Inserción inmediata posparto del dispositivo intrauterino para la anticoncepción
Resumen
Antecedentes
Las mujeres que desean comenzar la anticoncepción intrauterina (AIU) durante el período posparto quizá se beneficien de la inserción de AIU inmediatamente después del parto. La inserción después del alumbramiento reduce considerablemente el riesgo de embarazo posterior y elimina la necesidad de una visita de retorno para comenzar la anticoncepción. Sin la opción de la inserción inmediata, puede que muchas mujeres nunca regresen para recibir el servicio o pueden adoptar una anticoncepción menos efectiva.
Objetivos
El objetivo fue examinar los resultados de la inserción de AIU inmediatamente después del alumbramiento (en el transcurso de diez minutos), especialmente cuando se compara con la inserción en otros momentos posparto. El análisis se centró en la colocación con éxito de AIU (inserción), la expulsión posterior y el uso del método.
Métodos de búsqueda
Se hicieron búsquedas de ensayos hasta el 1 abril 2015. Las fuentes incluyeron PubMed (MEDLINE), Registro Cochrane Central de Ensayos Controlados (Cochrane Central Register of Controlled Trials) (CENTRAL), POPLINE, Web of Science, EMBASE, LILACS, ClinicalTrials.gov y ICTRP. Para la revisión original, los autores contactaron con investigadores para identificar otros ensayos.
Criterios de selección
Se buscaron los ensayos controlados aleatorios (ECA) con al menos un brazo de tratamiento que incluyeron la colocación inmediata de AIU (es decir, en el transcurso de diez minutos del alumbramiento). Los brazos de comparación podrían haber incluido inserción posparto temprana (desde los diez minutos del alumbramiento hasta el alta hospitalaria) o la inserción estándar (durante una visita posparto). Los ensayos también podrían haber comparado diferentes métodos de AIU o técnicas de inserción. El parto puede haber sido vaginal o por cesárea. Los resultados primarios fueron la colocación (inserción), la expulsión posterior y el uso del método cuando se evaluaron en los estudios.
Obtención y análisis de los datos
Para los datos dicotómicos, se utilizó el odds ratio (OR) de Mantel‐Haenszel con un intervalo de confianza (IC) del 95%. Estudios anteriores informaron principalmente los resultados como tasas de tablas de mortalidad. Los ensayos se agregaron al metanálisis si presentaban intervenciones y medidas de resultado similares. Un análisis de sensibilidad incluyó estudios con pruebas de calidad moderada o alta y datos de resultado suficientes.
Resultados principales
Se incluyeron 15 ensayos. Siete estudios informados desde 2010 hasta 2014 fueron añadidos a las ocho de la revisión original de 2001. Los ensayos más nuevos compararon la inserción inmediata después del alumbramiento (de diez minutos a 48 horas) o la inserción estándar (durante la visita posparto). De cuatro con informes completos, tres eran ensayos pequeños. Los otros tres estudios fueron resúmenes de congresos. Los ocho ensayos más antiguos examinaron la inserción inmediata de diferentes dispositivos o técnicas de inserción. La mayoría de los estudios se publicó en los años ochenta, algunos con un informe limitado.
El análisis de sensibilidad incluyó ensayos con suficientes datos de resultado y pruebas de calidad moderada o alta. Cuatro ensayos más nuevos que compararon los momentos de inserción cumplieron con los criterios de inclusión. Dos estudios utilizaron el sistema intrauterino que libera levonorgestrel (SIU‐LNG) después del parto vaginal. Los otros dos ensayos colocaron la AIU después de la cesárea; uno utilizó el dispositivo intrauterino CuT 380A (DIU) y el otro utilizó el SIU‐LNG.
Un ensayo piloto comparó la inserción inmediata versus la inserción temprana o estándar. En los grupos que compararon la inserción inmediata versus temprana (n = 30), a todas las pacientes se les insertó el SIU‐LNG. A los seis meses, los grupos tenían la misma tasa de expulsión y no difirieron significativamente en el uso de la AIU.
Para la inserción inmediata versus estándar, se realizaron metanálisis de cuatro ensayos. Las tasas de inserción no difirieron significativamente entre los brazos de estudio. Sin embargo, el ensayo de Uganda indicó que fue más probable que se lograra la inserción en el grupo inmediato, aunque la estimación fue poco precisa. En el metanálisis, fue más probable la expulsión a los seis meses en el grupo de inserción inmediata, pero el intervalo de confianza fue amplio (OR 4,89; IC del 95%: 1,47 a 16,32; participantes = 210; estudios = 4). El uso de la AIU a los seis meses fue más probable con la inserción inmediata que con la inserción estándar (OR 2,04; IC del 95%: 1,01 a 4,09; participantes = 243; estudios = 4). Los brazos de estudio no difirieron en el uso a los tres o 12 meses en los ensayos individuales pequeños.
Conclusiones de los autores
Los ensayos recientes compararon diferentes momentos de inserción después del parto vaginal o por cesárea. Las pruebas fueron limitadas porque los estudios con informes completos tuvieron en general tamaños de muestra pequeños. En general, la calidad de las pruebas fue moderada; en los resúmenes y los estudios más antiguos, el informe fue limitado. Los ensayos en curso se agregarán a las pruebas, aunque algunos son pequeños. Se necesitan ensayos con poder estadístico adecuado para calcular las tasas de expulsión y los efectos secundarios.
El efecto beneficioso de la anticoncepción efectiva inmediatamente después del parto puede superar la desventaja del aumento del riesgo de expulsión. Las visitas prenatales frecuentes durante el tercer trimestre brindan la oportunidad de discutir los métodos anticonceptivos efectivos y el momento deseado para su inicio. El seguimiento clínico puede ayudar a detectar la expulsión temprana, así como educar a las mujeres acerca de los signos y los síntomas de expulsión.
PICOs
Resumen en términos sencillos
Anticoncepción intrauterina poco después del parto
Las mujeres tienen dos alternativas principales para la anticoncepción intrauterina (AIU): una que libera la hormona levonorgestrel y una sin hormonas que contiene cobre. Comenzar el uso de la AIU inmediatamente después del parto y antes del alta hospitalaria puede ser bueno por muchas razones. La mujer sabe que no está embarazada, y el momento y el lugar son convenientes para comenzar un método que funciona bien. Se examinó si la AIU tendría mayores probabilidades de expulsarse sola si se colocaba inmediatamente después del parto de un recién nacido. En las mujeres que deseaban la AIU pero no se les colocó de inmediato, se estudió si regresaron posteriormente para la inserción.
Hasta el 1 de abril de 2015, se hicieron búsquedas computarizadas de ensayos aleatorios de AIU insertado en el transcurso de los diez minutos posteriores al alumbramiento (posparto). Se escribió a los investigadores para encontrar más estudios. Los ensayos podrían comparar diferentes momentos para la inserción, así como los tipos de AIU y las maneras de insertar el dispositivo.
Se encontraron 15 ensayos. Siete estudios recientes compararon la inserción de AIU inmediatamente después del parto versus la inserción temprana (antes del alta hospitalaria) o la inserción posterior (semanas después del alta). Cuatro tenían informes completos, aunque tres fueron estudios pequeños. Fue tan probable que la inserción se lograra cuando se colocó inmediatamente después del parto como cuando se planificó la colocación posterior, excepto en un estudio de Uganda. En general, en los cuatro ensayos, el AIU se expulsó con mayor frecuencia por sí solo cuando se colocó inmediatamente después del parto que cuando se insertó semanas después. El uso a los seis meses fue más probable con la inserción inmediatamente después del parto que semanas después. En los estudios únicos, los grupos no difirieron en el uso a los tres o a los 12 meses.
Ocho estudios más antiguos examinaron los tipos de AIU colocados inmediatamente después del parto. El diseño cambiante de la AIU no pareció afectar el hecho de si la AIU se mantuvo o si se utilizó más adelante. La inserción de la AIU manualmente o con un instrumento acorde no pareció tener influencia.
Se encontraron ensayos más nuevos con informes completos que compararon momentos de la colocación. Los estudios eran de buena calidad. Los ensayos en curso proporcionarán datos adicionales, aunque algunos tamaños de la muestra son pequeños. Estudios más grandes proporcionarían mejor información sobre si el AIU se expulsó solo y si ocurrieron efectos secundarios.
Authors' conclusions
Summary of findings
| Immediate insertion compared with early insertion for postpartum IUC | |||
| Patient or population: postpartum women with desire for contraceptive Setting: hospital or clinic Intervention: immediate postplacental insertion (within 10 minutes) Comparison: early insertion (10 minutes to 48 hours post delivery) | |||
| Outcomes | Relative effect | Participants | Quality of the evidence |
| Expulsion by 6 months | OR 1.00 (95% CI 0.20 to 5.04) | 30 | ⊕⊕⊕⊝ Moderate |
| IUC use at 6 months | OR 0.46 (95% CI 0.04 to 5.75) | 30 | ⊕⊕⊕⊝ |
| CI: Confidence interval; OR: Odds ratio | |||
| GRADE Working Group grades of evidence | |||
| Immediate insertion compared with standard insertion for postpartum IUC | |||
| Patient or population: postpartum women with desire for contraceptive Setting: hospital or clinic Intervention: immediate postplacental insertion (within 10 minutes) Comparison: standard insertion (at postpartum visit) | |||
| Outcomes | Relative effect | Participants | Quality of the evidence |
| Placement per protocol | OR 4.07 (95% CI 0.54 to 30.40); I2 = 68% | 243 (4 studies) | ⊕⊕⊕⊝ |
| Expulsion by 6 months | OR 4.89 (95% CI 1.47 to 16.32) | 210 (4 studies) | ⊕⊕⊕⊝ Moderate |
| IUC use at 6 months | OR 2.04 (95% CI 1.01 to 4.09) | 243 (4 studies) | ⊕⊕⊕⊝ |
| CI: Confidence interval; OR: Odds ratio | |||
| GRADE Working Group grades of evidence | |||
Background
Description of the condition
During the postpartum period, delay in initiating contraception is common. Effective contraception after childbirth helps lengthen birth intervals and thus improves the health of mothers and infants. Preventing unintended pregnancies helps avoid their financial, psychological, and health costs. A longer birth interval decreases the risk of major maternal complications including death, third‐trimester bleeding, puerperal endometritis, and anemia (Conde‐Agudelo 2000; WHO 2005). To reduce risk for poor maternal and infant health outcomes, the World Health Organization (WHO) has recommended waiting 24 months after a birth before attempting the next pregnancy (WHO 2005).
Intrauterine contraception (IUC) includes the levonorgestrel‐releasing intrauterine system (LNG‐IUS) and copper‐containing intrauterine devices (IUDs). The LNG‐IUS and the CuT 380A IUD provide contraceptive protection similar to that attained with tubal sterilization (Trussell 2011). Compared with sterilization, however, IUC use is simpler, less expensive, and immediately reversible. IUC use is not very common in 'more developed' regions globally, where the most prevalent forms of contraception are condoms and contraceptive pills at 18% each, among women who are married or in union (UN 2011). In 'less developed' areas, female sterilization leads at 21% and is followed by IUC use (15%) and contraceptive pills (7%) (UN 2011). These statistics are influenced by countries with large populations such as India and China, which are dominated by sterilization and IUC use, respectively.
How the intervention might work
Insertion of IUC immediately after delivery has several advantages. It may avoid the discomfort related to standard insertion and bleeding from insertion will be disguised by lochia. The woman is known to be not pregnant, and her motivation for contraception may be high. For women with limited access to medical care, postpartum care before discharge provides an opportunity to discuss contraception. Delay in initiating contraception is common in the postpartum period because of the challenges of caring for a new infant (Teal 2014a). Immediate placement of a long‐acting reversible contraceptive, such as IUC or an implant, results in higher use rates. In the USA, however, most insurance reimbursement policies for delivery‐related care do not allow separate billing for postpartum IUC or implants prior to discharge (Aiken 2014).
Women who delay getting IUC may experience an unintended pregnancy or may never return for the insertion (Allen 2009). An early study from Colombia showed that 95% of women interested in immediate postpartum IUD insertion had it done. Only 45% of those wishing later insertion ultimately had an IUD inserted. Although some women in the latter group may have been ambivalent and later decided against an IUD, the inconvenience and expense of a return visit probably deterred some women (Echeverry 1973). A US database review examined the return rate for IUC insertion when two visits were required (Bergin 2012). Only 54% of the women had IUC inserted; return rates were higher when the IUC order initiated at a gynecologic rather than an obstetric visit. Women delivering at a university hospital in the USA were surveyed about postpartum contraception (Glazer 2011). Nearly a fourth were interested in immediate IUC placement if available. Months later, only 5% were using IUC and nearly a fourth still wanted IUC.
Why it is important to do this review
For the original review in 2001, the randomized controlled trials (RCTs) of immediate postplacental insertion compared different devices or different insertion techniques. Many of the IUDs used in those studies are no longer marketed. None of the trials compared IUC insertion at different time points. In 2010, a conference abstract reported results of a trial of immediate postplacental versus standard insertion. Over subsequent years, several trials have compared insertion times for postpartum IUC. The recent studies have examined immediate IUC insertion after cesarean delivery, as well as after vaginal delivery. Investigators used the LNG‐IUS or the CuT 380A. Such trials could provide further evidence regarding subsequent expulsion and method use after immediate insertion of currently marketed IUC.
Objectives
Our aim was to examine the outcomes of IUC insertion immediately after placenta delivery (within 10 minutes), especially when compared with insertion at other postpartum times. We focused on successful placement (insertion), subsequent expulsion, and method use.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials that had at least one arm with immediate postplacental IUC insertion, defined as within 10 minutes of placenta delivery. Recent trials use the term 'postplacental' rather than 'postpartum' when referring to this timing of insertion.
Types of participants
We included studies of postpartum women of any age.
Types of interventions
Trials were eligible if they examined insertion of any type of IUC within 10 minutes of placenta delivery, either vaginal or cesarean. Comparisons could include the following:
-
different devices or different insertion techniques
-
immediate postplacental insertion (within 10 minutes of placenta delivery) versus
-
early postpartum insertion (10 minutes to hospital discharge) or
-
standard insertion (during a postpartum visit after hospital discharge), often referred to as delayed or interval insertion.
-
Types of outcome measures
Primary outcomes
Successful placement (insertion), subsequent expulsion, and method use at study assessment
Secondary outcomes
Pregnancy, perforation, infection, and other adverse events
Search methods for identification of studies
Electronic searches
We searched for trials until 1 April 2015. Sources included PubMed (MEDLINE), Cochrane Central Register of Controlled Trials (CENTRAL), Web of Science, EMBASE, POPLINE, and LILACS. We also searched for current trials via ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). We have provided search strategies in Appendix 1 and earlier strategies in Appendix 2.
Searching other resources
For the original review, the search began with several review articles. The authors also contacted other investigators to attempt to find studies they might have missed, including unpublished reports. In updating, we examined reference lists of relevant reports and reviews for additional trials or secondary articles.
Data collection and analysis
Selection of studies
We assessed all titles and abstracts identified during the search. Two authors examined the search results for potentially eligible studies. For studies that appeared to be eligible for this review, we obtained and examined the full‐text articles. We resolved discrepancies by discussion.
Data extraction and management
Two authors extracted the data. One author entered the data into Review Manager (RevMan 2014), and a second author checked accuracy. Extracted data included the study characteristics, risk of bias, and outcome data. The authors resolved discrepancies by discussion.
Assessment of risk of bias in included studies
The authors examined trials for methodological quality according to recommended principles (Higgins 2011). We considered factors such as study design, method used to generate the randomization sequence, allocation concealment, blinding, and losses to follow‐up and early discontinuation. We also examined the methods used for outcome assessment.
Measures of treatment effect
When the crude number of events was published for dichotomous outcomes, we calculated the Mantel‐Haenszel odds ratio (OR) with 95% confidence interval (CI). An example is the proportion of women with spontaneous expulsion. We used a fixed‐effect model for analysis within a single study. Fixed and random effects yield the same result if no heterogeneity exists, as when a comparison includes only one study. For meta‐analysis, we used random effects. Even when the interventions were similar across studies, the inclusion criteria could lead to differences in study populations. For the original review, the authors reported results primarily as gross cumulative event rates per 100 women. Most studies had insufficient sample sizes to assess rare events, such as uterine perforation.
Dealing with missing data
If reports were missing information or data needed for analysis, we wrote to the study investigators. However, we limited our requests to studies less than 10 years old, as well as older studies that had a report in the past five years. Investigators are unlikely to have access to data from older studies.
Assessment of heterogeneity
We conducted meta‐analyses of studies with similar intervention and outcome measures. We assessed heterogeneity, or degree of inconsistency, using the I2 statistic (Higgins 2003; Higgins 2011). Values greater than 50% may represent substantial heterogeneity, which could be related to clinical or methodological heterogeneity. However, the meta‐analyses we were able to conduct did not show such heterogeneity.
Data synthesis
We applied principles from GRADE (Grades of Recommendation, Assessment, Development and Evaluation) to assess the evidence quality and to address confidence in the effect estimates (Balshem 2011; Higgins 2011). Our assessment was based on the quality of evidence from individual studies, which could be rated as high, moderate, low, or very low. We considered the evidence from RCTs to be of high quality initially, then downgraded quality for each of the following: (1) no information on randomization sequence generation or allocation concealment, or one of those was clearly inadequate; (2) no blinding; (3) follow‐up less than six weeks for expulsion, less than three months later for method use, or less than six months for pregnancy; (4) losses greater than 20%; and (5) missing information for both blinding and losses.
Additional tables contain summaries of the various interventions, our quality assessment, and available outcome data. When the interventions and outcomes were similar, we conducted meta‐analysis as noted earlier. We created Summary of findings tables (Guyatt 2011). However, most trials differed in the experimental and comparison interventions. In addition, many reports did not provide sufficient outcome data for analysis, especially those had only a conference abstract. We did not conduct meta‐analysis of those trials and did not include them in the Summary of findings.
Sensitivity analysis
We examined a group of trials that had evidence of moderate or high quality and sufficient outcome data. We identified these studies in Additional tables and discussed the study results in Summary of main results.
Results
Description of studies
Results of the search
In 2014, we added the term 'postplacental' to the PubMed strategy and ran the search without date restriction. Some of the other databases included 'postplacenta' under key words. The database searches into April 2015 produced 247 citations (Figure 1). After 64 duplicates were removed (55 electronically and 8 by hand), we had 183 references. An abstract from a trial substudy, which was not eligible, led us to the original trial, for a total of 184 unduplicated citations. Upon screening titles and abstracts, we discarded 170 studies for not meeting eligibility criteria. Many were not RCTs or did not provide the relevant intervention. We reviewed the full text of 14 items for eligibility and excluded three items from two studies. Information on seven newly included trials came from seven primary reports (full text or abstract) plus four secondary reports or abstracts.

Study flow diagram (2015)
Searches of ClinicalTrials.gov and ICTRP yielded 28 unduplicated studies. We discarded 22 on the basis of title or summary, and added one to Studies awaiting classification and three to Ongoing studies. We excluded two trial listings with reasons.
Included studies
We included 15 RCTs in this review. The seven newer trials compared immediate postpartum insertion versus early or standard insertion and were published from 2010 to 2014. The eight studies from the original review examined immediate insertion of different devices, device modifications, or insertion techniques. Most of the eight early trials were published in the 1980s, except for one that was published in 1996. The interventions are described below and summarized in Table 1.
| Study | Delivery type | IUC | Inadequate | No blinding | Loss to follow‐up | Quality of evidencea |
| Immediate versus early insertion (10 minutes to 48 hours) | ||||||
| Vaginal | LNG‐IUS | _ | ‐1 | _ | Moderate | |
| Vaginal | CuT 380A | _ | ‐1 | Unclear | Moderate | |
| Vaginal or cesarean | CuT 380A | ‐1 | Unclear | Unclear | Low | |
| Immediate versus standard insertion (weeks) | ||||||
| Vaginal | LNG‐IUS (6 to 8 weeks) | _ | _ | _ | High | |
| Vaginal | LNG‐IUS (> 6 weeks) | _ | ‐1 | _ | Moderate | |
| Cesarean | LNG‐IUS (4 to 8 weeks) | _ | _ | ‐1 | Moderate | |
| Cesarean | CuT 380A (6 weeks) | _ | ‐1 | _ | Moderate | |
| Vaginal or cesarean | CuT 380A (4 to 12 weeks) | ‐1 | Unclear | Unclear | Low | |
| Immediate insertion: IUC types, modifications, or insertion techniques | ||||||
| Unclear | Multiload 250 versus CuT 200 | ‐1 | Unclear | _ | Moderate | |
| Vaginal | Copper 7 versus Lippes Loop D vs Nova‐T‐PP | _ | ‐1 | _ | Moderate | |
| Unclear | Nova‐T‐PP vs Nova T | ‐1 | Unclear | _ | Moderate | |
| Unclear | Progestasert vs CuT 200 | _ | Unclear | ‐1 | Moderate | |
| Vaginal | IPCS‐52 vs CuT 200 | _ | Unclear | ‐1 | Moderate | |
| Vaginal |
_ | Unclear | ‐1 | Moderate | ||
| Unclear | Delta Loop vs Delta T | _ | Unclear | _ | Moderate | |
| Vaginal | CuT 380A: hand vs ring‐forceps insertion | _ | _ | _ | High | |
aRCTs considered high quality initially, then downgraded for (1) no information on randomization sequence generation or allocation concealment, or one was clearly inadequate; (2) no blinding; (3) losses > 20%; (4) information missing for both blinding and losses. Follow‐up time not shown as all studies met criteria.
bNo full report; sources included conference abstracts and clinical trial listings.
The seven recent trials compared immediate versus early or standard insertion.
-
Two trials examined immediate insertion (within 10 minutes postplacental) versus early postpartum insertion (10 minutes to 48 hours) using the CuT 380A (Ahuja 2014; Singh 2014). Delivery was vaginal in Ahuja 2014, and Singh 2014 included both vaginal and cesarean delivery. Neither study had a full report.
-
Four trials compared immediate versus standard insertion (4 to 12 weeks postpartum).
-
Two used the CuT 380A (Ogburn 2013; Lester 2015). In Lester 2015, delivery was cesarean, and Ogburn 2013 included women with either vaginal or cesarean birth. Ogburn 2013 did not yet provide a full report.
-
Two used a levonorgestrel‐releasing intrauterine system (LNG‐IUS) (20 μg) (Chen 2010; Whitaker 2014). In Whitaker 2014, delivery was cesarean.
-
-
One trial examined immediate, early, and standard (after six weeks) insertion of the LNG‐IUS after vaginal delivery (Dahlke 2011).
The eight trials from the 2001 review examined different devices, device modifications, or insertion techniques.
-
A multicenter RCT studied the addition of absorbable chromic sutures tied around the superior arms of conventional IUDs (Cole 1984; Kisnisci 1985). For example, investigators tied two chromic sutures (size No. 2) to the lateral arms of a Copper T 220 C (TCu 220C) with the free ends of the sutures left 0.5 cm long and projecting caudad at a 45‐degree angle. This modification of the TCu 220C was termed the Delta T. Similarly, investigators tied three chromic sutures to the superior bar of a Lippes Loop D, thereafter termed the Delta Loop. Each modification was compared with the conventional device without sutures. The other interventions examined in these two reports were hand versus instrument insertion of the various devices (Cole 1984; Kisnisci 1985).
-
Two early trials focused on progesterone‐releasing IUDs.
-
Lavin 1983 compared the conventional Progestasert IUD, containing 38 mg progesterone, released into the uterus over a year, with the Copper T 200 (TCu 200). The investigators compared hand versus instrument insertion for each IUD type.
-
A prototype of a longer‐acting Progestasert contained 52 mg of progesterone (ICPS‐52), and was designed to provide three years of protection. This was compared with the TCu 200 in Apelo 1985.
-
-
In two trials, investigators modified a Nova T device to have two flexible arms, 2 cm in length, added to the base of the vertical stem; the arms pointed superiorly at a 45‐degree angle. This Nova T Postpartum (Nova‐T‐PP) and standard Nova T were otherwise identical.
-
Van Kets 1987 compared the modified (Nova‐T‐PP) and standard Nova T.
-
WHO 1980 compared the Nova‐T‐PP with the Lippes Loop D and with the Copper 7 (Gravigard).
-
-
Two trials examined other interventions, i.e.,
-
standard Multiload 250 (ML Cu 250) versus TCu 200 (Thiery 1980); and
-
hand versus ring‐forceps insertion of a standard CuT 380A (Xu 1996).
-
Excluded studies
We excluded several trials from the review. Data from Thiery 1983 were included in a larger report (Cole 1984). We also excluded a subgroup analysis of the Cole 1984 data (Chi 1985). Shikary 1987 examined standard postpartum insertions, and Stuart 2015 compared early versus standard insertion. Others were not randomized controlled trials or did not have a relevant outcome measure (Characteristics of excluded studies).
Risk of bias in included studies
Figure 2 shows the overall risk of bias for evidence in this review. Risk of bias by trial can be seen in Figure 3. We also summarized the quality of evidence from each trial in Table 1. Communication with researchers supplemented several of the early reports (WHO 1980; Lavin 1983; Apelo 1985; Kisnisci 1985; Xu 1996).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Of the reports from 2010 to 2014, four provided sample size calculations (Chen 2010; Ahuja 2014; Whitaker 2014; Lester 2015). A pilot study was not powered for the utilization outcome (Dahlke 2011). Those lacking information on sample size calculation were conference abstracts (Ogburn 2013; Singh 2014). For the initial review, only Xu 1996 provided a sample size calculation.
Allocation
Most trials used an appropriate method of generating the randomization sequence and mentioned some type of computer‐generated randomization scheme. Two conference abstracts (Ogburn 2013; Singh 2014) and an older report (Van Kets 1987) made no mention of randomization. Thiery 1980 mentioned a list of random numbers.
Many trials attempted to conceal the upcoming assignment from those involved with the trials, usually by using sequentially numbered, sealed, opaque envelopes. Two conference abstracts (Ogburn 2013; Singh 2014) and two older reports (Thiery 1980; Van Kets 1987) made no mention of allocation concealment.
Blinding
Three trials used some type of blinding: evaluators (Chen 2010), investigator and evaluators (Whitaker 2014), or participants and evaluators (Xu 1996). Four studies did not use any type of blinding, or the clinical trial listing stated that blinding was "not applicable" (WHO 1980; Dahlke 2011; Ahuja 2014; Lester 2015). The other eight made no mention of blinding.
Incomplete outcome data
Loss to follow‐up was high in Whitaker 2014 (33%) and Lavin 1983 (based on reported life‐table rates). Losses were differential across study arms in two trials (Cole 1984; Apelo 1985). In Cole 1984, four of six study groups had high losses, as did one of four in Apelo 1985. One arm in Kisnisci 1985 lost more than 20%. Two conference abstracts provided no information on losses (Ogburn 2013; Ahuja 2014).
Effects of interventions
See: Summary of findings for the main comparison ; Summary of findings 2
For trials that compared insertion times, Table 2 provides a summary of insertion rates. A summary of IUC use and expulsion can be found in Table 3. Those tables show sample sizes for trials as well.
| Study | N | Placement (insertion) per protocol (%) | ||
| Immediate | Early | Standard | ||
| 102 | 88 | _ | 90 | |
| 46 | 100 | 100 | 94 | |
| 42 | 95 | _ | 82 | |
| 68 | 100 | _ | 53 | |
| 156 | 87 | _ | 77 | |
| 263 | _ | _ | _ | |
| 200 | _ | _ | _ | |
aIn sensitivity analysis; sufficient outcome data and evidence of moderate or high quality (Table 1).
| Study | N | Use at | Expulsion by | ||||
| Immediate | Early | Standard | Immediate | Early | Standard | ||
| 102 | 84 | _ | 77 | 22d | _ | 4 | |
| 46 | 87 | 93 | 94 | 27 | 27 | 0 | |
| 42 | 70 | _ | 59 | 20 | _ | 0 | |
| 68 | 79 | _ | 47 | 3 | _ | 6 | |
| 156 | 57 | _ | 59 | _ | _ | _ | |
| 263 | 89 | 74 | _ | 9 | 24 | _ | |
| 200 | 84 | 77 | _ | 8 | 11 | _ | |
aUse based on women randomized; Ogburn 2013 assessed at 12 months.
bExpulsion based on IUC placed (Table 2); Ahuja 2014 assessed at 6 weeks.
cIn sensitivity analysis; sufficient outcome data and evidence of moderate or high quality (Table 1).
dExcludes 5 women with IUC placed 11 to 15 minutes postplacental; does not significantly affect results.
Immediate postplacental versus early insertion
Three trials compared immediate postplacental versus early postpartum (10 minutes to 48 hours) insertion. In a pilot study, the LNG‐IUS was placed after vaginal delivery (N = 46) (Dahlke 2011). The investigators also studied standard insertion; those results are presented in the next section. Two trials using the CuT 380A had only conference abstracts (Ahuja 2014; Singh 2014), so we presented the results separately below. Both were larger trials (N = 263 and N = 200, respectively).
In Dahlke 2011, all women in the immediate and early insertion groups had the LNG‐IUS placed (inserted) (Analysis 1.1). Of the 15 women with early insertion, 14 had the IUS placed within 30 minutes of placenta delivery. The study arms had the same expulsion rate by six months (Analysis 1.2). Utilization at three or six months did not differ significantly between the study groups (Analysis 1.3; Analysis 1.4), but this pilot study was not powered to detect such a difference.
Two trials used the CuT 380A IUD, but the conference abstracts provided limited data. Ahuja 2014 compared immediate versus early insertion after vaginal delivery, and Singh 2014 included women with either vaginal or cesarean delivery. Expulsion, assessed at six weeks in Ahuja 2014, was lower for the immediate group compared with the early group (9% versus 24%, reported P = 0.0037) (Analysis 1.5). In Singh 2014, the study arms did not differ significantly for expulsion by six months (reported P = 0.06) (Analysis 1.5). Continued use at six months was higher in the immediate group compared with the early group in Ahuja 2014 (reported P = 0.0054), but the groups were not significantly different in Singh 2014 (reported P = 0.13) (Analysis 1.6). No pregnancy occurred in Singh 2014. Removals for medical reasons were 5.2% in the immediate group and 7.4% in the early group (reported P > 0.05) (Singh 2014). No perforation or pelvic inflammatory disease occurred. In Ahuja 2014, complications leading to removals were 2.2% and were reportedly similar for the two groups.
Immediate postplacental versus standard insertion
Five trials compared immediate postplacental versus standard insertion. Four had full reports, and the time frames ranged from four to eight weeks postpartum. Two of those trials examined the LNG‐IUS after vaginal delivery (Chen 2010; Dahlke 2011). Dahlke 2011 was a small pilot study (N = 46), and Chen 2010 was mid‐sized (N = 102). Two other trials placed the IUC after cesarean section, using the CuT 380A (N = 68) (Lester 2015) or the LNG‐IUS (N = 42) (Whitaker 2014). Whitaker 2014 was terminated early because of slow recruitment and had a high loss to follow‐up (33% of 42 women randomized). Ogburn 2013 (N = 156) had only a conference abstract to date, so we have presented the results separately below.
Meta‐analysis of the four trials with full reports showed the following.
-
IUC placement according to the study plan (protocol) was considered successful insertion. The study arms did not differ significantly (Analysis 2.1). The substantial heterogeneity (I2 = 68%) was related to the Uganda study (Lester 2015). Insertion was more likely in the immediate group in that trial, but the CI was very wide. The US trials indicated little difference between the study arms, regardless of whether the IUC was inserted after vaginal or cesarean delivery, or whether the LNG‐IUS or the CuT 380A was used.
-
For expulsion, the denominator was the number of women with IUC placed according to study plan. Expulsion by six months was more likely with immediate insertion than with standard insertion (OR 4.89, 95% CI 1.47 to 16.32; participants = 210; studies = 4) (Analysis 2.2). However, the wide confidence interval indicates imprecision in the estimate.
-
For IUC use, the denominator was the number of women randomized. Use at six months was more likely with immediate insertion than with standard insertion (OR 2.04, 95% CI 1.01 to 4.09; participants = 243; studies = 4) (Analysis 2.3). However, the groups did not differ at three months in one small trial (Dahlke 2011) (Analysis 2.4) or at 12 months in another (Whitaker 2014) (Analysis 2.5).
Other results for these four trials included pregnancies and complications. Two studies found no pregnancies by six months (Chen 2010; Lester 2015). Complications were few. Two trials diagnosed one infection in each randomized arm (Chen 2010; Lester 2015). Two studies found no infection (Dahlke 2011; Whitaker 2014), and two reported no other adverse events (Whitaker 2014; Lester 2015).
Ogburn 2013, which had only a conference abstract, placed the CuT 380A after either vaginal or cesarean delivery. Immediate postplacental insertion was compared with standard, which ranged from 4 to 12 weeks postpartum. The study arms did not differ significantly for IUC placement (insertion) (Analysis 2.6). The groups were similar for use at 12 months (Analysis 2.7), and no major complication occurred.
Immediate postplacental insertion: different devices or insertion techniques
The eight trials included from the 2001 review examined immediate insertion of different devices or insertion techniques; none compared insertion times. Seven were published from 1980 to 1987, and one in 1996. The reported life‐table rates mentioned below are also shown in Data and analyses. For these trials, we summarized the results for insertion, use, and expulsion in Table 4.
| Study | IUC | N | Insertion | Placement | Use at | Expulsion by |
| Life‐table rates | ||||||
| TCu 200 | 269 | _ | _ | 85.0 | 9.4 | |
| Multiload 250 | 293 | _ | _ | 88.8 | 7.4 | |
| Copper 7 | 277 | _ | _ | 64.6 | 31.1 | |
| Lippes Loop D | 250 | _ | _ | 48.1 | 41.3 | |
| Nova‐T‐PP | 277 | _ | _ | 52.3 | 39.4 | |
| Nova‐T‐PP | 205 | _ | _ | 87.5 | 5.5 | |
| Nova T | 203 | _ | _ | 88.4 | 6.0 | |
| Progestasert | 100 | Hand | _ | 59.9 | 35.8 | |
| 100 | Inserter | _ | 57.2 | 35.2 | ||
| TCu 200 | 100 | Hand | _ | 86.3 | 9.0 | |
| 100 | Inserter | _ | 86.1 | 8.1 | ||
| IPCS‐52 mg | 50 | Hand | _ | 57.3 | 39.0 | |
| 50 | Inserter | _ | 79.6 | 14.2 | ||
| TCu 200 | 50 | Hand | _ | 81.9 | 14.1 | |
| 50 | inserter | _ | 89.7 | 10.3 | ||
| Delta T | 728 | _ | 99.9 | 81.8 | 11.6 | |
| TCu 220 | 718 | _ | 99.7 | 81.8 | 11.5 | |
| Delta Loop | 662 | _ | 99.7 | 78.5 | 15.7 | |
| Lippes Loop D | 648 | _ | 99.7 | 73.8 | 21.5 | |
| Delta T | 518 | Hand | 99.7 | 84.2 | 11.2 | |
| 517 | Mechanical | 99.7 | 82.6 | 11.5 | ||
| Delta Loop | 122 | _ | 99.2 | 96.3 | 3.7 | |
| Delta T | 124 | _ | 100.0 | 90.7 | 7.6 | |
| Percent | ||||||
| CuT 380A | 470 | Hand | _ | 81.3 | 13.0 | |
| 440 | Ring‐forceps | _ | 79.1 | 12.5 | ||
aLavin 1983 reported use and expulsion by 12 months.
bWHO 1980 excluded expulsions within 48 hours.
The addition of chromic sutures to conventional IUDs had little impact on clinical outcomes (Cole 1984) (Analysis 3.1 to Analysis 5.4). Virtually all participants received an IUD (Analysis 3.1; Analysis 4.1; Analysis 5.1). Women were randomly assigned to either a Delta Loop or a Lippes Loop D. The only significant finding noted by the investigators was a lower rate of expulsion with the Delta Loop at six months (15.7 versus 21.5 per 100 women). Gross six‐month continuation rates with the two devices were 78.5 and 73.8 per 100 women, respectively. Follow‐up rates were 62.7 and 66.1. Among women randomized to either the Delta T or the TCu 220C, the rates of expulsion at six months were 11.6 and 11.5 per 100 women, respectively. Six‐month continuation rates were 81.8 per 100 women for both groups, and follow‐up rates were 71.0 and 70.9. The technique of insertion for the Delta Loop device (hand versus inserter or forceps) had no significant impact on expulsion or continuation rates. Follow‐up rates were 84.2 for hand insertion and 83.0 for instrument insertion.
Kisnisci 1985 compared the Delta Loop and the Delta T (Analysis 6.1 to Analysis 6.6). These results from one center involved in Cole 1984 were published separately. Nearly all participants received an IUD. Expulsion rates per 100 women at 12 months were 3.7 for the Delta Loop and 7.6 for the Delta T. The 12‐month pregnancy rates were 2.1 and 0, respectively. The two devices had similar 12‐month rates of removal for pain or bleeding: 1.1 and 1.0 per 100 women. Likewise, continuation rates were comparable: 93.3 and 90.7 per 100 women, respectively. Follow‐up rates were 78.9% for the Delta Loop and 86.7% for the Delta T. The investigators noted that the groups did not differ significantly in the termination or event rates.
The TCu 200 was superior to the Progestasert for immediate postpartum insertion for the outcomes in this review (Lavin 1983) (Analysis 7.1 to Analysis 7.5). The Progestasert had significantly higher expulsion rates than the TCu 200, and the differences were independent of whether the devices had been introduced by hand or with an inserter. The 12‐month expulsion rates for hand insertion and instrument insertion were 9.0 and 8.1 for the TCu 200, and 35.8 and 35.2 for the Progestasert. The 12‐month continuation rates were reported to be significantly higher for the TCu 200 groups (86.3 when introduced by hand and 86.1 for inserter) than for the Progestasert groups (59.9 and 57.2, respectively). At 12 months, follow‐up rates were 69.7 and 67.7 for the TCu200 and Progestasert instrument‐insertion groups, respectively, versus 76.1 and 77.4 for the respective hand‐insertion groups; significance levels were not provided.
The TCu 200 also appeared to perform better than the prototype three‐year progesterone device (IPCS‐52) (Apelo 1985) (Analysis 8.1 to Analysis 8.9). The 12‐month rates for expulsion when hand‐introduced were 39.0 for IPCS‐52 and 19.9 for TCu 200. The comparable rates for the inserter‐introduced devices were 14.2 for the IPCS‐52 and 10.3 for the TCu 200. The 12‐month continuation rates per 100 women for the IPCS‐52 were 57.3 for hand insertion and 77.1 for instrument insertion. For the TCu 200, the continuation rates were 73.8 for hand inserted and 84.9 for instrument inserted. The 36‐month continuation rates were 52.3, 55.8, 67.7, and 62.9, respectively. The investigators tested for statistical significance after 36 months. The life‐table rates for expulsion at 36 months were 39.0 for the hand‐introduced IPCS‐52, 24.2 for the inserter‐introduced IPCS‐52, 19.9 for the hand‐introduced TCu 200, and 13.1 for the inserter‐introduced TCu 200. Investigators reported that expulsion for the hand‐inserted IPCS‐52 was significantly greater than for the hand‐inserted or instrument‐inserted TCu 200. Removals for bleeding and pain were uncommon with both devices and did not change between 12 and 36 months. For pooled data, the researchers noted that the TCu 200 had a significantly lower expulsion rate than the IPCS‐52, and instrument insertions had a significantly lower expulsion rate than hand insertions. At 12 months, follow‐up rates appeared lower for the TCu 200 group with hand insertion (71.1%) than for the group with instrument insertion (99.1%). For the IPCS‐52, both insertion groups had 83% follow‐up. At 36 months, when statistical testing was done, follow‐up ranged from 55% to 67% across the groups.
The modified Nova T was no better than the standard Nova T (Van Kets 1987) or the Copper 7 (WHO 1980).
-
A multicenter trial compared the Nova‐T‐PP, Lippes Loop D, Copper 7, and Lippes Loop (WHO 1980) (Analysis 9.1 to Analysis 9.4).
-
Spontaneous expulsion rates at six months for all three IUDs exceeded the predetermined stopping rules, so the trial terminated early. The 12‐month expulsion rates per 100 women were 41.3 for the Nova‐T‐PP, 44.1 for the Lippes Loop, and 34.8 for the Copper 7. The investigators stated that expulsion for the Copper 7 was significantly lower than for the Lippes Loop; no statistical testing was mentioned. Study procedures specified that women with expulsion within 48 hours after insertion were discontinued from the study and were excluded from the life‐table analysis. Early expulsion was significantly higher in the Lippes Loop group (reported P < 0.01).
-
Corresponding 12‐month pregnancy rates were 5.6, 12.1, and 7.2 per 100 women.
-
Total 12‐month discontinuation rates were high with all devices: 53.1, 60.9, and 47.7 per 100 women. The discontinuation rate at 12 months was significantly higher for the Lippes Loop (60.9) than for the Copper 7 (47.7) (reported P < 0.01).
-
Loss to follow‐up was 17.8% for Nova‐T‐PP, 16.2% for Lippes Loop, and 16.0% for Copper 7.
-
-
Outcomes with the modified and standard Nova T were more favorable in a single‐center trial (Van Kets 1987) than in a multicenter trial (WHO 1980). The 12‐month spontaneous expulsion rates per 100 women were low with both the Nova‐T‐PP (6.2) and standard Nova T (6.6). The 12‐month pregnancy rates were also low with both devices: 0.6 and 0.0, respectively. Continuation rates at 12 months were 87.4 for the Nova‐T‐PP and 78.2 for the Nova T. Loss to follow‐up was 6.2 for both groups at 12 months and 18 months. The investigators stated that none of these differences was statistically significant (Analysis 10.1 to Analysis 10.4).
Thiery 1980 noted that results for immediate insertion of the ML Cu 250 and the TCu 200 were similar (Analysis 11.1 to Analysis 11.4). Twelve‐month rates of expulsion per 100 women were 9.9 for the ML Cu 250 and 11.2 for the TCu 200. Corresponding pregnancy rates were 2.4 and 0.5. The 12‐month continuation rates were 77.3 and 77.2, respectively. For 12 months, rates for loss to follow‐up were 1.6 for the ML Cu250 and 2.0 for the TCu 200. At 24 months, the differences between the devices in pregnancy and expulsion rates reportedly showed "borderline" significance.
Xu 1996 showed hand versus instrument insertion of the CuT 380A IUD to be comparable. The study groups did not differ significantly for expulsion by six months (Analysis 12.1), nor for continuation at six months (Analysis 12.2). No pregnancies, perforations, or infections occurred. Loss to follow‐up by six months was 3% for the hand‐insertion group and 7% for the instrument‐insertion group.
Discussion
Summary of main results
Sensitivity analysis
In this synthesis, we included trials with sufficient outcome data and evidence of moderate or high quality (Table 1). We focused on the trials that compared IUC insertion times (Table 2; Table 3). Four of these newer trials met the criteria; three used the LNG‐IUS and one used the CuT 380A.
A pilot trial examined immediate postplacental versus early insertion of the LNG‐IUS, and also examined standard insertion. All women in the immediate and early groups received the IUC (N = 30). Expulsion and use data can be found in summary of findings Table for the main comparison. Expulsion rates were the same for both groups by six months. The study arms did not differ significantly for use at six months.
Data on immediate versus standard insertion came from meta‐analysis of four trials (N = 243). Results provide the basis for summary of findings Table 2.
-
For rates of insertion (IUC received), the study arms were not significantly different. However, the Uganda study showed that insertion was more likely for the immediate group, although the estimate was imprecise. The three US studies indicated little difference between the groups.
-
Expulsion by six months was more likely for the immediate group, but the confidence interval was wide. Two trials examined the LNG‐IUS after vaginal delivery. The other two placed the IUC after cesarean section; one used the CuT 380A and the other, the LNG‐IUS.
-
IUC use at six months was based on the women randomized. Use was more likely for women with immediate insertion than for those with standard insertion. Two studies inserted the IUC after vaginal delivery and two after cesarean section. In individual small trials, the study arms did not differ in use at 3 or 12 months.
Other studies
Three newer trials comparing insertion times used the CuT 380A (Table 1). The conference abstracts lacked design information and outcome data. However, these trials were larger than those with full reports. Two trials examined immediate versus early insertion. Expulsion by six weeks in one study was reported to be lower for the immediate group compared with the early group, and continued use at six months was higher in the immediate group. In the other trial with early insertion, the study arms did not differ significantly in expulsion or continuation at six months. The third trial studied immediate versus standard insertion. Insertion rates did not differ significantly between the study arms, nor did continued use at 12 months.
Eight early studies showed that modifications of IUDs for immediate postpartum insertion were not helpful (Table 4). Modifications included the addition of absorbable sutures to the superior arms of T‐shaped IUDs and Lippes Loops, and the addition of plastic arms to the vertical stem of a Nova T. The choice of insertion technique (hand versus instrument) also appeared to be clinically unimportant. Only one trial noted any significant difference (Apelo 1985), but a later trial with better methods found no substantial differences (Xu 1996).
Overall completeness and applicability of evidence
Three of the seven newer trials examined different insertion times for the LNG‐IUS and four compared insertion times for the CuT 380A. Three placed the IUC after vaginal delivery, two after cesarean delivery, and two after either type of delivery. The eight trials from the 2001 review compared different devices or insertion techniques with immediate insertion rather than different insertion times. Several of those trials examined immediate insertion after vaginal delivery, but others did not specify the type of delivery. Many of the IUDs examined in the earlier studies are no longer widely used. Overall, limited data were provided on safety (e.g., perforation and infection) and on other adverse events.
Immediate IUC insertion is common in many areas of the world. Of the trials comparing insertion times, four were conducted in the USA, two in India, and one in Uganda. The early trials of immediate insertion were conducted in Eastern and Western Europe, South America, and Asia. Three were multisite trials. Non‐randomized studies also provide evidence of how common immediate IUC insertion has become; some are discussed below (Agreements and disagreements with other studies or reviews). Feasibility studies show that IUC insertion may be acceptable in some settings, but conducting an RCT may not. In a study of early versus standard insertion in Malawi, nearly the number planned were recruited but less than half were randomized (Bryant 2013). In Kampala, Uganda, more than half the women screened opted out of the feasibility study for intra‐cesarean insertion (Lester 2015). Surveys of the women who opted out, as presented in a conference abstract, indicated that 41% wanted to ask their husband first, 16% did not like IUC, and 15% said their husband did not like IUC (Lester 2015).
Insertion of hormonal IUC may be delayed because of concerns about the effect on lactation. Another Cochrane review examined hormonal contraceptives and lactation (Lopez 2015). Two included studies did provide data on lactation with LNG‐IUS use. In a secondary analysis from Chen 2010 (Chen 2011), women who had immediate postpartum insertion stopped exclusive breastfeeding earlier than women who had standard insertion. The trial was not designed to measure this effect. The pilot study (Dahlke 2011) stated that breastfeeding at six months did not differ significantly across the immediate, early, and standard groups, although the standard group had the lowest rate. Most progestin‐only methods are considered category 3 for less than six weeks postpartum (WHO 2009). Category 3 means that the theoretical or proven risks usually outweigh the advantages of using the method. The method is not usually recommended unless more appropriate methods are unavailable or unacceptable. In the USA, for the first month postpartum, progestin‐only methods are category 2, meaning that the advantages of using the method generally outweigh the risks (CDC 2011).
Quality of the evidence
We summarized our assessment of the evidence quality (Table 1). Risk of bias is also summarized in Figure 2 and is shown by study in Figure 3. Overall, we considered the evidence to be of moderate quality. About a fourth of the reports lacked information on the randomization process and allocation concealment. Several trials had no blinding or no mention of blinding. The timing of insertion made blinding of participants unfeasible. Assessors could have been blinded, but the outcomes were often expulsion and IUC use. Standards for reporting trials were developed in the late 1990s, and the CONSORT statement was widely adopted in 2010 (Schulz 2010). The three recent trials for which we had only a conference abstract lacked information needed to assess risk of bias. For two of those trials, the investigators communicated that full reports were in progress.
Agreements and disagreements with other studies or reviews
Immediate postpartum insertion of IUC involves trade‐offs. Expulsion rates appear higher with postplacental and early postpartum insertion than with standard insertion. The net effect of these expulsions is not clear from published studies, for example, if detected and another contraceptive is started, accidental pregnancies might be avoided. In the two more recent studies that assessed pregnancy, none was reported. The earlier studies had varied pregnancy rates. Insertion of IUC immediately after delivery is convenient for both the woman and the clinician. Resumption of ovulation can be unpredictable after delivery, and IUC provides highly effective contraception during the puerperium.
Non‐randomized studies provide some information about expulsion and IUC use or continuation after immediate or early insertion. Over a five‐year period in India, 1037 women had a CuT 200B inserted immediately after vaginal (37%) or cesarean (63%) delivery (Shukla 2012). The expulsion rate was 11% by six months and most occurred in the first six weeks. A prospective case series of early LNG‐IUS insertion after vaginal delivery was conducted at a university medical center in the USA. With only 40 women enrolled, the expulsion rate was 38% (Stuart 2012). The results may not be reliable because of the small sample size. In Turkey, 268 women chose the timing for CuT 380A insertion after vaginal or cesarean delivery: immediate, early (up to 72 hours), or standard (Eroglu 2006). Complete expulsion was higher for the immediate (11%) and early (14%) groups compared with the standard insertion group (3.6%) at eight weeks. In Pakistan, 100 women had a CuT 380A or Multiload inserted immediately after vaginal or cesarean delivery (Alam 2014). Complete expulsion occurred in three women by 10 weeks.
Early trials included in this review indicated that immediate postpartum insertion of IUDs was safe and effective. Decades ago, WHO 1980 judged expulsion and pregnancy rates to be excessive for women who underwent immediate IUD insertion. However, variable clinical experience, rather than patient characteristics, may be responsible (Chi 1985; Kisnisci 1985; Thiery 1985). Two systematic reviews examined studies of various designs that compared different insertion times or routes (Kapp 2009; Sonalkar 2014). Evidence from studies of copper‐containing IUDs and the LNG‐IUS indicated that postplacental and early postpartum insertion was safe compared with standard insertion, but expulsion rates were higher.
Expulsion rates may be lower for postplacental insertion after cesarean delivery than after vaginal delivery (Kapp 2009; Sonalkar 2014). Recent studies examined immediate insertion after cesarean delivery but were uncontrolled. A study in Turkey examined the safety and efficacy of immediate insertion of the CuT 380A during cesarean section (Celen 2011). At six months, the cumulative expulsion rate was 10.6 per 100 women and the continuation rate was 81.6%. A feasibility study of immediate insertion after cesarean delivery assessed expulsion and acceptability among 90 women in the USA (Levi 2012). Among the 60% who returned for a six‐week visit or provided a six‐month phone interview, no expulsions were identified by examination or ultrasound nor were reported by the women. The investigators emphasized the importance of providing effective contraception before discharge, given that only 48% returned for the six‐week postpartum visit. A pilot study in Dakar, Senegal, examined cesarean insertion of IUC in 46 women (Gueye 2013). The expulsion rate at three months was 2.2%. In India, 564 women had intra‐cesarean insertion, and the expulsion rate was 9% by six months (Mishra 2014).

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
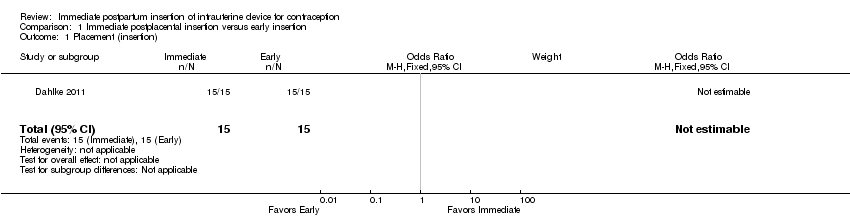
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 1 Placement (insertion).

Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 2 Expulsion by 6 months.

Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 3 Use at 3 months.

Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 4 Use at 6 months.
| Study | Time frame | Immediate insertion | Early insertion | Reported P |
| Ahuja 2014 | ‐‐‐ | N = 131 | N = 132 | ‐‐‐ |
| Ahuja 2014 | 6 weeks | 24.1% | 9.1% | .0037 |
| Singh 2014 | ‐‐‐ | N = 100 | N = 100 | ‐‐‐ |
| Singh 2014 | 6 months | 8.3% | 10.5% | .06 |
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 5 Expulsion.
| Study | Immediate insertion | Early insertion | Reported P |
| Ahuja 2014 | 88.9% | 74.1% | .0054 |
| Ahuja 2014 | N = 131 | N = 132 | ‐‐‐ |
| Singh 2014 | 83.5% | 77.1% | .13 |
| Singh 2014 | N = 100 | N = 100 | ‐‐‐ |
Comparison 1 Immediate postplacental insertion versus early insertion, Outcome 6 Use at 6 months.

Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 1 Placement (insertion) per protocol.
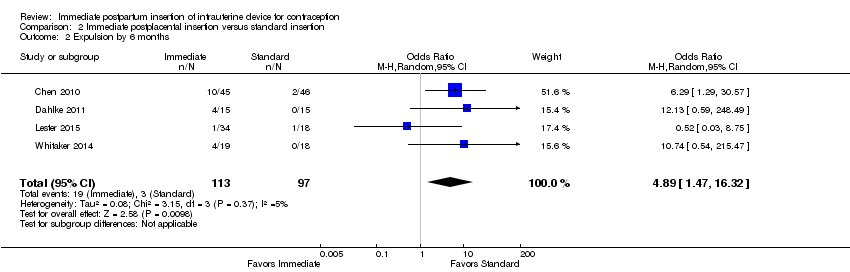
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 2 Expulsion by 6 months.

Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 3 Use at 6 months.
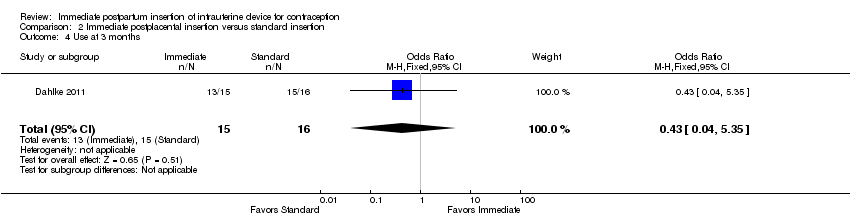
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 4 Use at 3 months.

Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 5 Use at 12 months.
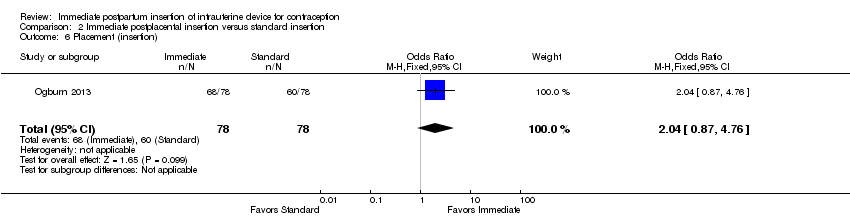
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 6 Placement (insertion).
| Study | Immediate insertion | Early insertion |
| Ogburn 2013 | N = 78 | N = 78 |
| Ogburn 2013 | 57% | 59% |
Comparison 2 Immediate postplacental insertion versus standard insertion, Outcome 7 Continued use at 12 months.
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 99.7% | 99.7% |
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 1 Insertion per protocol.
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 15.7 | 21.5 |
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 78.5 | 73.8 |
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Study | Delta Loop | Lippes Loop D |
| Cole 1984 | 62.7% | 66.1% |
Comparison 3 Immediate postpartum insertion: Delta Loop versus Lippes Loop D, Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
| Study | Delta T | TCu 220 C |
| Cole 1984 | 99.9% | 99.7% |
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 1 Insertion per protocol.
| Study | Delta T | TCu 220 C |
| Cole 1984 | 11.6 | 11.5 |
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Study | Delta T | TCu 220 C |
| Cole 1984 | 81.8 | 81.8 |
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Study | Delta T | TCu 220 C |
| Cole 1984 | 71.0% | 70.9% |
Comparison 4 Immediate postpartum insertion: Delta T versus TCu 220 C, Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
| Study | Delta Loop | Delta Loop |
| Cole 1984 | 99.7% | 99.7% |
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 1 Insertion per protocol.
| Study | Hand insertion | Instrument insertion |
| Cole 1984 | 11.2 | 11.5 |
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 2 Life‐table rates per 100 women for expulsion (6‐month).
| Study | Hand insertion | Instrument insertion |
| Cole 1984 | 84.2 | 82.6 |
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 3 Life‐table rates per 100 women for continuation (6‐month).
| Study | Delta Loop | Delta Loop |
| Cole 1984 | 84.2% | 83.0% |
Comparison 5 Immediate postpartum insertion: Delta Loop (hand versus instrument insertion), Outcome 4 Life‐table rates per 100 women for follow‐up (6‐month).
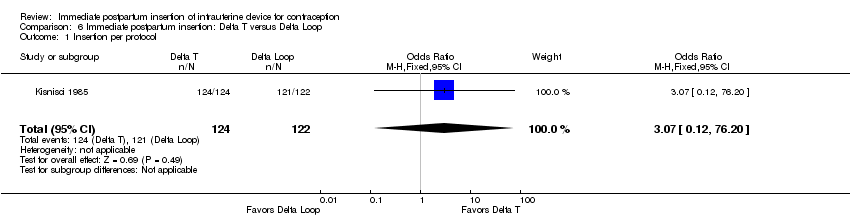
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 1 Insertion per protocol.
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 7.6 | 3.7 |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 2 Life‐table rates per 100 women for expulsion (12‐month).
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 0 | 2.1 |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 3 Life‐table rates per 100 women for pregnancy (12‐month).
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 1.0 | 1.1 |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 4 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month).
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 90.7 | 93.3 |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 5 Life‐table rates per 100 women for continuation (12‐month).
| Study | Delta T | Delta Loop |
| Kisnisci 1985 | 86.7% | 78.9% |
Comparison 6 Immediate postpartum insertion: Delta T versus Delta Loop, Outcome 6 Life‐table rates per 100 women for follow‐up (12‐month).
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 9.0 | 35.8 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 1 Hand insertion: Life‐table rates per 100 women for expulsion (12‐month).
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 8.1 | 35.2 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 2 Instrument insertion: Life‐table rates per 100 women for expulsion (12‐month).
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 86.3 | 59.9 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 3 Hand insertion: Life‐table rates per 100 women for continuation (12‐month).
| Study | TCu 200 | Progestasert |
| Lavin 1983 | 86.1 | 57.2 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 4 Instrument insertion: Life‐table rates per 100 women for continuation (12‐month).
| Study | Insertion method | TCu 200 | Progestasert |
| Lavin 1983 | Hand | 76.1 | 77.4 |
| Lavin 1983 | Instrument | 69.7 | 67.7 |
Comparison 7 Immediate postpartum insertion: TCu 200 versus Progestasert, Outcome 5 Life‐table rates per 100 women for follow‐up (12‐month).
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 19.9 | 10.3 | 39.0 | 14.2 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 5.5 | 0 | 3.2 | 5.2 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 2 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 73.8 | 84.9 | 57.3 | 77.1 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 3 Life‐table rates per 100 women for continuation (12‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 19.9 | 13.1 | 39.0 | 24.2 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 4 Life‐table rates per 100 women for expulsion (36‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 67.7 | 62.9 | 52.3 | 55.8 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 5 Life‐table rates per 100 women for continuation (36‐month) by device and insertion method.
| Study | TCu 200 (pooled) | IPCS‐52 (pooled) |
| Apelo 1985 | 16.4 | 31.3 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 6 Life‐table rates per 100 women for expulsion (36‐month) by device pooled.
| Study | Hand (pooled) | Inserter (pooled) |
| Apelo 1985 | 29.2 | 18.5 |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 7 Life‐table rates per 100 women for expulsion (36‐month) by method pooled.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 71.1% | 99.1% | 82.8% | 82.5% |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 8 Life‐table rates per 100 women for follow‐up (12‐month) by device and insertion method.
| Study | TCu 200 (hand) | TCu 200 (inserter) | IPCS‐52 (hand) | IPCS‐52 (inserter) |
| Apelo 1985 | 55.6% | 63.9% | 66.7% | 54.8% |
Comparison 8 Immediate postpartum insertion: TCu 200 versus IPCS‐52 mg, Outcome 9 Life‐table rates per 100 women for follow‐up (36‐month) by device and insertion method.
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 41.3 | 44.1 | 34.8 |
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 5.6 | 12.1 | 7.2 |
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 53.1 | 60.9 | 47.7 |
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 3 Life‐table rates per 100 women for discontinuation (12‐month).
| Study | Nova‐T‐PP | Lippes Loop | Copper 7 |
| WHO 1980 | 17.8% | 16.2% | 16.0% |
Comparison 9 Immediate postpartum insertion: Nova‐T‐PP versus Lippes Loop versus Copper 7, Outcome 4 Loss to follow‐up (unclear time frame).
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 6.2 | 6.6 |
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 0.6 | 0 |
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 87.4 | 78.2 |
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 3 Life‐table rates per 100 women for continuation (12‐month).
| Study | Nova‐T‐PP | Nova‐T |
| Van Kets 1987 | 6.2 | 6.2 |
Comparison 10 Immediate postpartum insertion: Nova‐T‐PP versus Nova‐T, Outcome 4 Life‐table rates per 100 women for loss to follow‐up (12‐month).
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 11.2 | 9.9 |
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 1 Life‐table rates per 100 women for expulsion (12‐month).
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 0.5 | 2.4 |
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 2 Life‐table rates per 100 women for pregnancy (12‐month).
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 77.2 | 77.3 |
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 3 Life‐table rates per 100 women for continuation (12‐month).
| Study | TCu 200 | ML Cu 250 |
| Thiery 1980 | 2.0 | 1.6 |
Comparison 11 Immediate postpartum insertion: TCu 200 versus ML Cu 250, Outcome 4 Life‐table rates per 100 women for loss to follow‐up (12‐month).

Comparison 12 Immediate postpartum insertion: CuT 380A (hand versus instrument insertion), Outcome 1 Expulsion by 6 months.
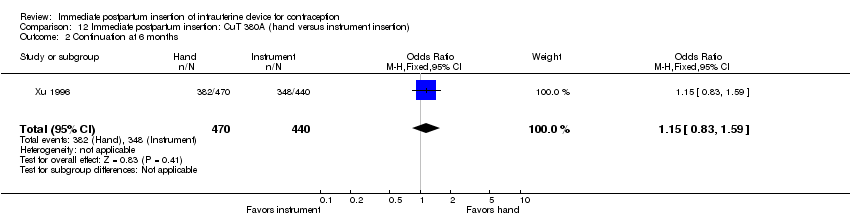
Comparison 12 Immediate postpartum insertion: CuT 380A (hand versus instrument insertion), Outcome 2 Continuation at 6 months.
| Immediate insertion compared with early insertion for postpartum IUC | |||
| Patient or population: postpartum women with desire for contraceptive Setting: hospital or clinic Intervention: immediate postplacental insertion (within 10 minutes) Comparison: early insertion (10 minutes to 48 hours post delivery) | |||
| Outcomes | Relative effect | Participants | Quality of the evidence |
| Expulsion by 6 months | OR 1.00 (95% CI 0.20 to 5.04) | 30 | ⊕⊕⊕⊝ Moderate |
| IUC use at 6 months | OR 0.46 (95% CI 0.04 to 5.75) | 30 | ⊕⊕⊕⊝ |
| CI: Confidence interval; OR: Odds ratio | |||
| GRADE Working Group grades of evidence | |||
| Immediate insertion compared with standard insertion for postpartum IUC | |||
| Patient or population: postpartum women with desire for contraceptive Setting: hospital or clinic Intervention: immediate postplacental insertion (within 10 minutes) Comparison: standard insertion (at postpartum visit) | |||
| Outcomes | Relative effect | Participants | Quality of the evidence |
| Placement per protocol | OR 4.07 (95% CI 0.54 to 30.40); I2 = 68% | 243 (4 studies) | ⊕⊕⊕⊝ |
| Expulsion by 6 months | OR 4.89 (95% CI 1.47 to 16.32) | 210 (4 studies) | ⊕⊕⊕⊝ Moderate |
| IUC use at 6 months | OR 2.04 (95% CI 1.01 to 4.09) | 243 (4 studies) | ⊕⊕⊕⊝ |
| CI: Confidence interval; OR: Odds ratio | |||
| GRADE Working Group grades of evidence | |||
| Study | Delivery type | IUC | Inadequate | No blinding | Loss to follow‐up | Quality of evidencea |
| Immediate versus early insertion (10 minutes to 48 hours) | ||||||
| Vaginal | LNG‐IUS | _ | ‐1 | _ | Moderate | |
| Vaginal | CuT 380A | _ | ‐1 | Unclear | Moderate | |
| Vaginal or cesarean | CuT 380A | ‐1 | Unclear | Unclear | Low | |
| Immediate versus standard insertion (weeks) | ||||||
| Vaginal | LNG‐IUS (6 to 8 weeks) | _ | _ | _ | High | |
| Vaginal | LNG‐IUS (> 6 weeks) | _ | ‐1 | _ | Moderate | |
| Cesarean | LNG‐IUS (4 to 8 weeks) | _ | _ | ‐1 | Moderate | |
| Cesarean | CuT 380A (6 weeks) | _ | ‐1 | _ | Moderate | |
| Vaginal or cesarean | CuT 380A (4 to 12 weeks) | ‐1 | Unclear | Unclear | Low | |
| Immediate insertion: IUC types, modifications, or insertion techniques | ||||||
| Unclear | Multiload 250 versus CuT 200 | ‐1 | Unclear | _ | Moderate | |
| Vaginal | Copper 7 versus Lippes Loop D vs Nova‐T‐PP | _ | ‐1 | _ | Moderate | |
| Unclear | Nova‐T‐PP vs Nova T | ‐1 | Unclear | _ | Moderate | |
| Unclear | Progestasert vs CuT 200 | _ | Unclear | ‐1 | Moderate | |
| Vaginal | IPCS‐52 vs CuT 200 | _ | Unclear | ‐1 | Moderate | |
| Vaginal |
_ | Unclear | ‐1 | Moderate | ||
| Unclear | Delta Loop vs Delta T | _ | Unclear | _ | Moderate | |
| Vaginal | CuT 380A: hand vs ring‐forceps insertion | _ | _ | _ | High | |
| aRCTs considered high quality initially, then downgraded for (1) no information on randomization sequence generation or allocation concealment, or one was clearly inadequate; (2) no blinding; (3) losses > 20%; (4) information missing for both blinding and losses. Follow‐up time not shown as all studies met criteria. | ||||||
| Study | N | Placement (insertion) per protocol (%) | ||
| Immediate | Early | Standard | ||
| 102 | 88 | _ | 90 | |
| 46 | 100 | 100 | 94 | |
| 42 | 95 | _ | 82 | |
| 68 | 100 | _ | 53 | |
| 156 | 87 | _ | 77 | |
| 263 | _ | _ | _ | |
| 200 | _ | _ | _ | |
| aIn sensitivity analysis; sufficient outcome data and evidence of moderate or high quality (Table 1). | ||||
| Study | N | Use at | Expulsion by | ||||
| Immediate | Early | Standard | Immediate | Early | Standard | ||
| 102 | 84 | _ | 77 | 22d | _ | 4 | |
| 46 | 87 | 93 | 94 | 27 | 27 | 0 | |
| 42 | 70 | _ | 59 | 20 | _ | 0 | |
| 68 | 79 | _ | 47 | 3 | _ | 6 | |
| 156 | 57 | _ | 59 | _ | _ | _ | |
| 263 | 89 | 74 | _ | 9 | 24 | _ | |
| 200 | 84 | 77 | _ | 8 | 11 | _ | |
| aUse based on women randomized; Ogburn 2013 assessed at 12 months. | |||||||
| Study | IUC | N | Insertion | Placement | Use at | Expulsion by |
| Life‐table rates | ||||||
| TCu 200 | 269 | _ | _ | 85.0 | 9.4 | |
| Multiload 250 | 293 | _ | _ | 88.8 | 7.4 | |
| Copper 7 | 277 | _ | _ | 64.6 | 31.1 | |
| Lippes Loop D | 250 | _ | _ | 48.1 | 41.3 | |
| Nova‐T‐PP | 277 | _ | _ | 52.3 | 39.4 | |
| Nova‐T‐PP | 205 | _ | _ | 87.5 | 5.5 | |
| Nova T | 203 | _ | _ | 88.4 | 6.0 | |
| Progestasert | 100 | Hand | _ | 59.9 | 35.8 | |
| 100 | Inserter | _ | 57.2 | 35.2 | ||
| TCu 200 | 100 | Hand | _ | 86.3 | 9.0 | |
| 100 | Inserter | _ | 86.1 | 8.1 | ||
| IPCS‐52 mg | 50 | Hand | _ | 57.3 | 39.0 | |
| 50 | Inserter | _ | 79.6 | 14.2 | ||
| TCu 200 | 50 | Hand | _ | 81.9 | 14.1 | |
| 50 | inserter | _ | 89.7 | 10.3 | ||
| Delta T | 728 | _ | 99.9 | 81.8 | 11.6 | |
| TCu 220 | 718 | _ | 99.7 | 81.8 | 11.5 | |
| Delta Loop | 662 | _ | 99.7 | 78.5 | 15.7 | |
| Lippes Loop D | 648 | _ | 99.7 | 73.8 | 21.5 | |
| Delta T | 518 | Hand | 99.7 | 84.2 | 11.2 | |
| 517 | Mechanical | 99.7 | 82.6 | 11.5 | ||
| Delta Loop | 122 | _ | 99.2 | 96.3 | 3.7 | |
| Delta T | 124 | _ | 100.0 | 90.7 | 7.6 | |
| Percent | ||||||
| CuT 380A | 470 | Hand | _ | 81.3 | 13.0 | |
| 440 | Ring‐forceps | _ | 79.1 | 12.5 | ||
| aLavin 1983 reported use and expulsion by 12 months. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placement (insertion) Show forest plot | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Expulsion by 6 months Show forest plot | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.20, 5.04] |
| 3 Use at 3 months Show forest plot | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.75] |
| 4 Use at 6 months Show forest plot | 1 | 30 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.04, 5.75] |
| 5 Expulsion Show forest plot | Other data | No numeric data | ||
| 6 Use at 6 months Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Placement (insertion) per protocol Show forest plot | 4 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 4.07 [0.54, 30.40] |
| 2 Expulsion by 6 months Show forest plot | 4 | 210 | Odds Ratio (M‐H, Random, 95% CI) | 4.89 [1.47, 16.32] |
| 3 Use at 6 months Show forest plot | 4 | 243 | Odds Ratio (M‐H, Random, 95% CI) | 2.04 [1.01, 4.09] |
| 4 Use at 3 months Show forest plot | 1 | 31 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.04, 5.35] |
| 5 Use at 12 months Show forest plot | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.63, 7.44] |
| 6 Placement (insertion) Show forest plot | 1 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.87, 4.76] |
| 7 Continued use at 12 months Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Insertion per protocol Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Insertion per protocol Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Insertion per protocol Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for expulsion (6‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (6‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for follow‐up (6‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Insertion per protocol Show forest plot | 1 | 246 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.07 [0.12, 76.20] |
| 2 Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for pregnancy (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) Show forest plot | Other data | No numeric data | ||
| 5 Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 6 Life‐table rates per 100 women for follow‐up (12‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Hand insertion: Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 2 Instrument insertion: Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Hand insertion: Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Instrument insertion: Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 5 Life‐table rates per 100 women for follow‐up (12‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Life‐table rates per 100 women for expulsion (12‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for removal due to bleeding or pain (12‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (12‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for expulsion (36‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 5 Life‐table rates per 100 women for continuation (36‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 6 Life‐table rates per 100 women for expulsion (36‐month) by device pooled Show forest plot | Other data | No numeric data | ||
| 7 Life‐table rates per 100 women for expulsion (36‐month) by method pooled Show forest plot | Other data | No numeric data | ||
| 8 Life‐table rates per 100 women for follow‐up (12‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| 9 Life‐table rates per 100 women for follow‐up (36‐month) by device and insertion method Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for discontinuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Loss to follow‐up (unclear time frame) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for loss to follow‐up (12‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Life‐table rates per 100 women for expulsion (12‐month) Show forest plot | Other data | No numeric data | ||
| 2 Life‐table rates per 100 women for pregnancy (12‐month) Show forest plot | Other data | No numeric data | ||
| 3 Life‐table rates per 100 women for continuation (12‐month) Show forest plot | Other data | No numeric data | ||
| 4 Life‐table rates per 100 women for loss to follow‐up (12‐month) Show forest plot | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Expulsion by 6 months Show forest plot | 1 | 910 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.71, 1.54] |
| 2 Continuation at 6 months Show forest plot | 1 | 910 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.83, 1.59] |

