Flumazenil versus placebo or no intervention for people with cirrhosis and hepatic encephalopathy
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Double‐blind, single‐centre, placebo‐controlled RCT. Cross‐over design: all participants underwent both intervention periods (received flumazenil and placebo). | |
| Participants | 13 participants with cirrhosis with no evidence of overt hepatic encephalopathy but with abnormal brainstem evoked potentials (5 participants) or prolonged Number Connection Test times (6 participants), or both at baseline corresponding to a diagnosis of minimal hepatic encephalopathy. Mean ± SD age: flumazenil/placebo: 54 ± 7 years. Proportion of men: 77%. Aetiology of cirrhosis: alcohol 77%; hepatitis B/C 15%. Proportion testing positive for benzodiazepines at baseline (Table 5): 0%. | |
| Interventions | Intervention comparison: intravenous bolus flumazenil 1 mg followed by 4 boluses of 0.5 mg every 30 minutes versus placebo (saline). Total dose of flumazenil: 3 mg. Washout period: 72 hours before cross‐over to the alternative arm. Cointerventions: none described. | |
| Outcomes | Outcomes included in meta‐analyses: none. | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | Not described. | |
| Country | Italy. | |
| Notes | Included data: RCT did not describe outcomes for first intervention period. Therefore, we were unable to include the trial in our meta‐analyses. The study report includes 2 tables containing data for the 5 participants with abnormal evoked potentials at baseline and the 6 participants with abnormal Number Connection Test times. There were no change in the group mean variables after flumazenil. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Concealed drug containers. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | Low risk | Funding from the Italian Liver Foundation. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, multi‐centre, placebo‐controlled RCT. Cross‐over design: investigators crossed‐over participants who did not respond to intervention during first period (remained in Grade III or IVa coma) to alternative intervention. | |
| Participants | 527 participants with cirrhosis and overt hepatic encephalopathy (Grades III or IVa; Table 2), admitted to an intensive care unit. Diagnostic criteria corresponded to acute hepatic encephalopathy. Precipitating factors are described (Table 4). Mean ± SD age (grade III/IVa): flumazenil: 56 ± 11.5/53 ± 12 years; placebo: 48 ± 20/55 ± 13.5 years. Proportion of men: 69%. Aetiology of cirrhosis: alcohol 40%; hepatitis B/C 59%. Proportion testing positive for benzodiazepines at baseline (Table 5): 10/527 (1.9%) participants. | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 1 mg given over 3 to 5 minutes versus placebo (isotonic saline). Total dose of flumazenil: 1 mg. Cointerventions: lactulose 30 mL every 6 hours via nasogastric tube; antibiotics were given to 22 participants with sepsis in the flumazenil group and 8 in the placebo group | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), and serious adverse events (Table 6) assessed for a maximum of 4 days after randomisation. | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | January 1993 to December 1997. | |
| Country | Italy. | |
| Notes | Included data: Serum benzodiazepines were detected in 10 participants (4 with Grade III and 6 with Grade IVa coma). The published paper provides no information about the distribution of these participants to flumazenil or placebo during the first intervention period. Therefore, we were unable to exclude these participants from the analyses. The trial reported on several serious adverse events (Table 6). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequential list of block‐randomised assignments. |
| Allocation concealment (selection bias) | Low risk | Concealed ampoules of flumazenil and placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel using placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment using placebo. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | Low risk | No information provided. |
| Other bias | Low risk | No other biases. |
| Overall assessment | Low risk | Low risk of bias. |
| Methods | Double‐blind, single‐centre, placebo‐controlled RCT. Cross‐over design: participants who did not respond after 10 minutes during the first period received the alternative intervention. | |
| Participants | 14 participants with cirrhosis experiencing 18 separate episodes of acute hepatic encephalopathy classified as Grade II to IV (Table 2). Precipitating factors are described (Table 4). Mean ± SD age: whole group 54.8 ± 7.7 years. Proportion of men: 71%. Aetiology of cirrhosis: alcohol 71%; hepatitis B/C 29%. Proportion testing positive for benzodiazepines at baseline (Table 5): 3/14 (21.4%) participants. | |
| Interventions | Intervention comparison: continuous intravenous infusion flumazenil 0.1 mg/mL at 1 mL/minute flumazenil versus placebo (sodium edetate 1 mg). Investigators stopped the infusion after 10 minutes if participants showed improvement in electroencephalography or coma grade. Total dose of flumazenil: 1 mg. Cointerventions: none described. | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), and serious adverse events (Table 6) assessed after maximum of 3 days. | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | May 1988 to May 1990. | |
| Country | France. | |
| Notes | Included data: the trial included 14 participants who between them experienced 18 episodes of acute hepatic encephalopathy. 1 participant entered the trial once and 1 entered the trial 3 times. We included data from the first intervention period in our analyses. The published report described the number of participants who died after the second treatment period only (Table 6). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Concealed drug vials. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | Outcome data were complete. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in original protocol or information in trial registries. |
| For‐profit funding | High risk | Hoffmann‐La Roche Ltd. supplied flumazenil and placebo. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, single‐centre, placebo‐controlled, parallel‐arm RCT. | |
| Participants | 40 participants with cirrhosis and hepatic encephalopathy classified as subclinical (corresponding to minimal; 10 participants) or overt Grade I to III (30 participants; Table 2). Type of overt hepatic encephalopathy (acute or chronic) not specified. Mean ± SD age: flumazenil: 44.5 ± 12.9 years; placebo: 43.7 ± 11.9 years. Proportion of men: 73%. Aetiology of cirrhosis: alcohol 0%; hepatitis B/C 100%. Proportion testing positive for benzodiazepines at baseline (Table 5): investigators did not screen for benzodiazepines. | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 1 mg/hour for 5 hours versus placebo (saline) administered similarly. Total dose of flumazenil: 5 mg. Cointerventions: lactulose 30 mL 6‐hourly | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), serious adverse events (Table 6), and Number Connection Test assessed after a maximum of 5 hours. | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | December 1999 to January 2002. | |
| Country | Turkey. | |
| Notes | Included data: the trial report included information about participants with minimal and overt hepatic encephalopathy. We have analysed these 2 groups separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Concealed drug containers. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants are included in analyses. |
| Selective reporting (reporting bias) | Low risk | Trial describes clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | Unclear risk | No information provided. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, single‐centre, placebo‐controlled RCT. Cross‐over design: investigators crossed over all participants to the alternative intervention. | |
| Participants | 10 participants with cirrhosis and no clinical evidence of overt hepatic encephalopathy; 5 participants had minimal hepatic encephalopathy based on the finding of either abnormal visual evoked potentials or Number Connection Test results. Age (range): 40 to 60 years. Proportion of men: 80%. Aetiology of cirrhosis: alcohol 30%; hepatitis B/C 70%. Proportion testing positive for benzodiazepines at baseline (Table 5): 0%. | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 1 mg over 2 minutes versus placebo. Total dose of flumazenil: 1 mg. Washout period: 4 hours. Cointerventions: none reported. | |
| Outcomes | Outcomes included in meta‐analyses: none. | |
| Neuropsychiatric assessment | At baseline and post infusion:
| |
| Inclusion period (date) | Not described. | |
| Country | The Netherlands. | |
| Notes | Included data: trial did not include separate information about the first allocation period. Therefore, we were unable to include the trial in our meta‐analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Low risk | Concealed drug containers. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | The trial describes clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | Unclear risk | No information provided. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, single‐centre, placebo‐controlled RCT. Cross‐over design: all participants were crossed over to alternative intervention. | |
| Participants | 10 participants with cirrhosis and subclinical (corresponding to minimal) hepatic encephalopathy diagnosed based on a score on the Digit Symbol Substitution test of < 1 SD of the age‐matched normative mean. Mean age ± SD: 53.9 ± 7.4 years. Proportion of men: 80%. Aetiology of cirrhosis: alcohol 60%; hepatitis B/C 20%. Proportion testing positive for benzodiazepines at baseline (Table 5): apparently not performed (not specifically stated). | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 0.2 mg over an unspecified time versus placebo (saline). Total dose of flumazenil: 0.2 mg. Washout period: 1 week. Cointerventions: none described. | |
| Outcomes | Outcomes included in meta‐analyses: mortality and serious adverse events (Table 6) assessed at end of the intervention. | |
| Neuropsychiatric assessment | At baseline:
At baseline and post infusion:
Duration of follow‐up and timing of tests not described. | |
| Inclusion period (date) | Not described. | |
| Country | UK. | |
| Notes | Included data: we received additional (unpublished) information about the trial methods and number of participants allocated to flumazenil/placebo during the first allocation period via email in 2003 when conducting the previous version of this review. The trial did not evaluate the number of participants with an overall improvement in hepatic encephalopathy. Therefore, we were unable to include the trial in our analyses of this outcome measure. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table. |
| Allocation concealment (selection bias) | Low risk | Serially numbered opaque sealed envelopes used in administration of concealed drug containers. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | Unclear risk | No information provided. |
| Other bias | High risk | Primary investigators described a significant drug by order and group by drug by order interaction. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, multi‐centre, parallel‐arm, placebo controlled RCT. | |
| Participants | 49 participants with cirrhosis and chronic overt hepatic encephalopathy (Grades I to III; Table 2). Mean age ± SD: flumazenil: 55.5 ± 9.4 years; placebo: 53.6 ± 10.3 years. Proportion of men: 69%. Aetiology of cirrhosis: alcohol 51%; hepatitis B/C 35%. Proportion testing positive for benzodiazepines at baseline (Table 5): 11% in flumazenil group; 5% in placebo group. | |
| Interventions | Intervention comparison: intravenous boluses of flumazenil 0.4 mg, 0.8 mg, and 1 mg at 1‐minute intervals followed by a 3‐hour infusion of flumazenil 1 mg/hour versus placebo (saline). Total dose of flumazenil: 5.2 mg. Cointerventions: none reported. | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), and serious adverse events (Table 6) assessed after a maximum of 4 weeks. | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | Not reported. | |
| Country | Switzerland (primary), France, Germany, Italy, Canada, the Netherlands, the UK, and Korea. | |
| Notes | Included data: authors reported intention‐to‐treat analyses including all participants randomised and a per‐protocol analysis excluding protocol violators (25 participants). We included data on all participants in our analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list of random numbers. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used in double‐blind administration of flumazenil and placebo. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | High risk | Support from Hoffmann‐La Roche Ltd. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, parallel‐arm, single‐centre, placebo‐controlled RCT. | |
| Participants | 12 participants with cirrhosis and an acute episode of hepatic encephalopathy defined as Grade IIIa with severely abnormal electroencephalography changes, but a Glasgow Coma Score of < 12 (Table 2). Proportion of men: not reported. Mean age ± SD: whole group 58.2 ± 5.4 years. Aetiology of liver disease: not reported. Proportion testing positive for benzodiazepines at baseline (Table 5): 0%. | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 0.2 mg/kg over 10 minutes versus placebo (saline). Total dose of flumazenil: 0.2 mg/kg. Cointerventions: none described. | |
| Outcomes | Outcomes included in meta‐analyses: mortality (Table 6) and serious adverse events (Table 6). | |
| Neuropsychiatric assessment | Baseline and post infusion:
The timing of assessments post infusion was not described. | |
| Inclusion period (date) | Not described. | |
| Country | France. | |
| Notes | Included data: the trial report did not specifically state the number of participants with (or without) improvement in hepatic encephalopathy separately for the allocation groups. Therefore, we were unable to include the trial in the analysis of this outcome measure. Article published in French (full translation available). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Concealed drug containers. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Trial report gave the impression that there were no missing outcome data although this was not specifically stated. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | Unclear risk | No information provided. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, cross‐over, single‐centre, placebo‐controlled RCT. | |
| Participants | 2 participants with cirrhosis and stable hepatic encephalopathy (Grade III). Description corresponded to chronic overt hepatic encephalopathy although this was not specifically stated. Proportion of men: not reported. Mean age: not reported. Aetiology of liver disease: alcohol 100%. Proportion testing positive for benzodiazepines at baseline (Table 5): apparently not performed (not specifically stated). | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 1 mg over 1 minute versus placebo. Total dose of flumazenil: 1 mg. Washout period: not specified. Cointerventions: not reported. | |
| Outcomes | Outcomes included in meta‐analyses: mortality and serious adverse events (Table 6) assessed for a maximum of 2 hours post interventions. | |
| Neuropsychiatric assessment | Clinical assessment of mental status: assessed after 2 hours (no specific score; timing not specified). | |
| Inclusion period (date) | Not described. | |
| Country | Germany. | |
| Notes | Included data: investigators described the design as cross‐over but did not provide data from the first intervention period. Therefore, we were unable to include the trial in our analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Trial described as double blind and placebo controlled. However, trial only reported as a letter and the type of placebo (or mode of administration) is not described. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Trial described as double blind and placebo controlled. However, trial only reported as a letter and the type of placebo (or mode of administration) is not clearly described. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Trial gave impression that participants survived although this was not specifically stated. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | Unclear risk | No information provided. |
| Other bias | High risk | Trial only included 2 participants. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, single‐centre, parallel‐arm placebo‐controlled RCT. | |
| Participants | 54 participants with cirrhosis and acute hepatic encephalopathy (Grade III or IV). Precipitating factors are described (Table 4). Mean age ± SD: flumazenil: 59.6 ± 6.0 years; placebo: 57.7 ± 5.4 years. Proportion of men: 54%. Aetiology of cirrhosis: hepatitis B/C 100%. Proportion testing positive for benzodiazepines at baseline (Table 5): 0%. | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 0.4 mg/mL at 10 mL/minute for 5 minutes versus placebo (saline). Total dose of flumazenil: 2 mg. Cointerventions: lactulose enemas; branch‐chain amino acids. | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), and serious adverse events (Table 6) assessed for maximum of 24 hours after intervention. | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | January 1997 to December 1997. | |
| Country | Italy. | |
| Notes | Included data: we included data on all participants in our analyses. Notes about the design: investigators repeated the intervention once after 3 hours in non‐responders (no improvement in neurological status) or immediately if they detected an improvement followed by a relapse. The report did not include information about the number of participants who received a second infusion. In the results section of the report the investigators stipulate that the second infusion was the same as the one first received. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Double‐blind administration of flumazenil and placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel using placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment using placebo. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants were included in the analyses. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | Unclear risk | No information provided. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, single‐centre, parallel‐arm RCT. | |
| Participants | 72 participants with overt hepatic encephalopathy (Grade III or IV) associated with cirrhosis (65%) or fulminant hepatic failure (35%). Diagnostic criteria for participants with cirrhosis corresponded to acute hepatic encephalopathy. Mean age ± SD: flumazenil: 55.4 ± 6.6 years; placebo: 56.8 ± 7.9 years. Proportion of men: 63%. Aetiology of cirrhosis: not reported. Proportion testing positive for benzodiazepines at baseline (Table 5): apparently not performed (not specifically stated). | |
| Interventions | Intervention comparison: slow intravenous injection flumazenil 0.5 mg followed by intravenous infusion of flumazenil 1 mg of over 30 minutes versus placebo (saline). Total dose of flumazenil: 1.5 mg. Cointerventions: lactulose enemas, L‐ornithine L‐aspartate | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), and serious adverse events (Table 6) assessed after a maximum of 2 weeks. | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | May 2006 to July 2008. | |
| Country | China. | |
| Notes | Included data: the trial report did not provide separate information on participants with cirrhosis and participants with acute liver failure. Therefore, we conducted a sensitivity analysis excluding this trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Double‐blind administration of flumazenil and placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants included in the analyses. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in original protocol or information in trial registries. |
| For‐profit funding | Unclear risk | No information provided. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, single‐centre, cross‐over, placebo‐controlled RCT. Cross‐over design: investigators only crossed over participants who remained in Grade IV coma, 24 hours after the first study period. | |
| Participants | 21 participants with cirrhosis and acute hepatic encephalopathy (Grade IV). Precipitating factors are described (Table 4). Mean age ± SD: flumazenil: 52.7 ± 5.4 years; placebo: 57.4 ± 9.0 years. Proportion of men: 81%. Aetiology of cirrhosis: alcohol 62%; hepatitis B/C 5%. Proportion testing positive for benzodiazepines at baseline (Table 5): 4/21 (19%) participants. | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 2 mg over 5 minutes versus placebo (saline). Total dose of flumazenil: 2 mg. Washout period: 24 hours. Cointerventions: lactulose 30 mL 4 times daily. | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), and serious adverse events (Table 6) assessed after a maximum follow‐up of 24 hours. | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | March 1988 to February 1992. | |
| Country | Canada. | |
| Notes | Included data: we only included data from the first treatment period in our analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers. |
| Allocation concealment (selection bias) | Low risk | Blinded administration of flumazenil or placebo. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants included in analyses. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | High risk | Technical assistance from Hoffmann‐La Roche Ltd., Canada and Nutley, NJ, USA. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, single‐centre, cross‐over, placebo‐controlled RCT. Cross‐over design: all participants (except 2 who underwent transplantation) received flumazenil and placebo. | |
| Participants | 18 participants with hepatic encephalopathy secondary to acute liver failure (28%) or cirrhosis (82%), who had an arterial blood ammonia > 30 μmol/L, and an abnormal electroencephalography despite at least 24 hours of treatment with a low protein diet and lactulose alone or with neomycin. Precipitating factors are described (Table 4). Mean age ± SD: whole group 48.56 ± 14.67 years. Proportion of men: 39%. Aetiology of cirrhosis: alcohol 38%; hepatitis B/C 15%. Proportion testing positive for benzodiazepines at baseline (Table 5): 0%. | |
| Interventions | Intervention comparison 1: First 9 participants: intravenous infusion flumazenil 0.1 mg/minute over 10 minutes; 4 hours later given a bolus injection flumazenil 0.5 mg followed by a continuous infusion of flumazenil 0.25 mg/hour for 3 days versus infusion vehicle alone. Total dose of flumazenil: 19.5 mg. Washout period: 24 hours. Intervention comparison 2: Second 9 participants: intravenous infusion of flumazenil 0.1 mg/minute over 10 minutes versus infusion vehicle alone. Total dose of flumazenil: 19.5 mg. Washout period 24 hours. Cointerventions: protein restriction, lactulose alone or with neomycin. | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), and serious adverse events (Table 6) assessed after a maximum of 3 days. | |
| Neuropsychiatric assessment | First 9 participants: baseline and post infusion:
Second 9 participants: baseline and post infusion:
| |
| Inclusion period (date) | February 1987 to February 1990. | |
| Country | The Netherlands. | |
| Notes | Included data: Two patients were withdrawn on day one of the study to undergo liver transplantation thus only 16 people took part in the full cross‐over study. The study involved people with hepatic encephalopathy associated with cirrhosis and with acute liver failure; the trial data were not provided separately for these two groups so no separate analysis can be performed by aetiology of the hepatic encephalopathy. We only include data from the first treatment period in our analyses | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Used concealed drug containers. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data and all participants were included in analyses. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in original protocol or information in trial registries. |
| For‐profit funding | High risk | Support provided by Hoffmann‐La Roche B.V., Mijdrecht, The Netherlands. |
| Other bias | Unclear risk | Investigators simplified the intervention regimen after inclusion of the first 9 participants as the second period of the 72‐hour infusion was too demanding for participants. The effect that this has on bias control was unclear. |
| Overall assessment | High risk | High risk of bias. |
| Methods | Double‐blind, single‐centre, parallel‐arm, placebo‐controlled RCT. | |
| Participants | 25 participants with cirrhosis and overt hepatic encephalopathy (Grade II to IV). Precipitating factors are described (Table 4). Mean age ± SD: flumazenil: 62.2 ± 2.7 years; placebo: 52.2 ± 3.3 years. Proportion of men: 69%. Aetiology of cirrhosis: alcohol 80%; hepatitis B/C 12%. Proportion testing positive for benzodiazepines at baseline (Table 5): not conducted (not specifically stated). | |
| Interventions | Intervention comparison: intravenous infusion flumazenil 1 mg over 5 minutes versus placebo (saline). Total dose of flumazenil: 1 mg. Cointerventions: intravenous branched‐chain amino acids. | |
| Outcomes | Outcomes included in meta‐analyses: mortality, hepatic encephalopathy (Table 1), and serious adverse events (Table 6) assessed for a maximum of 2 weeks (until death or discharge). | |
| Neuropsychiatric assessment | Baseline and post infusion:
| |
| Inclusion period (date) | April 1995 to March 1996. | |
| Country | China. | |
| Notes | Included data: all participants were included in the analyses. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers with stratified block randomisation. |
| Allocation concealment (selection bias) | Low risk | Administration of concealed drug containers with sealed, opaque, serially numbered envelopes. |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of participants and personnel. |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) | Unclear risk | Missing outcome data not described. |
| Selective reporting (reporting bias) | Low risk | Trial described clinically relevant outcomes. We had no access to information about outcomes described in the original protocol or information in trial registries. |
| For‐profit funding | High risk | Roche supplied the flumazenil. |
| Other bias | Low risk | No other biases. |
| Overall assessment | High risk | High risk of bias. |
RCT: randomised clinical trial; SD: standard deviation.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Prospective study including 14 participants with cirrhosis and overt hepatic encephalopathy. The investigators reported an improvement in mental status in 71% of participants within minutes of receiving intravenous flumazenil lasting for 1 to 2 hours. Participants also received lactulose . Six participants died. The study was excluded as it did not include a control group. | |
| Prospective study evaluating 7 children with fulminant hepatic failure awaiting emergency liver transplantation. The investigators reported that flumazenil injection led to a transient improvement in mental status in 1 child but had no effect on mental status in the remaining six. The study was excluded as none of the participants had hepatic encephalopathy associated with cirrhosis and it did not include a control group. | |
| Prospective study including 10 participants with alcohol‐related cirrhosis and overt hepatic encephalopathy classified as grade IV based on an assessment of mental status, electroencephalography, and visual evoked responses. The investigators reported an improvement in mental status in 8/10 participants. Six participants died within 1 year. The study was excluded as it did not include a control group. | |
| Prospective study including 17 participants (2 children) with hepatic encephalopathy associated with acute liver failure (9 participants) or cirrhosis (8 participants). Cointerventions included lactulose, branched‐chain amino acids, antibiotics, diuretics, histamine‐receptor antagonists, human albumin, and fresh frozen plasma. Transient improvement in the manifestations of hepatic encephalopathy was seen following flumazenil in 4 (44%) participants with fulminant hepatic failure and 5 (63%) with cirrhosis. Mortality was not reported. This study was excluded as it did not include a control group. | |
| Open, single‐centre, non‐randomised study which is included in the sensitivity analyses of serious adverse events. The study involved 22 participants with cirrhosis and overt hepatic encephalopathy (Grades I‐III using West Haven criteria) recruited between April 1996 and September 1997. Intervention comparison: 12 participants received an intravenous bolus of flumazenil 0.5 mg followed by an intravenous infusion of flumazenil in a dose of 1.0 mg over 4 hours for an unspecified period of time. The remaining 10 participants received Xing‐Nao‐Jing, a traditional Chinese medicine also given as an intravenous infusion. Total dose of flumazenil: 1.5 mg. Outcomes: The study report stated that 2 participants in the flumazenil group died of liver failure but the time of death in relation to the intervention was not specified and study did not specifically state if there were any deaths in control group. The article was published in Chinese but a translation was available. The study was excluded as it did not contain a control group. | |
| Double‐blind, cross‐over, placebo‐controlled, single‐centre randomised clinical trial including 20 liver transplant candidates with cirrhosis. The trial is included in the sensitivity analyses of serious adverse events. The main objective of trial was to evaluate the differential effects of flumazenil on cognitive function and anxiety in people with alcohol‐related (10 participants) or non‐alcohol‐related (10 participants) cirrhosis. None of the included participants had evidence of overt hepatic encephalopathy. The investigators evaluated a range of psychometric tests and reported the results as group mean values. No information was provided about the number of participants with abnormal test results. Proportion of men: 60%. Mean ± SD age: alcohol‐related cirrhosis: 47.7 ± 10.5 years; non‐alcoholic cirrhosis: 48.4 ± 11.7 years. Proportion testing positive for benzodiazepines at baseline: not tested Intervention: intravenous infusion flumazenil 0.1 mg/minute for 10 minutes then 0.05 mg/minute for 20 minutes versus placebo (saline). Total dose of flumazenil: 2 mg. Washout period: 60 minutes. Outcomes: The investigators reported changes in psychometric tests for participants with alcohol‐related or non‐alcohol‐related cirrhosis without providing an overall estimate of numbers with (or without) improved manifestations. The trial did not report any deaths or serious adverse events. The study was excluded because none of the participants had hepatic encephalopathy. | |
| Open, single‐centre, prospective, non‐randomised study included in the sensitivity analyses of serious adverse events at 48 hours. The study involved 25 participants with alcohol‐related cirrhosis and acute hepatic encephalopathy, 13 of whom received flumazenil. The proportion of men and the mean age of participants was not reported. Proportion testing positive for benzodiazepines at baseline: not mentioned Intervention: intravenous bolus of flumazenil 0.2 mg every ten minutes until improvement in clinical status up to a maximum total dose of 2 mg followed by a continuous maintenance infusion of 0.3 mg. per hour for 48 hours. Total dose of flumazenil: maximum 16.4 mg. Outcomes: .The Investigators reported that the mortality rates were similar in the flumazenil and control groups, but did not provide information on the number of participants who died. Published in French but a translation was available.The study was excluded as it was not randomised. | |
| Prospective study evaluating the effect of 30‐minute, incremental intravenous boluses of flumazenil in 11 participants with cirrhosis (6 stage 0,4 stage one, one stage 2 hepatic encephalopathy) in whom baseline somatosensory evoked potentials were abnormal. Four patients (36%) showed a clear improvement in evoked potentials with flumazenil. Mortality was not described. The study was excluded as it did not include a control group. | |
| Randomised clinical trial comparing intravenous flumazenil plus lactulose enemas versus flumazenil alone. A total of 20 participants (18 men) with cirrhosis and hepatic encephalopathy were included. The maximum dose of flumazenil was 9 mg. The dose was adjusted based on the clinical effect. Investigators assessed hepatic encephalopathy based on Conn Criteria and defined an improvement from Grade IV to I within 6 hours as clinically significant. None of the included participants died and all 12 in the flumazenil plus lactulose group and all 8 in the flumazenil group showed improved manifestations of hepatic encephalopathy. The study was excluded as there was no placebo arm. |
.
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Treatment of Hepatic Encephalopathy with Flumazenil and Change in Cortical Gamma Aminobutyric Acid Levels in MRS [magnetic resonance spectroscopy]. |
| Methods | Randomised clinical trial. |
| Participants | Participants with non‐alcoholic cirrhosis and hepatic encephalopathy. |
| Interventions | Flumazenil and placebo. |
| Outcomes | Recovery from hepatic encephalopathy and change in cortical gamma aminobutyric acid levels. |
| Starting date | November 2014. |
| Contact information | Deanna Martin, [email protected] and Amanda Brennan [email protected]. |
| Notes | Estimated completion date: June 2017. |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 11 | 842 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.16] |
| Analysis 1.1  Comparison 1 Flumazenil versus placebo, Outcome 1 All‐cause mortality. | ||||
| 1.1 Overt hepatic encephalopathy | 10 | 822 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.16] |
| 1.2 Minimal hepatic encephalopathy | 2 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality and bias control Show forest plot | 12 | 844 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.16] |
| Analysis 1.2 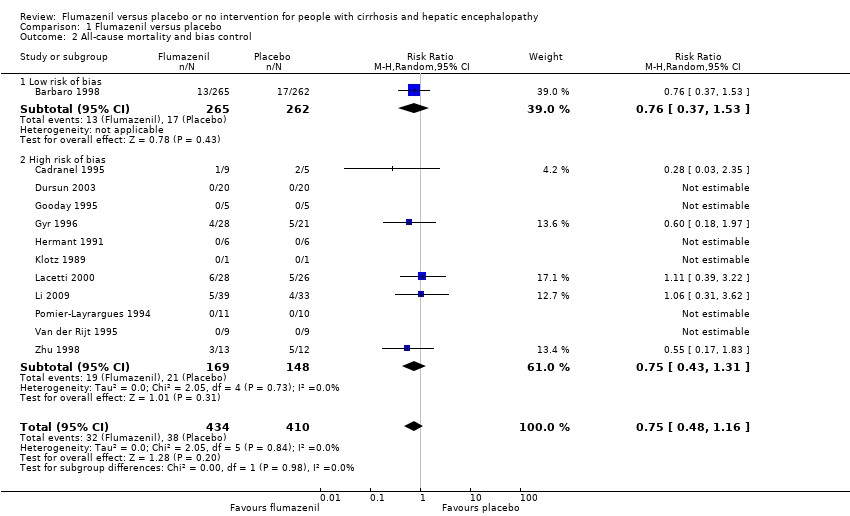 Comparison 1 Flumazenil versus placebo, Outcome 2 All‐cause mortality and bias control. | ||||
| 2.1 Low risk of bias | 1 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.37, 1.53] |
| 2.2 High risk of bias | 11 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.43, 1.31] |
| 3 All‐cause mortality and trial design Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Flumazenil versus placebo, Outcome 3 All‐cause mortality and trial design. | ||||
| 3.1 Cross‐over | 6 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.35, 1.34] |
| 3.2 Parallel‐arm | 6 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.45, 1.44] |
| 4 All‐cause mortality and duration of follow‐up Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Flumazenil versus placebo, Outcome 4 All‐cause mortality and duration of follow‐up. | ||||
| 4.1 ≤ 1 day | 5 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.38, 1.87] |
| 4.2 > 1 day | 6 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.42, 1.21] |
| 5 Hepatic encephalopathy Show forest plot | 9 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.71, 0.80] |
| Analysis 1.5 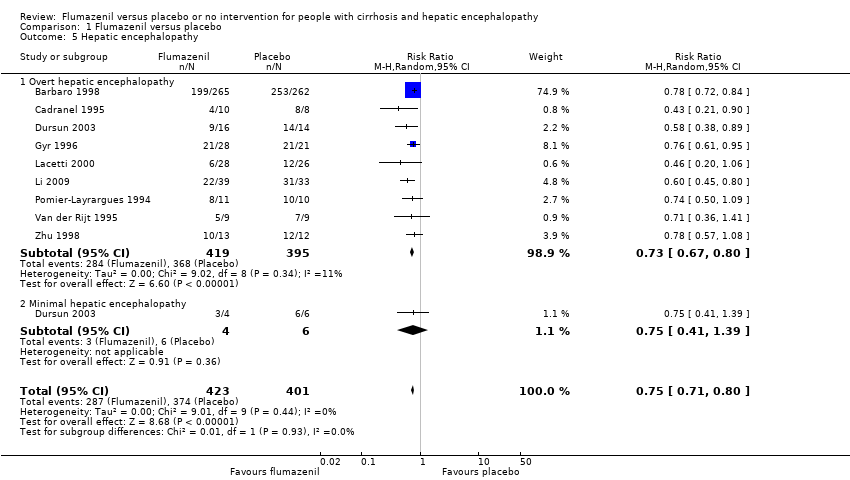 Comparison 1 Flumazenil versus placebo, Outcome 5 Hepatic encephalopathy. | ||||
| 5.1 Overt hepatic encephalopathy | 9 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.67, 0.80] |
| 5.2 Minimal hepatic encephalopathy | 1 | 10 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.39] |
| 6 Hepatic encephalopathy and bias control Show forest plot | 9 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.71, 0.80] |
| Analysis 1.6  Comparison 1 Flumazenil versus placebo, Outcome 6 Hepatic encephalopathy and bias control. | ||||
| 6.1 Low risk of bias | 1 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.72, 0.84] |
| 6.2 High risk of bias | 8 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.61, 0.78] |
| 7 Hepatic encephalopathy and trial design Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Flumazenil versus placebo, Outcome 7 Hepatic encephalopathy and trial design. | ||||
| 7.1 Cross‐over | 4 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.72, 0.83] |
| 7.2 Parallel‐arm | 5 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.79] |
| 8 Hepatic encephalopathy and duration of follow‐up Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.8 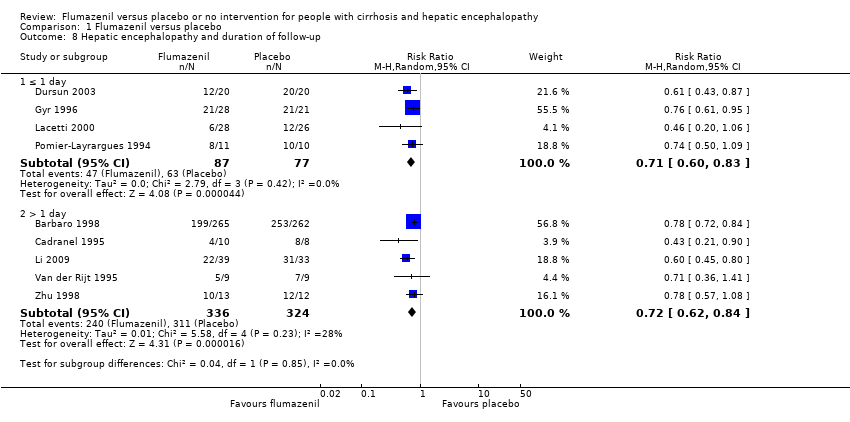 Comparison 1 Flumazenil versus placebo, Outcome 8 Hepatic encephalopathy and duration of follow‐up. | ||||
| 8.1 ≤ 1 day | 4 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.83] |
| 8.2 > 1 day | 5 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.62, 0.84] |
| 9 Hepatic encephalopathy and acute liver failure Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| Analysis 1.9  Comparison 1 Flumazenil versus placebo, Outcome 9 Hepatic encephalopathy and acute liver failure. | ||||
| 9.1 Cirrhosis | 7 | 734 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.71, 0.82] |
| 9.2 Acute liver failure or cirrhosis | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.47, 0.80] |
| 10 Number Connection Test Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Flumazenil versus placebo, Outcome 10 Number Connection Test. | ||||
| 11 All‐cause mortality and acute liver failure Show forest plot | 11 | 842 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.16] |
| Analysis 1.11 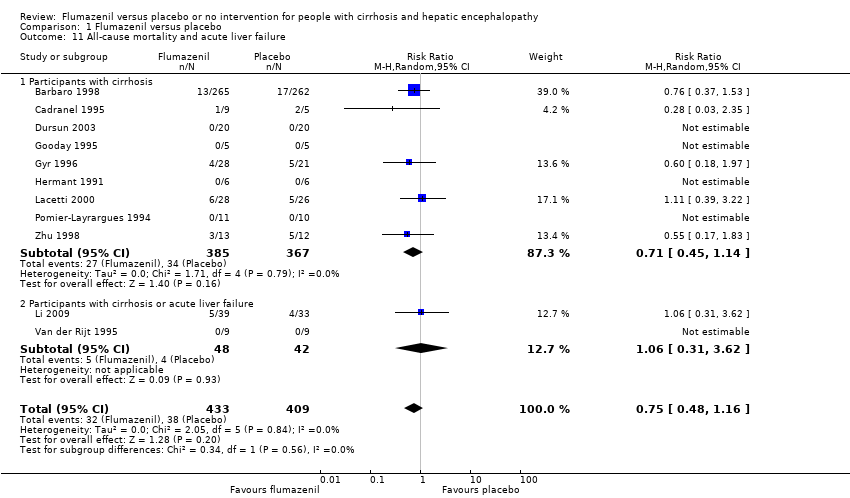 Comparison 1 Flumazenil versus placebo, Outcome 11 All‐cause mortality and acute liver failure. | ||||
| 11.1 Participants with cirrhosis | 9 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.14] |
| 11.2 Participants with cirrhosis or acute liver failure | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.31, 3.62] |
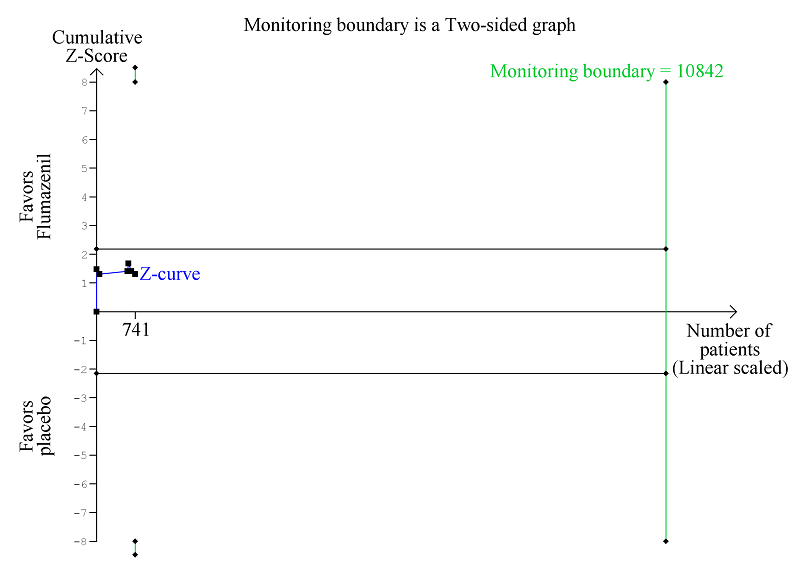
Trial Sequential Analysis of randomised clinical trials evaluating flumazenil versus placebo for people with hepatic encephalopathy. The outcome is all‐cause mortality. The original meta‐analysis included 11 randomised clinical trials with 842 participants. The Trial Sequential Analysis ignored three randomised clinical trials due to insufficient information size (Cadranel 1995; Gyr 1996; Zhu 1998). The analysis was made with alpha 3%, power 90%, relative risk reduction 20%, assumed control risk 10%, and diversity 10%. The blue line (Z‐curve) corresponds to the cumulative meta‐analysis, the black horizontal line is the conventional boundary (3% level of significance), and the inward sloping green line is the Trial Sequential Monitoring Boundary. Futility boundaries are ignored because the information is insufficient. The analysis found no evidence to support or refute a beneficial or harmful effect of flumazenil on mortality.
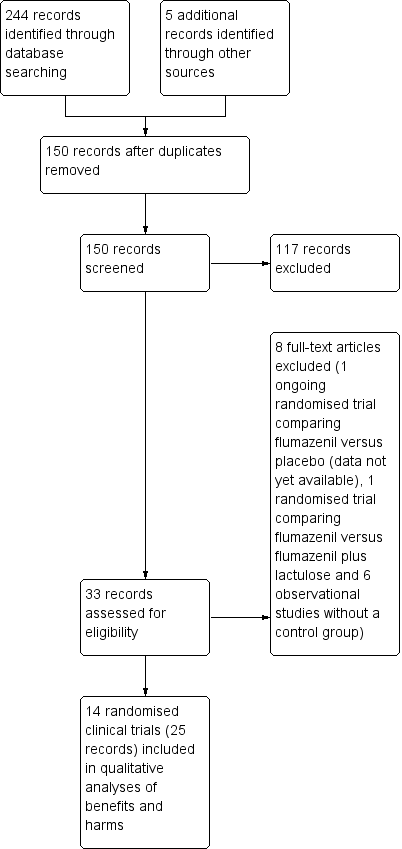
Study flow diagram for identification and selection of randomised clinical trials.
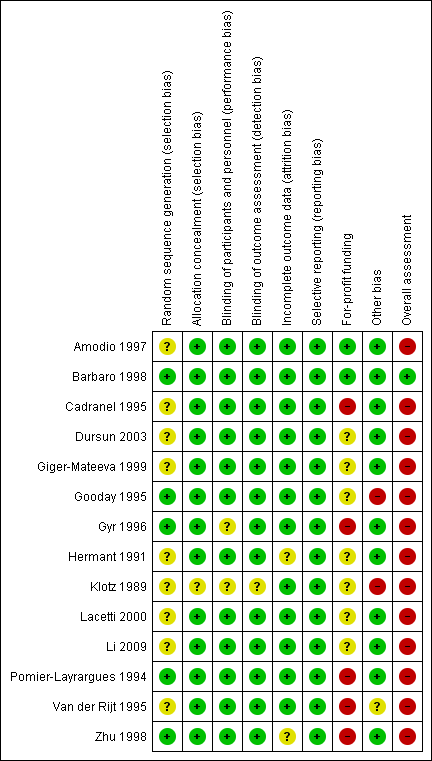
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Trial Sequential Analysis of randomised clinical trials evaluating flumazenil versus placebo for people with cirrhosis and hepatic encephalopathy. The outcome is hepatic encephalopathy. The original meta‐analysis included 11 randomised clinical trials with 824 participants. The Trial Sequential Analysis is made with alpha 3%, power 90%, relative risk reduction 20%, assumed control risk 60%, and diversity 10%. The blue line (Z‐curve) corresponds to the cumulative meta‐analysis, the black horizontal line is the conventional boundary (3% level of significance), and the inward sloping green line is the Trial Sequential Monitoring Boundary. The analysis found that the Z‐curve crossed the monitoring boundary before reaching the diversity‐adjusted required information size of 914 participants.

Comparison 1 Flumazenil versus placebo, Outcome 1 All‐cause mortality.

Comparison 1 Flumazenil versus placebo, Outcome 2 All‐cause mortality and bias control.

Comparison 1 Flumazenil versus placebo, Outcome 3 All‐cause mortality and trial design.

Comparison 1 Flumazenil versus placebo, Outcome 4 All‐cause mortality and duration of follow‐up.

Comparison 1 Flumazenil versus placebo, Outcome 5 Hepatic encephalopathy.

Comparison 1 Flumazenil versus placebo, Outcome 6 Hepatic encephalopathy and bias control.

Comparison 1 Flumazenil versus placebo, Outcome 7 Hepatic encephalopathy and trial design.

Comparison 1 Flumazenil versus placebo, Outcome 8 Hepatic encephalopathy and duration of follow‐up.

Comparison 1 Flumazenil versus placebo, Outcome 9 Hepatic encephalopathy and acute liver failure.

Comparison 1 Flumazenil versus placebo, Outcome 10 Number Connection Test.

Comparison 1 Flumazenil versus placebo, Outcome 11 All‐cause mortality and acute liver failure.
| Flumazenil versus placebo for people with cirrhosis and hepatic encephalopathy | ||||||
| Patient or population: people with hepatic encephalopathy Setting: hospital Intervention: flumazenil Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with flumazenil | |||||
| All‐cause mortality | Study population | RR 0.75 | 842 | ⊕⊕⊝⊝ | The only RCT with low risk of bias found no effect of flumazenil on all‐cause mortality (RR 0.76, 95% CI 0.37 to 1.53). The Trial Sequential Analysis found insufficient evidence to support or refute an intervention benefit/harm. | |
| 93 per 1000 | 70 per 1000 | |||||
| Hepatic encephalopathy | Study population | RR 0.75 | 824 | ⊕⊕⊝⊝ | The only RCT with a low risk of bias reported a beneficial effect of flumazenil on hepatic encephalopathy (RR 0.78, 95% CI 0.72 to 0.84; Barbaro 1998). The Trial Sequential Analysis found that flumazenil was associated with a beneficial effect on hepatic encephalopathy (Figure 1). The methods used to assess this outcome varied considerably (Table 1) and the duration of follow‐up was very short in the majority of RCTs. | |
| 933 per 1000 | 700 per 1000 | |||||
| Serious adverse events | See comment | See comment | Not estimable | 842 | ⊕⊕⊝⊝ | All‐cause mortality was the only serious adverse event reported for both the intervention and control group (Table 6). The narrative text in 4 RCTs described that causes of death included liver failure, progressive liver disease, and infections. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised clinical trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to risk of bias: only one RCT had a low risk of bias. 2 Downgraded due to imprecision: wide confidence intervals. | ||||||
| Trial | Type of hepatic encephalopathy | Neuropsychiatric assessment | Definition of overall improvement |
| Minimal | Number Connection Test and Brainstem Auditory Evoked Response. | Investigators did not define or assess the number of participants with an overall improvement. | |
| Overt | Mental status assessed using a clinical scale (Table 2), Modified Glasgow Coma Scale (Table 2), and electroencephalography (Table 3). | Improvement in clinical scores or electroencephalography. | |
| Overt | Mental status assessed using a clinical scale (Table 2), and electroencephalography (Table 3). | Improvement in clinical score or electroencephalography. | |
| Minimal or overt | Mental status assessed using a clinical scale (Table 2), Number Connection Test results, and electroencephalography (Table 3). | Improvement based on the clinical score and Number Connection Test results. | |
| Minimal | Number Connection Test and brainstem auditory evoked response. | Not defined. The investigators included a post‐hoc subjective assessment of alertness. | |
| Minimal | Simple and complex reaction time, verbal memory, psychomotor speed, short‐term and working memory. | Improvement in psychomotor speed evaluated using change in reaction time; Investigators did not define or assess the number of participants with overall improvement. . | |
| Overt | Mental status (Table 2), and electroencephalography (Table 3). | Clinically relevant improvement defined as a 2‐point improvement in clinical score at any time during treatment compared with baseline. The investigators also reported improvement defined using the clinical scale score (mean for all individual observations). | |
| Overt | Glasgow Coma Scale (Table 2), and electroencephalography. | A 2‐point improvement in the Glasgow Coma Score and electroencephalography. | |
| Overt | Clinical assessment (score not described). | Improvement in clinical status | |
| Overt | Mental status (scale not specified) and Glasgow Coma Scale (Table 2). | Investigators originally classified participants as Grade III to IV coma. Method of assessment not stipulated. The trial report defined 'clinically relevant improvement' as primary outcome defined as a 3‐point improvement in Glasgow Coma Score. | |
| Overt | Glasgow Coma Scale (Table 2), and electroencephalography | Improvement in the Glasgow Coma Score of ≥ 3 points. | |
| Overt | Modified Glasgow Coma Score (Table 2), and electroencephalography. | Improvement in ≥ 2 items on modified Glasgow Coma Score within 1 hour after the end of treatment. | |
| Overt | Clinical scale (Table 2). | A ≥ 1 ‐point decrease in severity of hepatic encephalopathy. | |
| Overt | Clinical scale (Table 2). | Overall improvement in hepatic encephalopathy based on clinical grade. | |
| RCT: randomised clinical trial. | |||
| Scale (Grippon 1988) used in Cadranel 1995 ; Barbaro 1998 . | |
| I | Euphoria or depression, mild confusion, slowness, disorder in sleep rhythm. |
| II | Drowsiness, inappropriate behaviour, accentuation of stage I. |
| III | Stupor; participant sleeps most of the time, but is rousable; incoherent speech; marked confusion. |
| IVa | Coma, co‐ordinated response to painful stimuli. |
| IVb | Coma, hyperextension, and pronosupination after painful stimuli. |
| IVc | Coma, no response to painful stimuli. |
| V | Clinical decerebration. |
| Scale (Fitz 1998) used in Dursun 2003 . | |
| Subclinical | Normal examination with subtle changes in psychometric or Number Connection Tests. |
| I | Impaired attention, irritability, depression, or personality changes. |
| II | Drowsiness, behavioural changes, sleep disorders, and poor memory. |
| III | Confusion, disorientation, somnolence, and amnesia. |
| Scale (Jones 1988) used in Gyr 1996 . | |
| ‐ | Clinical assessment criteria consisted of the anamnestic criterion: disorders of sleep pattern (insomnia, hypersomnia, inversion of sleep rhythm) in combination with assessment of the level of consciousness (1 to 4 as described below). Score items weighted so major disturbances of consciousness (portal systemic encephalopathy stage III and IV) were associated with scores of ≥ 11. Portal systemic encephalopathy stage II defined as scores of 5 to 10 and stage I of 3 to 4. |
| 1 | Light disturbance of consciousness if ≥ 1 of following symptoms were present: drowsiness (tendency to fall asleep but wake up spontaneously or in response to normal voice or light), intermittent or permanent disorientation, retardation of ability to perform mental tasks (serial subtractions of sevens), mood disorder, inappropriate behaviour. |
| 2 | Somnolence (arousable to physical stimuli such as mild prodding or shaking only). |
| 3 | Stupor (localised motor response to pain). |
| 4 | Coma (unarousability, no or unlocalised motor reactions to painful stimuli). |
| Scale (no reference provided in paper) used in Van der Rijt 1995 . | |
| 1 | Presence of ≥ 2 of following abnormalities: inverted sleep pattern, disturbed memory, impaired calculation (serial sevens), slowness of speech, or flapping tremor. |
| 2 | Presence of ≥ 2 of following: lethargy, time disorientation, or flapping tremor. |
| 3 | Presence of ≥ 2 of following: a state in which person had to be stimulated repetitively to open his/her eyes or execute commands, disorientation in terms of place and disorientation with respect to person. |
| 4 | Coma. |
| 1 | Trivial lack of awareness, euphoria or anxiety, shortened attention span, impaired performance of addition or subtraction. |
| 2 | Lethargy or apathy, minimal disorientation for time or place, subtle personality change, inappropriate behaviour. |
| 3 | Somnolence to semistupor, but responsive to verbal stimuli; confusion; gross disorientation. |
| 4 | Coma. |
| Glasgow Coma Scale (CGS) (Teasdale 1974) used in Hermant 1991 ; Lacetti 2000 ; Dursun 2003 ; Li 2009 . | |
| Scores | Eye opening (E):
Verbal response (V):
Motor response (M):
|
| Grading |
|
| Modified Glasgow Coma Scale (Pappas 1983) used in Pomier‐Layrargues 1994 ; Barbaro 1998 . | |
| Scores |
|
| Electroencephalography grading/Fischer classification (Nusinovici 1977 and Spehlman 1991) used in Hermant 1991 ; Pomier‐Layrargues 1994 ; Cadranel 1995 ; Barbaro 1998 . | |
| I | Irregular background activity (theta and alpha). |
| II | Continuous theta activity, bursts of delta waves. |
| III | Prevalent delta activity; polyphasic transients sharp and slow wave complexes. |
| IVa | Continuous delta activity; abundant sharp and slow wave complexes; electroencephalography reactivity present. |
| IVb | Slower activity (delta and some polyphasic transients); electroencephalography reactivity = 0. |
| IVc | Discontinuous activity with silent periods. |
| V | Flat. |
| Electroencephalography grading (Parsons‐Smith 1957) used in Dursun 2003 . | |
| A | Generalised suppression of alpha rhythm and its frequent replacement by faster potentials in all leads. The tracings in this grade are generally flat and featureless. |
| B | Alpha rhythm very unstable and disturbed by random waves at 5‐7 per second over both hemispheres. Rhythms most often seen over temporal lobes. In many cases with underlying fast activity. |
| C | Alpha rhythm still seen, but disturbed over both hemispheres by medium‐voltage 5‐6 per second waves. These occur in runs, are not paroxysmal, and do not usually block to eye opening although blocking may occur. Rhythms are particularly well seen over temporal and frontal lobes. |
| D | 5‐6 per second rhythms seen in grade C are now constant in all areas and replace all other cortical activity recorded on electroencephalogram. Appearance of this abnormality in a patient presenting with only slight neuropsychiatric symptoms is very striking. |
| E | 5 to 6 per second rhythms replaced by frontally preponderant bi‐lateral synchronous 2 per second rhythms, which spread backwards over hemispheres. At times, 6 per second rhythms might reappear, but special features of records are occurrence of these diencephalic discharges. |
| Electroencephalography grading (Kennedy 1973) used in Gyr 1996 . | |
| 0 | 8 to 12 per second basic rhythm, mean dominant frequency > 8 per second, % theta < 20. |
| 1 | Sudden shifts between normal alpha frequency (around 9 or 10 per second) and slow substitutes (6‐8 per second); mean dominant frequency > 7 per second, % theta > 35. |
| 2 | Diffuse slow activity posterior alpha rhythm seen occasionally, mean dominant frequency 5 to 7 per second, % theta > 60. |
| 3 | Dominant slow activity in all areas, mean dominant frequency 3 to 5 per second, % delta 70. |
| 4 | Bilaterally synchronous, 2‐3 per second waves, predominating over frontal lobes and spreading backwards to occipital lobes; occasional short‐lived appearance of faster rhythms (5 or 6 per second) or voltage depression, mean dominant frequency < 3 per second, % delta 70. |
| Electroencephalography grading (Markand 1984) used in Van der Rijt 1995 . | |
| 0 | Background activity consisting of alpha rhythm. |
| 1 | Alpha rhythm with some scattered theta waves. |
| 2 | Background activity of theta activity intermixed with some delta and alpha frequencies. |
| 3 | Background of delta polymorphic activity of high amplitude with spontaneous variability. |
| 4 | Delta activity of relatively small amplitude. |
| Trial | Participants (n) | Precipitating factors (n) |
| 527 | Gastrointestinal bleeding (352), surgery (95), sepsis (45), dehydration (6), unknown (29). | |
| 14 | Gastrointestinal bleeding (4), sepsis (7), alcoholic hepatitis (3), portal vein thrombosis (1), viral hepatitis (1), unknown (2). | |
| 54 | Gastrointestinal bleeding (31), sepsis (7), drugs (11), surgery (1). | |
| 21 | Gastrointestinal bleeding (7), sepsis (2), dehydration (1), surgery (2), none (9), portacaval shunting (4). | |
| 18 | Hepatitis (5), acute exacerbation in cirrhosis (2), partial hepatectomy (1). | |
| 25 | Gastrointestinal bleeding (13), protein overload (6), infection (2), wounds (1), unknown (3). | |
| n: number of participants. | ||
| Trial | Required period free of benzodiazepines before inclusion | Baseline screening for benzodiazepines | Screening method and detection level | Negative testing at baseline an inclusion criterion | Proportion testing positive for benzodiazepines at baseline |
| 2 weeks | Yes |
| No | 0% | |
| 4 days | Yes |
| No | 1.9% | |
| Not reported | Yes |
| No | 21.4% | |
| 3 days | No | Not reported | Not reported | Not reported | |
| 3 months | Yes |
| No | 0% | |
| 1 month | No | Not reported | Not reported | Not reported | |
| Yes, but length not specified | Yes |
Post‐hoc analysis
| No | 8.2% on screening tests Flumazenil 11%; placebo 5% 12/49 samples for more sensitive testing lost | |
| Not reported | Yes | Not reported | Yes | 0% | |
| Not reported | No | Not reported | Not reported | Not reported | |
| 2 weeks | Yes |
Post‐hoc analysis
| Yes | 0% | |
| Not reported | No | Not reported | N/A | N/A | |
| 3 days | Yes |
Post‐hoc analysis
| No | 19% | |
| Recent | Yes |
| Yes | 0% | |
| 7 days | No | Not reported | Not reported | Not reported |
| Trial | Number of participants | Included in analyses of serious adverse events | Data included in primary analysis | Serious adverse events |
| 13 | No | Cross‐over RCT. Data from the first treatment period not described. | Publication does not describe any deaths or other serious adverse events. | |
| 527 | Yes | Cross‐over RCT. Data from the first treatment period included. | Thirteen non‐responders in the flumazenil group and 17 non responders in the placebo group died 3 to 4 days (range 2‐6) after randomisation. The causes of dead were septic shock (20 participants); hypovolaemic shock (8 participants) and lactic acidosis (2 participants) but information was not provided on the number of deaths by cause in each group. | |
| 14 | Yes | Cross‐over RCT. Data from the first treatment period included. | One of 12 responders died from septic shock on day 4 and 2 of 6 non‐responders died from septic shock (day 2) and lactic acidosis (day 4) but information is not provided on the groups to which they a were allocated. | |
| 40 | Yes | Parallel‐arm RCT. We included all participants in the analyses. | Publication did not describe any deaths or other serious adverse events. | |
| 10 | No | Cross‐over RCT. Data from the first treatment period not described. | Publication did not describe any deaths or other serious adverse events. | |
| 10 | Yes | Cross‐over RCT. Data from the first treatment period included. | Publication did not describe any deaths or other serious adverse events. | |
| 49 | Yes | Parallel‐arm RCT. We included all participants in the analyses. | Four of 28 participants allocated to flumazenil and 5 of 21 allocated to placebo died within 4 weeks of the trial. One participant in the placebo group died with respiratory failure during the course of the study. The authors described participants as having severe liver disease suggesting that the cause of death in the remaining 8 participants may have been cirrhosis‐related although this is not specifically stated. The investigators classified the remaining adverse events viz flushing, nausea, vomiting, and irritability, which were experienced by 4 participants, as non‐serious. | |
| 12 | Yes | Parallel‐arm RCT. We included all participants in the analyses. | Publication did not describe any deaths or other serious adverse events. | |
| 2 | No | Cross‐over RCT. Data from the first treatment period were not described. | Publication does not describe any deaths or other serious adverse events. | |
| 54 | Yes | Parallel‐arm RCT. We include all participants in the analyses. | Six of 28 participants in the flumazenil group and 5 of 26 in the control group died. The causes of death were not provided. | |
| 72 | Yes | Parallel‐arm RCT. The included participants had hepatic encephalopathy associated with cirrhosis or acute liver failure. Data were not provide separately for the 2 groups. | Five of 39 participants in the flumazenil group and 4 of 33 participants in the control group died. The causes of death were not provided. | |
| 21 | Yes | Cross‐over RCT. Data from the first treatment period were included. | Publication did not describe any deaths or other serious adverse events. | |
| 18 | Yes | Cross‐over RCT. The included participants had hepatic encephalopathy associated with cirrhosis or acute liver failure. Data were not provide separately for the 2 groups.Data from the first treatment period were included. | Publication did not describe any deaths or other serious adverse events. Two participants with fulminant hepatic failure underwent orthotopic liver transplantation on day one of the study | |
| 25 | Yes | Parallel‐arm RCT. We included all participants in the analyses. | Three of 13 participants in the flumazenil group and 5 of 12 participants in the control group died. The causes of death were not provided | |
| RCT: randomised clinical trial. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 All‐cause mortality Show forest plot | 11 | 842 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.16] |
| 1.1 Overt hepatic encephalopathy | 10 | 822 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.16] |
| 1.2 Minimal hepatic encephalopathy | 2 | 20 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 All‐cause mortality and bias control Show forest plot | 12 | 844 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.16] |
| 2.1 Low risk of bias | 1 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.37, 1.53] |
| 2.2 High risk of bias | 11 | 317 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.43, 1.31] |
| 3 All‐cause mortality and trial design Show forest plot | 12 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Cross‐over | 6 | 592 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.35, 1.34] |
| 3.2 Parallel‐arm | 6 | 252 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.45, 1.44] |
| 4 All‐cause mortality and duration of follow‐up Show forest plot | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 ≤ 1 day | 5 | 176 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.38, 1.87] |
| 4.2 > 1 day | 6 | 666 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.42, 1.21] |
| 5 Hepatic encephalopathy Show forest plot | 9 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.71, 0.80] |
| 5.1 Overt hepatic encephalopathy | 9 | 814 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.67, 0.80] |
| 5.2 Minimal hepatic encephalopathy | 1 | 10 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.41, 1.39] |
| 6 Hepatic encephalopathy and bias control Show forest plot | 9 | 824 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.71, 0.80] |
| 6.1 Low risk of bias | 1 | 527 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.72, 0.84] |
| 6.2 High risk of bias | 8 | 297 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.61, 0.78] |
| 7 Hepatic encephalopathy and trial design Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 7.1 Cross‐over | 4 | 584 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.72, 0.83] |
| 7.2 Parallel‐arm | 5 | 240 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.59, 0.79] |
| 8 Hepatic encephalopathy and duration of follow‐up Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 ≤ 1 day | 4 | 164 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.60, 0.83] |
| 8.2 > 1 day | 5 | 660 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.62, 0.84] |
| 9 Hepatic encephalopathy and acute liver failure Show forest plot | 9 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 9.1 Cirrhosis | 7 | 734 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.71, 0.82] |
| 9.2 Acute liver failure or cirrhosis | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.47, 0.80] |
| 10 Number Connection Test Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 11 All‐cause mortality and acute liver failure Show forest plot | 11 | 842 | Risk Ratio (M‐H, Random, 95% CI) | 0.75 [0.48, 1.16] |
| 11.1 Participants with cirrhosis | 9 | 752 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.14] |
| 11.2 Participants with cirrhosis or acute liver failure | 2 | 90 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.31, 3.62] |

