Enfriamiento para los recién nacidos con encefalopatía isquémica hipóxica
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Single‐centre randomised controlled trial in Turkey | |
| Participants | Included 21 term infants with peripartum asphyxia (5‐minute Apgar score < 6, with acidosis on cord or arterial blood shortly after delivery (pH < 7.1 or base deficit > 10 mmol/L) and encephalopathy) without congenital abnormality (metabolic, malformations, chromosomal, congenital infection) or transitory drug depression | |
| Interventions | Hypothermia: temperature lowered in 11 infants by cooling cap for 72 hours (left external auditory canal temperature lowered to 33 to 33.5 °C and rectal temperature maintained at 36 to 36.5 °C by servo‐mechanism of radiant warmer). Infants re‐warmed at 0.5 °C/hour Standard care: 10 infants had rectal temperature maintained at 36 to 36.5 °C by servo‐mechanism of radiant warmer | |
| Outcomes | Primary outcome: platelet‐activating factor in cerebrospinal fluid | |
| Notes | Age at initiation of cooling included (1.9 hours), but not age at randomisation for infants allocated to standard care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated protocol number |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not specified |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up: complete to discharge |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Multicentre international randomised controlled trial | |
| Participants | Included 234 infants born at ≥ 36 weeks' gestation with clinical evidence of peripartum hypoxia‐ischaemia (Apgar score ≤ 5 at 10 minutes, continued need for resuscitation at 10 minutes, or severe acidosis (pH < 7 or base deficit ≥ 16 in cord blood or arterial/venous blood within 60 minutes of birth)) AND moderate or severe encephalopathy (Sarnat criteria) or clinical seizures AND moderate or severely abnormal background or seizures on amplitude integrated electroencephalography Excluded infants were older than 5.5 hours at randomisation, or had received prophylactic anticonvulsants, or had major congenital abnormalities, or had head trauma, or had severe growth restriction (< 1800 g birthweight), or were considered too critically unwell to benefit from intensive care, or equipment was unavailable, or were planned to participate in other trials | |
| Interventions | Hypothermia (N = 116): head cooling by cooling cap (Olympic Medical Cool Care System) on a radiant warmer servo‐controlled to infant's abdominal skin temperature adjusted to maintain rectal temperature at 34 to 35 °C for 72 hours. Infants re‐warmed at no more than 0.5 °C per hour Standard care (N = 118): radiant warmer servo‐controlled to infant's abdominal skin temperature adjusted to maintain rectal temperature at 36.8 to 37.2 °C | |
| Outcomes | Primary: combined frequency of mortality and severe neurodevelopmental disability in survivors at 18 months of age (gross motor function 3 to 5; Mental Development Index < 70 or bilateral cortical visual impairment) | |
| Notes | Randomised at 4.6 hours Several non‐cooled infants had elevation of body temperature greater than 38 °C Later reports: follow‐up at 7 to 8 years (Guillet 2011) as well as secondary analyses of primary outcome by severity of encephalopathy at randomisation (Wyatt 2007) and of the prognostic value of clinical assessment of encephalopathy (Gunn 2008) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate, with block randomisation by computer‐generated numbers in opaque sealed envelopes stratified by participating centre |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | 18‐month follow‐up in 218/234 (93%) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Multicentre randomised controlled trial in US | |
| Participants | Included 65 infants ≥ 35 weeks' gestation, > 2000 g birthweight, who were ≤ 6 hours of age with ≥ 1 clinical sign of a hypoxic‐Ischaemic insult (cord gas ≤ 7.0 or base deficit ≥ 13, initial infant gas pH < 7.1, Apgar score ≤ 5 at 10 minutes, continued resuscitation after 5 minutes, fetal bradycardia lasting ≥ 15 minutes, or postnatal hypoxic Ischaemic event with oxygen desaturation < 70% or arterial oxygen tension < 35 mmHg for 20 minutes with evidence of Ischaemic (chest compressions, hypotension, haemorrhage)) and 2 features of neonatal encephalopathy (posturing, seizures, autonomic dysfunction, or abnormalities of tone, reflexes or state of consciousness) Infants excluded with sepsis at birth (2 infants allocated to standard care), maternal chorioamnionitis, birthweight or head circumference < 10th centile for gestational age, or congenital abnormalities | |
| Interventions | Hypothermia: temperature lowered in 32 infants by application of ice to head and body for up to 2 hours and then maintained at 32.5 °C to 33.5 °C (rectal) on a servo‐controlled cooling blanket for 48 hours (Blanketrol II, Cincinnati Sub‐Zero). Re‐warmed by 0.5 °C per hour after 48 hours Standard care: 33 had rectal temperature maintained at 36.5 °C to 37.5 °C by servo‐controlled radiant warmer | |
| Outcomes | Efficacy and safety outcomes published in 2 consecutive reports. Primary outcomes in efficacy report included death or 12‐month neurodevelopmental outcome (Bayley Scales of Infant Development ‐ Psychomotor Development Index (PDI) and Mental Development Index (MDI), Cognitive Adaptive Test/Clinical Linguistic and Auditory Milestone Scale (CAT/CLAMS) or Vineland assessments). Primary outcomes in safety report included bradycardia, disseminated intravascular coagulopathy and sepsis. Additional data collected on short‐term adverse effects of cooling included coagulopathy, cardiac arrhythmias, persistent metabolic acidosis, sepsis/pneumonia within the first 7 days of life, hypokalaemia, necrotising enterocolitis, skin injury, extension of intracranial haemorrhage, persistent pulmonary hypertension of the newborn, and treatment with extracorporeal membrane oxygenation (ECMO) | |
| Notes | Randomised at 3.1 (standard care)/3.4 (hypothermia) hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequate, with web‐based centralised online blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Assessment of short‐term outcomes nearly complete (62/65), but incomplete 12‐month assessment (53/65 = 81%) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Single‐centre randomised controlled trial in New Zealand | |
| Participants | Included infants in the report of Gunn 1998 and additional infants randomised in the reports of Battin 2001 and Battin 2003. In total, the trials enrolled 31 infants of 37 weeks' gestation or greater with perinatal asphyxia (pH ≤ 7.09 or Apgar ≤ 6 at 5 minutes) plus evidence of encephalopathy (lethargy/stupor, hypotonia, abnormal reflexes including absent or weak suck. Infants with major congenital abnormalities were excluded | |
| Interventions | Hypothermia: 18 infants underwent cooling via cooling cap (Silclear tubing cap in first 17 infants, Olympic Medical Cool Care System for remainder), with target temperature determined by sequential randomisation with 6 infants in a minimal cooling group (36 °C to 36.5 °C), followed by 6 infants in a mild hypothermia group (35.5 °C to 35.9 °C), 6 infants at 34.5 °C to 35.4 °C Standard care: 15 infants had rectal temperature maintained at 36.8 °C to 37.2 °C with servo‐controlled radiant warmer (10 in initial study and 3 in follow‐up) | |
| Outcomes | Acute adverse events such as seizures or evidence of multi‐system involvement (hypotension (mean arterial pressure < 40 mmHg), bradycardia (< 80 beats/minute), cardiac arrhythmia, persistent pulmonary hypertension (requiring nitric oxide), meconium aspiration syndrome requiring respiratory support, infection, thrombocytopenia, hypoglycaemia, maximum acidosis during cooling, electrolyte imbalance (hyponatraemia, hypokalaemia), and acute renal failure. Short‐term outcomes (death, cranial ultrasound, electroencephalogram, head computerised tomography) and 18‐month neurodevelopmental follow‐up (Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) and Psychomotor Development Index (BSID PDI)) also assessed | |
| Notes | 40 infants reported, with 31 randomised. Non‐randomised infants (7 cooled to 34 °C to 35.5 °C, 2 controls) not included in review Randomised at a mean of 4.4 hours (hypothermia) and 3.8 hours (control) of age Not stated if deaths followed withdrawal of care | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Low risk, by sequential, computer‐generated numbers |
| Allocation concealment (selection bias) | Unclear risk | Adequate |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Unclear risk | Caregivers not blinded to treatment assignment for short‐term outcomes, but assessors of neurodevelopment at 12 months were blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Assessment of short‐term and 12‐month outcomes complete. Follow‐up at 18 months complete for 14/15 surviving cooled infants and 9/10 surviving control infants |
| Methods | Multicentre international study | |
| Participants | Included 221 infants 35 weeks' gestation or greater with moderate or severe hypoxic ischaemic encephalopathy (HIE) (defined as lethargy, stupor, coma, abnormal tone, seizure, or a combination) and evidence of peripartum HIE (at least 2 of: Apgar ≤ 5 at 10 minutes, continued need for mechanical ventilation after 10 minutes, with or without metabolic acidosis with cord or arterial pH of ≤ 7 with or without base deficit of ≥ 12 within 60 minutes of birth) Infants for whom hypothermia could not be initiated within 6 hours of life, who weighed < 2000 g, who had major congenital abnormalities suspected, who had overt bleeding, who required > 80% fraction of inspired oxygen (FiO2), who started hypothermia before randomisation, or for whom death was imminent were excluded | |
| Interventions | Hypothermia: 110 infants cooled by being exposed to the ambient environment (turning radiant warmer off) with refrigerated gel packs applied as required to maintain rectal temperature at 33 °C to 34 °C for 72 hours Infants re‐warmed by 0.5 °C every 2 hours Standard care: 111 standard care infants rectal temperature was maintained at 36.8 °C to 37.3 °C | |
| Outcomes | Primary outcomes: composite of mortality or major sensorineural disability at 2 years. Major sensorineural disability was defined as neuromotor delay (moderate or severe cerebral palsy, Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) and Psychomotor Development Index (BSID PDI) of less than ‐2 standard deviations (SD), or a disability index on the Gross Motor Function Classification System (GFMCS) of 2 to 5), developmental delay (BSID MDI, Cognitive Scale, or Language Composite Scale, score of less than ‐2 SD), blindness (vision worse than 20/200 in both eyes), deafness requiring amplification or worse, or a combination Secondary outcomes: mortality, major sensorineural disability and individual components (neuromotor delay, developmental delay, blindness, deafness, or a combination), and survival free of any sensorineural disability Adverse events recorded included cardiac arrhythmia requiring treatment, prolonged QT interval, hypotension requiring inotropic agents, overt bleeding, thrombosis or coagulopathy treated with fresh frozen plasma, with or without cryoglobulin, hypoxia in 100% O2 resulting in discontinuation of hypothermia, thrombocytopenia, oliguria, hepatic dysfunction, gastrointestinal bleeding or necrotising enterocolitis, sepsis and mortality | |
| Notes | Recruitment halted due to loss of equipoise Enrolled and randomised at 3.9 (cooled) and 4 (control) hours Additional data reported on magnetic resonance imaging findings (Cheong 2012) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequentially numbered, sealed envelopes with computer‐generated random numbers stratified by study centre |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Assessment of outcomes nearly complete (107/110 cooled and 101/111 control infants) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Single‐centre study in China | |
| Participants | Included 62 consecutive infants of 37 weeks' gestation or greater with peripartum hypoxia‐ischaemic (Apgar < 6 at 5 minutes with first postnatal arterial pH < 7.1 or base deficit > 15) and clinical encephalopathy quasi‐randomised within 6 hours of birth. Infants with major congenital abnormalities and severe hypoxaemia due to severe persistent fetal circulation were excluded | |
| Interventions | Hypothermia: 32 infants cooled by cooling cap device (SDL‐V, Tianyuan Scientific Development) shielded under radiant warmer with output to maintain rectal temperature at 34 °C to 35 °C for 72 hours. Infants re‐warmed spontaneously, radiant warmer used if temperature remained less than 36° C after 12 hours Standard care: 30 standard care infants had intermittent measurement of rectal temperature ‐ target temperature not stated | |
| Outcomes | Mortality, neuroimaging (computerised tomography scan) and neurobehavioural assessment at 7 to 10 days of life using Neonatal Behavioral Neurological Assessment score | |
| Notes | Enrolled at 3.6 (hypothermia)/3.8 (standard care) hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Inadequate, quasi‐randomised (alternate day allocation according to odd or even day of admission) |
| Allocation concealment (selection bias) | High risk | Inadequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | High risk | Follow‐up incomplete: analysis not intention to treat, and followed to 10 days of age |
| Selective reporting (reporting bias) | Low risk | |
| Overall risk of bias | High risk | |
| Methods | International multicentre study | |
| Participants | Included 129 neonates of at least 36 weeks' gestation with evidence of birth asphyxia (Apgar < 5 at 10 minute, need for continued resuscitation after 10 minutes, cord or arterial pH of ≤ 7 or base deficit of ≥ 16 within 60 minutes of birth or both) and clinical evidence of encephalopathy (hypotonia, abnormal reflexes, absent/weak suck, clinical seizures, or a combination) Excluded infants > 5.5 hours of age, administration of > 20 mg/kg phenobarbital, weight < 1800 g, head circumference < 3rd percentile (if other growth parameters > 3rd percentile), presence of major congenital anomalies, imperforate anus, presence of gross haemorrhage, or infants who were "in extremis" | |
| Interventions | Hypothermia: 64 infants cooled via a cooling mattress (Tecotherm TS Med 200, TecCom) with a target rectal temperature of 33.5 °C (range 33.0 °C to 34.0 °C) for 72 hours. Temperature was controlled by manual adjustment of cooling mattress (not servo‐controlled). Infants re‐warmed by less than 0.5 °C per hour Standard care: 65 infants received standard treatment with a target rectal temperature of 37 °C (range 36.5 °C to 37.5 °C) All infants received morphine (0.1 mg/kg) every 4 hours or an equivalent dose of fentanyl | |
| Outcomes | Primary outcome: death or severe disability (neurological functional score 3 to 5, development quotient (DQ) < 2 standard deviations (SD), with or without severe bilateral cortical visual impairment) at 18 to 21 months Secondary outcomes: death or severe disability within infants with moderate or severe hypoxic ischaemic encephalopathy, death, DQ < 2 SD, disabling cerebral palsy, bilateral cortical visual impairment or severe hearing loss Additional adverse events recorded included systemic hypotension, metabolic acidosis, seizures on electroencephalogram or clinical, intracranial haemorrhage, venous thrombosis, overt bleeding, coagulopathy, thrombocytopenia, haemoconcentration, systemic infection, arrhythmia, hypoglycaemia, hypocalcaemia, hyponatraemia, elevated liver enzymes, pathological renal function, need for ventilator support after initiation of intervention, need for inhaled nitric oxide or death during intervention | |
| Notes | Randomised at 4.6 (hypothermia) and 4.1 (control) hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation using numbered, sealed envelopes, stratified by centre and severity |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Assessment of short‐term outcomes nearly complete (62/64 cooled and 63/65 control infants), but incomplete follow‐up data (53/64 (83%) of cooled and 58/65 (89%) of control infants) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Multicentre randomised controlled trial within National Institute of Child Health and Human Development (NICHD) network in the US | |
| Participants | Included 208 infants ≥ 36 weeks' gestation < 6 hours of age with evidence of seizures or encephalopathy and either (a) pH ≤ 7.0 or base deficit ≥ 16 mmol/L on cord blood or blood gas within 1 hour of birth OR (b) if no blood gas or if pH 7.01 to 7.15 or base deficit 10 to 15.9 mmol/L then additional criteria required: acute perinatal event (late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, haemorrhage or cardiorespiratory arrest) AND either a 10‐minute Apgar score ≤ 5 or assisted ventilation initiated at birth and continued for at least 10 minutes. Criteria for encephalopathy included assessment of loss of consciousness, spontaneous activity, posture, primitive reflexes and autonomic nervous system abnormalities Excluded infants that were unable to be enrolled by 6 hours of age, had chromosomal or major congenital abnormalities, had growth restriction (birthweight ≤ 1800 g) or had consent refused by parent or neonatologist or who were moribund | |
| Interventions | Hypothermia: 102 infants were placed on a pre‐cooled infant blanket (Blanketrol II Hyper‐Hypothermia System, Cincinnati Sub‐Zero) servo‐controlled to a target oesophageal temperature of 33.5 °C for 72 hours (25th and 75th percentiles at 33.2 °C and 33.5 °C). Re‐warming occurred by 0.5 °C per hour Standard care: 106 infants received standard care with skin temperature servo‐controlled to abdominal skin temperature 36.5 °C to 37 °C (25th and 75th percentiles at 36.9 °C and 37.5 °C) All infants had abdominal and oesophageal temperature monitoring | |
| Outcomes | Primary outcome: composite of death or moderate/severe disability at 18 to 22 months according to Gross Motor Function Classification System (GMFCS), Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) and Psychomotor Development Index (BSID PDI), aided hearing loss or presence of persistent seizures (Moderate disability ‐ BSID MDI 70 to 84 and at least 1 of: GMFCS 2, hearing impaired without amplification or persistent seizure disorder. Severe disability ‐ BSID MDI < 70, GMFCS 3 to 5, aided hearing loss or blindness) Data collected on adverse events included those during the 72‐hour intervention (cardiac arrhythmia, persistent acidosis, major thrombosis or bleeding, skin changes, death) and those prior to hospital discharge (hypotension, persistent pulmonary hypertension, renal impairment, hepatic dysfunction, sepsis, hypoglycaemia, hypokalaemia, death, length of stay, feeding status and use of anticonvulsants at discharge) | |
| Notes | Initial mean temperature of hypothermic infants on cooling was 32.7 °C. Several non‐cooled infants had elevation of body temperature > 38 °C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation by telephone by data‐coordinating centre, stratified by centre and generated by random, permuted block algorithm |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Follow‐up: complete 18 to 22 months' follow‐up in 205/208 (98.6%) |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Multicentre randomised controlled trial in the US | |
| Participants | Included 19 infants ≥ 36 weeks' gestation and < 6 hours of age with evidence of seizures or encephalopathy and either (a) pH ≤ 7.0 or base deficit ≥ 16 mmol/L on cord blood or blood gas within 1 hour of birth OR (b) if no blood gas or if pH 7.01 to 7.15 or base deficit 10 to 15.9 mmol/L then additional criteria required: acute perinatal event (late or variable decelerations, cord prolapse, cord rupture, uterine rupture, maternal trauma, haemorrhage or cardiorespiratory arrest) AND either a 10‐minute Apgar score ≤ 5 or assisted ventilation initiated at birth and continued for at least 10 minutes. Criteria for encephalopathy included assessment of loss of consciousness, spontaneous activity, posture, primitive reflexes and autonomic nervous system abnormalities Excluded infants unable to be enrolled by 6 hours of age, had chromosomal or major congenital abnormalities, had growth restriction (birthweight ≤ 1800 g) or had consent refused by parent or neonatologist | |
| Interventions | Hypothermia: 9 infants were placed on a pre‐cooled infant blanket (Blanketrol II Hyper‐Hypothermia System, Cincinnati Sub‐Zero) servo‐controlled to a target oesophageal temperature of 34.5 °C for 72 hours. Re‐warming occurred by 0.5 °C per hour Standard care: 10 infants received standard care with skin temperature servo‐controlled to abdominal skin temperature 36.5 °C (oesophageal temperature 37.0 °C to 37.5 °C) All infants had abdominal and oesophageal temperature monitoring | |
| Outcomes | Primary outcomes: temperature, heart rate and diastolic blood pressure during cooling as well as adverse events such as cardiac arrhythmia, persistent acidosis, major thrombosis or bleeding, skin changes and death Secondary outcomes: adverse events (hypotension, persistent pulmonary hypertension, renal failure, hepatic dysfunction, disseminated intravascular coagulation), data on hospital course (days on oxygen, length of stay) and discharge status (need for gavage feeds, abnormal neurological examination, seizures requiring anticonvulsants or abnormal magnetic resonance imaging) | |
| Notes | Initial mean temperature of hypothermic infants on cooling was 32.9 °C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised randomisation by telephone by a data co‐ordinating centre, stratified by centre and generated by random, permuted block algorithm |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible |
| Blinding of outcome assessment (detection bias) | Low risk | N/A |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data complete |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Multicentre international randomised controlled trial | |
| Participants | Included 325 infants ≥ 36 weeks' gestation with an Apgar of < 5 or continued need for resuscitation at 10 minutes, a pH < 7 or base deficit ≥ 16 mmol/L within the 1st hour of life, and evidence of moderate‐to‐severe encephalopathy (lethargy, stupor or coma), and either hypotonia, abnormal reflexes, absent or weak suck, or clinical evidence of seizure. Additionally, infants had to have seizures or abnormal background for at least 3 minutes on amplitude‐integrated electroencephalogram (aEEG) | |
| Interventions | Hypothermia: 163 infants were cooled via cooling blanket (Tecotherm TS Med 200, TecCom) with a manually adjusted (non‐servo controlled) target rectal temperature of 33 °C to 34 °C (actual mean 33.5 °C). Re‐warming occurred by 0.5 °C per hour Standard care: 162 infants received standard care with skin temperature servo‐controlled to a target rectal temperature of 37 °C (actual mean 36.9 °C) All infants had continuous skin and rectal temperature monitoring. Uniform guidance provided on respiratory and circulatory care, fluid requirements, management of seizures and sedation | |
| Outcomes | Primary outcome: composite of death or severe disability at 18 months (as determined by Gross Motor Function Classification System (GMFCS) level III to V, Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) < 70, bilateral cortical visual impairment Secondary outcomes (at 18 months): death, severe disability at 18 months, survival without neurological abnormality, multiple neurodevelopmental disabilities, BSID MDI score, Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI) score, GMFCS score, cerebral palsy, hearing loss, no useful vision, seizures requiring anticonvulsants or microcephaly Adverse events recorded included the presence of intracranial haemorrhage, persistent hypotension, pulmonary haemorrhage, persistent pulmonary hypertension, prolonged blood coagulation time, culture‐confirmed sepsis, necrotising enterocolitis, thrombocytopenia, major venous thrombosis, renal failure requiring dialysis, pneumonia, pulmonary air leak and duration of hospitalisation | |
| Notes | Several non‐cooled infants had elevation of body temperature > 38 °C | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By telephone from data co‐ordinating centre or via web‐based system. Minimisation used to ensure balance by centre and degree of aEEG abnormality |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible. Uniform guidelines for care provided to minimise potential confounding |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Low risk | Both short‐term and follow‐up outcome data complete |
| Selective reporting (reporting bias) | Low risk | |
| Methods | Multicentre randomised controlled trial in China | |
| Participants | Included 194 infants ≥ 37 weeks' gestation and bodyweight ≥ 2.5 kg, admitted to neonatal intensive care unit (NICU) within 6 hours with clinical evidence of exposure to perinatal hypoxic ischaemia (Apgar score of ≤ 3 at 1 minute and or < 5 at 5 minute, cord pH < 7 or base deficit ≥ 16 mmol/L, or a need for resuscitation or ventilation at 5 minutes) or a diagnosis of encephalopathy (mild, moderate or severe) Excluded infants with major congenital anomalies, infection, other encephalopathy (neonatal stroke, central nervous system abnormality, intracranial haemorrhage), or severe anaemia | |
| Interventions | Hypothermia: 100 infants underwent head cooling (YJW608‐04B, Henyang Radio Manufactory) for 72 hours, with target a nasopharyngeal temperature of 34.0 °C, with additional use of a radiant warmer to target a rectal temperature of 34.5 °C to 35 °C. Following cooling, infants underwent spontaneous re‐warming Standard care: 94 infants cared for in servo‐controlled radiant warmers with rectal temperature target 36 °C to 37.5 °C. Laboratory and other monitoring parameters identical for both groups | |
| Outcomes | Primary outcome: composite of death or severe disability (Gessell Child Development Age Scale, developmental quotient (DQ) < 70, Gross Motor Function Classification System (GMFCS) score 3 to 5), death or severe disability at 18 months Secondary outcomes: death or survival with severe disability in infants with moderate‐to‐severe, moderate, or severe hypoxic ischaemic encephalopathy, DQ Adverse events recorded included major (severe arrhythmia, major venous thrombosis, refractory hypotension, moderate or severe scleroedema, severe bleeding, scleroedema) and minor (mild arrhythmia, mild scleroedema, renal dysfunction, liver dysfunction, thrombocytopenia, serum electrolyte or biochemical abnormalities) | |
| Notes | 6 infants treated with hypothermia and 5 controls had a temperature > 38 °C Onset of treatment occurred at a mean 4.1 hours of age for cooled infants and 4.0 hours of age for controls | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By numbered, sealed envelopes containing random computer‐generated numbers Stratified by centre in blocks of 6 |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of intervention: not possible. Uniform guidelines for laboratory and other monitoring provided |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors blinded to treatment allocation |
| Incomplete outcome data (attrition bias) | Unclear risk | Outcome data (both short and long term) incomplete on 16% of those cooled and 22% of those who received standard care. Of those infants for whom short‐term data were reported, follow‐up data were complete (93% of cooled and 94% of control infants followed up, including some infants whose follow‐up was by telephone or at local paediatrician) |
| Selective reporting (reporting bias) | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Case series (without controls) | |
| Case series (without controls) | |
| Case series (without controls) | |
| Retrospective cohort study with historical controls | |
| Retrospective cohort study with historical controls. Included deep hypothermia (33 °C) | |
| Case series (without controls) | |
| Case series (without controls) | |
| Case series (without controls) | |
| Case series (without controls) | |
| Case series (without controls) | |
| Retrospective cohort study with historical controls | |
| Non‐randomised prospective study employing cooling via extracorporeal membrane oxygenation (ECMO), cohorts treated at sequentially decreasing temperatures. Did not meet pre‐specified inclusion criteria for presence of perinatal asphyxia or encephalopathy | |
| Feasibility study using solid ice cap, halted after 4 patients cooled, technical data only | |
| Non‐randomised prospective pilot study employing cooling via extracorporeal membrane oxygenation (ECMO), cohorts treated at sequentially decreasing temperatures. Did not meet pre‐specified inclusion criteria for presence of perinatal asphyxia or encephalopathy | |
| Randomised controlled trial, outcomes included magnetic resonance imaging findings but not mortality, neurodevelopmental disability or adverse events | |
| Case series (without controls) | |
| Retrospective cohort study with historical controls | |
| Method of allocation not able to be determined, although described as 'random assignment'. Excluded as did not meet modified inclusion criteria (hypothermia initiated at up to 10 hours of life) | |
| Retrospective cohort study with historical controls | |
| Randomised controlled trial, but outcomes did not include data on pre‐specified outcomes | |
| Case series (without controls) | |
| Case series (without controls) | |
| Randomised controlled trial of whole body cooling using water bottles. Outcomes included rectal temperature during cooling period, neurological assessment up to day 17, seizures and death before discharge. Not included in analysis because study inclusion criteria do not meet the pre‐defined definition of peripartum asphyxia | |
| Infants enrolled from composite of TOBY and CoolCap and several pilot studies. Outcomes included magnetic resonance imaging findings but not mortality or neurodevelopmental disability. Excluded from review as it included infants from multiple randomised controlled trials (with previously reported data) and multiple modalities of cooling | |
| Open cohort series with historical controls | |
| Retrospective cohort study with historical controls | |
| Case series (without controls) | |
| Case series (without controls) | |
| Single‐centre randomised controlled trial (RCT), included infants from multiple international RCTs (CoolCap, TOBY) as well as additional infants. Outcomes included use of baseline amplitude‐integrated electroencephalogram to predict mortality and neurodevelopmental outcomes in normothermic and hypothermic infants. Mortality and the composite outcome of death or severe disability were reported. Excluded from review as it included infants from multiple RCTs (with previously reported data) and multiple modalities of cooling | |
| Case series (without controls) | |
| Case series (without controls) | |
| Safety and efficacy study of selective head cooling. Method of allocation not able to be determined, although described as 'random assignment'. No pre‐defined outcomes for this review were reported | |
| Study of the effects of selective head cooling on cardiac function. Method of allocation not able to be determined, although described as 'randomly divided' |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Single‐centre randomised controlled trial in India |
| Participants | Included infants with hypoxic ischaemic encephalopathy (criteria not specified in abstract). Other criteria (gestational age, age at enrolment, criteria for peripartum asphyxia) not stated and exclusion criteria not specified in abstract |
| Interventions | Hypothermia: infants underwent whole body cooling via cooling gel packs for target rectal temperature of rectal temperature 33 °C to 34 °C for 72 hours. Re‐warming protocol not specified Control treatment: standard care, not specified. Number of infants included not stated |
| Outcomes | Primary outcome was death or developmental delay at 6 months. Other outcomes reported not specified in abstract |
| Notes |
| Methods | Single‐centre randomised controlled trial in India |
| Participants | Included 35 infants with severe perinatal asphyxia (criteria not specified in letter). Other criteria (gestational age, age at enrolment, criteria for peripartum asphyxia) not stated and exclusion criteria not specified in letter |
| Interventions | Hypothermia: 20 Infants underwent whole body cooling (method not stated) with a target rectal temperature of rectal temperature 33.5 °C for 72 hours. Re‐warming protocol not specified Control treatment: 15 infants received standard care, treatment not specified |
| Outcomes | Primary outcome was death or abnormal neurological examination at time of discharge. Other outcomes reported not specified in abstract |
| Notes |
| Methods | Single‐centre randomised controlled trial in China Most likely a subset of trial of Zhou 2010 |
| Participants | Included 51 infants ≥ 37 weeks with a birthweight of ≥ 2.5 kg, admitted to the neonatal intensive care unit within 6 hours with clinical evidence of exposure to perinatal hypoxic ischaemia (Apgar score ≤ 3 at 1 minute and ≤ 5 at < 5 minutes, cord pH < 7 or base deficit ≥ 16 mmol/L, need for resuscitation or ventilation at 5 minutes, or a combination) or a diagnosis of encephalopathy (mild, moderate or severe). Excluded infants with major congenital abnormalities, intracranial haemorrhage and severe anaemia |
| Interventions | Hypothermia: 23 infants were treated via selective head cooling (YJW608‐04B, Henyang Radio Manufactory) for 72 hours, with target nasopharyngeal temperature of 34.0 °C, with additional use of a radiant warmer to target a rectal temperature of 34.5 °C to 35 °C. Re‐warming occurred over 8 hours Control treatment: 28 infants cared for in servo‐controlled radiant warmers with rectal temperature target 36 °C to 37.5 °C |
| Outcomes | Outcomes included cerebrospinal fluid levels of neuron‐specific enolase, S‐100 and amino acid neurotransmitters as well as Bayley Scales of Infant Development ‐ Mental Development Index (BSID MDI) < 70 and Bayley Scales of Infant Development ‐ Psychomotor Development Index (BSID PDI) < 70 at 12 months |
| Notes | Most likely a subset of trial of Zhou 2010. This needs to be confirmed 1 control infant died, unclear whether death was following withdrawal of support Age at treatment onset 4.1 hours of life (hypothermia) and 4.0 hours of life (controls) Blinding of assessment at follow‐up unclear |
| Methods | Single‐centre randomised controlled trial in India |
| Participants | Included 21 newborn infants with neonatal encephalopathy (Thompson score < 5). Other criteria (gestational age, age at enrolment, criteria for peripartum asphyxia) not stated. Exclusion criteria not specified |
| Interventions | Hypothermia: 11 Infants underwent whole body cooling via a mattress containing phase‐changing material for target rectal temperature of rectal temperature 33 °C to 34 ° C for 72 hours. Re‐warming protocol not specified Control treatment: 10 infants received standard care, treatment not specified |
| Outcomes | Primary and secondary outcomes not specified. Outcomes reported included: temperature, time to cooling and re‐warming, number of blanket changes required, heart rate, respiratory rate, blood pressure, platelet counts, C‐reactive protein, liver enzymes or coagulation profile and mortality |
| Notes | Excluded as detail on methods and results insufficient to warrant inclusion |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Xenon and Cooling Therapy in Babies at High Risk of Brain Injury Following Poor Condition at Birth: Randomised Pilot Study (The CoolXenon2 Study) |
| Methods | Randomised controlled single‐centre pilot study in UK |
| Participants | Includes: infants born at ≥ 36 weeks' gestation WITH clinical evidence of peripartum hypoxia‐ischaemia (Apgar score ≤ 5 at 10 minutes, continued need for resuscitation at 10 minutes, or severe acidosis (pH < 7 or base deficit ≥ 16 mmol/L in cord blood or arterial/venous blood within 60 minutes of birth)) AND abnormal amplitude‐integrated electroencephalogram background AND moderate or severe encephalopathy (Sarnat criteria) with 1 of hypotonia, abnormal reflexes, absent or weak suck, clinical seizures, or a combination. For xenon therapy, infants must be intubated with normal partial pressure of CO2 (pCO2), a positive end‐expiratory pressure of < 6 cmH2O and fraction of inspired oxygen (FiO2) < 40%, seizures under control, be > 2.3 kg in weight and < 5 hours old, have a birthweight greater than the 2nd percentile for age, have no major congenital anomalies and be haemodynamically stable with no evidence of infection Excludes: infants considered futile, infants not meeting above criteria |
| Interventions | All infants cooled using whole body hypothermia for 72 hours at 33.5 °C. Enrolled infants randomised to 50% Xenon inhalation for 18 hours (using a closed loop xenon‐delivery system (cooling protocol not specified) or to standard cooling therapy |
| Outcomes | Primary outcome: physiological changes during and within 24 hours after end treatment Secondary outcomes: Bayley III, measured at 18 or 24 months, magnetic resonance imaging within 14 days after treatment |
| Starting date | March 2010 |
| Contact information | Marianne Thoresen, M.D. |
| Notes | Currently recruiting, estimated completion April 2014 with 24 patients anticipated. Follows non‐randomised single‐centre CoolXenon study. All infants cooled |
| Trial name or title | Darbe Administration in Newborns Undergoing Cooling for Encephalopathy (DANCE trial) |
| Methods | Randomised controlled trial |
| Participants | Includes: infants > 36 weeks' gestation and < 12 hours old with evidence of moderate‐to‐severe perinatal hypoxic ischaemic encephalopathy and evidence of an acute perinatal event and either (a) pH ≤ 7.0 or base deficit ≥ 16 mmol/L on cord blood or blood gas within 1 hour of birth OR (b) if no blood gas or if pH 7.01 to 7.15 or base deficit 10 to 15.9 mmol/L then additional criteria required: acute perinatal event AND either a 10‐minute Apgar score ≤ 5 or assisted ventilation initiated at birth and continued for at least 10 minutes Excludes: infants with chromosomal or major congenital abnormalities, had growth restriction (birthweight ≤ 1800 g), had a central venous haematocrit > 65%, platelet count > 600,000/dL, neutropenia (absolute neutrophil count < 500 µL), had a maternal history of major vascular thrombosis or multiple fetal losses, or were receiving extracorporeal membrane oxygenation (ECMO), had a core temperature < 33.5 °C for > 1 hour prior to screening, or were determined to be critically ill and unlikely to benefit from intensive care by the attending neonatologist |
| Interventions | All infants undergoing cooling, hypothermia protocol not specified. Enrolled infants will be randomised to receive either: a) high‐dose darbepoetin (10 µg/kg/dose), b) low‐dose darbepoetin (2 µg/kg/dose) or c) placebo. All infants were given 2 doses of Darbe or placebo, with the first dose within 12 hours of delivery and the second dose at 7 days |
| Outcomes | Primary outcomes: presence of adverse events such as alterations in blood pressure, secondary infections, neutropenia, thrombotic/vascular events, haematological events (platelets, haematocrit level, polycythaemia), and hepatic/renal dysfunction Secondary outcomes: pharmacokinetic profile of darbepoetin |
| Starting date | June 2012 |
| Contact information | Mariana Baserga, M.D. |
| Notes | Not yet recruiting patients. Estimated completion March 2014 with 45 patients anticipated. Phase I/II dose safety and pharmacokinetic trial. All infants to be cooled |
| Trial name or title | Hypothermia Treatment in Hyperammonemia and Encephalopathy |
| Methods | Non‐randomised safety study, cohort study with historic controls |
| Participants | Includes: infants > 36 weeks' gestation and ≥ 2200 g birthweight who are up to 2 months of age with clinical signs and symptoms of a urea cycle disorder or propionic, methylmalonic, or isovaleric acidaemia and hyperammonaemia and encephalopathy requiring renal replacement therapy Excludes: hyperammonaemia due to other disorders (lysinuric protein intolerance, mitochondrial disorders, congenital lactic acidosis, and fatty acid oxidation disorders), unrelated serious co‐morbidities, genetic disease, intraventricular haemorrhage, traumatic brain injury, low birthweight (< 2200 g at > 36 weeks' gestation) and infants in extremis |
| Interventions | All enrolled infants will be cooled to 33.5 °C (± 1 °C ) for 72 hours and then re‐warmed by 0.5 °C every 3 hours over 18 hours. Patients will also receive standard of care therapy (renal replacement). Historic controls will also receive renal replacement therapy |
| Outcomes | Primary outcomes: presence of unexpected serious adverse events, feasibility Secondary outcome: time to normalisation of ammonia level |
| Starting date | August 2007 |
| Contact information | Uta Lichter‐Konecki, M.D., Ph.D. |
| Notes | Currently recruiting, estimated enrolment 24 patients, estimated completion July 2015 |
| Trial name or title | Safety and Efficacy of Oral Topiramate in Neonates With Hypoxic Ischemic Encephalopathy Treated With Hypothermia: a Pilot Study of the Neonatal Neuroprotection of Asphyxiated Tuscan Infants (NeoNATI) Network |
| Methods | Randomised controlled trial |
| Participants | Includes: infants > 36 weeks' gestation and birthweight > 1800 g with evidence of asphyxia (at least 1 of: Apgar score < 5 at 10 minutes, need for resuscitation at 10 minutes after birth, acidosis (pH < 7.0, base deficit > 16 mmol/L within 60 minutes from birth), moderate‐to‐severe encephalopathy (altered state of consciousness (irritability, lethargy, stupor or coma) and > 1 of: hypotonia; abnormal reflexes, including oculomotor or pupil abnormalities; absent or weak suck, clinical seizures), and an abnormal amplitude‐integrated electroencephalogram. Excludes: infants with congenital abnormalities, congenital viral infections or evidence encephalopathy other than hypoxic ischaemic encephalopathy |
| Interventions | All infants will undergo hypothermia. Enrolled infants randomised either to a treatment arm, receiving topiramate (10 mg/kg) once daily from the time of initiation of cooling and for 3 doses, or to standard care. Hypothermia protocol not specified |
| Outcomes | Primary outcome: neurological outcome at 6, 12 and 18 months of life Secondary outcomes: neuroradiological outcome at 3 and 12 months of life |
| Starting date | February 2010 |
| Contact information | Luca Filippi, M.D. |
| Notes | Currently recruiting, estimated enrolment 60 patients, estimated completion August 2013 |
| Trial name or title | Assessing the Neuro‐protective Effect of Mild Cooling in Neonates Receiving Extracorporeal Membrane Oxygenation (ECMO): a Randomised Controlled Trial (NEST Study) |
| Methods | Randomised controlled trial |
| Participants | Includes: infants of at least 35 weeks' gestation and 2000 g birthweight who are < 29 days of age at recruitment and who meet existing standard criteria for ECMO eligibility (evidence of severe cardiorespiratory failure, suffering from a condition that is potentially reversible, no more than 7 consecutive days of high‐pressure ventilation prior to referral for ECMO) Excludes: infants cooled prior to ECMO, those requiring ECMO for postoperative cardiac support, and those with an uncontrolled bleeding disorder, a congenital or acquired central nervous system disorder, or a congenital diaphragmatic hernia |
| Interventions | Infants receiving ECMO randomised to standard ECMO or ECMO with mild cooling |
| Outcomes | Primary outcome: cognitive score from the Bayley scales of Infant and toddler Development, 3rd edition (Bayley‐III) at age of 2 years (24 to 27 months) Secondary outcomes: death and multiple metrics of neurological dysfunction |
| Starting date | January 2005 |
| Contact information | David Field, M.D. |
| Notes | Excludes infants with hypoxic ischaemic encephalopathy (cooling is for prevention of ECMO‐associated morbidity). Completed, in follow‐up phase |
| Trial name or title | Evaluation of Systemic Hypothermia Initiated After 6 Hours of Age in Infants ≥36 Weeks Gestation With Hypoxic‐Ischemic Encephalopathy: A Bayesian Evaluation. A Protocol for the NICHD Neonatal Research Network |
| Methods | Multicentre randomised controlled trial |
| Participants | Incudes: infants meeting National Institute of Child Health and Human Development (NICHD) 2005 criteria for encephalopathy and perinatal asphyxia enrolled between 6 and 24 hours of age. Excludes: chromosomal or major congenital abnormalities, birthweight ≤ 1800 g, moribund infants, core temp < 34 °C for > 1 hour prior to screening or consent refused by parent or neonatologist |
| Interventions | Enrolled infants are randomised to either a hypothermia (with a target oesophageal temperature of 33.5 °C using pre‐cooled infant blanket) or control (37.0 °C) for 96 hours. Temperature monitoring and re‐warming as per NICHD 2005 protocol |
| Outcomes | Primary outcome: death or moderate or severe disability at 18 to 24 months Secondary outcomes: death, mild, moderate and severe disability, non‐central nervous system organ system dysfunction, presence of a do not resuscitate (DNR) order with or without withdrawal of support, presence of neonatal seizures, with and without EEG abnormalities |
| Starting date | April 2008 |
| Contact information | Abbot R. Laptook, M.D. and Rosemary D. Higgins, M.D. |
| Notes | Currently recruiting, enrolment of 168 subjects anticipated, anticipated completion March 2014 |
| Trial name or title | Optimizing Cooling Strategies at < 6 hours of Age for Neonatal Hypoxic‐Ischemic Encephalopathy |
| Methods | Multicentre randomised controlled trial |
| Participants | Incudes: infants ≥ 36 weeks' gestation < 6 hours of age with evidence of encephalopathy and either (a) pH ≤ 7.0 or base deficit ≥ 16 mmol/L on cord blood or blood gas within 1 hour of birth OR (b) if no blood gas or if pH 7.01 to 7.15 or base deficit 10 to 15.9 mmol/L then additional criteria required: acute perinatal event AND either a 10‐minute Apgar score ≤ 5 or assisted ventilation initiated at birth and continued for at least 10 minutes. Criteria for encephalopathy included assessment of loss of consciousness, spontaneous activity, posture, tone, primitive reflexes, and autonomic nervous system abnormalities. Excludes: unable to be enrolled by 6 hours of age, presence of chromosomal or major congenital abnormalities, presence of growth restriction (birthweight ≤ 1800 g), moribund infants, those with a core temp < 33.5 °C for > 1 hour prior to screening, or consent refused by parent or neonatologist |
| Interventions | Enrolled infants placed in 1 of 4 cooling groups using a cooling blanket: (1) cooling for 72 hours to 33.5 °C; (2) cooling for 120 hours to 33.5 °C; (3) cooling for 72 hours to 32.0 °C or (4) cooling for 120 hours to 32.0 °C, all followed by slow re‐warming. Temperatures monitored by both oesophageal and skin probes. Infants will be examined at 18 to 22 months corrected age to assess their neurodevelopmental outcomes |
| Outcomes | Primary outcome: death or moderate‐to‐severe disability at 18 to 22 months Secondary outcomes: death; mild, moderate and severe disability; withdrawal of care; acute adverse events; clinical neonatal seizures; severe neonatal brain abnormalities; magnetic resonance imaging between 7 and 14 days; cognitive outcome; cerebral palsy; visual impairment; hearing impairment; multi‐organ dysfunction |
| Starting date | September 2010 |
| Contact information | Seetha Shankaran, M.D. and Rosemary D. Higgins, M.D. |
| Notes | Currently recruiting, estimated enrolment 726 subjects, anticipated completion 2017. All infants cooled |
| Trial name or title | Neuroprotective Effects of Hypothermia Combined With Inhaled Xenon Following Perinatal Asphyxia |
| Methods | Randomised controlled trial |
| Participants | Includes: infants 36 to 43 weeks' gestation with at least 1 of: Apgar score of < 5 at 10 minutes, continued need for resuscitation at 10 minutes, a pH < 7 or base deficit ≥ 16 within the first hour of life, and evidence of moderate‐to‐severe encephalopathy (lethargy, stupor or coma), and hypotonia or abnormal primitive reflexes. Additionally, infants had to have abnormal or suppressed background or seizures for at least 3 minutes on amplitude‐integrated electroencephalogram Excludes: initiation of hypothermia after 6 hours, randomisation after 12 hours of age, oxygen requirement > 70%, presence of other serious congenital abnormalities or the infant's condition appears terminal |
| Interventions | Enrolled infants randomised to either a treatment arm with 30% inhaled xenon for 24 hours or a control arm receiving standard care for hypoxic ischaemic encephalopathy. All infants receive hypothermia as well as standard intensive care |
| Outcomes | Primary outcome: reduction in lactate/N‐acetylaspartate (Lac/NAA) ratio on magnetic resonance spectroscopy or preserved fractional anisotropy measured on diffusion weighted magnetic resonance imaging Secondary outcome: clinical outcomes at hospital discharge |
| Starting date | February 2012 |
| Contact information | Denis Azzopardi, M.D. |
| Notes | Currently recruiting, estimated enrolment 130 subjects, anticipated completion 2013. All infants cooled |
| Trial name or title | MRI Thermal Imaging of Infants Undergoing Cooling for HIE |
| Methods | Non‐randomised observational study |
| Participants | Includes: infants in the neonatal intensive care unit who are treated with hypothermia for hypoxic ischaemic encephalopathy and who are scheduled to have magnetic resonance imaging (MRI) for evaluation of the extent of their injury Excludes: infants too unstable to have MRI scan (on cardiac pressor medications or more than 40% oxygen) or too active to obtain MRI without sedation |
| Interventions | Enrolled infants will undergo MRI evaluation of the N‐acetylaspartate (NAA)‐H20 frequency shift (for measuring relative temperature changes) from at least 5 regions of the brain during cooling and again after re‐warming |
| Outcomes | Primary outcome: brain temperature during cooling Secondary outcome: brain temperature on re‐warming Uniformity and patterns of temperature will be analysed and variations by modality of cooling will also be explored. MRI findings (temperature distribution) will also be compared to the MRI injury patterns and infant outcomes in order to determine if distribution of cooling is related to outcome |
| Starting date | May 2010 |
| Contact information | William F Walsh, M.D. |
| Notes | Currently recruiting, estimated enrolment 10 subjects, anticipated completion 2013. All infants cooled |
| Trial name or title | Pilot Study of Head Cooling in Preterm Infants With Hypoxic Ischemic Encephalopathy |
| Methods | Non‐randomised feasibility study |
| Participants | Includes: intubated infants 32 0/7‐35 6/7 weeks who are < 6 hours old gestation meeting criteria for hypoxic ischaemic encephalopathy (HIE) (Apgar 0 to 3 at 1, 5 and 10 minutes, pH < 7.0, base deficit > 15, with or without need for continued resuscitation due to hypoxia at 10 minutes, AND a physical examination with evidence of hypotonia or lethargy or seizures indicative of evolving HIE) Excludes: infants in extremis on clinical examination or survival not expected, evidence of head trauma or skull fracture causing major intracranial haemorrhage, intraventricular haemorrhage, weight less than the 5th percentile for gestational age, imperforate anus, refusal of consent |
| Interventions | Enrolled infants to undergo hypothermia via Olympic Cool Cap for up to 72 hours, with body temperature maintained in the normal range (36.1 °C to 37 °C rectally). Infants tracked until discharge with follow‐up at 6, 12 and 24 months of age |
| Outcomes | Primary outcome: measurement of rectal temperature in relation to cap temperature |
| Starting date | February 2008 |
| Contact information | William F Walsh, M.D. |
| Notes | Currently recruiting, estimated enrolment 5 subjects, anticipated completion 2013. Feasibility study, all infants cooled |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or major disability in survivors assessed, by method of cooling Show forest plot | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.83] |
| Analysis 1.1  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 1 Death or major disability in survivors assessed, by method of cooling. | ||||
| 1.1 Selective head cooling with mild systemic hypothermia | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.92] |
| 1.2 Whole body cooling | 5 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.84] |
| 2 Mortality, by method of cooling Show forest plot | 11 | 1468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| Analysis 1.2  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 2 Mortality, by method of cooling. | ||||
| 2.1 Selective head cooling with mild systemic hypothermia | 5 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 2.2 Whole body cooling | 6 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.89] |
| 3 Major neurodevelopmental disability by method of cooling Show forest plot | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.63, 0.94] |
| Analysis 1.3 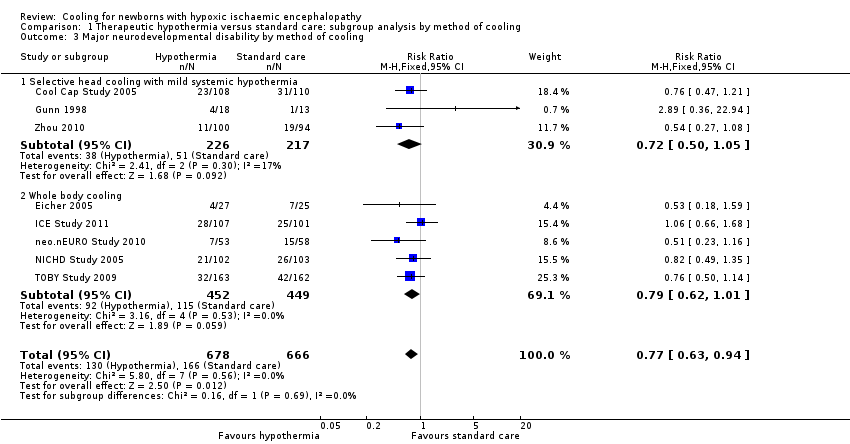 Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 3 Major neurodevelopmental disability by method of cooling. | ||||
| 3.1 Selective head cooling with mild systemic hypothermia | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 3.2 Whole body cooling | 5 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 4 Major neurodevelopmental disability in survivors assessed, by method of cooling Show forest plot | 8 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.80] |
| Analysis 1.4  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 4 Major neurodevelopmental disability in survivors assessed, by method of cooling. | ||||
| 4.1 Selective head cooling with mild systemic hypothermia | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.47, 0.94] |
| 4.2 Whole body cooling | 5 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.83] |
| 5 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by method of cooling Show forest plot | 6 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.94] |
| Analysis 1.5  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 5 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by method of cooling. | ||||
| 5.1 Selective head cooling with mild systemic hypothermia | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.29] |
| 5.2 Whole body cooling | 4 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.95] |
| 6 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by method of cooling Show forest plot | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.94] |
| Analysis 1.6  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 6 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by method of cooling. | ||||
| 6.1 Selective head cooling with mild systemic hypothermia | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.54, 1.36] |
| 6.2 Whole body cooling | 4 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.93] |
| 7 Neuromotor development (BSID PDI) in survivors assessed Show forest plot | 3 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐4.39, 5.94] |
| Analysis 1.7  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 7 Neuromotor development (BSID PDI) in survivors assessed. | ||||
| 7.1 Selective head cooling with mild systemic hypothermia | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐16.47, 2.87] |
| 7.2 Whole body cooling | 2 | 249 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐2.31, 9.91] |
| 8 Mental development (BSID MDI) in survivors assessed Show forest plot | 3 | 271 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [‐2.77, 7.71] |
| Analysis 1.8  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 8 Mental development (BSID MDI) in survivors assessed. | ||||
| 8.1 Selective head cooling with mild systemic hypothermia | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐10.30 [‐23.91, 3.31] |
| 8.2 Whole body cooling | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | 4.69 [‐0.98, 10.37] |
| 9 Cerebral palsy in survivors assessed Show forest plot | 7 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.54, 0.82] |
| Analysis 1.9  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 9 Cerebral palsy in survivors assessed. | ||||
| 9.1 Selective head cooling with mild systemic hypothermia | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.94] |
| 9.2 Whole body cooling | 4 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.52, 0.86] |
| 10 Blindness in survivors assessed Show forest plot | 7 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.01] |
| Analysis 1.10  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 10 Blindness in survivors assessed. | ||||
| 10.1 Selective head cooling with mild systemic hypothermia | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.23, 1.37] |
| 10.2 Whole body cooling | 5 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.17] |
| 11 Deafness in survivors assessed Show forest plot | 7 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.35, 1.26] |
| Analysis 1.11  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 11 Deafness in survivors assessed. | ||||
| 11.1 Selective head cooling with mild systemic hypothermia | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.36, 5.72] |
| 11.2 Whole body cooling | 5 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.25, 1.11] |
| 12 Outcome at 6 to 7 years of age Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 12 Outcome at 6 to 7 years of age. | ||||
| 12.1 Death or moderate‐to‐severe disability | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.04] |
| 12.2 Death | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.43, 0.94] |
| 12.3 Moderate‐to‐severe disability | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.57, 1.48] |
| 12.4 Cerebral palsy | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.31, 1.18] |
| 12.5 Blindness | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.03, 4.00] |
| 12.6 Deafness | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.26, 22.20] |
| 12.7 Seizures | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.25, 1.68] |
| 13 Sinus bradycardia Show forest plot | 8 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.59 [4.94, 27.17] |
| Analysis 1.13  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 13 Sinus bradycardia. | ||||
| 13.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.40 [2.05, 52.60] |
| 13.2 Whole body cooling | 4 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.06 [4.43, 32.85] |
| 14 Major arrhythmia Show forest plot | 8 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.12, 2.56] |
| Analysis 1.14  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 14 Major arrhythmia. | ||||
| 14.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.60] |
| 14.2 Whole body cooling | 4 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.99] |
| 15 Hypotension (mean arterial pressure < 40 mmHg) Show forest plot | 8 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| Analysis 1.15  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 15 Hypotension (mean arterial pressure < 40 mmHg). | ||||
| 15.1 Selective head cooling with mild systemic hypothermia | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.84, 1.36] |
| 15.2 Whole body cooling | 5 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.08] |
| 16 Hypotension requiring inotropic support Show forest plot | 6 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| Analysis 1.16 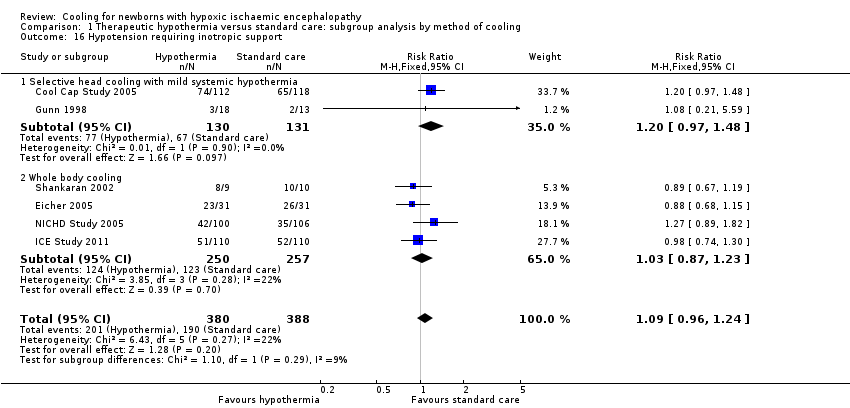 Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 16 Hypotension requiring inotropic support. | ||||
| 16.1 Selective head cooling with mild systemic hypothermia | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.97, 1.48] |
| 16.2 Whole body cooling | 4 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.87, 1.23] |
| 17 Anaemia requiring transfusion Show forest plot | 5 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.71, 1.43] |
| Analysis 1.17  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 17 Anaemia requiring transfusion. | ||||
| 17.1 Selective head cooling with mild systemic hypothermia | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.28, 1.80] |
| 17.2 Whole body cooling | 3 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.75, 1.58] |
| 18 Leukopenia Show forest plot | 4 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.85, 6.79] |
| Analysis 1.18  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 18 Leukopenia. | ||||
| 18.1 Selective head cooling with mild systemic hypothermia | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.22, 4.33] |
| 18.2 Whole body cooling | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.70 [1.02, 31.82] |
| 19 Thrombocytopenia Show forest plot | 8 | 1392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.05, 1.40] |
| Analysis 1.19  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 19 Thrombocytopenia. | ||||
| 19.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.09, 2.31] |
| 19.2 Whole body cooling | 5 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
| 20 Any coagulopathy Show forest plot | 7 | 1188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.93, 1.29] |
| Analysis 1.20  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 20 Any coagulopathy. | ||||
| 20.1 Selective head cooling with mild systemic hypothermia | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.56] |
| 20.2 Whole body cooling | 6 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.87, 1.30] |
| 21 Coagulopathy resulting in major thrombosis or haemorrhage Show forest plot | 4 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.58, 4.83] |
| Analysis 1.21  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 21 Coagulopathy resulting in major thrombosis or haemorrhage. | ||||
| 21.1 Selective head cooling with mild systemic hypothermia | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.16, 4.65] |
| 21.2 Whole body cooling | 2 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.62, 11.37] |
| 22 Hypoglycaemia Show forest plot | 7 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.60, 1.06] |
| Analysis 1.22  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 22 Hypoglycaemia. | ||||
| 22.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 22.2 Whole body cooling | 3 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.49, 0.98] |
| 23 Hypokalaemia Show forest plot | 5 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.08] |
| Analysis 1.23  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 23 Hypokalaemia. | ||||
| 23.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 23.2 Whole body cooling | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.15] |
| 24 Renal impairment Show forest plot | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| Analysis 1.24  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 24 Renal impairment. | ||||
| 24.1 Selective head cooling with mild systemic hypothermia | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 24.2 Whole body cooling | 3 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.51, 1.06] |
| 25 Oliguria Show forest plot | 6 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| Analysis 1.25  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 25 Oliguria. | ||||
| 25.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.24] |
| 25.2 Whole body cooling | 3 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.36] |
| 26 Sepsis Show forest plot | 8 | 1222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.26] |
| Analysis 1.26  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 26 Sepsis. | ||||
| 26.1 Selective head cooling with mild systemic hypothermia | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.25, 2.48] |
| 26.2 Whole body cooling | 5 | 940 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.30] |
| 27 Persistent pulmonary hypertension Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.27  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 27 Persistent pulmonary hypertension. | ||||
| 27.1 Whole body cooling | 4 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.94, 1.97] |
| 28 Treated with inhaled nitric oxide Show forest plot | 4 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.72, 1.92] |
| Analysis 1.28  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 28 Treated with inhaled nitric oxide. | ||||
| 28.1 Selective head cooling with mild systemic hypothermia | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.41, 7.90] |
| 28.2 Whole body cooling | 3 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.66, 1.88] |
| 29 Hepatic dysfunction Show forest plot | 6 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.05] |
| Analysis 1.29  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 29 Hepatic dysfunction. | ||||
| 29.1 Selective head cooling with mild systemic hypothermia | 2 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 29.2 Whole body cooling | 4 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.14] |
| 30 Gastric tube feeds at discharge Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.30 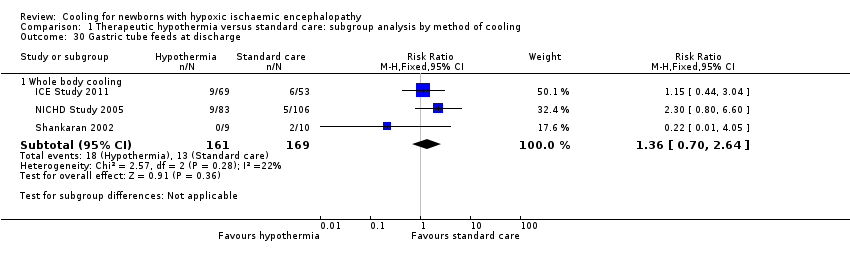 Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 30 Gastric tube feeds at discharge. | ||||
| 30.1 Whole body cooling | 3 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.70, 2.64] |
| 31 Seizures during initial hospitalisation Show forest plot | 8 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| Analysis 1.31  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 31 Seizures during initial hospitalisation. | ||||
| 31.1 Selective head cooling with mild systemic hypothermia | 3 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.10] |
| 31.2 Whole body cooling | 5 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 32 Seizures or need for anticonvulsant treatment at follow‐up Show forest plot | 4 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| Analysis 1.32  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 32 Seizures or need for anticonvulsant treatment at follow‐up. | ||||
| 32.1 Selective head cooling with mild systemic hypothermia | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.43, 2.00] |
| 32.2 Whole body cooling | 3 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.37] |
| 33 MRI abnormalities Show forest plot | 3 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.89] |
| Analysis 1.33  Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 33 MRI abnormalities. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or major disability in survivors assessed Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1  Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 1 Death or major disability in survivors assessed. | ||||
| 1.1 Infants with moderate encephalopathy | 5 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.84] |
| 1.2 Infants with severe encephalopathy | 5 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.93] |
| 2 Mortality Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.2  Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 2 Mortality. | ||||
| 2.1 Infants with moderate encephalopathy | 5 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.88] |
| 2.2 Infants with severe encephalopathy | 5 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.93] |
| 3 Major disability in survivors assessed Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.3  Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 3 Major disability in survivors assessed. | ||||
| 3.1 Infants with moderate encephalopathy | 5 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 3.2 Infants with severe encephalopathy | 5 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or major disability in survivors assessed Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.1  Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 1 Death or major disability in survivors assessed. | ||||
| 1.1 Intermediate aEEG | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 1.2 Severe aEEG | 2 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.03] |
| 2 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.2  Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 2 Mortality. | ||||
| 2.1 Intermediate aEEG | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.15] |
| 2.2 Severe aEEG | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.69, 2.72] |
| 3 Major disability in survivors assessed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 3.3  Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 3 Major disability in survivors assessed. | ||||
| 3.1 Intermediate aEEG | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 3.2 Severe aEEG | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.63, 2.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or major disability in survivors assessed, by quality of follow‐up Show forest plot | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.83] |
| Analysis 4.1  Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 1 Death or major disability in survivors assessed, by quality of follow‐up. | ||||
| 1.1 High‐quality follow‐up at 18 to 24 months | 7 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 1.2 Lower‐quality follow‐up at 12 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.41, 0.92] |
| 2 Major neurodevelopmental disability, by quality of follow‐up Show forest plot | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.63, 0.94] |
| Analysis 4.2  Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 2 Major neurodevelopmental disability, by quality of follow‐up. | ||||
| 2.1 High‐quality follow‐up at 18 to 24 months | 7 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.96] |
| 2.2 Lower‐quality follow‐up at 12 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.18, 1.59] |
| 3 Major neurodevelopmental disability in survivors assessed, by quality of follow‐up Show forest plot | 8 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.80] |
| Analysis 4.3  Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 3 Major neurodevelopmental disability in survivors assessed, by quality of follow‐up. | ||||
| 3.1 High‐quality follow‐up at 18 to 24 months | 7 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.82] |
| 3.2 Lower‐quality follow‐up at 12 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.97] |
| 4 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up Show forest plot | 6 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.94] |
| Analysis 4.4  Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 4 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up. | ||||
| 4.1 High‐quality follow‐up at 18 to 24 months | 5 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 0.99] |
| 4.2 Lower‐quality follow‐up at 12 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.97] |
| 5 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up Show forest plot | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.94] |
| Analysis 4.5  Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 5 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up. | ||||
| 5.1 High‐quality follow‐up at 18 to 24 months | 5 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.95] |
| 5.2 Lower‐quality follow‐up at 12 months | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.68] |

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 1 Death or major disability in survivors assessed, by method of cooling.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 2 Mortality, by method of cooling.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 3 Major neurodevelopmental disability by method of cooling.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 4 Major neurodevelopmental disability in survivors assessed, by method of cooling.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 5 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by method of cooling.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 6 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by method of cooling.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 7 Neuromotor development (BSID PDI) in survivors assessed.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 8 Mental development (BSID MDI) in survivors assessed.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 9 Cerebral palsy in survivors assessed.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 10 Blindness in survivors assessed.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 11 Deafness in survivors assessed.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 12 Outcome at 6 to 7 years of age.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 13 Sinus bradycardia.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 14 Major arrhythmia.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 15 Hypotension (mean arterial pressure < 40 mmHg).

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 16 Hypotension requiring inotropic support.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 17 Anaemia requiring transfusion.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 18 Leukopenia.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 19 Thrombocytopenia.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 20 Any coagulopathy.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 21 Coagulopathy resulting in major thrombosis or haemorrhage.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 22 Hypoglycaemia.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 23 Hypokalaemia.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 24 Renal impairment.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 25 Oliguria.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 26 Sepsis.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 27 Persistent pulmonary hypertension.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 28 Treated with inhaled nitric oxide.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 29 Hepatic dysfunction.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 30 Gastric tube feeds at discharge.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 31 Seizures during initial hospitalisation.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 32 Seizures or need for anticonvulsant treatment at follow‐up.

Comparison 1 Therapeutic hypothermia versus standard care: subgroup analysis by method of cooling, Outcome 33 MRI abnormalities.

Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 1 Death or major disability in survivors assessed.

Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 2 Mortality.

Comparison 2 Therapeutic hypothermia versus standard care: subgroup analysis by baseline severity of encephalopathy, Outcome 3 Major disability in survivors assessed.

Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 1 Death or major disability in survivors assessed.

Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 2 Mortality.

Comparison 3 Therapeutic hypothermia versus standard care: subgroup analysis by baseline amplitude‐integrated electroencephalogram (aEEG) findings, Outcome 3 Major disability in survivors assessed.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 1 Death or major disability in survivors assessed, by quality of follow‐up.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 2 Major neurodevelopmental disability, by quality of follow‐up.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 3 Major neurodevelopmental disability in survivors assessed, by quality of follow‐up.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 4 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up.

Comparison 4 Therapeutic hypothermia versus standard care: subgroup analysis by quality of follow‐up, Outcome 5 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or major disability in survivors assessed, by method of cooling Show forest plot | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.83] |
| 1.1 Selective head cooling with mild systemic hypothermia | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.92] |
| 1.2 Whole body cooling | 5 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.66, 0.84] |
| 2 Mortality, by method of cooling Show forest plot | 11 | 1468 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.64, 0.88] |
| 2.1 Selective head cooling with mild systemic hypothermia | 5 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.59, 1.04] |
| 2.2 Whole body cooling | 6 | 942 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.89] |
| 3 Major neurodevelopmental disability by method of cooling Show forest plot | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.63, 0.94] |
| 3.1 Selective head cooling with mild systemic hypothermia | 3 | 443 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.50, 1.05] |
| 3.2 Whole body cooling | 5 | 901 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.62, 1.01] |
| 4 Major neurodevelopmental disability in survivors assessed, by method of cooling Show forest plot | 8 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.80] |
| 4.1 Selective head cooling with mild systemic hypothermia | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.47, 0.94] |
| 4.2 Whole body cooling | 5 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.53, 0.83] |
| 5 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by method of cooling Show forest plot | 6 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.94] |
| 5.1 Selective head cooling with mild systemic hypothermia | 2 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.51, 1.29] |
| 5.2 Whole body cooling | 4 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.56, 0.95] |
| 6 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by method of cooling Show forest plot | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.94] |
| 6.1 Selective head cooling with mild systemic hypothermia | 2 | 153 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.54, 1.36] |
| 6.2 Whole body cooling | 4 | 514 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.93] |
| 7 Neuromotor development (BSID PDI) in survivors assessed Show forest plot | 3 | 271 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐4.39, 5.94] |
| 7.1 Selective head cooling with mild systemic hypothermia | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐6.80 [‐16.47, 2.87] |
| 7.2 Whole body cooling | 2 | 249 | Mean Difference (IV, Fixed, 95% CI) | 3.80 [‐2.31, 9.91] |
| 8 Mental development (BSID MDI) in survivors assessed Show forest plot | 3 | 271 | Mean Difference (IV, Fixed, 95% CI) | 2.47 [‐2.77, 7.71] |
| 8.1 Selective head cooling with mild systemic hypothermia | 1 | 21 | Mean Difference (IV, Fixed, 95% CI) | ‐10.30 [‐23.91, 3.31] |
| 8.2 Whole body cooling | 2 | 250 | Mean Difference (IV, Fixed, 95% CI) | 4.69 [‐0.98, 10.37] |
| 9 Cerebral palsy in survivors assessed Show forest plot | 7 | 881 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.54, 0.82] |
| 9.1 Selective head cooling with mild systemic hypothermia | 3 | 312 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.46, 0.94] |
| 9.2 Whole body cooling | 4 | 569 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.52, 0.86] |
| 10 Blindness in survivors assessed Show forest plot | 7 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.38, 1.01] |
| 10.1 Selective head cooling with mild systemic hypothermia | 2 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.23, 1.37] |
| 10.2 Whole body cooling | 5 | 588 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.35, 1.17] |
| 11 Deafness in survivors assessed Show forest plot | 7 | 720 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.35, 1.26] |
| 11.1 Selective head cooling with mild systemic hypothermia | 2 | 144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.36, 5.72] |
| 11.2 Whole body cooling | 5 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.25, 1.11] |
| 12 Outcome at 6 to 7 years of age Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Death or moderate‐to‐severe disability | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.64, 1.04] |
| 12.2 Death | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.43, 0.94] |
| 12.3 Moderate‐to‐severe disability | 1 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.57, 1.48] |
| 12.4 Cerebral palsy | 1 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.31, 1.18] |
| 12.5 Blindness | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.03, 4.00] |
| 12.6 Deafness | 1 | 113 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.38 [0.26, 22.20] |
| 12.7 Seizures | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.25, 1.68] |
| 13 Sinus bradycardia Show forest plot | 8 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.59 [4.94, 27.17] |
| 13.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.40 [2.05, 52.60] |
| 13.2 Whole body cooling | 4 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 12.06 [4.43, 32.85] |
| 14 Major arrhythmia Show forest plot | 8 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.12, 2.56] |
| 14.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.60] |
| 14.2 Whole body cooling | 4 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 3.99] |
| 15 Hypotension (mean arterial pressure < 40 mmHg) Show forest plot | 8 | 1221 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.92, 1.09] |
| 15.1 Selective head cooling with mild systemic hypothermia | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.84, 1.36] |
| 15.2 Whole body cooling | 5 | 939 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.90, 1.08] |
| 16 Hypotension requiring inotropic support Show forest plot | 6 | 768 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| 16.1 Selective head cooling with mild systemic hypothermia | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.97, 1.48] |
| 16.2 Whole body cooling | 4 | 507 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.87, 1.23] |
| 17 Anaemia requiring transfusion Show forest plot | 5 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.71, 1.43] |
| 17.1 Selective head cooling with mild systemic hypothermia | 2 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.28, 1.80] |
| 17.2 Whole body cooling | 3 | 489 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.75, 1.58] |
| 18 Leukopenia Show forest plot | 4 | 537 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.40 [0.85, 6.79] |
| 18.1 Selective head cooling with mild systemic hypothermia | 2 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.22, 4.33] |
| 18.2 Whole body cooling | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.70 [1.02, 31.82] |
| 19 Thrombocytopenia Show forest plot | 8 | 1392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [1.05, 1.40] |
| 19.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.09, 2.31] |
| 19.2 Whole body cooling | 5 | 937 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.98, 1.33] |
| 20 Any coagulopathy Show forest plot | 7 | 1188 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.93, 1.29] |
| 20.1 Selective head cooling with mild systemic hypothermia | 1 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.89, 1.56] |
| 20.2 Whole body cooling | 6 | 958 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.87, 1.30] |
| 21 Coagulopathy resulting in major thrombosis or haemorrhage Show forest plot | 4 | 689 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.58, 4.83] |
| 21.1 Selective head cooling with mild systemic hypothermia | 2 | 261 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.16, 4.65] |
| 21.2 Whole body cooling | 2 | 428 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [0.62, 11.37] |
| 22 Hypoglycaemia Show forest plot | 7 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.60, 1.06] |
| 22.1 Selective head cooling with mild systemic hypothermia | 4 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.63, 1.74] |
| 22.2 Whole body cooling | 3 | 554 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.49, 0.98] |
| 23 Hypokalaemia Show forest plot | 5 | 738 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.79, 1.08] |
| 23.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.79, 1.16] |
| 23.2 Whole body cooling | 2 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.69, 1.15] |
| 24 Renal impairment Show forest plot | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.02] |
| 24.1 Selective head cooling with mild systemic hypothermia | 3 | 272 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.80, 1.10] |
| 24.2 Whole body cooling | 3 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.51, 1.06] |
| 25 Oliguria Show forest plot | 6 | 865 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.76, 1.19] |
| 25.1 Selective head cooling with mild systemic hypothermia | 3 | 455 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.24] |
| 25.2 Whole body cooling | 3 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.70, 1.36] |
| 26 Sepsis Show forest plot | 8 | 1222 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.26] |
| 26.1 Selective head cooling with mild systemic hypothermia | 3 | 282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.25, 2.48] |
| 26.2 Whole body cooling | 5 | 940 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.30] |
| 27 Persistent pulmonary hypertension Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 27.1 Whole body cooling | 4 | 614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.94, 1.97] |
| 28 Treated with inhaled nitric oxide Show forest plot | 4 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.72, 1.92] |
| 28.1 Selective head cooling with mild systemic hypothermia | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [0.41, 7.90] |
| 28.2 Whole body cooling | 3 | 395 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.66, 1.88] |
| 29 Hepatic dysfunction Show forest plot | 6 | 975 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.74, 1.05] |
| 29.1 Selective head cooling with mild systemic hypothermia | 2 | 424 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.12] |
| 29.2 Whole body cooling | 4 | 551 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.67, 1.14] |
| 30 Gastric tube feeds at discharge Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 Whole body cooling | 3 | 330 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.70, 2.64] |
| 31 Seizures during initial hospitalisation Show forest plot | 8 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 31.1 Selective head cooling with mild systemic hypothermia | 3 | 273 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.85, 1.10] |
| 31.2 Whole body cooling | 5 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.78, 1.00] |
| 32 Seizures or need for anticonvulsant treatment at follow‐up Show forest plot | 4 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.59, 1.31] |
| 32.1 Selective head cooling with mild systemic hypothermia | 1 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.43, 2.00] |
| 32.2 Whole body cooling | 3 | 511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.37] |
| 33 MRI abnormalities Show forest plot | 3 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.60, 0.89] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or major disability in survivors assessed Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Infants with moderate encephalopathy | 5 | 472 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.84] |
| 1.2 Infants with severe encephalopathy | 5 | 283 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.72, 0.93] |
| 2 Mortality Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Infants with moderate encephalopathy | 5 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.41, 0.88] |
| 2.2 Infants with severe encephalopathy | 5 | 285 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.64, 0.93] |
| 3 Major disability in survivors assessed Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Infants with moderate encephalopathy | 5 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.50, 0.90] |
| 3.2 Infants with severe encephalopathy | 5 | 115 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.50, 1.12] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or major disability in survivors assessed Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Intermediate aEEG | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 1.2 Severe aEEG | 2 | 123 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.67, 1.03] |
| 2 Mortality Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Intermediate aEEG | 1 | 171 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.49, 1.15] |
| 2.2 Severe aEEG | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.69, 2.72] |
| 3 Major disability in survivors assessed Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Intermediate aEEG | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.19, 0.98] |
| 3.2 Severe aEEG | 1 | 26 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.63, 2.94] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or major disability in survivors assessed, by quality of follow‐up Show forest plot | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.68, 0.83] |
| 1.1 High‐quality follow‐up at 18 to 24 months | 7 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 1.2 Lower‐quality follow‐up at 12 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.41, 0.92] |
| 2 Major neurodevelopmental disability, by quality of follow‐up Show forest plot | 8 | 1344 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.63, 0.94] |
| 2.1 High‐quality follow‐up at 18 to 24 months | 7 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.64, 0.96] |
| 2.2 Lower‐quality follow‐up at 12 months | 1 | 52 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.18, 1.59] |
| 3 Major neurodevelopmental disability in survivors assessed, by quality of follow‐up Show forest plot | 8 | 917 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.55, 0.80] |
| 3.1 High‐quality follow‐up at 18 to 24 months | 7 | 889 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.56, 0.82] |
| 3.2 Lower‐quality follow‐up at 12 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.97] |
| 4 Neuromotor delay (BSID PDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up Show forest plot | 6 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.94] |
| 4.1 High‐quality follow‐up at 18 to 24 months | 5 | 629 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 0.99] |
| 4.2 Lower‐quality follow‐up at 12 months | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.37 [0.14, 0.97] |
| 5 Developmental delay (BSID MDI more than 2 SD below mean) in survivors assessed, by quality of follow‐up Show forest plot | 6 | 667 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.94] |
| 5.1 High‐quality follow‐up at 18 to 24 months | 5 | 638 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.59, 0.95] |
| 5.2 Lower‐quality follow‐up at 12 months | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.19, 1.68] |

