Inserción inmediata posparto del dispositivo intrauterino para la anticoncepción
Information
- DOI:
- https://doi.org/10.1002/14651858.CD003036.pub3Copy DOI
- Database:
-
- Cochrane Database of Systematic Reviews
- Version published:
-
- 26 June 2015see what's new
- Type:
-
- Intervention
- Stage:
-
- Review
- Cochrane Editorial Group:
-
Cochrane Fertility Regulation Group
- Copyright:
-
- Copyright © 2015 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Article metrics
Altmetric:
Cited by:
Authors
Contributions of authors
Initial review (2001): David A Grimes (formerly of FHI 360) and Kenneth F Schulz (FHI 360) developed the proposal, conducted the literature search, extracted the data, and performed the analysis. Huib van Vliet and Nancy Stanwood were co‐authors who contributed to the writing and revising of the review. At the time, N Stanwood was with the University of Rochester Medical Center (NY).
2005 to 2010: LM Lopez wrote the plain language summary, entered data from original studies into tables, and reviewed search results. In 2010, she incorporated data from an abstract.
2015: LM Lopez conducted the searches, marked the data for extraction from the included studies, and wrote the results and discussion. A Bernholc entered study characteristics and outcome data, and drafted summary tables. G Stuart and D Hubacher clarified technical and clinical issues in the text, and commented on the responses to the peer reviewers. All authors reviewed and commented on the manuscript.
Sources of support
Internal sources
-
No sources of support supplied
External sources
-
National Institute of Child Health and Human Development, USA.
2000 to 2015: Support for conducting the review and updates at FHI 360
-
US Agency for International Development, USA.
2000 to 2010: Support for conducting the review and updates at FHI 360
2014: This report is made possible by the generous support of the American people through the United States Agency for International Development (USAID) under the terms of The Evidence Project, cooperative agreement no. AID‐OAA‐A‐13‐00087. The findings and conclusions are the sole responsibility of the authors and do not necessarily reflect the views of USAID or the United States Government.
Declarations of interest
Family Health International, now known as FHI 360, conducted two trials (Cole 1984: Kisnisci 1985). An employee of FHI 360 was involved in two trials (Lavin 1983; Apelo 1985). Authors of this review are employed at FHI 360, but none were involved in those trials.
Acknowledgements
Carol Manion of FHI 360 assisted with the literature searches for the initial review and updates.
Version history
| Published | Title | Stage | Authors | Version |
| 2015 Jun 26 | Immediate postpartum insertion of intrauterine device for contraception | Review | Laureen M Lopez, Alissa Bernholc, David Hubacher, Gretchen Stuart, Huib AAM Van Vliet | |
| 2010 May 12 | Immediate post‐partum insertion of intrauterine devices | Review | David A Grimes, Laureen M Lopez, Kenneth F Schulz, Huib AAM Van Vliet, Nancy L. Stanwood | |
| 2001 Apr 23 | Immediate post‐partum insertion of intrauterine devices | Review | David A Grimes, Kenneth F Schulz, Huib HAAM Van Vliet, Nancy L. Stanwood, Laureen M Lopez | |
Keywords
MeSH
Medical Subject Headings (MeSH) Keywords
Medical Subject Headings Check Words
Female; Humans; Pregnancy;
PICOs

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 1 Placement (insertion).

Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 2 Expulsion by 6 months.

Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 3 Use at 3 months.

Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 4 Use at 6 months.
| Study | Time frame | Immediate insertion | Early insertion | Reported P |
| Ahuja 2014 | ‐‐‐ | N = 131 | N = 132 | ‐‐‐ |
| Ahuja 2014 | 6 weeks | 24.1% | 9.1% | .0037 |
| Singh 2014 | ‐‐‐ | N = 100 | N = 100 | ‐‐‐ |
| Singh 2014 | 6 months | 8.3% | 10.5% | .06 |
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 5 Expulsion.
| Study | Immediate insertion | Early insertion | Reported P |
| Ahuja 2014 | 88.9% | 74.1% | .0054 |
| Ahuja 2014 | N = 131 | N = 132 | ‐‐‐ |
| Singh 2014 | 83.5% | 77.1% | .13 |
| Singh 2014 | N = 100 | N = 100 | ‐‐‐ |
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 6 Use at 6 months.

Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 1 Placement (insertion) per protocol.
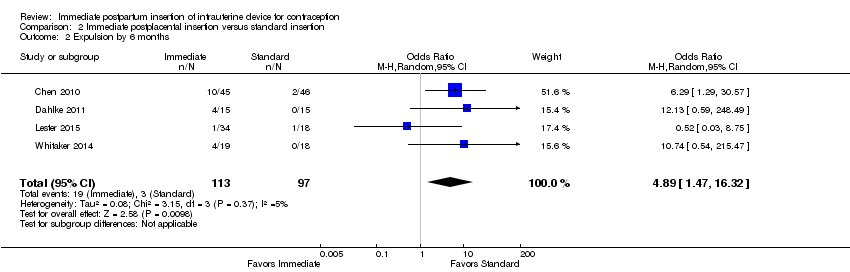
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 2 Expulsion by 6 months.

Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 3 Use at 6 months.
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 4 Use at 3 months.

Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 5 Use at 12 months.
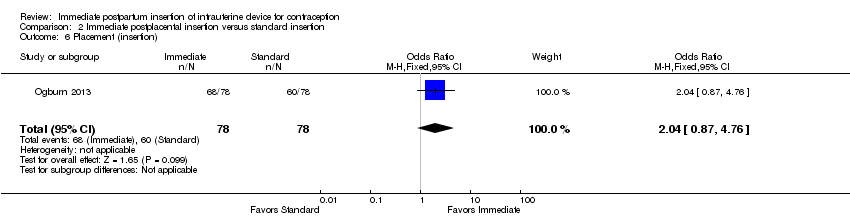
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 6 Placement (insertion).
| Study | Immediate insertion | Early insertion |
| Ogburn 2013 | N = 78 | N = 78 |
| Ogburn 2013 | 57% | 59% |
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 7 Continued use at 12 months.
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 99.7% | 99.7% |
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 1 Insertion per protocol.
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 15.7 | 21.5 |
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 78.5 | 73.8 |
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 62.7% | 66.1% |
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
| Study | Delta T | TCu 220 C |
| Cole 1984 | 99.9% | 99.7% |
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 1 Insertion per protocol.
| Study | Delta T | TCu 220 C |
| Cole 1984 | 11.6 | 11.5 |
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Study | Delta T | TCu 220 C |
| Cole 1984 | 81.8 | 81.8 |
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Study | Delta T | TCu 220 C |
| Cole 1984 | 71.0% | 70.9% |
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
| Study | Delta Loop | Delta Loop |
| Cole 1984 | 99.7% | 99.7% |
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 1 Insertion per protocol.
| Study | Hand insertion | Instrument insertion |
| Cole 1984 | 11.2 | 11.5 |
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Study | Hand insertion | Instrument insertion |
| Cole 1984 | 84.2 | 82.6 |
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Study | Delta Loop | Delta Loop |
| Cole 1984 | 84.2% | 83.0% |
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
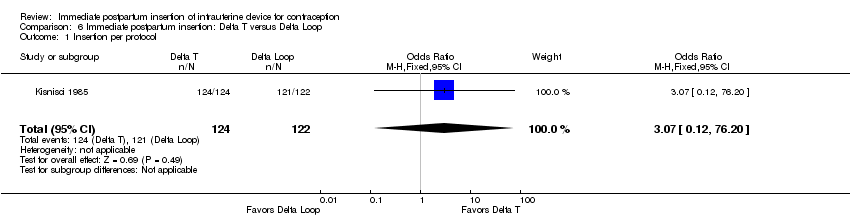
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 1 Insertion per protocol.
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 7.6 | 3.7 |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 2 Life‐table rates per 100 women for expulsion (12‐month).
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 0 | 2.1 |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 3 Life‐table rates per 100 women for pregnancy (12‐month).
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 1.0 | 1.1 |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 4 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month).
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 90.7 | 93.3 |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 5 Life‐table rates per 100 women for continuation (12‐month).
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 86.7% | 78.9% |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 6 Life‐table rates per 100 women for follow‐up (12‐month).
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 9.0 | 35.8 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 1 Hand insertion: Life‐table rates per 100 women for expulsion (12‐month).
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 8.1 | 35.2 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 2 Instrument insertion: Life‐table rates per 100 women for expulsion (12‐month).
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 86.3 | 59.9 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 3 Hand insertion: Life‐table rates per 100 women for continuation (12‐month).
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 86.1 | 57.2 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 4 Instrument insertion: Life‐table rates per 100 women for continuation (12‐month).
| Study | Insertion method | TCu 200 | Progestasert |
| Lavin 1983 | Hand | 76.1 | 77.4 |
| Lavin 1983 | Instrument | 69.7 | 67.7 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 5 Life‐table rates per 100 women for follow‐up (12‐month).
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 19.9 | 10.3 | 39.0 | 14.2 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 5.5 | 0 | 3.2 | 5.2 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 2 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 73.8 | 84.9 | 57.3 | 77.1 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 3 Life‐table rates per 100 women for continuation (12‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 19.9 | 13.1 | 39.0 | 24.2 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 4 Life‐table rates per 100 women for expulsion (36‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 67.7 | 62.9 | 52.3 | 55.8 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 5 Life‐table rates per 100 women for continuation (36‐month) by device and insertion method.
| Study | TCu 200 (pooled) | IPCS‐52 (pooled) |
| Apelo 1985 | 16.4 | 31.3 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 6 Life‐table rates per 100 women for expulsion (36‐month) by device pooled.
| Study | Hand (pooled) | Inserter (pooled) |
| Apelo 1985 | 29.2 | 18.5 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 7 Life‐table rates per 100 women for expulsion (36‐month) by method pooled.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 71.1% | 99.1% | 82.8% | 82.5% |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 8 Life‐table rates per 100 women for follow‐up (12‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 55.6% | 63.9% | 66.7% | 54.8% |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 9 Life‐table rates per 100 women for follow‐up (36‐month) by device and insertion method.
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 41.3 | 44.1 | 34.8 |
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 5.6 | 12.1 | 7.2 |
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 53.1 | 60.9 | 47.7 |
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 3 Life‐table rates per 100 women for discontinuation (12‐month).
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 17.8% | 16.2% | 16.0% |
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 4 Loss to follow‐up (unclear time frame).
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 6.2 | 6.6 |
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 0.6 | 0 |
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 87.4 | 78.2 |
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 3 Life‐table rates per 100 women for continuation (12‐month).
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 6.2 | 6.2 |
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 4 Life‐table rates per 100 women for loss to follow‐up (12‐month).
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 11.2 | 9.9 |
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 0.5 | 2.4 |
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 77.2 | 77.3 |
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 3 Life‐table rates per 100 women for continuation (12‐month).
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 2.0 | 1.6 |
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 4 Life‐table rates per 100 women for loss to follow‐up (12‐month).

Comparison 12 Immediate postpartum insertion: CuT 380A (hand versus instrument insertion), Outcome 1 Expulsion by 6 months.
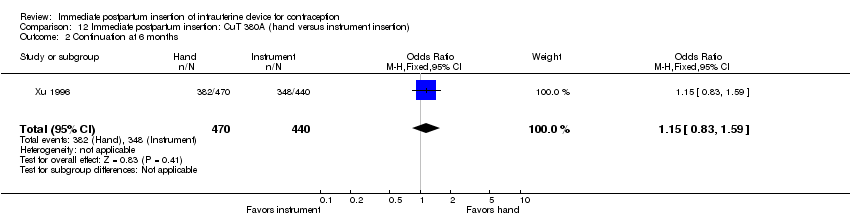
Comparison 12 Immediate postpartum insertion: CuT 380A (hand versus instrument insertion), Outcome 2 Continuation at 6 months.
| Immediate insertion compared with early insertion for postpartum IUC | |||
| Patient or population: postpartum women with desire for contraceptive Setting: hospital or clinic Intervention: immediate postplacental insertion (within 10 minutes) Comparison: early insertion (10 minutes to 48 hours post delivery) | |||
| Outcomes | Relative effect | Participants | Quality of the evidence |
| Expulsion by 6 months | OR 1.00 (95% CI 0.20 to 5.04) | 30 | ⊕⊕⊕⊝ Moderate |
| IUC use at 6 months | OR 0.46 (95% CI 0.04 to 5.75) | 30 | ⊕⊕⊕⊝ |
| CI: Confidence interval; OR: Odds ratio | |||
| GRADE Working Group grades of evidence | |||
| Immediate insertion compared with standard insertion for postpartum IUC | |||
| Patient or population: postpartum women with desire for contraceptive Setting: hospital or clinic Intervention: immediate postplacental insertion (within 10 minutes) Comparison: standard insertion (at postpartum visit) | |||
| Outcomes | Relative effect | Participants | Quality of the evidence |
| Placement per protocol | OR 4.07 (95% CI 0.54 to 30.40); I2 = 68% | 243 (4 studies) | ⊕⊕⊕⊝ |
| Expulsion by 6 months | OR 4.89 (95% CI 1.47 to 16.32) | 210 (4 studies) | ⊕⊕⊕⊝ Moderate |
| IUC use at 6 months | OR 2.04 (95% CI 1.01 to 4.09) | 243 (4 studies) | ⊕⊕⊕⊝ |
| CI: Confidence interval; OR: Odds ratio | |||
| GRADE Working Group grades of evidence | |||
| Study | Delivery type | IUC | Inadequate | No blinding | Loss to follow‐up | Quality of evidencea |
| Immediate versus early insertion (10 minutes to 48 hours) | ||||||
| Vaginal | LNG‐IUS | _ | ‐1 | _ | Moderate | |
| Vaginal | CuT 380A | _ | ‐1 | Unclear | Moderate | |
| Vaginal or cesarean | CuT 380A | ‐1 | Unclear | Unclear | Low | |
| Immediate versus standard insertion (weeks) | ||||||
| Vaginal | LNG‐IUS (6 to 8 weeks) | _ | _ | _ | High | |
| Vaginal | LNG‐IUS (> 6 weeks) | _ | ‐1 | _ | Moderate | |
| Cesarean | LNG‐IUS (4 to 8 weeks) | _ | _ | ‐1 | Moderate | |
| Cesarean | CuT 380A (6 weeks) | _ | ‐1 | _ | Moderate | |
| Vaginal or cesarean | CuT 380A (4 to 12 weeks) | ‐1 | Unclear | Unclear | Low | |
| Immediate insertion: IUC types, modifications, or insertion techniques | ||||||
| Unclear | Multiload 250 versus CuT 200 | ‐1 | Unclear | _ | Moderate | |
| Vaginal | Copper 7 versus Lippes Loop D vs Nova‐T‐PP | _ | ‐1 | _ | Moderate | |
| Unclear | Nova‐T‐PP vs Nova T | ‐1 | Unclear | _ | Moderate | |
| Unclear | Progestasert vs CuT 200 | _ | Unclear | ‐1 | Moderate | |
| Vaginal | IPCS‐52 vs CuT 200 | _ | Unclear | ‐1 | Moderate | |
| Vaginal |
_ | Unclear | ‐1 | Moderate | ||
| Unclear | Delta Loop vs Delta T | _ | Unclear | _ | Moderate | |
| Vaginal | CuT 380A: hand vs ring‐forceps insertion | _ | _ | _ | High | |
| aRCTs considered high quality initially, then downgraded for (1) no information on randomization sequence generation or allocation concealment, or one was clearly inadequate; (2) no blinding; (3) losses > 20%; (4) information missing for both blinding and losses. Follow‐up time not shown as all studies met criteria. | ||||||
| Study | N | Placement (insertion) per protocol (%) | ||
| Immediate | Early | Standard | ||
| 102 | 88 | _ | 90 | |
| 46 | 100 | 100 | 94 | |
| 42 | 95 | _ | 82 | |
| 68 | 100 | _ | 53 | |
| 156 | 87 | _ | 77 | |
| 263 | _ | _ | _ | |
| 200 | _ | _ | _ | |
| aIn sensitivity analysis; sufficient outcome data and evidence of moderate or high quality (Table 1). | ||||
| Study | N | Use at | Expulsion by | ||||
| Immediate | Early | Standard | Immediate | Early | Standard | ||
| 102 | 84 | _ | 77 | 22d | _ | 4 | |
| 46 | 87 | 93 | 94 | 27 | 27 | 0 | |
| 42 | 70 | _ | 59 | 20 | _ | 0 | |
| 68 | 79 | _ | 47 | 3 | _ | 6 | |
| 156 | 57 | _ | 59 | _ | _ | _ | |
| 263 | 89 | 74 | _ | 9 | 24 | _ | |
| 200 | 84 | 77 | _ | 8 | 11 | _ | |
| aUse based on women randomized; Ogburn 2013 assessed at 12 months. | |||||||
| Study | IUC | N | Insertion | Placement | Use at | Expulsion by |
| Life‐table rates | ||||||
| TCu 200 | 269 | _ | _ | 85.0 | 9.4 | |
| Multiload 250 | 293 | _ | _ | 88.8 | 7.4 | |
| Copper 7 | 277 | _ | _ | 64.6 | 31.1 | |
| Lippes Loop D | 250 | _ | _ | 48.1 | 41.3 | |
| Nova‐T‐PP | 277 | _ | _ | 52.3 | 39.4 | |
| Nova‐T‐PP | 205 | _ | _ | 87.5 | 5.5 | |
| Nova T | 203 | _ | _ | 88.4 | 6.0 | |
| Progestasert | 100 | Hand | _ | 59.9 | 35.8 | |
| 100 | Inserter | _ | 57.2 | 35.2 | ||
| TCu 200 | 100 | Hand | _ | 86.3 | 9.0 | |
| 100 | Inserter | _ | 86.1 | 8.1 | ||
| IPCS‐52 mg | 50 | Hand | _ | 57.3 | 39.0 | |
| 50 | Inserter | _ | 79.6 | 14.2 | ||
| TCu 200 | 50 | Hand | _ | 81.9 | 14.1 | |
| 50 | inserter | _ | 89.7 | 10.3 | ||
| Delta T | 728 | _ | 99.9 | 81.8 | 11.6 | |
| TCu 220 | 718 | _ | 99.7 | 81.8 | 11.5 | |
| Delta Loop | 662 | _ | 99.7 | 78.5 | 15.7 | |
| Lippes Loop D | 648 | _ | 99.7 | 73.8 | 21.5 | |
| Delta T | 518 | Hand | 99.7 | 84.2 | 11.2 | |
| 517 | Mechanical | 99.7 | 82.6 | 11.5 | ||
| Delta Loop | 122 | _ | 99.2 | 96.3 | 3.7 | |
| Delta T | 124 | _ | 100.0 | 90.7 | 7.6 | |
| Percent | ||||||
| CuT 380A | 470 | Hand | _ | 81.3 | 13.0 | |
| 440 | Ring‐forceps | _ | 79.1 | 12.5 | ||
| aLavin 1983 reported use and expulsion by 12 months. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placement (insertion) Show forest plot | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Expulsion by 6 months Show forest plot | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.20, 5.04] |
| 3 Use at 3 months Show forest plot | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.75] |
| 4 Use at 6 months Show forest plot | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.75] |
| 5 Expulsion Show forest plot | Other data | No numeric data | ||
| 6 Use at 6 months Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placement (insertion) per protocol Show forest plot | 4 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 4.07 [0.54, 30.40] |
| 2 Expulsion by 6 months Show forest plot | 4 | 210 | Odds Ratio (M‐H, Random, 95% CI) | 4.89 [1.47, 16.32] |
| 3 Use at 6 months Show forest plot | 4 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [1.01, 4.09] |
| 4 Use at 3 months Show forest plot | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 5.35] |
| 5 Use at 12 months Show forest plot | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.63, 7.44] |
| 6 Placement (insertion) Show forest plot | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.87, 4.76] |
| 7 Continued use at 12 months Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Insertion per protocol Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Insertion per protocol Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Insertion per protocol Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Insertion per protocol Show forest plot | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.12, 76.20] |
| 2 Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for pregnancy (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) Show forest plot | Other data | No numeric data | ||
| 5 Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 6 Life‐table rates per 100 women for follow‐up (12‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hand insertion: Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 2 Instrument insertion: Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Hand insertion: Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Instrument insertion: Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 5 Life‐table rates per 100 women for follow‐up (12‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Life‐table rates per 100 women for expulsion (12‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (12‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for expulsion (36‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 5 Life‐table rates per 100 women for continuation (36‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 6 Life‐table rates per 100 women for expulsion (36‐month) by device pooled Show forest plot | Other data | No numeric data | ||
| 7 Life‐table rates per 100 women for expulsion (36‐month) by method pooled Show forest plot | Other data | No numeric data | ||
| 8 Life‐table rates per 100 women for follow‐up (12‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 9 Life‐table rates per 100 women for follow‐up (36‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for discontinuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Loss to follow‐up (unclear time frame) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for loss to follow‐up (12‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for loss to follow‐up (12‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Expulsion by 6 months Show forest plot | 1 | 910 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.54] |
| 2 Continuation at 6 months Show forest plot | 1 | 910 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.83, 1.59] |


