Bifosfonatos para el mieloma múltiple: un metanálisis en red actualizado
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Study design: Parallel, not double‐blind Study length: not reported Study conducted during: not reported (study started in 1999) | |
| Participants | Bisphosphonates: enrolled 196, analyzed 196. Sex (M/F): Bisphosphonates: 109/87 Age: mean(SD): Bisphosphonates: 59 (8) Inclusion criteria: Stage (Durie 2005): I‐III Osteolytic lesion: NR Creatinine: NS Calcium: NS Other criteria: No cytotoxic chemotherapy prior to entry | |
| Interventions | Pamidronate: 90 mg IV, every 4 weeks; Indefinitely. Pamidronate and thalidomide: 400 mg orally, dose reduction to a minimum dose of 50 mg was allowed for treatment‐related toxicity. | |
| Outcomes | Total skeletal‐related events; total mortality; response rates; ONJ. | |
| Notes | SRE: bone lesion requiring a specific therapy (chemotherapy, irradiation or surgery). Funding: Supported by a major grant from the Programme Hospitalier de Recherche Clinique and by the Swiss Group for Clinical Cancer Research (SAKK). COI statement included: The authors declare no competing financial interests. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) | High risk | Study does not involve any blinding. |
| Blinding of participants and personnel (performance bias) | High risk | Study does not involve any blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Study does not involve any blinding. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, not double‐blind; not placebo‐controlled study Study length: not reported Study conducted during: Mar 1999 ‐ Dec 2001 | |
| Participants | Bisphosphonates: enrolled 46, analyzed 46. Sex (M/F): Bisphosphonates: 26/20 Mean age (range): Bisphosphonates: 67.3 (43‐75) Inclusion criteria: Stage (Durie 2005): III Osteolytic lesion: At least one Creatinine: NS Calcium: NS Other criteria: No cytotoxic chemotherapy prior to entry | |
| Interventions | Zoledronate: 4 mg IV, every 4 weeks; indefinitely. Control: no zoledronate. | |
| Outcomes | Total mortality; PFS. | |
| Notes | SRE: appearance of a new lytic lesion (excluding skull), after patient began zoledronate or progression of previous bone lesion according to criteria of Union Internationale Centre le Cancer. Funding: Not reported. COI statement included: Not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Study does not involve any blinding. |
| Blinding of participants and personnel (performance bias) | High risk | Study does not involve any blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Study does not involve any blinding. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, open‐label; not placebo‐controlled study Study length: not reported Study conducted during: Jun 2002 – Dec 2007 | |
| Participants | Bisphosphonates: enrolled 151, analyzed 151. Sex (M/F): Bisphosphonates: 71/80 Mean age (range): Bisphosphonates: 56.4 (29‐65) Inclusion criteria: Stage (Durie 2005): IIB‐ IIIB Osteolytic lesion: NS Creatinine: creatinine clearance > 30 mL/min Calcium: NS Other criteria: Adult patients at least 18 years but less than 65 years of age with untreated symptomatic multiple myeloma and measurable paraprotein in serum and urine, Eastern Cooperative Oncology Group performance status 0–2, and adequate renal (no end‐stage renal failure and creatinine clearance > 30 mL/min), hematologic (platelet count > 50×109/L, neutrophil count > 0.75×109/L), and liver function were eligible | |
| Interventions | Zoledronate: IV; 4 mg (or dose‐adjusted based on creatinine clearance) once every 28 days for 24 months. Control: no zoledronate. | |
| Outcomes | SRE; overall survival *$; progression free survival *$; | |
| Notes | PFS is defined as time from the start of high‐dose induction therapy (or time from the start of next treatment) to time of progression, relapse, or death. The primary endpoints were PFS and OS at 10 years, and the secondary endpoints included overall response, rates of complete response and very good partial response, and safety. Funding: This study was supported entirely with resources from the Mexican Institute of Social Security. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation, but the study was not sponsored by Novartis or by any other pharmaceutical company. ProEd Communications, Inc., provided medical editorial assistance with this manuscript. COI statement included: Aside from support from Novartis Pharmaceuticals Corporation for medical editorial assistance, all authors declare that they have no financial conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) | High risk | Open‐label study |
| Blinding of participants and personnel (performance bias) | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | Open‐label study |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: Nov 1983 – Feb 1987 (enrollment in the high dose arm was stopped in Jun 1984) | |
| Participants | Bisphosphonates: enrolled 98, analyzed 92. Sex (M/F): Bisphosphonates: 60/32 Mean age (SD/range): not reported Inclusion criteria: Stage (Durie 2005): I‐III Osteolytic lesion: NR Creatinine: < 3 mg/dL Calcium: Normal or elevated Other criteria: No cytotoxic chemotherapy prior to entry | |
| Interventions | Etidronate: capsules (20 mg/kg for 28 days every other 28 days: but this arm was discontinued); enrollment took place for: 5 mg/kg until death or discontinuation. | |
| Outcomes | Vertebral index; total mortality*; pain; | |
| Notes | SRE: bone progression (appearances of new lesions or worsening of existing ones)$; mortality* (from the date of randomization); calcium reported as a dichotomous variable. Funding: Supported by Norwich Eaton Pharmaceuticals Inc. and The National Cancer Institute of Canada. COI statement included: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: Aug 1990 – Jun 1993 | |
| Participants | Bisphosphonates: enrolled 205, analyzed 198. Sex (M/F): Bisphosphonates: 108/88 Mean age (SD): Bisphosphonates: 64 (10) Inclusion criteria: Stage (Durie 2005): III only Osteolytic lesion: at least one Creatinine: < 5 mg/dL Calcium: NS Other criteria: No bone specific treatment prior to entry | |
| Interventions | Pamidronate: 90 mg in 500 mL of 5% dextrose in water, every 4 weeks for 24 months. | |
| Outcomes | SRE (total); | |
| Notes | SRE: pathologic fracture or radiation treatment/surgery on bone or spinal cord compression. Pain control assessment: Bone pain reported by authors at 29 months. Funding: Supported by a grant from the Pharmaceuticals Division, Ciba–Geigy Corporation, Summit, N.J. COI statement included: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are adequately described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Blinding of outcome assessment (detection bias) | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: Sept 1990 – Jul 1995 | |
| Participants | Bisphosphonates: enrolled 152, analyzed 152. Sex (M/F): Bisphosphonates: 83/69 Mean age (SD): Bisphosphonates: 69 (NR) Inclusion criteria: Stage (Durie 2005): II‐III Osteolytic lesion: NS Creatinine: < 2.8 mg/dL Calcium: Normal or elevated Other criteria: No cytotoxic chemotherapy prior to entry | |
| Interventions | Pamidronate: 75 mg capsules orally bid (total 300mg /day); for at least 2 years. | |
| Outcomes | Total mortality*$; SRE; | |
| Notes | SRE: bone fracture other than vertebral or surgery or increase in number of osteolytic lesions + vertebral collapse. Funding: not reported. COI statement included: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: Jan 1985 – Jun 1990 | |
| Participants | Bisphosphonates: enrolled 49, analyzed 39. Sex (M/F): Bisphosphonates: 22/27 Mean age (SD): Bisphosphonates: 65.6 (9.8) Inclusion criteria: Stage (Durie 2005): II‐III Osteolytic lesion: NS Creatinine: < 2.8 mg/dL Calcium: Normal or elevated Other criteria: No cytotoxic chemotherapy prior to entry | |
| Interventions | Etidronate: 10 mg/kg orally daily with lunch; for 4 months. | |
| Outcomes | Total mortality *$ ;SRE (total); | |
| Notes | SRE: new extraspinal osteolytic bone lesions or fractures or vertebral index; total mortality: total number of deaths reported in the text. Funding: Supported in part by Nativelle, Issy‐les‐Moulineaux, France and by INSERM ; etudes biochimiques et therpaeuteques du myeloma multiple, by association pour la researche contre le cancer and by Ligue nationale de lute contre le cancer. COI statement included: not reported. Pain control assessment: Analgesic use at 4 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) | High risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) | High risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: not reported | |
| Participants | Bisphosphonates: enrolled 7, analyzed 7. Sex (M/F): not reported Mean age (SD): Not reported Inclusion criteria: Stage (Durie 2005): NS Osteolytic lesion: NS Creatinine: < 1.8 mg/dL Calcium: Normal or elevated Other criteria: none | |
| Interventions | Clodronate: 1600 mg/day orally; for 24 months. | |
| Outcomes | SRE; | |
| Notes | SRE: new osteolytic lesions or fractures or vertebral index ($); Pain control assessment: Pain index at 12 months. Funding: Clodronate: Dichloromthylene diphosphonate (CI2MDP) was provided by Procter and Gamble Inc, USA. COI statement included: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, open‐label; not blinded, not placebo‐controlled phase IV study Study length: not reported Study conducted during: Jun 2010 – Jul 2012 | |
| Participants | Bisphosphonates: enrolled 51, analyzed 51. The original plan was to enroll 96 patients per‐group. After enrollment of 75 patients an interim analysis was performed which suggested a beneficial effect with zoledronate. No treatment: 49 enrolled, analyzed 49. Sex (M/F): Bisphosphonates: 30/21 Mean age (range): 68 (40 – 87) Inclusion criteria: Stage (Durie 2005): Asymptomatic patients; stage not specified Osteolytic lesion: NS Creatinine: NS Calcium: NS Other criteria: Confirmed biochemical relapse of MM after an initial response, without symptoms derived from the disease | |
| Interventions | Zoledronic acid: 4 mg in a 15‐minute intravenous infusion every 4 weeks, for a total of 12 doses, plus standard supportive care. No treatment: supportive care only. | |
| Outcomes | Time to new treatment, overall survival, response rate, time to clinical symptoms, skeletal‐related events, time to a skeletal‐related event, hypercalcemia, osteonecrosis of jaw and renal dysfunction. | |
| Notes | SRE: bone fracture (vertebral and non‐vertebral), requirement for bone radiotherapy, requirement for bone surgery, or hypercalcemia. Funding: supported by an unrestricted grant from Novartis Farmaceutica S.A., Barcelona, Spain and sponsored by GEM/PETHEMA. Part of the work was also supported by grants PS09/01450 and PI12/02311 from the Spanish “Institutode Salud Carlos III (ISCIII)” and Fondo Europeo de DesarrolloRegional (FEDER), the Spanish Ministry of Economy andCompetitiveness and the European Regional Development Fund(ERDF) “Una manera de hacer Europa” (Innocampus; CEI‐2010‐1‐0010), the grant RD12/0036/0069 from “Red Temáticade Investigación Cooperativa en Cáncer (RTICC), and grant GCB‐120981SAN from the “Asociación Española Contra elCáncer (AECC)”. COI statement included: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Study does not involve any blinding. |
| Blinding of participants and personnel (performance bias) | High risk | Study does not involve any blinding. |
| Blinding of outcome assessment (detection bias) | High risk | Study does not involve any blinding. |
| Incomplete outcome data (attrition bias) | Low risk | The process for early stoppage is clearly described |
| Other bias | High risk | It is unclear if the interim analysis was pre‐planned or post‐hoc |
| Intention to treat Analysis | Low risk | Benefits data on accrued patients are analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind, comparing 30 mg versus 90 mg pamidronate Study length: median follow‐up: 3.4 years (range: 1.1 – 5.7) Study conducted during: Jan 2001 – Aug 2005 | |
| Participants | Pamidornate 30 mg: enrolled 252, analyzed 198. No treatment: 49 enrolled, analyzed 49. Sex (M/F): Pamidronate 90 mg: 149/101 Mean age (range): not reported Inclusion criteria: Stage (Durie 2005): I‐III Osteolytic lesion: NS Creatinine: < 400 µmol/L Calcium: NS Other criteria: No prior treatment with bisphosphonates | |
| Interventions | Pamidronate: 90 mg in 500 mL of 5% dextrose in water, every 4 weeks for at least 36 months. | |
| Outcomes | SRE (total); | |
| Notes | SRE: pathologic fracture or radiation treatment/surgery on bone or spinal cord compression. Funding: Nordic Cancer Union and Novartis Healthcare. COI statement included: “PG has received grant support from Janssen‐Cilag, a speaker’s bureau from Celgene, and fee as chairman of the data monitoring committee for BioInvent. AW has received grant support from Janssen‐Cilag, fees for consultancy from Janssen‐Cilag and Pharmion, and payment for advisory board participation from Novartis. HH‐H has received speakers fees. The Nordic Myeloma Study Group has received grant support from Janssen‐Cilag, Celgene, Amgen, and Nordpharma. All other authors declared no conflicts of interest.” | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are adequately described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blind. |
| Blinding of participants and personnel (performance bias) | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Blinding of outcome assessment (detection bias) | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, placebo‐controlled study Study length: not reported Study conducted during: not reported (stay started in 1989) | |
| Participants | Total: 170; 13 withdrawn after treatment. premature termination in additional 75. Sex (M/F): not available for the entire study cohort Mean age (range): not reported Inclusion criteria: Stage (Durie 2005): I‐III Osteolytic lesion: NR Creatinine: < 2.5 mg/dL Calcium: NS Other criteria: none | |
| Interventions | Clodronate: 1600 mg/day orally; for 12 months. | |
| Outcomes | SRE; | |
| Notes | SRE: bone progression ($); effect on pain characterized as the number of patients without pain or no need for therapy. Pain control assessment: Analgesic use OR presence of pain at 9 months. Funding: not reported. COI statement included: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, not double‐blind, placebo‐controlled study Study length: not reported Study conducted during: not reported (study started in 1989) | |
| Participants | Bisphosphonates: analyzed 23; Placebo: analyzed 23. Sex (M/F): Bisphosphonates: 10/13 Mean age (SD): Bisphosphonates: 60 (10) Inclusion criteria: Stage (Durie 2005): II‐III Osteolytic lesion: NS Creatinine: unclear Calcium: NS Other criteria: none | |
| Interventions | Pamidronate 60 mg IV, every 4 weeks; indefinitely. | |
| Outcomes | Total mortality, vertebral fractures. | |
| Notes | Funding: not reported COI statement included: not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: not reported Study conducted during: 1986 and 1989 | |
| Participants | Bisphosphonates (clodronate): enrolled 168, analyzed 168. Sex (M/F): Bisphosphonates (clodronate): 84/84 Age: mean(SD): Bisphosphonates: Not reported Inclusion criteria: Stage (Durie 2005): NR Osteolytic lesion: NR Creatinine: NS Calcium: NS Other criteria: No prior use of bisphosphonates and capacity to tolerate systemic chemotherapy | |
| Interventions | Clodronate 2400 mg capsules orally daily. | |
| Outcomes | SRE (total); total mortality; vertebral fractures; non‐vertebral fractures; calcium** | |
| Notes | Total mortality reported as a total number of deaths. Pain control assessment: Pain index at 12 months. Funding: Supported by Huhtamaki Oy, Leiras, Turku and the Finnish Cancer Foundation. COI statement included: NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blind. |
| Blinding of participants and personnel (performance bias) | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Blinding of outcome assessment (detection bias) | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, not double‐blind; placebo‐controlled study Study length: NR Study conducted during: NR | |
| Participants | Bisphosphonates (pamidronate): enrolled 16, analyzed 16. Sex (M/F): Bisphosphonates: NR Age: mean(SD): Bisphosphonates: NR Inclusion criteria: Stage (Durie 2005): II‐II Osteolytic lesion: NS Creatinine: NS Calcium: NS Other criteria: Verbal rating scale > II | |
| Interventions | Pamidronate 90 mg daily IV. | |
| Outcomes | Pain (continuous data). | |
| Notes | Pain control assessment: Visual analogue scale. Funding: NR. COI statement included: NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: NR Study conducted during: 1986 to 1992 | |
| Participants | Bisphosphonates: enrolled/analyzed 264. Sex (M/F): Bisphosphonates: 1.33 ratio Age: Median (Inter‐quartile range) Bisphosphonates: 62 (55‐67) Inclusion criteria: Stage (Durie 2005): II‐II Osteolytic lesion: at least one Creatinine: any Calcium: normal or elevated Other criteria: No cytotoxic chemotherapy prior to entry | |
| Interventions | Clodronate 1600 mg orally daily. | |
| Outcomes | Total mortality*; SRE; total fractures; vertebral fractures; non‐vertebral fracture; pain; calcium.*** | |
| Notes | SRE: event‐free survival (pathological fractures or hypercalcemia), calculated from survival curves; outcome on calcium also reported as a dichotomous variable on the number of patients with hypercalcemia; pain calculated as the number of patients with maximal pain over 24 months. Pain control assessment: Severe pain at 24 months. Funding: Drug provided by Leiras Oy, Finland and MRC grant. COI statement included: NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blind. |
| Blinding of participants and personnel (performance bias) | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Blinding of outcome assessment (detection bias) | Low risk | Methods for blinding of participants and personnel are adequately described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blind; placebo‐controlled study Study length: NR Study conducted during: 1994 to 1996 | |
| Participants | Bisphosphonates: enrolled 107, analyzed 99. Sex (M/F): Bisphosphonates: 53/46 Age: Mean (SD) Bisphosphonates: 62.9 (NR) Inclusion criteria: Stage (Durie 2005): II‐II Osteolytic lesion: at least one Creatinine: ≤ 3 mg/dL Calcium: normal Other criteria: No bone specific treatment prior to entry | |
| Interventions | Ibandronate 2 mg IV every month. | |
| Outcomes | SRE (total)/year; | |
| Notes | SRE: pathological fractures or vertebral fractures, hypercalcemia, severe bone pain, and bone radiotherapy or surgery. Funding: Roche Diagnostics GmbH, Mannheim, Germany. COI statement included: NR. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | Low risk | Study is double‐blinded. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Study is double‐blinded but who was blinded is not mentioned. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, open‐label; comparing zoledronate versus clodronate Study length: NR Study conducted during: 1994 to 1996 | |
| Participants | Zoledronate: analyzed 981. Sex (M/F): Bisphosphonates: 53/46 Age: Mean (SD) Bisphosphonates: NR Inclusion criteria: Stage (Durie 2005): I‐III (International Staging System) Osteolytic lesion: NS Creatinine: <5.65 mg/dL Calcium: NS Other criteria: No previous or concurrent active malignancies, No acute renal failure (serum creatinine > 500 µmol/L and unresponsive to 72 hours of rehydration | |
| Interventions | Zoledronate 4 mg IV every 3‐4 weeks. | |
| Outcomes | Mortality; SREs; complete response; vertebral fractures, other fractures; hypercalcemia; renal failure; very good partial response; treatment‐related toxicities. | |
| Notes | SRE: vertebral fractures, other fractures, spinal cord compression, need for radiation or surgery to bone lesions, and new osteolytic bone lesions were recorded until disease progression. Complete response: negative immunofixation (100% M‐protein reduction) very good partial response: at least 90% M‐protein reduction with positive immunofixation. Funding: UK MRC, unrestricted educational grants from Novartis, Schering Health Care, Chugai, Pharmion, Celgene, and Ortho Biotech for trial coordination and the laboratory studies. Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals. COI statement included: "F.E. Davies has honoraria from Speakers Bureau of Celgene and is a consultant/advisory board member of Celgene and Novartis. G. Cook is a consultant/advisory board member of and has honoraria from Speakers Bureau of Celgene. R.G. Owen has honoraria from Speakers Bureau of Celgene and Ortho Biotech, United Kingdom. G.H. Jackson has honoraria from Speakers Bureau of Celgene and is a consultant/advisory board member of Celgene and J&J. No potential conflicts of interest were disclosed by the other authors." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are adequately described. |
| Allocation concealment (selection bias) | Low risk | Methods of allocation concealment are adequately described. |
| Blinding (performance bias and detection bias) | High risk | Open‐label study. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, not double‐blind; not placebo‐controlled Study length: NR Study conducted during: 1996 to 2001 | |
| Participants | Bisphosphonates: analyzed 45. Sex (M/F): Bisphosphonates: 26/19 Age: Median (range) Bisphosphonates: 67 (47‐79) Inclusion criteria: Stage (Durie 2005): I‐II Osteolytic lesion: Any Creatinine: NS Calcium: NS Other criteria: No cytotoxic chemotherapy prior to entry | |
| Interventions | Pamidronate 60 mg IV, every month. | |
| Outcomes | Total skeletal‐related events; PFS, adverse events. | |
| Notes | SRE: single/multiple osteolytic lesions, pathological fractures and/or hypercalcemia. Funding: NR COI statement included: No. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel, not double‐blind; not placebo‐controlled Study length: NR Study conducted during: 1996 to 2001 | |
| Participants | Bisphosphonates: enrolled 81, analyzed 81. Sex (M/F): Bisphosphonates: 43/38 Age: Median (range) Bisphosphonates: 66 (41‐82) Inclusion criteria: Stage (Durie 2005): I Osteolytic lesion: Any Creatinine: < 1.2 mg/dL Calcium: < 10 mg/dL Other criteria: No cytotoxic chemotherapy prior to entry | |
| Interventions | Zoledronate 4 mg IV, every month. | |
| Outcomes | SRE (total); PFS; ONJ. | |
| Notes | SRE: single/multiple osteolytic lesions, pathological fractures and/or hypercalcemia. The trial was prematurely stopped due to ONJ case in patient receiving zoledronate. Funding: NR. COI statement included: No. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are described. |
| Allocation concealment (selection bias) | Low risk | Methods used for concealment of allocation are described. |
| Blinding (performance bias and detection bias) | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | Low risk | Alpha and beta errors are prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel, double‐blinded; double dummy; stratified; not placebo‐controlled Study length: 25 months Study conducted during: 1998 to 2000 | |
| Participants | Zoledronate: enrolled 564, analyzed 561 Sex (M/F): Zoledronate: NR Age: Mean (SD) Zoledronate: 58 (NR) Inclusion criteria: Stage (Durie 2005): III Osteolytic lesion: at least one Creatinine: <= 3 mg/dL Calcium: <= 12 mg/dL Other criteria: Serum bilirubin ≤ 2.5 mg/dL. No prior treatment with bisphosphonates within 12 months of the screening visit | |
| Interventions | Zoledronate 4 mg IV, every 4 weeks. | |
| Outcomes | SREs | |
| Notes | SREs were defined as pathologic fracture, spinal cord compression, radiation therapy to bone, and surgery to bone. Funding: Novartis Pharmaceuticals Corporation. COI statement included: "Dr. Seaman, Dr. Chen, and Dr. Reitsma are employed by Novartis Pharmaceuticals and may own stock in the company; Dr. Coleman has received honoraria from Novartis; and Dr. Hussein has received a research grant from Novartis." | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods used for generation of sequence of randomization are described. |
| Allocation concealment (selection bias) | Low risk | Methods used for concealment of allocation are described. |
| Blinding (performance bias and detection bias) | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are adequately described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel; open‐label; placebo‐controlled Study length: 48 months Study conducted during: 2000 to 2008 | |
| Participants | Bisphosphonates: enrolled72; analyzed 71. Sex (M/F): Zoledronate: 30/42 Age: Mean (SD) Zoledronate: 58.8 (12.02) Inclusion criteria: Stage (Durie 2005): Asymptomatic patients stage I Osteolytic lesion: at least one Creatinine: NS Calcium: NS Other criteria: Patients with evidence of paraprotein in the serum or urine and bone marrow infiltration with plasma cells which represent more than 10% of the nucleated cells. | |
| Interventions | Zoledronate 4 mg IV (or dose‐adjusted based on creatinine clearance) monthly. | |
| Outcomes | Days of PFS; SREs (defined as: pathologic fracture, initiation of radiotherapy or surgery on bone, spinal cord compression or hypercalcemia); adverse events. | |
| Notes | PFS was defined as time from date of randomization to death from any cause or one of the following events:
The trial was stopped early due to slow recruitment. Funding: Novartis Pharmaceuticals Corporation. COI statement included: No. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | Unclear risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Study is not blinded |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | Low risk | Dropouts are described. |
| Other bias | Unclear risk | Alpha and beta errors are not prespecified. However, a total sample size of 220 patients was specified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel; not double‐blind; not placebo‐controlled Study length: NR Study conducted during: NR | |
| Participants | Bisphosphonates: enrolled/analyzed 32. Sex (M/F): Pamidronate: 18/14 Age: Median (range) Pamidronate: 68 (55‐78) Inclusion criteria: Stage (Durie 2005): Stage I‐III Osteolytic lesion: NS Creatinine: <5 mg/dL Calcium: NS Other criteria: No prior treatment with any kind of bisphosphonate within 3 months before enrollment or calcitonine or mithramycin within 2 weeks before enrollment , or treatment with corticosteroids for any reason except part of the patient's chemotherapeutic regimen. | |
| Interventions | Pamidronate: 90 mg IV monthly. | |
| Outcomes | Total mortality;* | |
| Notes | Data provided by the authors of the article. Pain control assessment: Opiate usage Funding: NR COI statement included: No. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | High risk | Benefits data are not analyzed according to ITT principle. |
| Methods | Study design: Parallel; Not double‐blind; not placebo‐controlled Study length: NR Study conducted during: 1999 to 2001 | |
| Participants | Pamidronate: enrolled 23, analyzed 23. Sex (M/F): Pamidronate: 12/11 Age: Median (range) Pamidronate: 66 (55‐78) Inclusion criteria: Stage (Durie 2005): Stage II‐III Osteolytic lesion: at least one Creatinine: >4 mg/dL Calcium: NS Other criteria: No prior treatment with any kind of bisphosphonate within 3 months before enrollment or calcitonine or mithramycin within 2 weeks before enrollment , or treatment with corticosteroids for any reason except part of the patient's chemotherapeutic regimen. | |
| Interventions | Pamidronate: 90 mg IV monthly. | |
| Outcomes | Hypocalcemia, hypercalcemia.**** | |
| Notes | Funding: NR. COI statement included: No. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Blinding methods are not described. |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
| Methods | Study design: Parallel; not double‐blind; not placebo‐controlled Study length: NR Study conducted during: 2004 to 2009 | |
| Participants | Bisphosphonates: enrolled/analyzed 33. Sex (M/F): Clodronate: 23/10 Age: Mean (SD) Clodronatee: 59.9 (8.1) Inclusion criteria: Stage (Durie 2005): Stage II‐III Osteolytic lesion: NS Creatinine: NS Calcium: NS Other criteria: NS | |
| Interventions | Clodronate: During the intermittent period of chemotherapy, clodronate injection 300 mg (Bonefos®, Bayer Schering Pharma, Leverkusen, Germany) was administered for 5 days in 250 mL glucose injection through slow intravenous drip; after that, Bonefos® capsules were administered orally at 1600 mg every day in the morning. | |
| Outcomes | Bone metabolic markers | |
| Notes | Other notes: This study did not report any outcome of interest for this systematic review. Funding: NR. COI statement included: No. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Methods used for generation of sequence of randomization are not described. |
| Allocation concealment (selection bias) | High risk | Methods used for concealment of allocation are not described. |
| Blinding (performance bias and detection bias) | High risk | Study is not blinded |
| Blinding of participants and personnel (performance bias) | High risk | Who was blinded is not described. |
| Blinding of outcome assessment (detection bias) | High risk | Who was blinded is not described. |
| Incomplete outcome data (attrition bias) | High risk | Dropouts are not described. |
| Other bias | High risk | Alpha and beta errors are not prespecified. |
| Intention to treat Analysis | Low risk | Benefits data are analyzed according to ITT principle. |
COI: conflict of interest; ITT: intention‐to‐treat; IV: intravenous; MM: multiple myeloma; ONJ: osteonecrosis of the jaw; OS: overall survival; PFS:progression‐free survival; SD: standard deviation; SRE: skeletal‐related events.
* mortality data obtained from authors; *$ mortality data derived using the Tierney method
# total number of deaths reported in Berenson 1996
$ defined by reviewers
**hypercalcemia defined as > 2.65 mmol/L
&hypercalcemia defined as > 2.75 mmol/L
***hypercalcemia defined as > 3.00 mmol/L
**** hypercalcemia defined as presence of symptoms or serum calcium concentration, corrected for the serum albumin concentration, of at least 12.0 mg/dL or 3.0 mmol/L
! Data obtained from (author Fontana et al) and data from previous publication (abstract) were used
‐‐‐‐‐‐‐‐‐‐
The most common adverse effect that was reported was related to gastrointestinal symptoms (abdominal pain, diarrhea, pancreatitis). The number of patients with highest number of gastrointestinal symptoms was recorded and combined in the final analysis (since often it was not clear whether the same patients had one or more gastrointestinal symptoms). Effects on other organs (blood, kidney, liver, etc) were sporadically reported, and therefore not systematically extracted. However, the narrative summary was presented in the review.
__________
Effect on pain was non uniformly described. Data were extractable from 8 trials. (Study by Brincker et al reported data as the number of pain episodes instead of the number of patients with pain. Paper by Belch et al did not report data in an extractable form.) Study by McCloskey et al reported effect on back pain only, while other studies reported effect on 'pain' without specifying site of pain. The study by Lahtinen et al also reported pain according to its severity. However, we extracted data on the number of patients with pain on bisphosphonates vs. placebo, except in the study by McCloskey et al, where the effect on pain refers to patients without 'marked improvement in back pain'.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Nonrandomized study | |
| Nonrandomized study | |
| Nonrandomized study | |
| Nonrandomized and a combination therapy | |
| Observational phase IV study | |
| Nonrandomized and a combination therapy | |
| Cost‐effectiveness study | |
| Phase II denosumab randomized controlled trial | |
| Denosumab randomized controlled trial | |
| Duplicate publication | |
| Enrolled only 9 patients. Only one pathological fracture was reported among 6 patients enrolled in zoledronate arm and one pathological fracture among 3 patients enrolled in pamidronate arm. | |
| Study addressing data from denosumab randomized controlled trials | |
| No data of interest | |
| Nonrandomized and a combination therapy | |
| Nonrandomized and a combination therapy | |
| Observational study | |
| Nonrandomized study | |
| Prognostic study | |
| Combination therapy | |
| Denosumab randomized controlled trial | |
| Phase II denosumab randomized controlled trial | |
| Nonrandomized study | |
| Patients in both the study arms received the same dose of zoledronate |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Prolonged protection from bone disease in multiple myeloma (Magnolia) |
| Methods | An open‐label phase 3 multicenter international randomised trial |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Active Comparator: zoledronic acid (treatment with zoledronic acid for 4 years) Placebo Comparator: no treatment (treatment with zoledronic acid withheld after two years) |
| Outcomes | Primary Outcome Measures:
Secondary Outcome Measures:
|
| Starting date | January 2015 |
| Contact information | Name: Thomas Lund, MD Ph.D. Phone: +45 21450256; Email: [email protected] |
| Notes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 14 | 2706 | Hazard Ratio (Random, 95% CI) | 0.90 [0.76, 1.07] |
| Analysis 1.1  Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 1 Mortality. | ||||
| 1.1 Etidronate | 2 | 244 | Hazard Ratio (Random, 95% CI) | 1.24 [0.86, 1.80] |
| 1.2 Clodronate | 3 | 885 | Hazard Ratio (Random, 95% CI) | 0.93 [0.66, 1.29] |
| 1.3 Pamidronate | 5 | 977 | Hazard Ratio (Random, 95% CI) | 0.85 [0.67, 1.07] |
| 1.4 Ibandronate | 1 | 198 | Hazard Ratio (Random, 95% CI) | 1.07 [0.69, 1.64] |
| 1.5 Zoledronate | 3 | 402 | Hazard Ratio (Random, 95% CI) | 0.57 [0.43, 0.75] |
| 2 Progression‐free survival Show forest plot | 7 | 908 | Hazard Ratio (Random, 95% CI) | 0.75 [0.57, 1.00] |
| Analysis 1.2  Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 2 Progression‐free survival. | ||||
| 2.1 Clodronate | 1 | 26 | Hazard Ratio (Random, 95% CI) | 0.63 [0.17, 2.34] |
| 2.2 Pamidronate | 1 | 177 | Hazard Ratio (Random, 95% CI) | 1.24 [0.66, 2.33] |
| 2.3 Zoledronate | 5 | 705 | Hazard Ratio (Random, 95% CI) | 0.70 [0.52, 0.95] |
| 3 Vertebral fractures Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| Analysis 1.3  Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 3 Vertebral fractures. | ||||
| 3.1 Clodronate | 3 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.89] |
| 3.2 Pamidronate | 3 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.40, 1.20] |
| 3.3 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.61, 1.81] |
| 4 Non‐vertebral fractures Show forest plot | 6 | 1389 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.68, 1.56] |
| Analysis 1.4  Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 4 Non‐vertebral fractures. | ||||
| 4.1 Clodronate | 3 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.31] |
| 4.2 Pamidronate | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.95, 2.87] |
| 4.3 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.79, 1.98] |
| 5 Total skeletal‐related events Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.88] |
| Analysis 1.5  Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 5 Total skeletal‐related events. | ||||
| 5.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.39, 1.39] |
| 5.2 Clodronate | 1 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.65, 0.89] |
| 5.3 Pamidronate | 3 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.59, 0.91] |
| 5.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.35] |
| 5.5 Zoledronate | 4 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.89] |
| 6 Pain Show forest plot | 8 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.95] |
| Analysis 1.6 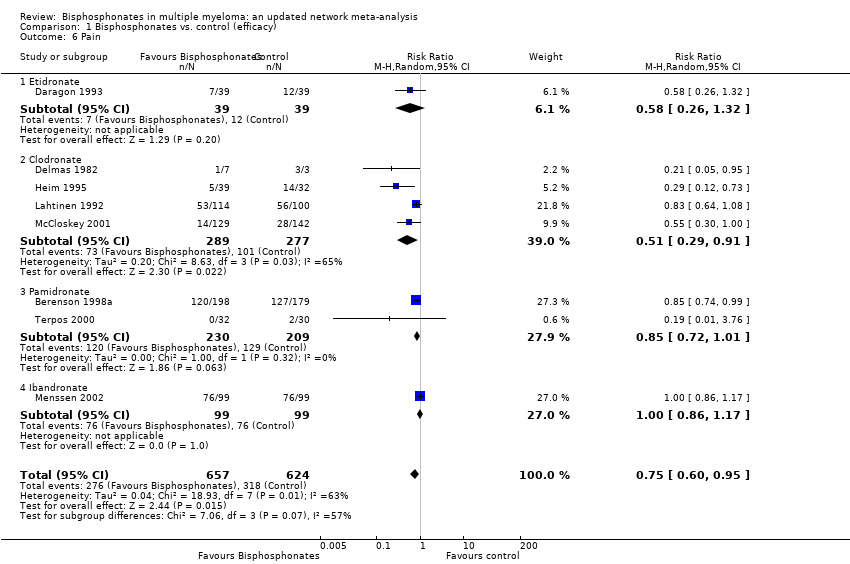 Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 6 Pain. | ||||
| 6.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.32] |
| 6.2 Clodronate | 4 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.29, 0.91] |
| 6.3 Pamidronate | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 6.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.86, 1.17] |
| 7 Incidence of hypercalcemia Show forest plot | 10 | 2174 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.56, 1.09] |
| Analysis 1.7  Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 7 Incidence of hypercalcemia. | ||||
| 7.1 Etidronate | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.73, 2.38] |
| 7.2 Clodronate | 3 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.45, 1.31] |
| 7.3 Pamidronate | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.31, 1.33] |
| 7.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.42] |
| 7.5 Zoledronate | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Osteonecosis of jaw Show forest plot | 6 | 1284 | Risk Ratio (M‐H, Random, 95% CI) | 4.61 [0.99, 21.35] |
| Analysis 2.1  Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 1 Osteonecosis of jaw. | ||||
| 1.1 Pamidronate | 2 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [0.13, 74.69] |
| 1.2 Zoledronate | 4 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 5.21 [0.91, 29.90] |
| 2 Gastrointestinal toxicity (grade III/IV) Show forest plot | 7 | 1829 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.95, 1.59] |
| Analysis 2.2  Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 2 Gastrointestinal toxicity (grade III/IV). | ||||
| 2.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.94] |
| 2.2 Clodronate | 2 | 872 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.82, 1.72] |
| 2.3 Pamidronate | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.90, 1.88] |
| 2.4 Zoledronate | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.23] |
| 3 Hypocalcaemia Show forest plot | 3 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.49, 9.74] |
| Analysis 2.3 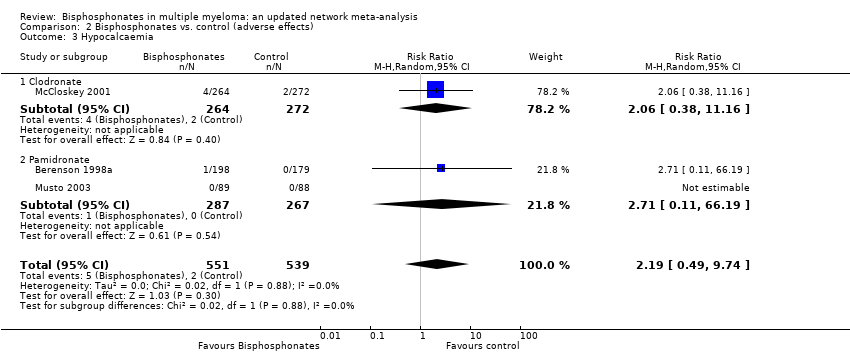 Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 3 Hypocalcaemia. | ||||
| 3.1 Clodronate | 1 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.38, 11.16] |
| 3.2 Pamidronate | 2 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.11, 66.19] |
| 4 Renal dysfunction Show forest plot | 2 | 414 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐9.75, 9.03] |
| Analysis 2.4  Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 4 Renal dysfunction. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Allocation concealment (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| Analysis 3.1 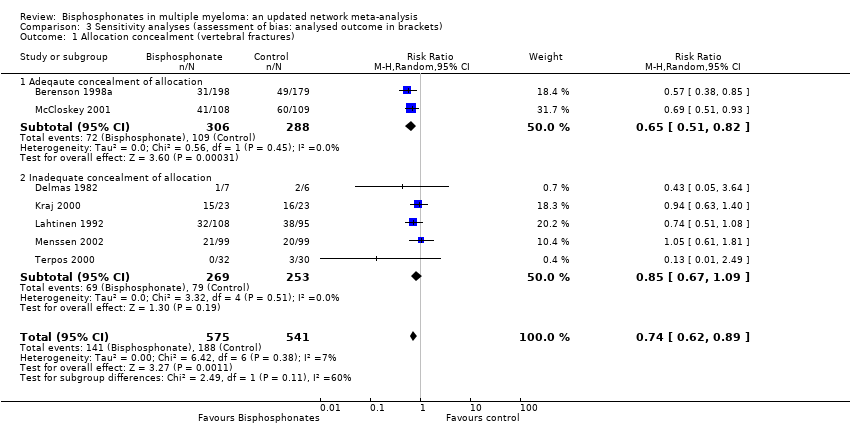 Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 1 Allocation concealment (vertebral fractures). | ||||
| 1.1 Adeqaute concealment of allocation | 2 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.51, 0.82] |
| 1.2 Inadequate concealment of allocation | 5 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 2 Blinding (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| Analysis 3.2  Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 2 Blinding (vertebral fractures). | ||||
| 2.1 Double‐blind | 5 | 1008 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.85] |
| 2.2 Not blinded | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.08, 3.72] |
| 3 Randomization method (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| Analysis 3.3  Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 3 Randomization method (vertebral fractures). | ||||
| 3.1 Randomization method is described | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.85] |
| 3.2 Randomization method is NOT described | 6 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.94] |
| 4 Type of data analysis (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| Analysis 3.4  Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 4 Type of data analysis (vertebral fractures). | ||||
| 4.1 Intention‐to‐treat analysis | 3 | 463 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.55, 1.22] |
| 4.2 Per protocol analysis | 4 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.89] |
| 5 Description of withdrawals and drop outs (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| Analysis 3.5  Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 5 Description of withdrawals and drop outs (vertebral fractures). | ||||
| 5.1 Withdrawals and dropouts well described | 3 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.55, 0.82] |
| 5.2 Withdrawals and dropouts NOT described | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.29] |
| 6 Alpha error (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| Analysis 3.6  Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 6 Alpha error (vertebral fractures). | ||||
| 6.1 Alpha error pre‐specified | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 6.2 Alpha error NOT pre‐specified | 6 | 913 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.94] |
| 7 Beta error (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| Analysis 3.7  Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 7 Beta error (vertebral fractures). | ||||
| 7.1 Beta error pre‐specified | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 7.2 Beta error NOT pre‐specified | 6 | 913 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.94] |
| 8 Gastrointestinal toxicity (grade III/IV: oral vs IV bisphosphonates)) Show forest plot | 7 | 1829 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.95, 1.59] |
| Analysis 3.8  Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 8 Gastrointestinal toxicity (grade III/IV: oral vs IV bisphosphonates)). | ||||
| 8.1 Oral route | 4 | 1250 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.89, 1.70] |
| 8.2 Intervenous route | 3 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.81, 1.90] |

Bisphosphonate chemical structures

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
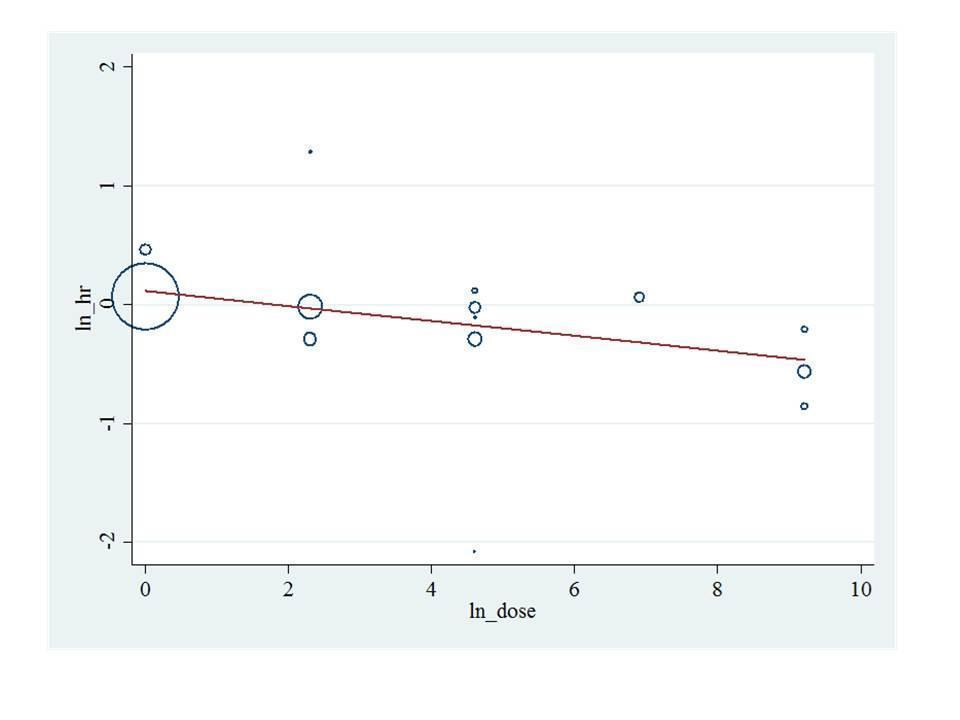
Bisphosphonate potency metaregression for overall survival. HR: Hazard ratio.

Randomized controlled trial (RCT) network for overall survival (OS), progression free survival (PFS) and skeletal related events (SREs).

A: Ranking probabilities of competing bisphosphonates. The size of each bar corresponds to the probability of each treatment to be at a specific rank. OS: Overall survival; PFS: Progression‐free survival; SRE: Skeletal‐related events; Osteonecrosis; GI: Gastrointestinal toxicity; Hyper: Hypercalcemia.
B: Surface under the cumulative ranking curve (SUCRA) plots for each treatment. The outcomes are listed on the horizontal axis. SUCRA for each outcome are on the vertical axis.

Funnel plot of comparison: 1 Bisphosphonates vs. control (efficacy), outcome: 1.6 Pain.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 1 Mortality.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 2 Progression‐free survival.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 3 Vertebral fractures.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 4 Non‐vertebral fractures.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 5 Total skeletal‐related events.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 6 Pain.

Comparison 1 Bisphosphonates vs. control (efficacy), Outcome 7 Incidence of hypercalcemia.

Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 1 Osteonecosis of jaw.

Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 2 Gastrointestinal toxicity (grade III/IV).

Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 3 Hypocalcaemia.

Comparison 2 Bisphosphonates vs. control (adverse effects), Outcome 4 Renal dysfunction.

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 1 Allocation concealment (vertebral fractures).

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 2 Blinding (vertebral fractures).

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 3 Randomization method (vertebral fractures).

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 4 Type of data analysis (vertebral fractures).

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 5 Description of withdrawals and drop outs (vertebral fractures).

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 6 Alpha error (vertebral fractures).

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 7 Beta error (vertebral fractures).

Comparison 3 Sensitivity analyses (assessment of bias: analysed outcome in brackets), Outcome 8 Gastrointestinal toxicity (grade III/IV: oral vs IV bisphosphonates)).
| Bisphosphonates in multiple myeloma | |||||
| Patient or population: patients with multiple myeloma Control: no treatment/placebo | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | |
| Assumed risk | Corresponding risk | ||||
| Control | Bisphosphonates | ||||
| Overall survival## | Medium‐risk population# | HR 0.90 | 2706 | ⊕⊕⊕⊝ | |
| 410 per 1000 | 378 per 1000 | ||||
| Progression‐free survival### | Medium‐risk population# | HR 0.75 | 908 | ⊕⊕⊝⊝ | |
| 470 per 1000 | 379 per 1000 | ||||
| Vertebral fractures | Medium‐risk population# | RR 0.74 | 1116 | ⊕⊕⊕⊝ | |
| 360 per 1000 | 266 per 1000 | ||||
| Non‐vertebral fractures | Medium‐risk population# | RR 1.03 | 1389 | ⊕⊕⊕⊝ | |
| 140 per 1000 | 144 per 1000 | ||||
| Skeletal‐related events | Medium‐risk population# | RR 0.74 | 2141 | ⊕⊕⊕⊝ | |
| 400 per 1000 | 296 per 1000 | ||||
| Pain | Medium‐risk population | RR 0.75 | 1281 | ⊕⊝⊝⊝ | |
| 540 per 1000 | 410 per 1000 | ||||
| Osteonecrosis of jaw | Medium‐risk population# | RR 4.61 (0.99 to 21.35) | 1284 | ⊕⊕⊝⊝ low10,11 | |
| NE | 0 per 1000 | ||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||
| GRADE Working Group grades of evidence | |||||
| 1 A total of 20 RCTs were included in the direct meta‐analysis. Only 35% (7/20) of trials had adequate allocation concealment. Only 20% (4/20) of trials reported methods of randomization. Similarly, 15% (3/20) of trials reported blinding procedures and personnel who were blinded to the intervention assignment. However, sensitivity analyses based on the methodological quality domains did not change the estimates. Hence, the assessment of studies’ limitations may represent the poor quality of reporting rather than true biased estimates. 9 Downgraded the quality of evidence by one level due to variation in assessment of pain based on blinding of the assessors. Only 15% (3/20) of trials reported blinding procedures and personnel who were blinded to the intervention assignment. Moreover, we found that RCTs with double‐blinding showed no significant benefit of bisphosphonates over placebo for amelioration of pain, while non‐blinded RCTs favored bisphosphonates over placebo for pain relief. We also downgraded the quality of evidence by one level due to imprecision. 10 Downgraded the quality of evidence by one level due to the potential for publication bias.The Osteonecrosis of jaw data were extractable from 30% (6/20) of studies eligible for direct meta‐analysis. 11 Downgraded the quality of evidence by one level due to imprecision.All included RCTs and also the pooled estimate have wide confidence intervals. # The moderate control risk was calculated via GRADEpro software based on average risk in the control arm of the included studies. ## We have calculated and presented overall mortality instead of OS. The expected events represent a median timeline of 5 years. ### PFS events represent death or progress or relapse. The expected events represent a median timeline of 5 years. | |||||
| Type of bisphosphonates | Bisphosphontes | Relative potency |
| Nonaminobisphosphonates | Etidronate | 1 |
| Clodronate | 10 | |
| Tiludronate | 10 | |
| Aminobisphosphonates | Pamidronate | 100 |
| Alendronate | 500 | |
| Ibandronate | 1,000 | |
| Risendronate | 2,000 | |
| Zoledronate | 10,000 | |
| Based on information from (Drake 2008; Dunford 2001). | ||
| Study ID | Adverse events (gastrointestinal symptoms) | Adverse events (hypocalcemia) | Adverse events (serum creatinine) | Adverse events (osteonecrosis of the jaw) |
| No | No | No | No | |
| Yes | Yes | No | No | |
| Yes | No | No | No | |
| No | No | No | No | |
| Yes | No | Yes | No | |
| No | No | No | No | |
| Yes | No | Yes | No | |
| Yes | Yes | No | No | |
| Yes | No | No | No | |
| No | Yes | No | No | |
| No | No | No | No | |
| No | No | No | Yes | |
| No | No | No | Yes | |
| No | No | No | Yes | |
| No | No | No | No | |
| No | No | No | No | |
| No | No | No | No | |
| Yes | No | No | Yes | |
| No | No | No | No | |
| No | No | No | Yes | |
| No | No | No | Yes | |
| Yes | No | No | Yes | |
| No | No | No | No | |
| No | No | No | Yes |
| MTC method (REM) | ||||||||
| Outcome | Treatment1 | Treatment2 | NRCTs | Patients | HR/RR | 95% LCRL | 95% UCRL | Quality of the evidence (GRADE) |
| OS | PL | CLO | 16 | 5260 | 1.19 | 0.88 | 1.63 | ⊕⊕⊕⊝ moderate |
| OS | ETI | CLO | 16 | 5260 | 1.48 | 0.96 | 2.51 | ⊕⊕⊕⊝ moderate |
| OS | IBAN | CLO | 16 | 5260 | 1.34 | 0.60 | 2.62 | ⊕⊕⊕⊝ moderate |
| OS | PAM 90 mg | CLO | 16 | 5260 | 1.04 | 0.64 | 1.64 | ⊕⊕⊕⊝ moderate |
| OS | ZOL | CLO | 16 | 5260 | 0.78 | 0.52 | 1.14 | ⊕⊕⊕⊝ moderate |
| OS | PAM 30 mg | CLO | 16 | 5260 | 1.04 | 0.48 | 2.09 | ⊕⊕⊝⊝ low* |
| OS | ETI | PL | 16 | 5260 | 1.25 | 0.88 | 1.95 | ⊕⊕⊝⊝ low* |
| OS | IBAN | PL | 16 | 5260 | 1.13 | 0.54 | 2.06 | ⊕⊕⊝⊝ low* |
| OS | PAM 90 mg | PL | 16 | 5260 | 0.87 | 0.60 | 1.23 | ⊕⊕⊕⊝ moderate |
| OS | ZOL | PL | 16 | 5260 | 0.67 | 0.46 | 0.91 | ⊕⊕⊕⊝ moderate |
| OS | PAM 30 mg | PL | 16 | 5260 | 0.87 | 0.44 | 1.64 | ⊕⊕⊝⊝ low* |
| OS | IBAN | ETI | 16 | 5260 | 0.94 | 0.37 | 1.80 | ⊕⊕⊝⊝ low* |
| OS | PAM 90 mg | ETI | 16 | 5260 | 0.73 | 0.38 | 1.14 | ⊕⊕⊝⊝ low* |
| OS | ZOL | ETI | 16 | 5260 | 0.56 | 0.29 | 0.87 | ⊕⊕⊕⊝ moderate |
| OS | PAM 30 mg | ETI | 16 | 5260 | 0.72 | 0.30 | 1.40 | ⊕⊕⊝⊝ low* |
| OS | PAM 90 mg | IBAN | 16 | 5260 | 0.87 | 0.39 | 1.74 | ⊕⊕⊝⊝ low* |
| OS | ZOL | IBAN | 16 | 5260 | 0.67 | 0.29 | 1.31 | ⊕⊕⊝⊝ low* |
| OS | PAM 30 mg | IBAN | 16 | 5260 | 0.87 | 0.32 | 2.06 | ⊕⊕⊝⊝ low* |
| OS | ZOL | PAM 90 mg | 16 | 5260 | 0.79 | 0.46 | 1.26 | ⊕⊕⊝⊝ low* |
| OS | PAM 30 mg | PAM 90 mg | 16 | 5260 | 1.00 | 0.57 | 1.74 | ⊕⊕⊝⊝ low* |
| OS | PAM 30 mg | ZOL | 16 | 5260 | 1.35 | 0.62 | 2.76 | ⊕⊕⊝⊝ low* |
| PFS | PL | PAM 90 mg | 9 | 3472 | 0.84 | 0.30 | 1.88 | ⊕⊝⊝⊝ very low *^ |
| PFS | ZOL | PAM 90 mg | 9 | 3472 | 0.59 | 0.20 | 1.39 | ⊕⊝⊝⊝ very low *^ |
| PFS | CLO | PAM 90 mg | 9 | 3472 | 0.66 | 0.16 | 1.71 | ⊕⊝⊝⊝ very low *^ |
| PFS | PAM 30 mg | PAM 90 mg | 9 | 3472 | 1.04 | 0.38 | 2.16 | ⊕⊝⊝⊝ very low *^ |
| PFS | ZOL | PL | 9 | 3472 | 0.70 | 0.46 | 1.03 | ⊕⊝⊝⊝ very low *^ |
| PFS | CLO | PL | 9 | 3472 | 0.77 | 0.30 | 1.47 | ⊕⊝⊝⊝ very low *^ |
| PFS | PAM 30 mg | PL | 9 | 3472 | 1.55 | 0.34 | 4.29 | ⊕⊝⊝⊝ very low *^ |
| PFS | CLO | ZOL | 9 | 3472 | 1.10 | 0.45 | 1.95 | ⊕⊝⊝⊝ very low *^ |
| PFS | PAM 30 mg | ZOL | 9 | 3472 | 2.30 | 0.45 | 6.78 | ⊕⊝⊝⊝ very low *^ |
| PFS | PAM 30 mg | CLO | 9 | 3472 | 2.38 | 0.43 | 8.15 | ⊕⊝⊝⊝ very low *^ |
| SREs | PL | CLO | 13 | 5727 | 1.27 | 0.81 | 1.84 | ⊕⊕⊝⊝ low* |
| SREs | ETI | CLO | 13 | 5727 | 1.01 | 0.37 | 2.20 | ⊕⊕⊝⊝ low* |
| SREs | PAM 90 mg | CLO | 13 | 5727 | 0.90 | 0.51 | 1.38 | ⊕⊕⊝⊝ low* |
| SREs | IBAN | CLO | 13 | 5727 | 1.37 | 0.68 | 2.55 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | CLO | 13 | 5727 | 0.72 | 0.41 | 1.02 | ⊕⊕⊝⊝ low* |
| SREs | PAM 30 mg | CLO | 13 | 5727 | 0.89 | 0.44 | 1.62 | ⊕⊕⊝⊝ low* |
| SREs | ETI | PL | 13 | 5727 | 0.79 | 0.33 | 1.61 | ⊕⊕⊝⊝ low* |
| SREs | PAM 90 mg | PL | 13 | 5727 | 0.71 | 0.49 | 0.96 | ⊕⊕⊕⊝ moderate |
| SREs | IBAN | PL | 13 | 5727 | 1.08 | 0.60 | 1.86 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | PL | 13 | 5727 | 0.57 | 0.37 | 0.76 | ⊕⊕⊕⊝ moderate |
| SREs | PAM 30 mg | PL | 13 | 5727 | 0.71 | 0.38 | 1.23 | ⊕⊕⊝⊝ low* |
| SREs | PAM 90 mg | ETI | 13 | 5727 | 1.06 | 0.40 | 2.25 | ⊕⊕⊝⊝ low* |
| SREs | IBAN | ETI | 13 | 5727 | 1.61 | 0.55 | 3.79 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | ETI | 13 | 5727 | 0.84 | 0.31 | 1.76 | ⊕⊕⊝⊝ low* |
| SREs | PAM30mg | ETI | 13 | 5727 | 1.06 | 0.35 | 2.57 | ⊕⊕⊝⊝ low* |
| SREs | IBAN | PAM 90 mg | 13 | 5727 | 1.56 | 0.80 | 2.90 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | PAM 90 mg | 13 | 5727 | 0.81 | 0.52 | 1.14 | ⊕⊕⊝⊝ low* |
| SREs | PAM 30 mg | PAM 90 mg | 13 | 5727 | 1.00 | 0.60 | 1.70 | ⊕⊕⊝⊝ low* |
| SREs | ZOL | IBAN | 13 | 5727 | 0.56 | 0.26 | 0.98 | ⊕⊕⊕⊝ moderate |
| SREs | PAM 90 mg | IBAN | 13 | 5727 | 0.70 | 0.29 | 1.44 | ⊕⊕⊝⊝ low* |
| SREs | PAM 30 mg | ZOL | 13 | 5727 | 1.28 | 0.68 | 2.51 | ⊕⊕⊝⊝ low* |
| Pain | ETI | CLO | 8 | 1281 | 2.15 | 0.22 | 9.56 | ⊕⊝⊝⊝ very low *^ |
| Pain | IBAN | CLO | 8 | 1281 | 4.13 | 0.57 | 16.99 | ⊕⊝⊝⊝ very low *^ |
| Pain | PAM 90 mg | CLO | 8 | 1281 | 1.76 | 0.57 | 16.99 | ⊕⊝⊝⊝ very low *^ |
| Pain | IBAN | ETI | 8 | 1281 | 4.07 | 0.23 | 19.62 | ⊕⊝⊝⊝ very low *^ |
| Pain | PAM 90 mg | ETI | 8 | 1281 | 1.75 | 0.11 | 7.64 | ⊕⊝⊝⊝ very low *^ |
| Pain | PAM 90 mg | IBAN | 8 | 1281 | 0.75 | 0.06 | 3 | ⊕⊝⊝⊝ very low *^ |
| Vertebral fractures | PL | CLO | 8 | 3076 | 1.50 | 0.87 | 2.62 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | IBAN | CLO | 8 | 3076 | 1.76 | 0.56 | 4.45 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | PAM 90 mg | CLO | 8 | 3076 | 1.07 | 0.45 | 2.07 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | ZOL | CLO | 8 | 3076 | 0.59 | 0.22 | 1.17 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | IBAN | PL | 8 | 3076 | 1.16 | 0.41 | 2.56 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | PAM90mg | PL | 8 | 3076 | 0.72 | 0.35 | 1.18 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | ZOL | PL | 8 | 3076 | 0.42 | 0.12 | 0.94 | ⊕⊕⊕⊝ moderate |
| Vertebral fractures | PAM90mg | IBAN | 8 | 3076 | 0.76 | 0.21 | 1.91 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | ZOL | IBAN | 8 | 3076 | 0.45 | 0.08 | 1.29 | ⊕⊕⊝⊝ low* |
| Vertebral fractures | ZOL | PAM 90 mg | 8 | 3076 | 0.64 | 0.17 | 1.68 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | PL | CLO | 7 | 3349 | 1.47 | 0.65 | 3.10 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | IBAN | CLO | 7 | 3349 | 2.13 | 0.44 | 7.20 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | PAM 90 mg | CLO | 7 | 3349 | 3.17 | 0.52 | 10.88 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | ZOL | CLO | 7 | 3349 | 0.82 | 0.24 | 2.32 | ⊕⊕⊝⊝ low* |
| Nonvertebral fractures | IBAN | PL | 7 | 3349 | 1.46 | 0.40 | 3.98 | ⊕⊕⊝⊝ low* |
| Non vertebral fractures | PAM 90 mg | PL | 7 | 3349 | 2.01 | 0.46 | 6.32 | ⊕⊕⊝⊝ low* |
| Non vertebral fractures | ZOL | PL | 7 | 3349 | 0.66 | 0.13 | 2.30 | ⊕⊕⊝⊝ low* |
| Non‐vertebral fractures | PAM 90 mg | IBAN | 7 | 3349 | 1.98 | 0.25 | 7.66 | ⊕⊕⊝⊝ low* |
| Non‐vertebral fractures | ZOL | IBAN | 7 | 3349 | 0.64 | 0.07 | 2.82 | ⊕⊕⊝⊝ low* |
| Non‐vertebral fractures | ZOL | PAM 90 mg | 7 | 3349 | 0.49 | 0.04 | 2.14 | ⊕⊕⊝⊝ low* |
| Hypercalcemia | PL | CLO | 11 | 4146 | 1.64 | 0.71 | 3.58 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ETI | CLO | 11 | 4146 | 2.59 | 0.51 | 8.80 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | IBAN | CLO | 11 | 4146 | 1.27 | 0.20 | 4.53 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | PAM90mg | CLO | 11 | 4146 | 1.14 | 0.32 | 3.05 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | CLO | 11 | 4146 | 1.04 | 0.32 | 2.47 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ETI | PL | 11 | 4146 | 1.55 | 0.40 | 4.27 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | IBAN | PL | 11 | 4146 | 0.76 | 0.16 | 2.27 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | PAM 90 mg | PL | 11 | 4146 | 0.70 | 0.26 | 1.40 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | PL | 11 | 4146 | 0.73 | 0.16 | 1.92 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | IBAN | ETI | 11 | 4146 | 0.68 | 0.08 | 2.53 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | PAM 90 mg | ETI | 11 | 4146 | 0.62 | 0.11 | 1.92 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | ETI | 11 | 4146 | 0.65 | 0.08 | 2.28 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | PAM 90 mg | IBAN | 11 | 4146 | 1.42 | 0.22 | 4.79 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | IBAN | 11 | 4146 | 1.50 | 0.15 | 5.74 | ⊕⊝⊝⊝ very low *$ |
| Hypercalcemia | ZOL | PAM 90 mg | 11 | 4146 | 1.23 | 0.21 | 4.05 | ⊕⊝⊝⊝ very low *$ |
| GIToxicity | PL | CLO | 8 | 3789 | 0.87 | 0.45 | 1.49 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ETI | CLO | 8 | 3789 | 1.14 | 0.01 | 7.59 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | PAM 90 mg | CLO | 8 | 3789 | 1.18 | 0.45 | 2.49 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ZOL | CLO | 8 | 3789 | 0.86 | 0.35 | 1.74 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ETI | PL | 8 | 3789 | 1.32 | 0.01 | 8.73 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | PAM 90 mg | PL | 8 | 3789 | 1.36 | 0.69 | 2.39 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ZOL | PL | 8 | 3789 | 1.07 | 0.38 | 2.39 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | PAM 90 mg | ETI | 8 | 3789 | 15.96 | 0.14 | 102.19 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ZOL | ETI | 8 | 3789 | 12.63 | 0.10 | 81.10 | ⊕⊕⊝⊝ low* $$ |
| GIToxicity | ZOL | PAM 90 mg | 8 | 3789 | 0.86 | 0.24 | 2.27 | ⊕⊕⊝⊝ low* $$ |
| ONJ | PL | PAM 90 mg | 8 | 3746 | 1.10 | 0.04 | 5.99 | ⊕⊝⊝⊝ very low *^ |
| ONJ | ZOL | PAM 90 mg | 8 | 3746 | 6.19 | 0.09 | 38.16 | ⊕⊝⊝⊝ very low *^ |
| ONJ | CLO | PAM 90 mg | 8 | 3746 | 0.77 | 0.01 | 4.94 | ⊕⊝⊝⊝ very low *^ |
| ONJ | PAM 30 mg | PAM 90 mg | 8 | 3746 | 0.44 | 0.05 | 1.83 | ⊕⊝⊝⊝ very low *^ |
| ONJ | ZOL | PL | 8 | 3746 | 5.70 | 0.72 | 21.26 | ⊕⊝⊝⊝ very low *^ |
| ONJ | CLO | PL | 8 | 3746 | 0.71 | 0.04 | 3.43 | ⊕⊝⊝⊝ very low *^ |
| ONJ | PAM 30 mg | PL | 8 | 3746 | 2.19 | 0.03 | 13.55 | ⊕⊝⊝⊝ very low *^ |
| ONJ | CLO | ZOL | 8 | 3746 | 0.13 | 0.02 | 0.44 | ⊕⊝⊝⊝ very low ** |
| ONJ | PAM 30 mg | ZOL | 8 | 3746 | 0.77 | 0.00 | 5.09 | ⊕⊝⊝⊝ very low *^ |
| ONJ | PAM 30 mg | CLO | 8 | 3746 | 11.14 | 0.04 | 76.15 | ⊕⊝⊝⊝ very low *^ |
| REM: Random effects model, for multiple treatment comparison method: sigma˜Unif(0,1); ONJ: osteonecrosis of the jaw; PL: Placebo; #RCTS: Number of randomized controlled trials; LCRL: Lower credibility limit; UCRL: Upper credibility limit; OS: Overall survival; PFS: Progression‐free survival; SREs: Skeletal‐related events; HR: Hazard ratio; RR: Risk ratio; ETI: Etidronate; CLO: Clodronate; PAM 90 mg: Pamidronate 90 mg: PAM 30 mg: Pamidronate 30 mg; IBAN: Ibandronate: ZOL: Zoledronate; *Randomized controlled trial with direct (head‐to‐head) comparison of zoledronate versus clodronate (Morgan 2010).* Imprecision; ^ Contributing direct evidence of low quality;$ For the contributing direct evidence, the pooled estimate along with individual studies have wide confidence intervals. Therefore, we downgraded the quality of evidence by two levels resulting in low quality evidence. $$ For the contributing direct evidence, individual studies have wide confidence intervals. Therefore, we downgraded the quality of evidence by one level resulting in moderate quality evidence.**The results from head to head RCT comparing zoledronate with clodronate showed no difference in risk of ONJ (Morgan 2010). However, the results from network meta‐analysis showed an increased risk of ONJ with zoledronate over clodronate which is indicative of incoherence. | ||||||||
| Study | Study design | Type of bisphosphonate | Total number of patients | Number of patients with ONJ | Route, dose, frequency | Treatment duration | ONJ frequency |
| Retrospective study | Pamidronate | 17 | 3 | Not reported | Not reported | 17.65% | |
| Zoledronate | 34 | 2 | 5.88% | ||||
| Pamidronate + zoledronate | 33 | 17 | 51.51% | ||||
| Retrospective study | Zoledronate | 300 | 14 | Not clear | Median: 18 months Range: 1‐121 months | 5% | |
| Not clear | Zoledronate | 64 | 7 | Not reported | Not clear | 9% | |
| Prospective study | Zoledronate | 32 | 5 | 15 minute infusion of 4 mg IV zoledronate once a month | Mean duration: 26.5 months, SD 18.7 months | 15% | |
| Retrospective study | Pamidronate | 20 | 0 | Not clear | 23 months | 0% | |
| Zoledronate | 37 | 5 | Not clear | 28 months | 11.9% | ||
| Pamidronate + zoledronate | 42 | 2 | Not clear | 47 months | 4.55% | ||
| Pamidronate | 93 | 7 | Not reported | 39 months ONJ patients (11‐76) vs 28 (4.5‐123) months without ONJ | 7.5% | ||
| Zoledronate | 33 | 1 | 3% | ||||
| Pamidronate + zoledronate | 66 | 6 | 9.1% | ||||
| Ibandronate | 1 | 0 | 0% | ||||
| Ibandronate + zoledronate | 4 | 1 | 25% | ||||
| Clodronate + zoledronate | 1 | 0 | 0% | ||||
| Alendronate + zoledronate | 1 | 0 | 0% | ||||
| Retrospective study | Pamidronate | 49 | 1 | 90 mg monthly | 28 months | 2% | |
| Zoledronate | 64 | 6 | 4 mg monthly | 12 months (7‐28) | 9.3% | ||
| Pamidronate + zoledronate | 30 | 7 | 43.5 months (24‐59) | 23.3% | |||
| Retrospective study | Zoledronate | 225 | 6 | Not reported | 10 months (4‐35) | 2.7% | |
| Retrospective study from 1991, prospective from 2001‐2006 | Pamidronate | 78 | 1 | 90 mg | 24 months (4‐120) | 1.28% | |
| Pamidronate | 91 | 6 | 4 mg 4‐6 weeks | 6.59% | |||
| Pamidronate + zoledronate | 85 | 21 | 24.71% | ||||
| ONJ: Osteonecrosis of the jaw; SD: standard deviation; IV: intravenous. | |||||||
| Study_ID | Reason for exclusion |
| No multiple myeloma patients with ONJ | |
| No extractable data for multiple myeloma patients (abstract) | |
| American Society of Hematology 2004 (abstract no 4933): Approximately 600 multiple myeloma patients. Teported frequency: 7 patients. Excluded due to imprecise reporting (e.g. approximately 600 multiple myeloma patients) | |
| ONJ: Osteonecrosis of the jaw. | |
| Study | Total number of patients | Clodronate | Pamidronate | Zoledronate | Pamidronate /zoledronate | Not specified | Others |
| 9 | 2 | 7 | |||||
| 1 | 1 | ||||||
| 1 | 1 | ||||||
| 1 | 1 | ||||||
| 9 | 2 | 4 | 3 | ||||
| 1 | 1 | ||||||
| 1 | 1 | ||||||
| 21 | 12 | 1 | 8 | ||||
| 1 | 1 | ||||||
| 7 | 2 | 5 | |||||
| 3 | 3 | ||||||
| 5 | 2 | 3 | |||||
| 22 | 17 | 4 | 1(A) | ||||
| 2 | 1 | 1 | |||||
| 13 | 13 | ||||||
| 101 | 18 | 78 | 5 | ||||
| 1 | 1 | ||||||
| 5 | 1 | 4 | |||||
| 2 | 2 | ||||||
| 1 | 1 | ||||||
| 21 | 1 | 19 | 1(A) | ||||
| 1 | 1 | ||||||
| 1 | 1 | ||||||
| 2 | 1 | 1 | |||||
| 6 | 6 | ||||||
| 2 | 2 | ||||||
| 101 | 30 | 31 | 23 | 17(16:A,1:U) | |||
| 1 | 1 | ||||||
| 3 | 1 | 2 | |||||
| 33 | 6 | 19 | 7 | 1(P,I,Z) | |||
| 2 | 1 | 1 | |||||
| 7 | 4 | 2 | 1 | ||||
| 1 | 1 | ||||||
| 3 | 1 | 2 | |||||
| 1 | 1 | ||||||
| 4 | 2 | 2 | |||||
| 2 | 2 | ||||||
| 4 | 4 | ||||||
| 35 | 3 | 14 | 18 | ||||
| 3 | 2 | 1 | |||||
| 28 | 14 | 4 | 10 | ||||
| 1 | 1 | ||||||
| 3 | 1 | 1 | 1(P/C) | ||||
| 1 | 1 | ||||||
| 6 | 6 | ||||||
| 2 | 2 | ||||||
| 34 | 3 | 20 | 5 | 6 (:I/Z,3:A,1:U) | |||
| 1 | 1 | ||||||
| 1 | 1 | ||||||
| 11 | 11 | ||||||
| 23 | 23 | ||||||
| 6 | 6 | ||||||
| 1 | 1 | ||||||
| 1 | 1 | ||||||
| 56 | 56 | ||||||
| 9 | 1 | 1 | 7 | ||||
| 27 | 27 | ||||||
| 1 | 1 | ||||||
| 12 | 2 | 8 | 2 | ||||
| 2 | 2 | ||||||
| 10 | 10 | ||||||
| Total | 676 | 3 | 128 | 270 | 113 | 130 | 32 |
| A: Alendronate; C: Clodronate; I: Ibandronate; P: Pamidronate; R: Risedronate; Z: Zoledronate; MM: multiple myeloma; U: Unknown. | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mortality Show forest plot | 14 | 2706 | Hazard Ratio (Random, 95% CI) | 0.90 [0.76, 1.07] |
| 1.1 Etidronate | 2 | 244 | Hazard Ratio (Random, 95% CI) | 1.24 [0.86, 1.80] |
| 1.2 Clodronate | 3 | 885 | Hazard Ratio (Random, 95% CI) | 0.93 [0.66, 1.29] |
| 1.3 Pamidronate | 5 | 977 | Hazard Ratio (Random, 95% CI) | 0.85 [0.67, 1.07] |
| 1.4 Ibandronate | 1 | 198 | Hazard Ratio (Random, 95% CI) | 1.07 [0.69, 1.64] |
| 1.5 Zoledronate | 3 | 402 | Hazard Ratio (Random, 95% CI) | 0.57 [0.43, 0.75] |
| 2 Progression‐free survival Show forest plot | 7 | 908 | Hazard Ratio (Random, 95% CI) | 0.75 [0.57, 1.00] |
| 2.1 Clodronate | 1 | 26 | Hazard Ratio (Random, 95% CI) | 0.63 [0.17, 2.34] |
| 2.2 Pamidronate | 1 | 177 | Hazard Ratio (Random, 95% CI) | 1.24 [0.66, 2.33] |
| 2.3 Zoledronate | 5 | 705 | Hazard Ratio (Random, 95% CI) | 0.70 [0.52, 0.95] |
| 3 Vertebral fractures Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 3.1 Clodronate | 3 | 433 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.56, 0.89] |
| 3.2 Pamidronate | 3 | 485 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.40, 1.20] |
| 3.3 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.61, 1.81] |
| 4 Non‐vertebral fractures Show forest plot | 6 | 1389 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.68, 1.56] |
| 4.1 Clodronate | 3 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.42, 1.31] |
| 4.2 Pamidronate | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 1.65 [0.95, 2.87] |
| 4.3 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.79, 1.98] |
| 5 Total skeletal‐related events Show forest plot | 10 | 2141 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.63, 0.88] |
| 5.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.39, 1.39] |
| 5.2 Clodronate | 1 | 204 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.65, 0.89] |
| 5.3 Pamidronate | 3 | 950 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.59, 0.91] |
| 5.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.80, 1.35] |
| 5.5 Zoledronate | 4 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.28, 0.89] |
| 6 Pain Show forest plot | 8 | 1281 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.60, 0.95] |
| 6.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.26, 1.32] |
| 6.2 Clodronate | 4 | 566 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.29, 0.91] |
| 6.3 Pamidronate | 2 | 439 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.72, 1.01] |
| 6.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 1.0 [0.86, 1.17] |
| 7 Incidence of hypercalcemia Show forest plot | 10 | 2174 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.56, 1.09] |
| 7.1 Etidronate | 1 | 166 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.73, 2.38] |
| 7.2 Clodronate | 3 | 831 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.45, 1.31] |
| 7.3 Pamidronate | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.31, 1.33] |
| 7.4 Ibandronate | 1 | 198 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.27, 1.42] |
| 7.5 Zoledronate | 2 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.19 [0.01, 3.91] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Osteonecosis of jaw Show forest plot | 6 | 1284 | Risk Ratio (M‐H, Random, 95% CI) | 4.61 [0.99, 21.35] |
| 1.1 Pamidronate | 2 | 573 | Risk Ratio (M‐H, Random, 95% CI) | 3.06 [0.13, 74.69] |
| 1.2 Zoledronate | 4 | 711 | Risk Ratio (M‐H, Random, 95% CI) | 5.21 [0.91, 29.90] |
| 2 Gastrointestinal toxicity (grade III/IV) Show forest plot | 7 | 1829 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.95, 1.59] |
| 2.1 Etidronate | 1 | 78 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.01, 7.94] |
| 2.2 Clodronate | 2 | 872 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.82, 1.72] |
| 2.3 Pamidronate | 3 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.90, 1.88] |
| 2.4 Zoledronate | 1 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.06, 15.23] |
| 3 Hypocalcaemia Show forest plot | 3 | 1090 | Risk Ratio (M‐H, Random, 95% CI) | 2.19 [0.49, 9.74] |
| 3.1 Clodronate | 1 | 536 | Risk Ratio (M‐H, Random, 95% CI) | 2.06 [0.38, 11.16] |
| 3.2 Pamidronate | 2 | 554 | Risk Ratio (M‐H, Random, 95% CI) | 2.71 [0.11, 66.19] |
| 4 Renal dysfunction Show forest plot | 2 | 414 | Mean Difference (IV, Random, 95% CI) | ‐0.36 [‐9.75, 9.03] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Allocation concealment (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 1.1 Adeqaute concealment of allocation | 2 | 594 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.51, 0.82] |
| 1.2 Inadequate concealment of allocation | 5 | 522 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.67, 1.09] |
| 2 Blinding (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 2.1 Double‐blind | 5 | 1008 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.58, 0.85] |
| 2.2 Not blinded | 2 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.08, 3.72] |
| 3 Randomization method (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 3.1 Randomization method is described | 1 | 377 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.85] |
| 3.2 Randomization method is NOT described | 6 | 739 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.65, 0.94] |
| 4 Type of data analysis (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 4.1 Intention‐to‐treat analysis | 3 | 463 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.55, 1.22] |
| 4.2 Per protocol analysis | 4 | 653 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.56, 0.89] |
| 5 Description of withdrawals and drop outs (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 5.1 Withdrawals and dropouts well described | 3 | 797 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.55, 0.82] |
| 5.2 Withdrawals and dropouts NOT described | 4 | 319 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.68, 1.29] |
| 6 Alpha error (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 6.1 Alpha error pre‐specified | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 6.2 Alpha error NOT pre‐specified | 6 | 913 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.94] |
| 7 Beta error (vertebral fractures) Show forest plot | 7 | 1116 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.62, 0.89] |
| 7.1 Beta error pre‐specified | 1 | 203 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.51, 1.08] |
| 7.2 Beta error NOT pre‐specified | 6 | 913 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.59, 0.94] |
| 8 Gastrointestinal toxicity (grade III/IV: oral vs IV bisphosphonates)) Show forest plot | 7 | 1829 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.95, 1.59] |
| 8.1 Oral route | 4 | 1250 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.89, 1.70] |
| 8.2 Intervenous route | 3 | 579 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [0.81, 1.90] |