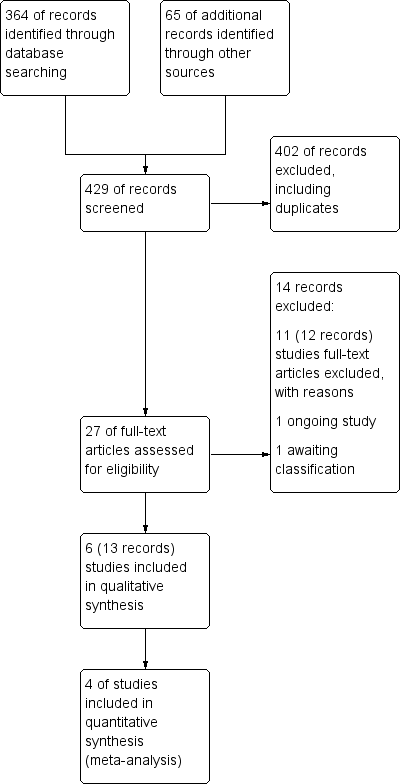
Antibióticos para las exacerbaciones del asma
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Design: randomised double‐blind placebo‐controlled trial Duration: trial endpoint of 5 days. Post‐treatment follow‐up carried out for 3 to 8 weeks Setting: trial participants initially treated in an emergency department setting. Trial carried out in the United States | |
| Participants | Population: 43 children with an acute exacerbation of asthma were randomised to receive clarithromycin (n = 22) or placebo (n = 21), in both cases in addition to normal care Age: participants ranged in age from 4 to 15 years. Age range in the clarithromycin group was 5 to 15 years, and age range in the placebo group was 4 to 15 years Inclusion criteria: presentation for evaluation within 72 hours of the start of an acute exacerbation of asthma Exclusion criteria: children with diagnosed bacterial infection needing antibiotics; children with contraindications to clarithromycin administration or with drug interactions with clarithromycin; renal impairment; pregnancy; treatment with antibiotics or systemic steroids within 2 weeks before presentation; chronic lung conditions (other than asthma) or chronic systemic illnesses. Participants were also excluded following randomisation if they did not attend follow‐up visits 1 and 2 in the specified periods Percentage withdrawn: withdrawal from the clarithromycin group was 36.4%; withdrawal from the placebo group was 33.3% Allowed medication: none recorded Disallowed medication: none recorded | |
| Interventions | Clarithromycin group: participants received 15 mg/kg, in 2 divided doses, to a maximum of 500 mg twice daily for 5 days Placebo group: participants received a placebo twice daily for 5 days. No further information given | |
| Outcomes | Primary endpoints were comparison of nasal cytokine and chemokine concentrations, and serum cytokines, between the 2 arms. The secondary endpoint was a comparison between the 2 groups on the presence or absence of Chlamydia pneumoniae and Mycoplasma pneumoniae infection at each of the 2 follow‐up visits | |
| Notes | Type of publication: peer‐reviewed Funding: study supported in part by grants from Abbott Laboratories Inc and Children's Medical Centre of Dallas Research Advisory Committee Contact: unsuccessful attempts made to contact study authors to seek further information about clinical outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study authors described the study as randomised but gave no further details |
| Allocation concealment (selection bias) | Unclear risk | "Abbott Laboratories Inc (Abbott Park, IL) provided formulations of clarithromycin and placebo to the Children's Medical Center at Dallas pharmacy for randomisation and distribution to patients" However, it is not clear if packs were identical, and if investigators would be able determine assignment |
| Blinding of participants and personnel (performance bias) | Low risk | Study authors stated that it was a "double‐blind placebo controlled" study |
| Blinding of outcome assessment (detection bias) | Unclear risk | No specific details were given regarding outcome assessor blinding |
| Incomplete outcome data (attrition bias) | High risk | More than 50% of participants did not complete follow‐up; therefore status is unknown |
| Selective reporting (reporting bias) | High risk | No prospective registration was identified, and not all evaluated outcomes were reported numerically, e.g. "No clinical differences were demonstrated for clarithromycin therapy vs placebo on visit 3" |
| Other bias | Low risk | No other source of bias was identified |
| Methods | Design: randomised double‐blind placebo‐controlled trial Duration: unclear Setting: trial participants recruited on admittance to hospital. Trial carried out in the United Kingdom | |
| Participants | Population: 60 adults with 71 exacerbations of asthma admitted to hospital between February 1979 and December 1980. Participants were randomised to receive amoxicillin (n = 37) or placebo (n = 34), in addition to normal care Age: 13 to 82 years old. Mean age in the amoxicillin group was 41.2 years, with a range from 13 to 82 years. Mean age in the placebo group was 37.4 years, with a range from 19 to 77 years Inclusion criteria: admission to hospital with an acute exacerbation of asthma, with FEV1 of 1.5 L or less, or PEFR of 150 L/min or less, or both, on admission Exclusion criteria: participants whose chest X‐rays showed signs of pneumonia; those who had a penicillin allergy Percentage withdrawn: 2 participants (5.9%) in the placebo arm withdrawn for 'slow clinical progress' Allowed medication: none recorded Disallowed medication: none recorded | |
| Interventions | Amoxicillin group: 500 mg amoxicillin given 3 times daily, in addition to usual care Placebo group: 'treated with identical placebos' | |
| Outcomes | Median length of hospital stay; physician assessment (scale of 4 to 12); participant assessment (VAS); percentage predicted PEFR; percentage predicted FEV1; percentage predicted FVC; days taken to reach 50% of final observed improvement (participant and physician scores) | |
| Notes | Type of publication: peer‐reviewed Funding: not reported Contact: no attempt made to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but no further details given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) | Low risk | Described as "double‐blind placebo‐controlled" |
| Blinding of outcome assessment (detection bias) | Unclear risk | No specific details given regarding outcome assessor blinding. For patient‐reported outcomes, the risk is likely low, as the blinded participant is the outcome assessor |
| Incomplete outcome data (attrition bias) | Low risk | 2 patients dropped out of the placebo group before discharge owing to slow clinical progress, but trialists report including them in a sensitivity analysis with the worst possible outcomes, and this had no impact on overall results |
| Selective reporting (reporting bias) | Unclear risk | No prospective registration identified, but all outcomes described in methods clearly reported. Study authors used medians and ranges and non‐parametric tests, so data could not be combined in meta‐analyses |
| Other bias | Unclear risk | Participants could be included more than once in the trial, as the episode, rather than the individual, was the unit of randomisation: 60 participants experienced 71 exacerbations during the trial |
| Methods | Design: randomised double‐blind placebo‐controlled trial Duration: trial endpoint 10 days. Post‐treatment follow‐up carried out for 6 weeks Setting: trial participants initially treated in an urgent care clinic, emergency room, or in‐patient hospital setting, and were then followed up after discharge at home. This was a multi‐centre, international study | |
| Participants | Population: 278 adults with acute exacerbations of asthma were randomised to receive telithromycin (n = 134) or placebo (n = 136). In both cases, treatment was given in addition to normal care Age: 17 to 68 years old. Mean age in the telithromycin group was 39.5 years, with a range from 17 to 64 years; mean age in the placebo group was 39.6 years, with a range from 17 to 68 years Inclusion criteria: adults between 18 and 55 years of age with a diagnosis of asthma for over 6 months, who sought medical help for an acute exacerbation of asthma, were enrolled within 24 hours after presentation. Inclusion criteria included increased wheeze and dyspnoea, with PEF < 80% of predicted value; ability to complete a diary of asthma symptoms and perform a home test of PEF; and ability to give written informed consent Exclusion criteria: need for immediate intensive care; known allergic cause of the acute episode; known lower respiratory tract disease, apart from asthma; smoking history of 10 or more pack‐years; need for use of regular OCS; use of any antibiotic within 30 days before enrolment; obvious infection requiring antibiotic treatment Percentage withdrawn: withdrawal from the telithromycin group was 5.97%, and withdrawal from the placebo group was 5.15% Allowed medication: participants were able to continue their usual treatment for asthma during the study. Participants who began taking an additional ICS within 3 days before or after the exacerbation received a dose increase at the investigator's discretion; those who required OCS for the exacerbation were prescribed prednisolone at 30 mg/d for 7 days Disallowed medication: none recorded | |
| Interventions | Telithromycin group: 800 mg telithromycin a day, given orally in the form of two 400‐mg capsules once daily for 10 days, in addition to usual care Placebo group: 2 placebo capsules, identical to telithromycin capsules, given once daily in addition to usual care | |
| Outcomes | Primary outcomes were change from baseline asthma symptom scores and PEFR in the morning over the 10‐day treatment period, using daily diaries of participants. Asthma symptoms were measured by a modified diary card symptom score in which participants rated their symptoms on a 7‐point scale (with 0 meaning no symptoms and 6 meaning severe symptoms). Clinic pulmonary function tests were secondary outcomes | |
| Notes | Type of publication: peer‐reviewed Funding: industry: Sanofi‐Aventis. "All authors had full access to the data, and no limits were placed by the study sponsor with respect to statements made in this report" Contact: trial lead author contacted for additional methodological details and outcome data; response received in September 2017 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | On a centrally randomised basis with use of computer‐generated codes, participants were assigned in a 1:1 ratio |
| Allocation concealment (selection bias) | Low risk | On a centrally randomised basis with use of computer‐generated codes, participants were assigned in a 1:1 ratio |
| Blinding of participants and personnel (performance bias) | Low risk | Participants were assigned in a 1:1 ratio to receive oral telithromycin (two 400 mg capsules daily) or placebo (2 capsules identical in appearance) for 10 days. Correspondence with lead author confirmed all participants, trial personnel, and outcome assessors were masked throughout |
| Blinding of outcome assessment (detection bias) | Low risk | No specific details were given in the trial report regarding outcome assessor blinding. Correspondence with lead author confirmed that all participants, trial personnel, and outcome assessors were masked throughout |
| Incomplete outcome data (attrition bias) | Unclear risk | Almost all participants were included in the safety analysis. Dropout overall was reasonably balanced, although more participants withdrew from the intervention arm owing to adverse events (8 vs 3), and more from the placebo arm owing to lack of efficacy (2 vs 0). More data for symptom score and PEFR were missing |
| Selective reporting (reporting bias) | Unclear risk | Several outcomes (health status at follow‐up (6 weeks); need for additional medications (e.g. ICS, OCS, bronchodilator use); time to next acute exacerbation of asthma) listed in the prospective trial registration were not fully reported. The lead author provided the following explanation: "the time‐to‐next‐acute‐exacerbation and need for additional medications data were not included because acquisition of such data in the setting of an acute exacerbation study, not unexpectedly, was so incomplete that a decision was taken not to analyse them" |
| Other bias | Low risk | No other source of bias was identified |
| Methods | Design: randomised double‐blind placebo‐controlled trial Duration: trial endpoint 10 days. Post‐treatment follow‐up carried out for 6 weeks Setting: multi‐centre study based in the United Kingdom. Participants recruited from 30 secondary care hospitals and 1 primary care centre | |
| Participants | Population: 199 adults with an acute exacerbation of asthma were randomised to receive azithromycin (n = 97) or an identical placebo (n = 102), both in addition to normal care. Participants were recruited at a wide range of hospitals across the United Kingdom Age: mean age of participants in the azithromycin arm of the study was 39.1 years; mean age of those in the placebo arm was 36.2 years Inclusion criteria: adults aged 18 to 55 with any smoking history, aged 56 to 65 with less than a 20 pack‐year smoking history, or older than 65 with a less than 5 pack‐year smoking history, with a documented history of having asthma for over 6 months and recruitment within 48 hours of presentation to medical care with an acute deterioration in asthma control requiring a course of oral or systemic corticosteroids or both, and PEF or FEV1 < 80% of predicted value Exclusion criteria: use of oral or systemic antibiotics within 28 days before enrolment; need for intensive care; significant lung disease other than asthma; long‐term use of over 20 mg of OCS daily; known QT‐interval prolongation; history of bradyarrhythmias or tachyarrhythmias or uncompensated heart failure; taking drugs known to prolong QT interval Percentage withdrawn: withdrawal from the azithromycin group was 10.3%, and withdrawal from the placebo group was 12.7% Allowed medication: none recorded Disallowed medication: none recorded other than those listed in the exclusion criteria | |
| Interventions | Azithromycin group: 2 x 250 mg azithromycin capsules taken once daily for 3 days, in addition to normal care Placebo group: placebo identical in appearance to azithromycin treatment given once daily, in addition to normal care | |
| Outcomes | Primary outcome was diary card summary symptom score. Secondary outcomes included quality of life, measured by the acute AQLQ and the mini AQLQ; pulmonary function tests including FEV1, FVC, FEV1/FVC, FEF, FEF50, PEF and time to 50% reduction in symptom score | |
| Notes | Type of publication: peer‐reviewed Funding: "This study was funded by the Efficacy and Mechanisms Evaluation programme of the MRC, in partnership with the NIHR (Funders Reference No. 10/60/27). The trial was supported by the NIHR Comprehensive Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London. Dr Johnston is an NIHR senior investigator and was supported by European Research Council FP7 Advanced Grant 233015, a Chair from Asthma UK (CH11SJ), and MRC Centre grant G1000758. The funders' had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication" Contact: trial lead author contacted for additional methodological details and outcome data; response received in September 2017 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was web based via access to a secure Imperial College London server and was performed using the InForm ITM System. Randomisation lists were generated by an ICTU statistician. Details such as block size were kept confidential and held separately by the ICTU |
| Allocation concealment (selection bias) | Low risk | The identity of study medications was blinded and medications were packaged and supplied by Sharp Clinical Services (Crickhowell, UK) with code‐break envelopes. Overencapsulated azithromycin capsules and placebo capsules were placed into child‐resistant tamper‐evident containers, and a randomised label applied to each container |
| Blinding of participants and personnel (performance bias) | Low risk | This was a double‐blind trial; therefore, all participants and care providers and those assessing outcomes were blinded to study treatment. Members of the trial team managing and analysing the data were also blind to the treatment received. Researchers imposed no requirement for unblinding during the AZALEA study; therefore no participants were unblinded before statistical analysis took place |
| Blinding of outcome assessment (detection bias) | Low risk | This was a double‐blind trial; therefore, all participants and care providers and those assessing outcomes were blinded to study treatment. Members of the trial team managing and analysing the data were also blind to the treatment received. Researchers imposed no requirement for unblinding during the AZALEA study; therefore no participants were unblinded before statistical analysis took place |
| Incomplete outcome data (attrition bias) | Unclear risk | All participants were included in the safety analysis, but only 80% of participants attended all 4 study visits and some data for symptom score and PEFR are missing |
| Selective reporting (reporting bias) | Low risk | Prospectively registered trial; outcomes reported as planned |
| Other bias | Low risk | No other source of bias identified |
| Methods | Design: randomised open‐label study Duration: trial endpoint 21 days. Post‐treatment follow‐up carried out for 12 weeks Setting: participants recruited from the population of patients of the Allergy Department of the 2nd Pediatric Clinic at the University of Athens, Greece. They were treated in the hospital, then discharged home or continued in hospital according to their clinical needs | |
| Participants | Population: 40 children with acute exacerbations of asthma were randomised to receive clarithromycin (n = 18), in addition to normal care, or to receive just normal care (n = 22) Age: children aged 6 to 14 participated in the study. Mean age of participants in the clarithromycin arm was 9.1 years, and mean age of those in the control arm was 8.4 years Inclusion criteria: children given a diagnosis of intermittent or mild persistent asthma, from the population followed up in the Allergy Department, 2nd Pediatric Clinic at the University of Athens, were invited to participate. If they experienced an acute asthma exacerbation, according to the judgement of their parents, with confirmation by the study physician, and wished to participate, they were included in the study Exclusion criteria: any additional chronic condition, apart from allergic rhinitis; children unable to follow study procedures Percentage withdrawn: percentage of participants withdrawn was 0% in both arms of the study Allowed medication: none recorded Disallowed medication: none recorded | |
| Interventions | Clarithromycin group: participants received 15 mg of clarithromycin per kg of body weight once daily for 3 weeks, plus normal care Control group: participants received just normal care | |
| Outcomes | Primary outcome was symptom‐free days during the 12‐week follow‐up period. Secondary outcomes were number and severity of periods with loss of asthma control, time to loss of control, duration and severity of the index exacerbation, PEFR variability, and lung function during the follow‐up period | |
| Notes | Type of publication: peer‐reviewed Funding: none recorded Contact: no attempt made to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerized randomisation table, blinded to patients and to the study physician, was used to allocate children" to study arms |
| Allocation concealment (selection bias) | Low risk | "A computerized randomisation table, blinded to patients and to the study physician, was used to allocate children" to study arms |
| Blinding of participants and personnel (performance bias) | High risk | This was an open‐label study |
| Blinding of outcome assessment (detection bias) | High risk | This was an open‐label study |
| Incomplete outcome data (attrition bias) | Low risk | "In fact, no patient/parent dropped out of the study after randomisation" |
| Selective reporting (reporting bias) | Unclear risk | No prospective registration was identified, but all outcomes described in the methods were clearly reported. Study authors used medians and interquartile ranges for non‐normal data, so these could not be combined in meta‐analyses |
| Other bias | Low risk | No other source of bias identified |
| Methods | Design: randomised double‐blind placebo‐controlled trial Duration: trial endpoint 7 days; post‐treatment follow‐up lasted between 1 and 3 weeks, varying between participants Setting: study carried out at the Children’s Orthopedic Hospital and Medical Center, in Seattle, Washington, USA, between September 1971 and July 1972 | |
| Participants | Population: 50 children with acute exacerbations of asthma were recruited. They were randomised to receive hetacillin (n = 20) or placebo (n = 24), both in addition to normal care Age: age range of study participants was from 1 to 18 years Inclusion criteria: children admitted to this study were hospitalised at Children’s Orthopedic Hospital and Medical Center between September 1971 and July 1972 for status asthmaticus. This was considered to be a lack of response of severe bronchospasm to 3 subcutaneous injections of 1:1000 aqueous epinephrine given at 15‐minute intervals Exclusion criteria: evidence of bacterial disease, specifically any of the following findings: otitis media, purulent pharyngitis, or fever; lobular pulmonary infiltrate on admission chest X‐ray; recent receipt of antibiotics Percentage withdrawn: 6 excluded ‐ 3 because they developed signs and symptoms suggesting bacterial disease, and 3 others because of failure to administer the study preparation (hetacillin or placebo). This gives an overall withdrawal percentage of 12% Allowed medication: all participants were treated via the same protocol, which included intravenous fluid, aminophylline, oral theophylline compounds, hydrocortisone, oral prednisone, nebulised isoproterenol, and phenylephrine and oxygen Disallowed medication: none recorded, aside from recent use of antibiotics as mentioned in the exclusion criteria | |
| Interventions | Hetacillin (ampicillin) group: 100 mg/kg/24 h IV followed after 24 hours by 225 mg oral 4 times daily for 6 days Placebo group: identically packaged to hetacillin and administered on the same schedule | |
| Outcomes | Hospital follow‐up evaluation: (a) vital signs (pulse, respirations, blood pressure) at least every hour for 12 hours, then as desired by house officer; (b) pulmonary index at 1, 12, 24 hours; (c) FVC and FEV1 at 1, 12, and 24 hours when possible; (d) chest X‐rays and blood gases repeated as needed. Follow‐up after discharge: visit to private physician or allergy clinic scheduled 1 to 3 weeks after discharge, so information on medications and complications, physical examination, pulmonary function tests, and convalescent serum could be obtained | |
| Notes | Type of publication: peer‐reviewed Funding: supported in part by Public Health Service training grant S‐TO1‐A10011 from the National Institute of Allergy and Infectious Disease, and in part by a grant from Bristol Laboratories Contact: no attempt made to contact study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study was described as 'pre‐randomised', but no further details were given |
| Allocation concealment (selection bias) | Unclear risk | Study was described as 'pre‐randomised', but no further details were given |
| Blinding of participants and personnel (performance bias) | Low risk | Study authors stated that it was 'double‐blind placebo‐controlled' |
| Blinding of outcome assessment (detection bias) | Unclear risk | No specific details were given regarding outcome assessor blinding |
| Incomplete outcome data (attrition bias) | High risk | Fifty asthma admissions were initially included in the study. Six were excluded ‐ 3 because of development of signs and symptoms suggesting bacterial disease, and 3 because of inadvertent failure to administer the study preparation (hetacillin or placebo). Distribution between study arms of those excluded because of suspected bacterial infection and because of protocol violations is not reported |
| Selective reporting (reporting bias) | High risk | No prospective registration was identified. Not all evaluated outcomes were reported numerically so they could not be included in meta‐analysis (e.g. graphically displayed only) |
| Other bias | Unclear risk | Baseline imbalance between arms was detected including difference in mean number of days of wheezing before admission (2.6 in hetacillin group; 5.8 in placebo group) |
AQLQ: asthma quality of life questionnaire; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; FEF: forced expiratory flow; FEF50: forced expiratory flow at 50% expiration; ICS: inhaled corticosteroids; MRC: Medical research Council; NIHR: National Instiutute for Health Research; OCS: oral corticosteroids; PEF: peak expiratory flow; PEFR: peak expiatory flow rate; QT: QT interval is a measure of the time between the start of the Q wave and the end of the T wave in the heart's electrical cycle; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Commentary on a study of long‐term prophylactic antibiotic use | |
| German commentary on a study of long‐term prophylactic antibiotic use | |
| Study of long‐term prophylactic antibiotic use in adult smokers with asthma | |
| Study of long‐term prophylactic antibiotic use | |
| Study of long‐term prophylactic antibiotic use | |
| Study of long‐term prophylactic antibiotic use | |
| Study of long‐term prophylactic antibiotic use | |
| Study of long‐term prophylactic antibiotic use | |
| Study involved young children (aged 1 to 3 years) with asthma‐like symptoms (i.e. without a diagnosis of asthma). Wheeze in this age group generally is not considered to be the same entity as asthma | |
| Study involved children with chronic rather than acute asthma | |
| Study of long‐term prophylactic antibiotic use |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomized controlled study within the ABC cohort (Asthma Begins in Childhood) |
| Participants | Participants in the ABC cohort study. Resident of Copenhagen, Sjælland, Møn, Lolland, or Falster. Both parents are Danish‐speaking. Parents agree to enrol the child. The child is at least 1 year old and has had 1 of the following asthma symptoms: 5 episodes within 6 mdr (1 episode: 3 consecutive days with lower airway symptoms) or 4 weeks daily lung symptoms or acute severe asthma |
| Interventions | Intervention: azithromycin (oral suspension, 40 mg/mL) Control: placebo oral suspension, same volume as active drug |
| Outcomes | Primary endpoint(s)
Secondary endpoint(s): no secondary endpoints |
| Notes | Study Title: antibiotics as a treatment of repeated asthmatic symptoms in children ‐ a randomised, controlled study within the ABC cohort (Asthma Begins in Childhood) Date of first registration: 04/10/2010; last refreshed: 20/09/2016, with status currently no longer recruiting Registered on EU clinical trial register No contact details available |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Azithromycin for children hospitalised with asthma |
| Methods | Randomised controlled trial; double‐blind parallel‐group |
| Participants | Children aged 4 to 12 years, with admission diagnosis of asthma at the Children's Hospital at Montefiore and history of persistent asthma (as defined by National Heart, Lung, and Blood Institute) |
| Interventions | Intervention: azithromycin suspension at 10 mg/kg/dose (max 500 mg), once daily for 3 days Control: placebo suspension, same volume as active drug once daily for 3 days |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | October 2013 |
| Contact information | Lindsey C Douglas, MD Division of Hospital Medicine, Assistant Professor, Albert Einstein College of Medicine, Montefiore Medical Center, New York, United States |
| Notes | Study currently recruiting participants. Information last verified May 2017 |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptom score Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.60, ‐0.08] | |
| Analysis 1.1  Comparison 1 Antibiotics versus placebo/usual care, Outcome 1 Symptom score. | ||||
| 1.1 Adults | 2 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.60, ‐0.08] | |
| 1.2 Children | 0 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 All adverse events Show forest plot | 3 | 506 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.43] |
| Analysis 1.2  Comparison 1 Antibiotics versus placebo/usual care, Outcome 2 All adverse events. | ||||
| 2.1 Adults | 2 | 462 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.45] |
| 2.2 Children | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.12, 5.18] |
| 3 Serious adverse events Show forest plot | 3 | 502 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| Analysis 1.3 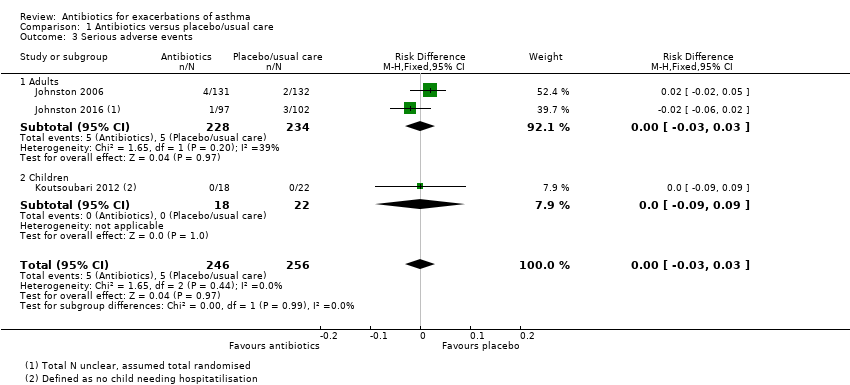 Comparison 1 Antibiotics versus placebo/usual care, Outcome 3 Serious adverse events. | ||||
| 3.1 Adults | 2 | 462 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| 3.2 Children | 1 | 40 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 4 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Antibiotics versus placebo/usual care, Outcome 4 Length of hospital stay (days). | ||||
| 4.1 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 PEF (GIV) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 23.42 [5.23, 41.60] | |
| Analysis 1.5  Comparison 1 Antibiotics versus placebo/usual care, Outcome 5 PEF (GIV). | ||||
| 5.1 Adults | 2 | Mean Difference (Fixed, 95% CI) | 23.42 [5.23, 41.60] | |
| 5.2 Children | 0 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 PEF Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.6  Comparison 1 Antibiotics versus placebo/usual care, Outcome 6 PEF. | ||||
| 6.1 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Forest plot of comparison: 1 Antibiotics versus placebo/usual care, outcome: 1.3 Serious adverse events.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotics versus placebo/usual care, Outcome 1 Symptom score.

Comparison 1 Antibiotics versus placebo/usual care, Outcome 2 All adverse events.

Comparison 1 Antibiotics versus placebo/usual care, Outcome 3 Serious adverse events.

Comparison 1 Antibiotics versus placebo/usual care, Outcome 4 Length of hospital stay (days).

Comparison 1 Antibiotics versus placebo/usual care, Outcome 5 PEF (GIV).

Comparison 1 Antibiotics versus placebo/usual care, Outcome 6 PEF.
| Antibiotics compared to placebo/usual care for acute asthma | ||||||
| Patient or population: acute asthma exacerbation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo/usual care | Risk with antibiotics | |||||
| ICU/HDU admission ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | One respiratory arrest in the placebo group in Shapiro 1974. No other studies reported this outcome |
| Symptom score at 10 days. Measured on a 7‐point scale (0 to 6) ; lower score denotes fewer symptoms | Mean symptom score at 10 days ranged from 2 to 2.20 points | MD 0.34 points lower (0.60 lower to 0.08 lower) | ‐ | (2 RCTs) | ⊕⊕⊕⊝a,d MODERATE | |
| All adverse events | 42 per 100 | 41 per 100 | OR 0.99 | 506 | ⊕⊕⊝⊝ | 2 studies in adults and 1 small old study in children with status asthmaticus |
| Serious adverse events Duration 3 days to 3 weeks | 2 per 100 | 2 per 100 | RD 0.00 | 502 | ⊕⊕⊝⊝ | Anticipated absolute effects were calculated using the figures in Figure 1. This is a re‐presentation of the results, but to 4 dp, which allows the calculation to be done |
| Mortality ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | No deaths were reported in any of the studies |
| Length of hospital stay, days | Mean length of hospital stay was 2.6 days | MD 0.1 days lower | ‐ | 43 | ⊕⊝⊝⊝ | 1 study reported medians and IQRs and found no significant differences, although data were skewed |
| Relapse after index presentation ‐ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| PEFR (GIV) Duration 10 days | Mean PEFR (GIV) ranged from 19.6 to 26.9 L/min (mean difference from baseline) | MD 23.42 L/min (mean difference from baseline) higher | ‐ | 469 | ⊕⊕⊕⊝ | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| a‐1 indirectness. Studies mostly recruited from hospital or emergency department. Therefore this review may represent more severe exacerbations and does not apply to people attending the GP and requesting antibiotics. The review does not apply to people who have already received a course of antibiotics. bNo downgrade for risk of bias. One small study excluded 6 participants post hoc, but excluding this study from the meta‐analysis did not affect the results. cNo downgrade. I2 = 0. Different antibiotics were given in each study. dNo downgrade. Only six RCTs have been published on antibiotics for asthma exacerbation. This strongly suggests that unpublished data exist or that clinical trials are seriously lacking for this common intervention. e‐1 imprecision. Confidence intervals include the possibility of important benefit and risk of harm. f‐1 indirectness. Studies mostly recruited from hospital or emergency department. Therefore this review may represent more severe exacerbations and does not apply to people attending the GP and requesting antibiotics. The review does not apply to people who have already received a course of antibiotics. One small study recruited children with status asthmaticus in 1974, when asthma management was different. g‐1 imprecision. Few events. h‐1 risk of bias. Study before good reporting standards introduced. Concerns over study, which excluded six participants, and it is not clear from which arm they were excluded. i‐1 indirectness. Participants were all children with status asthmaticus, and the study was conducted before current asthma management had been introduced (e.g. they all received IV adrenaline). j‐1 imprecision. One small study was included. | ||||||
| Study ID | Total n | Country | Age range (years) | Duration of follow‐up | Intervention comparison |
| 43 | USA | 4‐15 | 3‐8 weeks | Clarithromycin (15 mg/kg) vs placebo | |
| 71 | UK | 13‐82 | Unclear | Amoxicillin (300 mg 3 days) vs placebo | |
| 278 | International (multi‐centre) | 17‐68 | 6 weeks | Telithromycin (800 mg/d) vs placebo | |
| 199 | UK | Mean (SD) = 39.9 (14.82) | 6 weeks | Azithromycin (500 mg/d) vs placebo | |
| 40 | Greece | 6‐14 | 12 weeks | Clarithromycin (15 mg/kg/d for 3/52) vs placebo | |
| 50 | USA | 1‐18 | 7 days and 1 to 3 weeks | Hetacillin (ampicillin 100 mg/kg/24 h IV followed by 900 mg PO/d for 6/7) vs placebo |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptom score Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.60, ‐0.08] | |
| 1.1 Adults | 2 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.60, ‐0.08] | |
| 1.2 Children | 0 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 All adverse events Show forest plot | 3 | 506 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.69, 1.43] |
| 2.1 Adults | 2 | 462 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.69, 1.45] |
| 2.2 Children | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.12, 5.18] |
| 3 Serious adverse events Show forest plot | 3 | 502 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| 3.1 Adults | 2 | 462 | Risk Difference (M‐H, Fixed, 95% CI) | 0.00 [‐0.03, 0.03] |
| 3.2 Children | 1 | 40 | Risk Difference (M‐H, Fixed, 95% CI) | 0.0 [‐0.09, 0.09] |
| 4 Length of hospital stay (days) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 PEF (GIV) Show forest plot | 2 | Mean Difference (Fixed, 95% CI) | 23.42 [5.23, 41.60] | |
| 5.1 Adults | 2 | Mean Difference (Fixed, 95% CI) | 23.42 [5.23, 41.60] | |
| 5.2 Children | 0 | Mean Difference (Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 PEF Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Adults | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Children | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |