Angioplastia con balón, con y sin stent, versus tratamiento médico para pacientes con hipertensión y estenosis de la arteria renal
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Multi‐centre, randomised controlled clinical trial Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no, 38 patients (5%) had withdrawn or been lost to follow‐up. 20 from the BA group and 18 from the MT group. No reasons given for participants being lost to follow‐up Blinding of outcome measurement: unclear (but primary outcome was serum creatinine measurement) | |
| Participants | 806 patients between 42 and 88 years (mean age 70.5 years) 403 in each group 37% women Inclusion criteria: uncontrolled or refractory hypertension OR unexplained renal dysfunction with evidence of substantial anatomical atherosclerotic stenosis in at least one renal artery that was considered potentially suitable for endovascular revascularisation. Physician uncertain as to whether revascularisation would provide a worthwhile clinical benefit Exclusion criteria: previous revascularisation, non‐atheromatous cardiovascular disease, surgical intervention required, If the medical team felt it was likely that revascularisation would be required within 6 months 53 centres in the United Kingdom, 3 in Australia, 1 in New Zealand Enrolment period: between September 2000 through October 2007 | |
| Interventions | No run‐in period Patients assigned to BA ± stent insertion underwent this within 4 weeks of randomisation Decision to stent was decided by local practitioners. Renal protection devices were not used Follow‐up was at 1 to 3 months, 6 to 8 months, and 1 year after randomisation followed by annual follow up for 5 years. Median follow‐up was 34 months Antihypertensive regime was not fixed and BP was managed according to local hospital protocols 403 patients were assigned to revascularisation (335 underwent attempted revascularisation 18 of these were failed procedures due to a failure to cross the stenosis (13) or lesion undilatable (5); 68 were not revascularised due to minimal stenosis (33), patient not suitable for revascularisation (6), consent withdrawn (6), other or unknown (13) 403 assigned to MT, 24 (6%) crossed over to revascularisation | |
| Outcomes | Renal function (measured by the reciprocal of serum Cr) BP Time to renal and major cardiovascular events and mortality Complications | |
| Notes | Revascularisation was attempted in 335 out of 403 patients and was deemed to be technically successful in 317 95% undergoing revascularisation received a stent 103 participants had bilateral disease | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computerized minimized‐randomisation procedure" |
| Allocation concealment (selection bias) | Low risk | "Randomization was determined by means of a telephone call to the central trial office or through an online randomizations system" |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind for this type of intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Primary outcome based on serum creatinine measurements. No details provided with regard to the measurement of BP |
| Incomplete outcome data (attrition bias) | Low risk | 38 patients (5%) had withdrawn or been lost to follow‐up. 20 from the BA group and 18 from the MT group |
| Selective reporting (reporting bias) | Low risk | Reported on all appropriate outcomes |
| Other bias | High risk | Only 335 of the 403 (83.1%) participants allocated to balloon angioplasty underwent attempted revascularisation Patients were enrolled in the trial only if their clinician was uncertain as to whether revascularisation would be of clinical benefit. Therefore patients who otherwise met the study criteria but were felt to be likely to benefit from angioplasty were excluded from the study |
| Methods | Multi‐centre randomised controlled trial Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no, 143 patients (15%) either withdrew or were lost to follow‐up, 62 from the stent group and 81 from the MT group. 16 participants enrolled at a single site were also excluded due to concerns regarding the informed consent and eligibility of participants enrolled at that site Blinding of outcome measurement: Yes (a single endpoint committee whose members were unaware of the group assignments adjudicated all endpoints) | |
| Participants | 947 participants (mean age 69 ± 9 years) 467 assigned to stent + MT, 480 to MT alone 49% women in stent group, 51% women in MT group Inclusion criteria: atherosclerotic renal artery stenosis (defined as stenosis of 80% to 99% or 60% to 79% with a systolic pressure gradient of > 20 mmHg) and elevated blood pressure (defined as SBP > 155 mmHg) or chronic kidney disease (defined as an MDRD eGFR < 60 mL/min/1.73m2), or both Exclusion criteria: renal artery stenosis due to fibromuscular dysplasia, chronic kidney disease from a cause other than ischaemic nephropathy or associated with a serum creatinine of > 354 µmol/L (4 mg/dL), kidney length of < 7 cm and a lesion that could not be treated with the use of a single stent 112 centres Enrolment period: between May 2005 through January 2010 | |
| Interventions | No run‐in period Patients assigned to revascularisation underwent stent insertion. Embolic protection devices were used Median follow‐up was 43 months (interquartile range 31 to 55) Antihypertensive regime was fixed (candesartan ± hydrochlorothiazide and combination amlodipine‐atorvastatin) and BP treatment was managed according to study protocol (target BP < 140/90 if no co‐existing conditions or < 130/80 if diabetes or CKD) 467 patients were assigned to revascularisation (442 underwent revascularisation and 25 did not. Of the 25 who did not undergo stenting, 13 did not meet the lesion criteria, 3 could not have the stent delivered and 9 did not have the stent procedure attempted 480 assigned to MT. 19 (4%) crossed over to revascularisation. These were included in the intention‐to‐treat analysis in the MT alone group | |
| Outcomes | Adverse cardiovascular and renal events (composite endpoint of death from cardiovascular or renal causes, myocardial infarction, stroke, hospitalisation for congestive heart failure, progressive renal insufficiency or the need for renal replacement therapy Secondary outcomes included the individual components of the primary endpoint as well as all‐cause mortality and blood pressure | |
| Notes | The original trial enrolment criteria were modified during the trial. Initially all participants were required to have a systolic BP > 155 mmHg and be on at least 2 antihypertensive medications but later on this requirement was relaxed and participants without hypertension with CKD were also recruited Crossovers from MT to stenting were only approved if acute anuric renal failure, complete occlusion of all renal arteries and at least one kidney > 8 cm in length All participants who underwent randomisation were included in an intention‐to‐treat analysis with the exception of the 16 participants (9 in each group) who were enrolled at a single site at which scientific integrity issues were identified Embolic protection devices were used during all stent procedures | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was performed by means of an interactive voice randomisation system with the use of a permuted block design." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was performed by means of an interactive voice randomisation system with the use of a permuted block design." |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind for this type of intervention |
| Blinding of outcome assessment (detection bias) | Low risk | "A single end point committee whose members were unaware of the group assignments adjudicated all end points" |
| Incomplete outcome data (attrition bias) | Low risk | Clear reporting of participant dropouts and reasons; performed analyses by intention‐to‐treat with all randomised participants |
| Selective reporting (reporting bias) | Low risk | Reported on all appropriate outcomes |
| Other bias | Unclear risk | Possibly underpowered; 1080 participants would need to be enrolled, but due to slow recruitment only 947 were enrolled |
| Methods | Multi‐centre, randomised controlled clinical trial | |
| Participants | 106 patients between 18 and 75 years (mean age 60 years) | |
| Interventions | No run‐in period | |
| Outcomes | Mean office BP (mean of 3 office BP measurements by standard sphygmomanometry) | |
| Notes | The following outcomes could be ascertained from this study: mean differences in office BP, number and DDD of antihypertensive drugs given, mean difference in serum creatinine, restenosis of vessels, and complications. Results are presented after 3 months (before 44% of patients in the MT group underwent BA) and after 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer randomisation |
| Allocation concealment (selection bias) | Low risk | "Randomisation was performed by computer…without investigators’ knowledge of patients’ groups at the time of assignment" |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind for this particular intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | BP measured using an automated device |
| Incomplete outcome data (attrition bias) | Low risk | 2 lost to follow‐up in the MT group (1 of the 2 had crossed over to undergo BA) |
| Selective reporting (reporting bias) | Low risk | Reported on all relevant outcomes |
| Other bias | High risk | Patients assigned to MT group underwent BA at 3 months if diastolic pressure was > 95 mmHg despite treatment with 3 or more drugs – 22 out of 50 in the control group underwent BA |
| Methods | Multi‐centre, randomised controlled clinical trial | |
| Participants | 49 patients younger than 75 years (mean age 59 years) 30% in BA and 46% in MT had ostial stenosis | |
| Interventions | 2 to 6 week run‐in period on standardised antihypertensive regimen (nifedipine SR 20 mg bid; if necessary idem plus clonidine 0.15 mg bid; if necessary idem plus prazosine 2.5 mg once daily) | |
| Outcomes | Mean ambulatory BP | |
| Notes | The following outcomes could be ascertained from this study: mean differences in ambulatory BP, number and DDD of antihypertensive drugs given, mean difference in creatinine clearance, restenosis (defined as totally occluded vessels), and complications | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided about how the random sequence was generated |
| Allocation concealment (selection bias) | Low risk | Randomisation was stratified by centre and sealed, numbered envelopes opened in sequential order were used |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind for this particular intervention |
| Blinding of outcome assessment (detection bias) | Low risk | Primary endpoint was ambulatory blood pressure at termination |
| Incomplete outcome data (attrition bias) | Low risk | 1 patient in the MT group was withdrawn due to the development of symptomatic hypotension |
| Selective reporting (reporting bias) | Low risk | Reported all appropriate outcomes |
| Other bias | Low risk | No apparent other sources of bias |
| Methods | Randomised controlled trial Blinding of randomisation: unknown Blinding of intervention: unknown Complete follow‐up: unknown Blinding of outcome measurement: unknown | |
| Participants | 52 patients with mean age of 72 years Inclusion criteria: patients with stable renal failure (creatinine clearance > 30 mL/min), hypertension and haemodynamically significant atherosclerotic ostial renal artery stenosis Exclusion criteria: unknown Number of centres:unknown 51.5% had bilateral stenosis Mean stenosis was 80% | |
| Interventions | Medical therapy versus medical therapy plus renal artery stenting 28 participants were assigned to BA + stent insertion 24 participants were assigned to MT 100% participants in the intervention arm underwent stenting 1.9% crossed over from control to the intervention arm Follow‐up duration was 43 months | |
| Outcomes | Reduction by 20% in estimated GFR Need for renal replacement therapy Death | |
| Notes | The results of this trial have not yet been fully published (NITER) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind for this particular intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Randomised controlled trial Blinding of randomisation: unknown Blinding of intervention: unknown Complete follow‐up: unknown Blinding of outcome measurement: unknown | |
| Participants | 67 participants with a mean age of 67 years Inclusion criteria: patients with renal artery stenosis and an indication for revascularisation. Detailed inclusion criteria: unknown Exclusion criteria: unknown Number of centres: 13 sites in 4 countries | |
| Interventions | Medical therapy versus medical therapy plus renal artery stenting 34 participants were assigned to BA + stent insertion 33 participants were assigned to MT Follow‐up duration was 32 months | |
| Outcomes | Change in eGFR between baseline and 12 months | |
| Notes | The results of this trial have not yet been fully published (RADAR). The published abstract states that this study was terminated prematurely but does not give any reasons for this. It also states that 89 patients were enrolled but data is presented for only 67. The reason for this is again not given No information regarding cardiovascular or renal complications were provided | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Information not available |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind for this particular type of intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Information not available |
| Incomplete outcome data (attrition bias) | Unclear risk | Information not available |
| Selective reporting (reporting bias) | Unclear risk | Information not available |
| Other bias | Unclear risk | Information not available |
| Methods | Multi‐centre, randomised controlled clinical trial | |
| Participants | 55 patients between 40 and 75 years (mean age 61 years) Exclusion criteria: age < 40 or > 75 years, serum creatinine < 500 µmol/L, stroke or myocardial infarction within the previous 3 months | |
| Interventions | 4‐week run‐in period on fixed antihypertensive regimen (recommended regimen was atenolol, bendrofluazide, and calcium antagonist in any combination of at least two of these) | |
| Outcomes | Office BP (measurement with either a standard or a Hawksley random zero sphygmomanometer in the sitting position, no indication if mean of multiple or single BP‐measurement) Complications | |
| Notes | The following outcomes could be ascertained from this study: | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Details of randomisation not provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Not possible to blind for this particular intervention |
| Blinding of outcome assessment (detection bias) | Low risk | BP was measured by an "observer unaware of the allocation to intervention or medical therapy" |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients in the BA group and 4 in the MT group lost to follow‐up, no reasons given |
| Selective reporting (reporting bias) | Low risk | Reported on relevant outcomes |
| Other bias | High risk | Of the 25 participants assigned to BA 5 (20%) had a surgical intervention (3 nephrectomies, 2 vein bypasses) |
| Methods | Multi‐centre randomised controlled trial Blinding of randomisation: yes Blinding of intervention: no Complete follow‐up: no, 2 participants from the stent group and 2 from the MT group were lost to follow‐up. No reasons given for participants being lost to follow‐up Blinding of outcome measurement: unclear (but primary outcome was serum creatinine measurement) | |
| Participants | 140 patients (mean age 66.5 yrs) 55% women Inclusion criteria: patients with impaired renal function (CrCl < 80 mL/min per 1.73 m2 CG formula) with ostial stenosis of > 50% Exclusion criteria: CrCl < 15 mL/min, renal artery diameter < 4 mm, renal size < 8 cm, diabetes mellitus with proteinuria or malignant hypertension 48% had bilateral stenosis 10 centres – 9 in Netherlands, 1 in France Enrolment period: June 2000 and December 2005 | |
| Interventions | No run‐in period 74 were assigned to MT 64 were assigned to BA with stent insertion – 46 of these underwent the allocated treatment. 18 did not (1 BA only, 2 declined stent insertion, 1 died prior to stent insertion, 12 had stenosis < 50% at angiography, 2 technical failure to place stent). All 46 had successful stent insertion with a residual stenosis of < 20%. An intention‐to‐treat analysis was carried out with follow‐up data available for 62 of the intervention group and 74 of the MT group 1 patient from MT group crossed over due to refractory HTN Participants were followed up until they reached the primary end point or for 2 years with follow up visits at 1 month, then 3 months and then 3 monthly for 2 years. Fasting serum Cr and 3 x sitting BP measured at each visit | |
| Outcomes | Worsening of renal function (defined as a > 20% decline in Cockcroft‐Galt estimated Cr clearance) BP Incidence of refractory or malignant HTN Pulmonary oedema, CV morbidity, CV mortality, total mortality Complications | |
| Notes | 16 patients in the MT group and 10 in the stent group reached the primary endpoint. Event free survival did not differ significantly between groups. A by protocol analysis comparing the 90 patients who received MT to the 50 who received an intervention revealed similar results | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated permuted block randomisation" |
| Allocation concealment (selection bias) | Low risk | "Study personnel were unaware of the permuted block size" |
| Blinding of participants and personnel (performance bias) | High risk | Unable to blind for this type of intervention |
| Blinding of outcome assessment (detection bias) | Unclear risk | Primary outcome based on serum creatinine measurements. No details provided with regards to the measurement of BP |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients lost to follow‐up and 6 deaths in the MT group; 2 patients lost to follow‐up and 5 deaths in the BA group |
| Selective reporting (reporting bias) | Low risk | Reported all appropriate outcomes |
| Other bias | High risk | Only 46 out of the 64 participants allocated to balloon angioplasty underwent revascularisation |
BA: balloon angioplasty
bid: twice daily
BP: blood pressure
CrCl: creatinine clearance
CV: cardiovascular
DDD: defined daily doses (defined as average maintenance dose per day for adults)
HTN: hypertension
MT: medical therapy
ns: non‐significant
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| This study consisted of a comparison between angioplasty and surgical intervention. There was no medical treatment group | |
| This study was not randomised |
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | NCT01208714 Medical and Endovascular Treatment of Atherosclerotic Renal Artery Stenosis (METRAS) study |
| Methods | Multicentre randomised controlled trial |
| Participants | 120 participants with renal artery stenosis and hypertension |
| Interventions | Optimum medical therapy alone compared to stenting with optimum medical treatment |
| Outcomes | Primary outcomes include change in eGFR Secondary outcomes include blood pressure, need for renal replacement therapy, cardiovascular events and quality of life |
| Starting date | 2012 |
| Contact information | GP Rossi ‐ [email protected] |
| Notes | 5‐year follow‐up planned |
| Trial name or title | NCT00127738 Renal Atherosclerotic revascularization Evaluation (RAVE Study) |
| Methods | Single centre randomised pilot study |
| Participants | 20 participants with renal artery stenosis and an indication for revascularisation |
| Interventions | Optimum medical therapy alone compared to angioplasty ± stenting with optimum medical therapy |
| Outcomes | Primary outcomes include doubling of serum creatinine, need for renal replacement therapy, death Secondary outcomes include cardiovascular disease, blood pressure, antihypertensive medications |
| Starting date | 2007 |
| Contact information | Sheldon W Tobe ‐ [email protected] |
| Notes | This study will also aim to assess the renal resistance index to see if this is a useful tool for identifying patients with particularly severe renal artery stenosis who may be most likely to benefit from angioplasty |
eGFR: estimated glomerular filtration rate
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in systolic BP at 2 years or end of follow‐up Show forest plot | 5 | 1743 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐3.45, 1.30] |
| Analysis 1.1 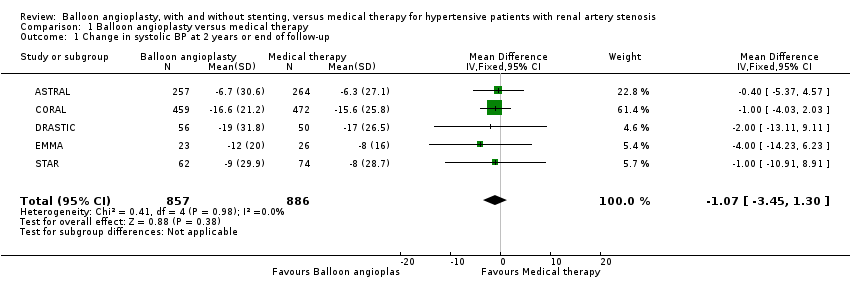 Comparison 1 Balloon angioplasty versus medical therapy, Outcome 1 Change in systolic BP at 2 years or end of follow‐up. | ||||
| 2 Change in diastolic BP at 2 years or end of follow‐up Show forest plot | 4 | 809 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐3.72, ‐0.27] |
| Analysis 1.2  Comparison 1 Balloon angioplasty versus medical therapy, Outcome 2 Change in diastolic BP at 2 years or end of follow‐up. | ||||
| 3 Serum creatinine at 2 years or end of follow up Show forest plot | 3 | 725 | Mean Difference (IV, Fixed, 95% CI) | ‐7.99 [‐22.60, 6.62] |
| Analysis 1.3  Comparison 1 Balloon angioplasty versus medical therapy, Outcome 3 Serum creatinine at 2 years or end of follow up. | ||||
| 4 Number of antihypertensive drugs Show forest plot | 3 | 1717 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.34, ‐0.03] |
| Analysis 1.4  Comparison 1 Balloon angioplasty versus medical therapy, Outcome 4 Number of antihypertensive drugs. | ||||
| 5 Cardiovascular adverse events Show forest plot | 7 | 2110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.75, 1.11] |
| Analysis 1.5  Comparison 1 Balloon angioplasty versus medical therapy, Outcome 5 Cardiovascular adverse events. | ||||
| 6 Renal adverse events Show forest plot | 7 | 2104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.75, 1.38] |
| Analysis 1.6 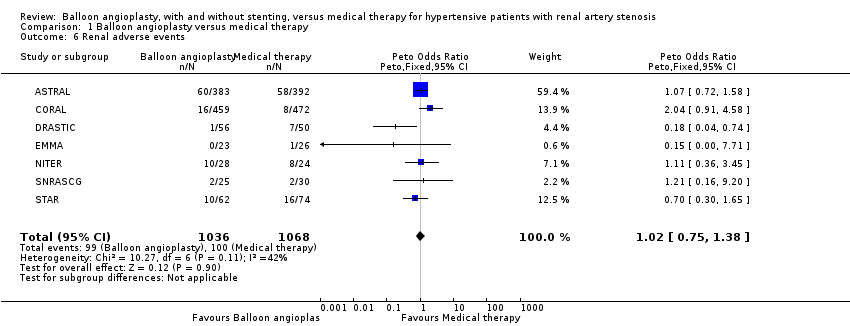 Comparison 1 Balloon angioplasty versus medical therapy, Outcome 6 Renal adverse events. | ||||
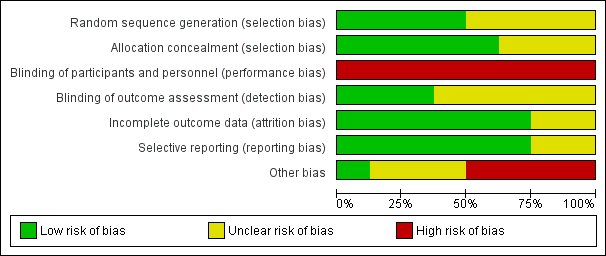
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
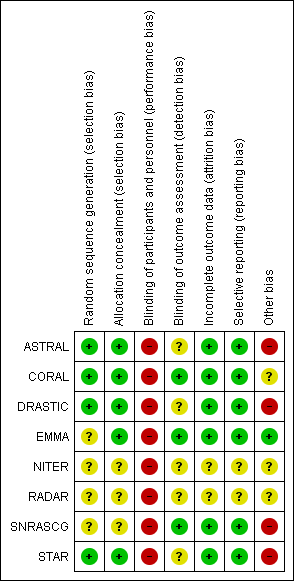
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Balloon angioplasty versus medical therapy, Outcome 1 Change in systolic BP at 2 years or end of follow‐up.

Comparison 1 Balloon angioplasty versus medical therapy, Outcome 2 Change in diastolic BP at 2 years or end of follow‐up.

Comparison 1 Balloon angioplasty versus medical therapy, Outcome 3 Serum creatinine at 2 years or end of follow up.

Comparison 1 Balloon angioplasty versus medical therapy, Outcome 4 Number of antihypertensive drugs.

Comparison 1 Balloon angioplasty versus medical therapy, Outcome 5 Cardiovascular adverse events.

Comparison 1 Balloon angioplasty versus medical therapy, Outcome 6 Renal adverse events.
| Complications | ASTRAL | CORAL | DRASTIC | EMMA | NITER | SNRASCG | STAR |
| Angina/myocardial infarction/heart failure | 112 in BA, 131 in MT | 40 in BA, 37 in MT | 0 in BA, 2 in MT | 0 in any group | Data not available | 4 in each group | 4 in BA, 6 in MT (defined as CAD + HF) |
| Cholesterol embolisation | 3 in BA, 0 in MT | 6 in BA, 0 in MT | 0 in BA, 1 in MT | 0 in any group | Data not available | 0 in any group | 1 in BA, 0 in MT |
| Non‐procedure‐related symptomatic hypotension | Data not available | Data not available | 0 in any group | 0 in BA, 1 in MT | Data not available | 0 in any group | Data not available |
| Stroke | 24 in BA, 23 in MT | 16 in BA, 23 in MT | 0 in any group | 0 in any group | 3 in BA, 4 in MT | 1 in BA, 4 in MT | 0 in BA, 1 in MT (defined as cerebro vascular disease) |
| Death | 103 in BA, 106 in MT | 63 in BA, 76 in MT | 0 in any group | 0 in any group | 3 in BA, 3 in MT | 2 in BA, 4 in MT | 6 in MT, 5 in BA |
| BA: balloon angioplasty group | |||||||
| Complications | ASTRAL | CORAL | DRASTIC | EMMA | NITER | SNRASCG | STAR |
| > 50% increase in serum creatinine or | Data not available | Data not available | 1 in BA, 3 in MT | 0 in BA, 1 in MT | Data not available | 0 in any group | 10 in BA, 16 in MT (data provided for 20% fall in CrCl only) |
| Renal failure | 55 in BA, 54 in MT (defined as AKI + dialysis for end stage renal disease) | 16 in BA, 8 in MT (defined as permanent RRT) | 0 in any group | 0 in any group | 10 in BA, 8 in MT (but unclear how worsening renal failure was defined) | 2 in each group | Data not available |
| Total occlusion of stenotic artery | 5 in BA, 4 in MT | Data not available | 0 in BA, 4 in MT | 0 in any group | Data not available | 0 in any group | Data not available |
| AKI: acute kidney injury | |||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Change in systolic BP at 2 years or end of follow‐up Show forest plot | 5 | 1743 | Mean Difference (IV, Fixed, 95% CI) | ‐1.07 [‐3.45, 1.30] |
| 2 Change in diastolic BP at 2 years or end of follow‐up Show forest plot | 4 | 809 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐3.72, ‐0.27] |
| 3 Serum creatinine at 2 years or end of follow up Show forest plot | 3 | 725 | Mean Difference (IV, Fixed, 95% CI) | ‐7.99 [‐22.60, 6.62] |
| 4 Number of antihypertensive drugs Show forest plot | 3 | 1717 | Mean Difference (IV, Fixed, 95% CI) | ‐0.18 [‐0.34, ‐0.03] |
| 5 Cardiovascular adverse events Show forest plot | 7 | 2110 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.75, 1.11] |
| 6 Renal adverse events Show forest plot | 7 | 2104 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.75, 1.38] |


