer; HT: hormone therapy; n/s: not significant. | ||||
| Study | Intervention (number of cycles) | Control (number of cycles) | Clinical pregnancy rate | P value |
| Clomiphene‐induced ovulation (n = 35) | HT (n = 52) | 3/35 vs 5/52 | n/s | |
| Clomiphene‐induced ovulation (n = 32) | HT plus GnRHa trigger (n = 37) | 2/32 vs 6/37 | n/s | |
| GnRHa: gonadotrophin releasing hormone agonist; HT: hormone therapy; n/s: not significant. | ||||
| Study | Intervention (number of embryo transfer) | Control (number of embryo transfer) | Clinical pregnancy rate | P value |
| Natural cycle FET | HT FET | 27.6% vs 25% | n/s | |
| FET: frozen‐thawed embryo transfer; HT: hormone therapy; n/s: not significant. | ||||
| Study | Intervention (number of cycles/embryo transfer) | Control (number of cycles/embryo transfer) | Endometrial thickness | P value |
| Clomiphene‐induced ovulation (n = 67) | HT alone or HT plus GnRHa suppression (n = 37) | 9.7 vs 9.8 | n/s | |
| Natural cycle FET (n = 332) | HMG FET (n = 340) | 8.9 vs 8.9 | n/s | |
| FET: frozen‐thawed embryo transfer; GnRHa: gonadotrophin releasing hormone agonist; HMG: human menopausal gonadotrophin; HT: hormone therapy; n/s: not significant. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical pregnancy rate per woman Show forest plot | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.40, 2.80] |
| 2 Multiple pregnancy rate per woman Show forest plot | 1 | 21 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.09, 68.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman Show forest plot | 1 | 159 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.39, 1.53] |
| 2 Clinical pregnancy rate per woman Show forest plot | 1 | 159 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.45, 1.71] |
| 3 Multiple pregnancy rate per woman Show forest plot | 1 | 159 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.13, 2.50] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman Show forest plot | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.16, 1.93] |
| 2 Miscarriage rate per woman Show forest plot | 1 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.01, 4.13] |
| 3 Ongoing pregnancy rate per woman Show forest plot | 1 | 168 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.44 [1.03, 5.76] |
| 4 Clinical pregnancy rate per woman Show forest plot | 1 | 60 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.32, 3.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman Show forest plot | 1 | 959 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.88, 2.05] |
| 2 Ongoing pregnancy rate per woman Show forest plot | 1 | 959 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.80, 1.83] |
| 3 Clinical pregnancy rate per woman Show forest plot | 1 | 959 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.87, 1.70] |
| 4 Cycle cancellation rate per woman Show forest plot | 1 | 959 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.52, 0.95] |
| 5 Endometrial thickness Show forest plot | 1 | 959 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.13, 0.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman Show forest plot | 1 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.66, 1.87] |
| 2 Miscarriage rate per woman Show forest plot | 1 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.25, 2.19] |
| 3 Clinical pregnancy rate per woman Show forest plot | 1 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.64, 1.78] |
| 4 Endometrial thickness Show forest plot | 1 | 236 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.54, 0.14] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman Show forest plot | 1 | 75 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.10 [0.04, 0.30] |
| 2 Miscarriage rate per woman Show forest plot | 6 | 991 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.37, 1.12] |
| 3 Ongoing pregnancy rate per woman Show forest plot | 1 | 106 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.61, 4.85] |
| 4 Clinical pregnancy rate per woman Show forest plot | 5 | 872 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.25] |
| 5 Cycle cancellation rate per woman Show forest plot | 3 | 636 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.73 [0.79, 9.38] |
| 6 Endometrial thickness Show forest plot | 3 | 625 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐0.41, 0.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical pregnancy rate per woman Show forest plot | 1 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.45, 2.62] |
| 2 Cycle cancellation rate per woman Show forest plot | 1 | 175 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.49, 2.00] |
| 3 Endometrial thickness Show forest plot | 1 | 175 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.31, 0.31] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman Show forest plot | 1 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.49 [1.07, 5.80] |
| 2 Miscarriage rate per woman Show forest plot | 1 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.35, 5.09] |
| 3 Multiple pregnancy rate per woman Show forest plot | 1 | 209 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.31, 6.48] |


