"Coasting" (interrupción de las gonadotrofinas) para la prevención del síndrome de hiperestimulación ovárica
Appendices
Appendix 1. CGF specialised register search strategy
From inception until 6 July 2016
Keywords CONTAINS "superovulation" or "superovulation induction" or "ovarian hyperstimulation" or "ovarian hyperstimulation syndrome" or "ovarian stimulation syndrome" or "*Ovulation Induction"or"OHSS" or Title CONTAINS "IVF" or "superovulation" or "superovulation induction" or "ovarian hyperstimulation" or "ovarian hyperstimulation syndrome" or"OHSS" or "ovarian stimulation syndrome" or "*Ovulation Induction"
AND
Keywords CONTAINS "coasting" or "reduced dose" or Title CONTAINS "coasting" or "reduced dose" (17 hits)
Appendix 2. CENTRAL search strategy
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials (OVID platform)
From inception until 6 July 2016
1 exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp ovulation induction/ or exp superovulation/ (2137)
2 (in vitro fertili#ation or IVF).tw. (3100)
3 (intracytoplasmic sperm injection$ or ICSI).tw. (1143)
4 (ov$ induc$ or ovar$ stimulat$).tw. (1511)
5 superovulation.tw. (141)
6 OHSS.tw. (223)
7 Ovar$ hyperstimulat$.tw. (693)
8 exp Ovarian Hyperstimulation Syndrome/ (142)
9 or/1‐8 (4860)
10 (coasting or coast).tw. (221)
11 (reduc$ adj5 gonadotrophin$).tw. (33)
12 (reduc$ adj5 gonadotropin$).tw. (70)
13 (withdraw$ adj5 gonadotropin$).tw. (7)
14 (withhold$ adj5 gonadotrophin$).tw. (2)
15 (withhold$ adj5 gonadotropin$).tw. (0)
16 (withdraw$ adj5 gonadotrophin$).tw. (2)
17 (Taper$ adj5 (gonadotrophin$ or gonadotropin$)).tw. (0)
18 ((reduc$ or withdraw$ or withhold$ or taper$) adj5 GnRH).tw. (125)
19 or/10‐18 (441)
20 9 and 19 (99)
Appendix 3. MEDLINE search strategy
Database: Ovid MEDLINE(R) In‐Process and Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1950 to 6 July 2016>
Search Strategy:
1 exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp ovulation induction/ or exp superovulation/ (36459)
2 (in vitro fertili#ation or IVF).tw. (26556)
3 (intracytoplasmic sperm injection$ or ICSI).tw. (7729)
4 (ov$ induc$ or ovar$ stimulat$).tw. (14368)
5 superovulation.tw. (1743)
6 OHSS.tw. (1274)
7 Ovar$ hyperstimulat$.tw. (4127)
8 exp Ovarian Hyperstimulation Syndrome/ (1861)
9 or/1‐8 (55478)
10 (coasting or coast).tw. (17689)
11 (reduc$ adj5 gonadotrophin$).tw. (199)
12 (reduc$ adj5 gonadotropin$).tw. (624)
13 (withdraw$ adj5 gonadotropin$).tw. (58)
14 (withhold$ adj5 gonadotrophin$).tw. (22)
15 (withhold$ adj5 gonadotropin$).tw. (17)
16 (withdraw$ adj5 gonadotrophin$).tw. (36)
17 (Taper$ adj5 (gonadotrophin$ or gonadotropin$)).tw. (1)
18 ((reduc$ or withdraw$ or withhold$ or taper$) adj5 GnRH).tw. (1071)
19 or/10‐18 (19579)
20 9 and 19 (379)
21 randomised controlled trial.pt. (406339)
22 controlled clinical trial.pt. (91305)
23 randomized.ab. (329363)
24 placebo.tw. (171267)
25 clinical trials as topic.sh. (177494)
26 randomly.ab. (237642)
27 trial.ti. (144959)
28 (crossover or cross‐over or cross over).tw. (65362)
29 or/21‐28 (1009451)
30 (animals not (humans and animals)).sh. (3990166)
31 29 not 30 (930198)
32 20 and 31 (81)
Appendix 4. Embase search strategy
From inception until 6 July 2016 (Ovid platform)
1 exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp ovulation induction/ or exp superovulation/ (55935)
2 (in vitro fertili#ation or IVF).tw. (36892)
3 (intracytoplasmic sperm injection$ or ICSI).tw. (12480)
4 (ov$ induc$ or ovar$ stimulat$).tw. (19154)
5 superovulation.tw. (1896)
6 OHSS.tw. (2064)
7 Ovar$ hyperstimulat$.tw. (5646)
8 exp Ovarian Hyperstimulation Syndrome/ (6868)
9 or/1‐8 (77862)
10 coasting.tw. (276)
11 (reduc$ adj3 gonadotrophin$).tw. (126)
12 (reduc$ adj3 gonadotropin$).tw. (373)
13 (withdrawal adj3 gonadotropin$).tw. (48)
14 (withhold$ adj3 gonadotrophin$).tw. (24)
15 (withhold$ adj3 gonadotropin$).tw. (18)
16 (withdrawal adj3 gonadotrophin$).tw. (21)
17 (Taper$ adj5 (gonadotrophin$ or gonadotropin$)).tw. (1)
18 ((reduc$ or withdraw$ or withhold$ or taper$) adj5 GnRH).tw. (1210)
19 or/10‐18 (1994)
20 9 and 19 (428)
21 Clinical Trial/ (847849)
22 Randomized Controlled Trial/ (377981)
23 exp randomization/ (67354)
24 Single Blind Procedure/ (20623)
25 Double Blind Procedure/ (122094)
26 Crossover Procedure/ (43745)
27 Placebo/ (260138)
28 Randomi?ed controlled trial$.tw. (120388)
29 Rct.tw. (17651)
30 random allocation.tw. (1431)
31 randomly allocated.tw. (22837)
32 allocated randomly.tw. (2036)
33 randomly divided.tw. (46545)
34 (allocated adj2 random).tw. (731)
35 Single blind$.tw. (16081)
36 Double blind$.tw. (152770)
37 ((treble or triple) adj blind$).tw. (462)
38 placebo$.tw. (217599)
39 prospective study/ (299537)
40 or/21‐39 (1514455)
41 case study/ (32879)
42 case report.tw. (286546)
43 abstract report/ or letter/ (931036)
44 or/41‐43 (1244071)
45 40 not 44 (1474939)
46 20 and 45 (119)
Appendix 5. CINAHL search strategy
From inception until 6 July 2016 (EBSCO platform)
| # | Query | Results |
| S18 | S11 AND S17 | 17 |
| S17 | S12 OR S13 OR S14 OR S15 OR S16 | 52 |
| S16 | TX ((reduc* or withdraw* or withhold* or taper*) N5 GnRH*) | 19 |
| S15 | TX withhold* N3 gonadotrop?in* | 1 |
| S14 | TX (reduc* N3 gonadotropin*) | 14 |
| S13 | TX (reduc* N3 gonadotrophin*) | 2 |
| S12 | TX coasting | 18 |
| S11 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 | 6,214 |
| S10 | (MM "Ovarian Hyperstimulation Syndrome") | 137 |
| S9 | TX Ovar* hyperstimulat* | 341 |
| S8 | TX OHSS | 77 |
| S7 | TX superovulation | 18 |
| S6 | TX (ov* induc* or ovar* stimulat*) | 3,094 |
| S5 | TX (IVF or ICSI) | 1,296 |
| S4 | TX (intracytoplasmic sperm injection) | 245 |
| S3 | TX (in vitro fertili#ation) | 2,970 |
| S2 | (MM "Ovulation Induction") | 240 |
| S1 | (MM "Fertilization in Vitro") | 1,494 |
Appendix 6. PsycINFO search strategy
From inception until 6 July 2016
1 exp reproductive technology/ (1466)
2 (in vitro fertili?ation or IVF).tw. (676)
3 (intracytoplasmic sperm injection$ or ICSI).tw. (68)
4 OHSS.tw. (6)
5 Ovar$ hyperstimulat$.tw. (10)
6 or/1‐5 (1714)
7 coasting.tw. (34)
8 (reduc$ adj3 gonadotrophin$).tw. (3)
9 (reduc$ adj3 gonadotropin$).tw. (15)
10 (withdrawal adj3 gonadotropin$).tw. (1)
11 (withhold$ adj3 gonadotrophin$).tw. (0)
12 (withhold$ adj3 gonadotropin$).tw. (0)
13 (withdrawal adj3 gonadotrophin$).tw. (0)
14 or/7‐13 (53)
15 6 and 14 (0)

Study flow diagram: July 2016 search for 2017 review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 3 Coasting versus no coasting, outcome: 3.1 OHSS.

Forest plot of comparison: 1 Coasting versus EUFA, outcome: 1.1 OHSS.

Forest plot of comparison: 5 Coasting versus cabergoline, outcome: 5.1 OHSS.

Comparison 1 Coasting versus no coasting, Outcome 1 OHSS.
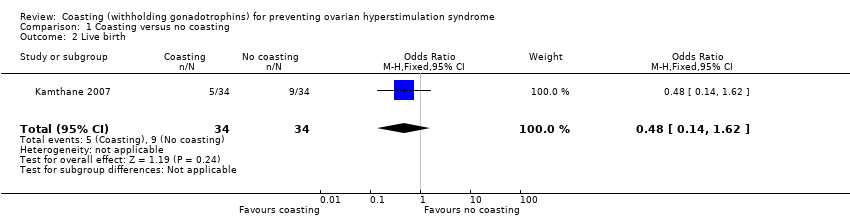
Comparison 1 Coasting versus no coasting, Outcome 2 Live birth.

Comparison 1 Coasting versus no coasting, Outcome 3 Clinical pregnancy.
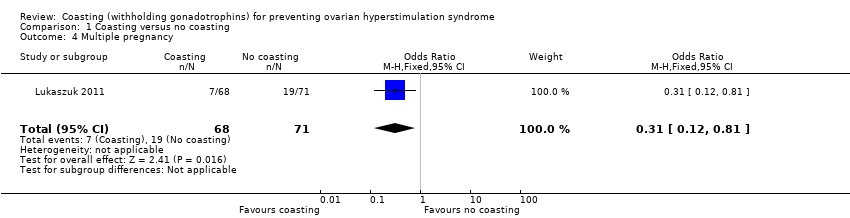
Comparison 1 Coasting versus no coasting, Outcome 4 Multiple pregnancy.
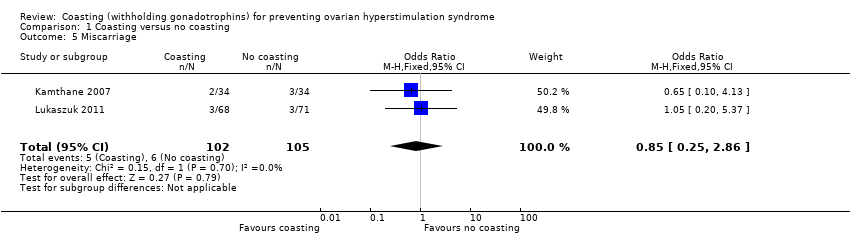
Comparison 1 Coasting versus no coasting, Outcome 5 Miscarriage.

Comparison 1 Coasting versus no coasting, Outcome 6 Number of oocytes retrieved.
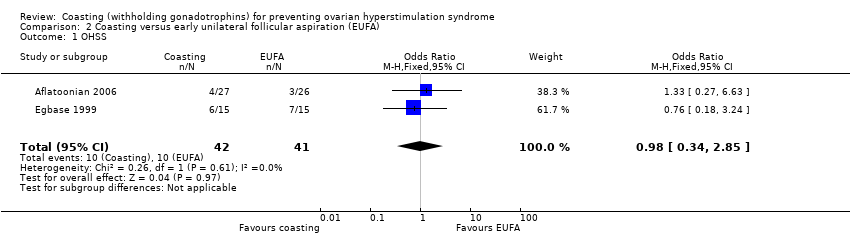
Comparison 2 Coasting versus early unilateral follicular aspiration (EUFA), Outcome 1 OHSS.
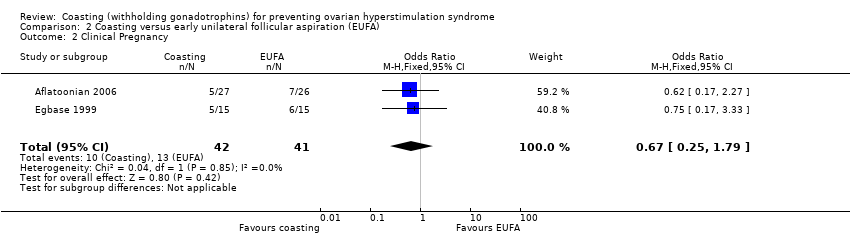
Comparison 2 Coasting versus early unilateral follicular aspiration (EUFA), Outcome 2 Clinical Pregnancy.
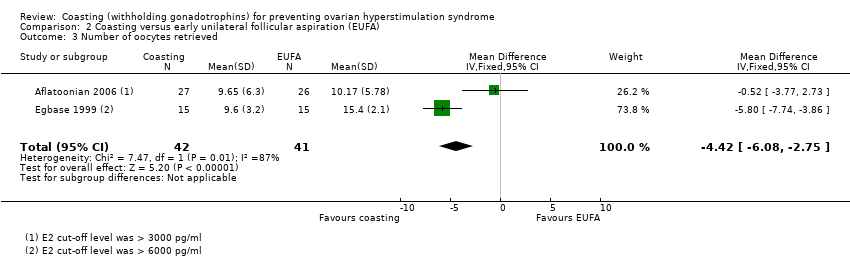
Comparison 2 Coasting versus early unilateral follicular aspiration (EUFA), Outcome 3 Number of oocytes retrieved.

Comparison 3 Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist), Outcome 1 OHSS.
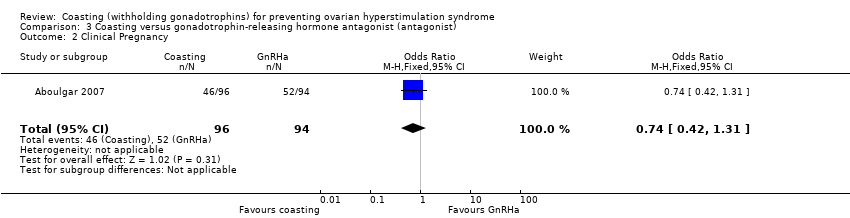
Comparison 3 Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist), Outcome 2 Clinical Pregnancy.

Comparison 3 Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist), Outcome 3 Multiple pregnancy.
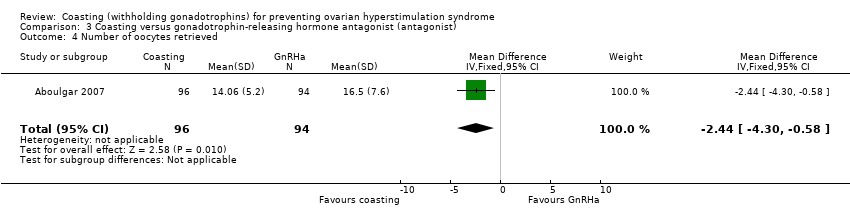
Comparison 3 Coasting versus gonadotrophin‐releasing hormone antagonist (antagonist), Outcome 4 Number of oocytes retrieved.
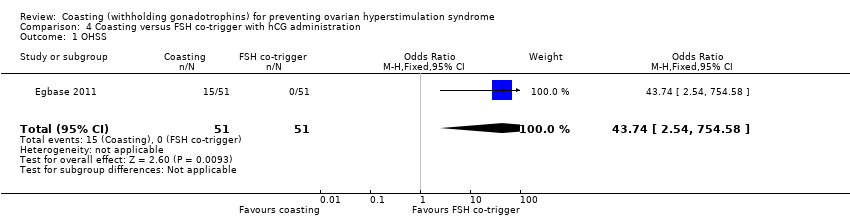
Comparison 4 Coasting versus FSH co‐trigger with hCG administration, Outcome 1 OHSS.

Comparison 4 Coasting versus FSH co‐trigger with hCG administration, Outcome 2 Clinical pregnancy.
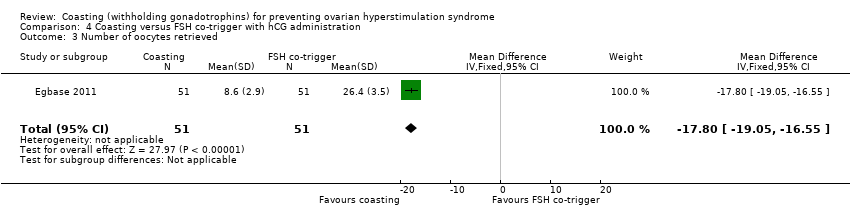
Comparison 4 Coasting versus FSH co‐trigger with hCG administration, Outcome 3 Number of oocytes retrieved.

Comparison 5 Coasting versus cabergoline, Outcome 1 OHSS.
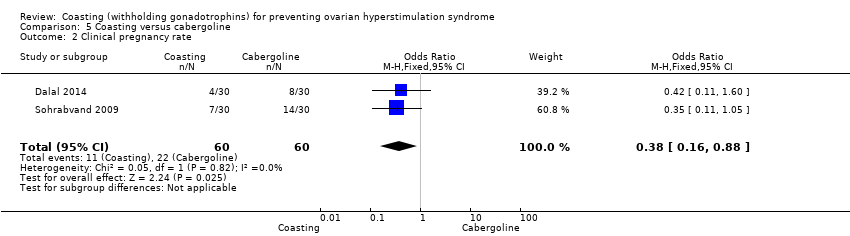
Comparison 5 Coasting versus cabergoline, Outcome 2 Clinical pregnancy rate.

Comparison 5 Coasting versus cabergoline, Outcome 3 Number of oocytes retrieved.
| Coasting versus no coasting for prevention of ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with no coasting | Risk with coasting | |||||
| OHSS | 457 per 1000 | 85 per 1000 | OR 0.11 | 207 | ⊕⊕⊝⊝ | |
| Live birth | 265 per 1000 | 147 per 1000 | OR 0.48 | 68 | ⊕⊝⊝⊝ | |
| Clinical pregnancy | 390 per 1000 | 344 per 1000 | OR 0.82 | 207 | ⊕⊕⊝⊝ | |
| Multiple pregnancy | 268 per 1000 | 102 per 1000 | OR 0.31 | 139 | ⊕⊝⊝⊝ | |
| Miscarriage | 57 per 1,000 | 49 per 1,000 (15 to 148) | OR 0.85 (0.25 to 2.86) | 207 | ⊕⊕⊝⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias: one study did not clearly describe the methods used, studies not blinded 2 Downgraded one level for serious imprecision: few events, wide confidence intervals, or both 3 Downgraded two levels for very serious imprecision: very few events, very wide confidence intervals, or both | ||||||
| Coasting versus early unilateral follicular aspiration for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with early unilateral follicular aspiration (EUFA) | Risk with coasting | |||||
| OHSS | 244 per 1000 | 240 per 1000 | OR 0.98 | 83 | ⊕⊝⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 317 per 1000 | 237 per 1000 | OR 0.67 | 83 | ⊕⊝⊝⊝ | |
| Multiple pregnancy | No data available | |||||
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for serious risk of bias: one study did not clearly describe methods, lack of blinding 2 Downgraded two levels for very serious imprecision: very few events and very wide confidence intervals | ||||||
| Coasting versus gonadotrophin‐releasing hormone antagonist for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with gonadotrophin‐releasing hormone antagonist | Risk with coasting | |||||
| OHSS | Not estimable | Not estimable | not estimable | 190 | ⊕⊕⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 553 per 1000 | 478 per 1000 | OR 0.74 | 190 | ⊕⊕⊝⊝ | |
| Multiple pregnancy | 181 per 1000 | 156 per 1000 | OR 0.84 | 98 | ⊕⊕⊝⊝ | |
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: method of sequence generation not reported, lack of blinding 2 Downgraded two levels due to very serious imprecision: no OHSS occurred in either group. Few events for multiple pregnancy. 3 Downgraded one level due to serious imprecision. Wide confidence intervals, few events | ||||||
| Coasting versus follicle stimulating hormone (FSH) administration at time of hCG trigger in preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with FSH co‐trigger with hCG administration | Risk with Coasting | |||||
| OHSS | Not estimable | Not estimable | OR 43.74 | 102 | ⊕⊝⊝⊝ | |
| Live birth | No data available | |||||
| Clinical Pregnancy | 510 per 1000 | 489 per 1000 | OR 0.92 | 102 | ⊕⊕⊝⊝1,3 | |
| Multiple pregnancy | No data available | |||||
| Miscarriage | No data available | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to serious risk of bias: method of sequence generation not reported, lack of blinding 2 Downgraded two levels due to very serious imprecision: only 15 events, all in one arm. 3 Downgraded one level due to serious imprecision: very wide confidence intervals | ||||||
| Coasting compared to cabergoline for preventing ovarian hyperstimulation syndrome (OHSS) | ||||||
| Population: Women undergoing assisted reproduction | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with cabergoline | Risk with Coasting | |||||
| OHSS | 100 per 1000 | 180 per 1000 | OR 1.98 | 120 | ⊕⊝⊝⊝1,2 | |
| Live birth | Not reported | |||||
| Clinical pregnancy rate | 367 per 1000 | 180 per 1000 | OR 0.38 (0.16 to 0.88) | 120 | ⊕⊕⊝⊝1 | |
| Multiple pregnancy | Not reported | |||||
| Miscarriage | Not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the mean risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded two levels due to very serious risk of bias: one study did not clearly define method, method of sequence generation not reported, lack of blinding 2 Downgraded one level due to serious imprecision: very few events and/or wide confidence interval. | ||||||
| Classification | Size of ovaries | Grade | Symptoms |
| Mild | 5 to 10 cm | grade 1 | abdominal tension and discomfort |
| grade 2 | grade 1 signs plus nausea, vomiting, diarrhoea, or a combination | ||
| Moderate | > 10 cm | grade 3 | grade 2 signs plus ultrasound evidence of ascites |
| Severe | > 12 cm | grade 4 | grade 3 signs plus clinical evidence of ascites, pleural effusion and dyspnoea, or a combination |
| grade 5 | grade 4 signs plus haemoconcentration increased blood viscosity, hypovolaemia, decreased renal perfusion, oliguria | ||
| Severe | Critical |
| Variably enlarged ovary | Variably enlarged ovary |
| Massive ascites ± hydrothorax | Tense ascites ± hydrothorax |
| Hct > 45% (> 30% increment over baseline value) | Hct > 55% |
| WBC > 15,000 | WBC > 35,000 |
| Oliguria | |
| Creatinine 1.0 to 1.5 | Creatinine > 1.6 |
| Creatinine clearance > 50 ml/min | Creatinine clearance < 50ml/min |
| Liver dysfunction | Renal failure |
| Anasarca | Thromboembolic phenomena |
| Adult respiratory distress syndrome (ARDS) | |
| Moderate | Severe Grade A | Severe Grade B | Severe Grade C |
| Discomfort, pain, nausea, distension, ultrasonic evidence of ascites and enlarged ovaries, normal haematological and biological profile | Dyspnoea, oliguria, nausea, vomiting, diarrhoea, abdominal pain, clinical evidence of ascites, marked distension of abdomen or hydro‐thorax, US showing large ovaries and marked ascites, normal biochemical profile | Grade A plus massive tension ascites, markedly enlarged ovaries, severe dyspnoea and marked oliguria, increased haematocrit, elevated serum creatinine and liver dysfunction | Complications such as respiratory distress syndrome, renal shut‐down, or venous thrombosis |
| Authors | E2 at coasting | E2 at hCG | Number and follicle size | Coasting time |
| > 3000 pg/mL or > 11,000 pmol/L* | < 3000 pg/mL or < 11,000 pmol/L* | > 29 follicles at least 30% > 15 mm | 3 to 11 days (mean 6.1) | |
| ≥ 3000 pg/ml or ≥ 11,000 pmol/l* | < 3000 pg/ml or < 11,000 pmol/l* | at least 3 follicles of 15.6 ± 1.4 mm | 1.9 ± 0.9 days | |
| > 3000 pg/ml or > 11,000 pmol/l* | < 3000 pg/mL or < 11,000 pmol/L* | 5 follicles at least 16 mm, two of which are at least 19 mm | 1 to 5 days | |
| > 2500 pg/ml or > 9000 pmol/l* | < 2500 pg/ml or < 9000 pmol/l* | ≥ 20 follicles > 15 mm | 1 to 6 days (mean 1.94) | |
| > 2700 pg/ml or > 10,000 pmol/l* | no values given | many immature follicles < 3 at 18 mm | 3 days | |
| > 2500 pg/ml or > 9000 pmol/l* | < 2500 pg/ml or < 9000 pmol/l* | ≥ 20 follicles > 14 mm | 1 to 6 days (mean 1.94) | |
| > 6000 pg/ml or > 22,000 pmol/l* | < 3000 pg/ml or 11,000 pmol/l* | > 15 follicles, each of > 18 mm in each ovary | 4.9 ± 1.6 days | |
| > 2700 pg/ml or > 10,000 pmol/l* | < 2700 pg/ml or < 10,000 pmol/l* | > 25 follicles, at least 3 follicles > 17 mm | 3 to 6 days (mean 4.3) | |
| > 3000 pg/ml or > 11,000 pmol/l* | 25% decline < 2250 pg/ml or 8250 pmol/l* | > 3 follicles of > 18 mm | 3 to 5 days (mean 3.4 ± 0.1) | |
| > 3600 pg/ml or > 13,000 pmol/l* | < 2700 pg/ml or < 10,000 pmol/l* | at least 25% of the follicles > 15 mm | 2 to 9 days (mean 3.4 ± 1.6) | |
| > 3000 pg/ml or > 11,000 pmol/l* | < 5500 pg/ml or < 20,000 pmol/l* | > 20 follicles at least 15 mm | 2.8 days | |
| > 4000 pg/ml or > 14,684 pmol/l* | < 4000 pg/ml or < 14,684 pmol/l* | > 20 follicles, at least 30% of them >15 mm | 2.9 ± 0.33 days | |
| * conversion factor to SI unit, 3.671 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 2 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.05, 0.24] |
| 2 Live birth Show forest plot | 1 | 68 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.14, 1.62] |
| 3 Clinical pregnancy Show forest plot | 2 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.44] |
| 4 Multiple pregnancy Show forest plot | 1 | 139 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.12, 0.81] |
| 5 Miscarriage Show forest plot | 2 | 207 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.25, 2.86] |
| 6 Number of oocytes retrieved Show forest plot | 2 | 207 | Mean Difference (IV, Fixed, 95% CI) | ‐3.86 [‐4.38, ‐3.33] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.34, 2.85] |
| 2 Clinical Pregnancy Show forest plot | 2 | 83 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.25, 1.79] |
| 3 Number of oocytes retrieved Show forest plot | 2 | 83 | Mean Difference (IV, Fixed, 95% CI) | ‐4.42 [‐6.08, ‐2.75] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Clinical Pregnancy Show forest plot | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.42, 1.31] |
| 3 Multiple pregnancy Show forest plot | 1 | 190 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.39, 1.80] |
| 4 Number of oocytes retrieved Show forest plot | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | ‐2.44 [‐4.30, ‐0.58] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 43.74 [2.54, 754.58] |
| 2 Clinical pregnancy Show forest plot | 1 | 102 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.43, 2.01] |
| 3 Number of oocytes retrieved Show forest plot | 1 | 102 | Mean Difference (IV, Fixed, 95% CI) | ‐17.80 [‐19.05, ‐16.55] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 OHSS Show forest plot | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.98 [0.69, 5.68] |
| 2 Clinical pregnancy rate Show forest plot | 2 | 120 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.16, 0.88] |
| 3 Number of oocytes retrieved Show forest plot | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | ‐4.30 [‐7.88, ‐0.72] |

