Drugs for nocturnal enuresis in children (other than desmopressin and tricyclics)
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT (double‐blind crossover trial) | |
| Participants | No. of children (boys): 20 (6) | |
| Interventions | A (20): diclofenac sodium 50 mg orally Duration of treatment: 30 days each arm | |
| Outcomes | Mean DRY nights (SEM): A: 21.7 (1.4), B: 3.65 (3.1) | |
| Notes | Diclofenac sodium is a prostaglandin synthesis inhibitor | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT (double‐blind crossover trial) | |
| Participants | No. of children (boys): 19 (7) | |
| Interventions | A (19): indomethacin suppository 50 mg | |
| Outcomes | Mean DRY nights during trial (SD): A: 19.5 (10.5), B: 3.6 (5.7) | |
| Notes | Groups comparable at baseline as crossover | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT | |
| Participants | No. of children (boys): 78 (48) | |
| Interventions | A (16): imipramine 1mg/kg/day | |
| Outcomes | No. improving >50%: A: 2/16, B: 4/20, C: 2/30, D: 6/12 Adverse events: 8 had mild gastrointestinal symptoms, but not clear which groups | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (divided by random selection) | |
| Participants | No. of children: 100 | |
| Interventions | A (34): meprobamate (3x/ day; dosage depends on age: 5‐9 years = 200 mg; 10‐14 = 400 mg) Duration of treatment: 3 months | |
| Outcomes | Mean wet nights during trial: A: 3.26, B: 3.21 | |
| Notes | No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (divided by random selection) | |
| Participants | No. of children: 150 | |
| Interventions | A (41): hydroxyzine chloride age 5‐9, 10 mg 2x/day; age 10‐14 10‐20 mg 3x/day Duration of treatment: 3 months (minimum 2) | |
| Outcomes | No. not achieving 14 dry nights during trial: A: 33/41, B: 34/44, C: 38/39 | |
| Notes | Hydroxyzine is a tranquillizer, methylphenidate is a stimulant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (double blind study) | |
| Participants | No. of children (boys): 50 (28) | |
| Interventions | A (25): atropine sulphate 0.15 mg + ephedrine sulphate 7.5 mg: age <10 years = 2 tablets, > 10 = 4 tablets Duration of treatment: 1 month | |
| Outcomes | No. not achieving 14 dry nights: A: 18/25, B: 18/25 | |
| Notes | Groups comparable on baseline wetting | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | No. of children 118 (33 properly included in trial) | |
| Interventions | A (16): Alarm (+ amphetamine for some children if not wakened by bell) | |
| Outcomes | No. not achieving 21 dry nights: A: 6/16, B: 14/17 | |
| Notes | Successful treatment requires the families to understand the commitment involved | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (double blind placebo controlled, stratified by sex) | |
| Participants | No. of children (boys): 241 (140) | |
| Interventions | A (121): chlordiazepoxide (5 mg) + amitriptyline (12.5 mg) Duration of treatment: 8 weeks | |
| Outcomes | No. not achieving 14 dry nights: A: 109/111, B: 104/104 | |
| Notes | Chlordiazepoxide used to suppress emotional stimuli | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (crossover trial) | |
| Participants | Number of children (boys): 69 (37) | |
| Interventions | A (45): Chlorprotixine (Truxal) 5mg Duration of treatment: 6 weeks each arm of trial | |
| Outcomes | Number of children achieving 100% dry nights: | |
| Notes | Danish language (translated) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT (double‐blind crossover trial) | |
| Participants | No. of children (boys): 55 (33) | |
| Interventions | A (28): triclofos 0.5 gm | |
| Outcomes | Mean wet nights in 4th week: A: 4.1, B: 4.5 | |
| Notes | Data from first arm of trial used | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT (crossover trial) | |
| Participants | Number of children (boys): 11 (8) | |
| Interventions | A (10): phenmetrazine (Preludin) 25mg (half tablet for young children; 2 tablets for adults) | |
| Outcomes | Mean (SD) number of wet nights per month: | |
| Notes | Very small sample | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | CCT (alternate allocation to groups) | |
| Participants | No. of children: 25 (boys A:6, B:5) | |
| Interventions | A (13): tranquillisers ‐ meprobamate (400mg daily and hydroxyzine 1mg kg daily) | |
| Outcomes | Mean (SD) frequency of wetting: A: 6.5 (1.19), B: 1.9 (2.11) | |
| Notes | No details of blinding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | RCT (double‐blind crossover trial) | |
| Participants | No. of children (boys): 23 (15) | |
| Interventions | A (23): Cetiprin 200 mg (emepronium bromide) Duration of treatment: 6 weeks each arm Follow up: none | |
| Outcomes | Results given relative to placebo arm: 2.7 more dry nights with Cetiprin than placebo; and 4.3 more dry nights with Cetiprin than during baseline observation (not statistically significant, data not provided). | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | CCT (alternate allocation) | |
| Participants | No. of children (boys): A+B 10 (8); C+D 8 | |
| Interventions | A (5): Alarm + methedrine 5mg | |
| Outcomes | No. not achieving 14 dry nights: A: 0/5, B: 0/5, C: 0/3, D: 4/5 | |
| Notes | Groups A+C and B+D combined for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | RCT | |
| Participants | No. of children (boys): 73 (42) | |
| Interventions | A (18): piracetam (20mg/kg at bedtime, maximum 800 mg) Duration of treatment: 8 weeks | |
| Outcomes | No. not achieving 14 dry nights during trial: A: 16/18, B: 8/15, C: 4/12, D: 9/14 | |
| Notes | Trialists concluded that piracetam was ineffective | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | No. of children: 50 | |
| Interventions | A (28): diazepam 15 mg per day increased to 25 mg if no response | |
| Outcomes | Mean wet nights per week in 4th week: A: 1.04 (SD 2.53), B: 5.91 (2.43) | |
| Notes | Results from first 4 weeks only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (double‐blind randomised crossover with 1 week washout) | |
| Participants | No. of children (boys): 18 (8) | |
| Interventions | A (18): imipramine hydrochloride (25 or 50mg) | |
| Outcomes | Mean number (SD) of wet nights over 28 days: A:9.2 (6.72), B:17.4 (5.8) | |
| Notes | Unclear if intention to treat | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | CCT (double‐blind to children and staff but alternate allocation to groups) | |
| Participants | No. of children: 65 reported | |
| Interventions | A (33): propantheline bromide 15 mg for 4 days, then 30 mg for 4 days, then 45 mg for 6 days | |
| Outcomes | Mean wet nights per week during trial: A: 4.7, B: 5.36 (difference stated to be significant, P<0.01) | |
| Notes | No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | RCT (crossover trial) | |
| Participants | Number of children (boys): 41 (25) | |
| Interventions | A (30): oxybutynin (2 x 5mg tablets at supper time) | |
| Outcomes | Mean difference in frequency of wet nights while taking oxybutynin rather than placebo was ‐1.87 (not significant) | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT (no details) | |
| Participants | Number of children: 58 | |
| Interventions | A (29): oxybutynine (5mg 3 times a day) | |
| Outcomes | Wet nights in 4th week: A: 1.1 (SD 1.7), B: 6.8 (0.5) | |
| Notes | Data estimated from graph | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | CCT (alternate allocation by last digit of hospital number) | |
| Participants | Number of children (boys): 15 (12) | |
| Interventions | A (9): propantheline 30 mg (1 tablet for 4 weeks, 2 tablets for 4 weeks then crossed over to B | |
| Outcomes | Mean wet nights during trial: A: 2.11, B: 1.75 | |
| Notes | No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | RCT (double‐blind) | |
| Participants | No. of children 88 (boys 62%) | |
| Interventions | A (41): furosemide (Lasix) 40mg | |
| Outcomes | At end of study mean percentage wet nights per week: A: 73%, B: 49%, C: 67% | |
| Notes | Danish paper | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | CCT (randomisation using case record numbers) | |
| Participants | No. of children: 62 (43 boys) plus 22 (15) on placebo | |
| Interventions | A (32): desmopressin 10.5 ‐ 24.5 mcg intranasally | |
| Outcomes | No. not achieving 14 dry nights: A: 11/32; B: 20/30; C: 21/22 | |
| Notes | Extra information supplied by authors (method of randomisation, results confirmed, no side effects) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
| Methods | RCT (randomised double‐blind crossover) | |
| Participants | No. of children: 69 | |
| Interventions | A (61): imipramine | |
| Outcomes | Mean wet nights per week in last 3 weeks of each drug: A: 2.4, B: 3.24, C: 4.36, D: 4.37 | |
| Notes | No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT (double‐blind crossover trial) | |
| Participants | No. of children: Trial 1: 53, Trial 2: 24 | |
| Interventions | Trial 1 Trial 2 | |
| Outcomes | Trial 1: 'an increase in dry nights with A only slightly greater than with Placebo B' | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT (method not specified) | |
| Participants | No. of children: 85 | |
| Interventions | A (31): desmopressin 20 mcg, intranasally | |
| Outcomes | Mean DRY nights/2 weeks (SE) on treatment: A: 31, 11.8 (0.5); B: 29, 8.9 (0.8); C: 25, 3.8 (0.8) | |
| Notes | Parallel groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (double‐blind crossover trial) | |
| Participants | No. of children (boys): 14 (6) | |
| Interventions | A (14): Indoramin 20 mg | |
| Outcomes | Mean DRY nights in 2 weeks: A: 3.61, B: 4.03, C: 3.3 | |
| Notes | Indoramin = alpha‐adrenoceptor blocker | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
| Methods | RCT (double‐blind, parallel groups, placebo controlled randomised trial) | |
| Participants | No. of children (boys): 87 (64) | |
| Interventions | A (44): atomoxetine, increasing dose over first 3 days until 1.5 mg/kg in 2 divided doses | |
| Outcomes | Dry nights per week (mean): A: 2.98, B: 1.61 (P= 0.010) | |
| Notes | Research funded by drug company | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (divided at random into 4 groups) | |
| Participants | No. of children (boys): 77 (48) | |
| Interventions | A (14): imipramine 0.9‐1.5 mg/kg/day | |
| Outcomes | Cure defined as >90% reduction in wet nights | |
| Notes | Groups comparable at baseline on age, sex and disease severity but groups of unequal sizes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | No. of children: 29 | |
| Interventions | A (9): oxybutynin 0.5 mg/kg | |
| Outcomes | Mean DRY nights in 2 weeks: A: 4.7 (SD 3.9), B: 6.2 (3.8), C: 4.3 (4) | |
| Notes | Groups comparable at baseline on age, urinalysis, dry nights, family history, socioeconomic conditions, but group A older | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT | |
| Participants | No. of children (boys): 300 (167) | |
| Interventions | A (78): propantheline (3 x 15mg) | |
| Outcomes | No. cured: A: 7/78, B: 10/72, C: 6/83 | |
| Notes | Groups comparable at baseline on age, sex | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (medications on double‐blind basis) Systematic baseline measure of wetting: Yes | |
| Participants | No. of children: 23 Age range 4 to 10 years | |
| Interventions | A (3): amphetamine sulphate (2.5mg) Duration of treatment: 5 weeks FU after 4 weeks | |
| Outcomes | Mean number of wet nights in final week of treatment: A+B:4.1, C:3.5, D:1.7 | |
| Notes | No SDs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
| Methods | RCT (groups randomly assigned) | |
| Participants | No. of children (boys): 41 (25) | |
| Interventions | A (14): imipramine 25mg | |
| Outcomes | Imipramine significantly better than amitriptyline, chlordiazepoxide clinidium and piracetam on therapeutic index score: A: 1.57, B: 1.25, C: 1.3, D: 0.33 | |
| Notes | No useable data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
cc = cubic centilitres; Excl = Exclusion criteria; FU = follow up; Incl = Inclusion criteria; m = month(s); NSAID = non‐steroidal anti‐inflammatory drug; OP = hospital outpatient department; RCT = Randomised controlled trial; SD = Standard Deviation; SEM = standard error of the mean; w = week(s); y = year(s);
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| RCT: No | |
| RCT: Yes but excluded as all children had detrusor instability | |
| RCT: No | |
| RCT: Yes but excluded as only 27/71 children had nocturnal enuresis | |
| RCT: No | |
| RCT: Yes but participants all had daytime wetting as well as bedwetting, and some adults were included | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: Yes but children had diurnal enuresis | |
| RCT: Yes but children had vesicoureteric reflux and detrusor instability | |
| RCT: No | |
| RCT: Yes but children had detrusor instability and urge incontinence | |
| RCT: Yes (double blind placebo controlled crossover trial) but excluded as some children allocated to more than one trial, and doses sometimes doubled, therefore no useable data | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: Yes but excluded because children were moved between trial arms, data therefore unreliable | |
| RCT: No | |
| RCT: Yes, but in enuretic children with urodynamically proven urge incontinence | |
| RCT: Yes (double blind crossover) but in children with neurogenic bladders | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT : No. 2 studies, using crossover design but not randomised. | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: Yes (double‐blind crossover study) but in enuretic children with 'uninhibited vesical activity' (assumed to be detrusor overactivity) | |
| RCT: Yes (double blind crossover trial) but many of the children had unstable bladder or diurnal enuresis or both | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: No | |
| RCT: Yes |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1 Number of wet nights per week Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.1 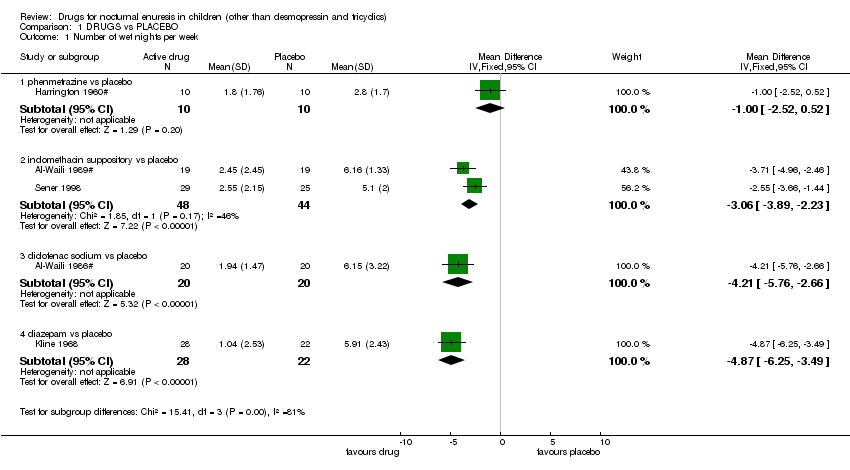 Comparison 1 DRUGS vs PLACEBO, Outcome 1 Number of wet nights per week. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.1 phenmetrazine vs placebo | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐2.52, 0.52] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.2 indomethacin suppository vs placebo | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐3.06 [‐3.89, ‐2.23] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.3 diclofenac sodium vs placebo | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.21 [‐5.76, ‐2.66] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1.4 diazepam vs placebo | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐4.87 [‐6.25, ‐3.49] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 Number of wet nights per week (no SDs) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.2
Comparison 1 DRUGS vs PLACEBO, Outcome 2 Number of wet nights per week (no SDs). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.1 amphetamine sulphate/ephedrine + atropine vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.2 furosemide vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.3 meprobamate vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.4 propantheline bromide vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.5 emepronium vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.6 indoramin 20 mg vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.7 indoramin 10 mg vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2.8 propantheline vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3 Number not achieving 14 consecutive dry nights Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.3  Comparison 1 DRUGS vs PLACEBO, Outcome 3 Number not achieving 14 consecutive dry nights. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.1 chlorprotixine vs placebo | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.2 indomethacin suppository vs placebo | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.79] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.3 diclofenac vs placebo | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.70] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.4 meprobamate vs placebo | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.35] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.5 hydroxyzine chloride vs placebo | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.6 methylphenidate hydrochloride vs placebo | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.94] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.7 atropine sulphate + ephedrine sulphate vs placebo | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.41] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.8 chlordiazepoxide + amitriptyline vs placebo | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.95, 1.01] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.9 piracetam vs placebo | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.91, 2.11] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.10 propantheline vs placebo | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.08] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.11 oxybutinin vs placebo | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.24] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.12 diazepam vs placebo | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.11, 0.46] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.13 propantheline vs placebo | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.15, 11.64] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3.14 atomoxetine vs placebo | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.95] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4 Number failing or relapsing after end of treatment Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.4  Comparison 1 DRUGS vs PLACEBO, Outcome 4 Number failing or relapsing after end of treatment. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.1 atropine sulphate + ephedrine sulphate vs placebo | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.80, 1.06] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.2 chlordiazepoxide + amitriptyline vs placebo | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.03] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4.3 oxybutinin vs placebo | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.62] | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5 Number of wet nights per week after treatment stopped (no SDs) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Analysis 1.5
Comparison 1 DRUGS vs PLACEBO, Outcome 5 Number of wet nights per week after treatment stopped (no SDs). | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5.1 propantheline bromide vs placebo | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||||||||||||||||||||||||||
| 1 Number of wet nights per week Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||
| Analysis 2.1  Comparison 2 DRUG ‐ DRUG COMPARISONS, Outcome 1 Number of wet nights per week. | ||||||||||||||||||||||||||||||||||||||||
| 1.1 meprobamate + hydroxyzine vs imipramine | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 4.6 [3.24, 5.96] | ||||||||||||||||||||||||||||||||||||
| 1.2 ephedrine sulphate vs imipramine | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 2.05 [1.02, 3.08] | ||||||||||||||||||||||||||||||||||||
| 1.3 indomethacin suppository vs desmopressin (nasal drops) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.45 [0.53, 2.37] | ||||||||||||||||||||||||||||||||||||
| 1.4 oxybutinin vs pseudoephedrine | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐0.95, 2.45] | ||||||||||||||||||||||||||||||||||||
| 1.5 oxybutinin vs indomethacin | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.02, 1.62] | ||||||||||||||||||||||||||||||||||||
| 1.6 oxybutinin vs dicyclomine | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐6.34, ‐5.06] | ||||||||||||||||||||||||||||||||||||
| 1.7 pseudoephedrine vs indomethacin | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐2.67, 0.77] | ||||||||||||||||||||||||||||||||||||
| 2 Number of wet nights per week (no SDs) Show forest plot | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||
| Analysis 2.2
Comparison 2 DRUG ‐ DRUG COMPARISONS, Outcome 2 Number of wet nights per week (no SDs). | ||||||||||||||||||||||||||||||||||||||||
| 2.1 furosemide vs imipramine | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||
| 2.2 triclofos vs ephedrine | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||
| 2.3 emepronium vs imipramine | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||
| 2.4 emepronium vs imipramine‐N‐oxide | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||
| 2.5 indoramin 20 mg vs indoramin 10 mg | Other data | No numeric data | ||||||||||||||||||||||||||||||||||||||
| 3 Number not achieving 14 consecutive dry nights Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||
| Analysis 2.3  Comparison 2 DRUG ‐ DRUG COMPARISONS, Outcome 3 Number not achieving 14 consecutive dry nights. | ||||||||||||||||||||||||||||||||||||||||
| 3.1 meprobamate + hydroxyzine vs imipramine | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.04, 2.69] | ||||||||||||||||||||||||||||||||||||
| 3.2 hydroxyzine chloride vs methylphenidate hydrochloride | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.30] | ||||||||||||||||||||||||||||||||||||
| 3.3 triclofos vs ephedrine | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.07] | ||||||||||||||||||||||||||||||||||||
| 3.4 diclofenac vs desmopressin (nose drops) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.13, 3.33] | ||||||||||||||||||||||||||||||||||||
| 3.5 oxybutinin vs imipramine | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.65, 2.39] | ||||||||||||||||||||||||||||||||||||
| 3.6 oxybutinin vs imipramine + oxybutinin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.95, 3.71] | ||||||||||||||||||||||||||||||||||||
| 3.7 propantheline vs propantheline + phenobarbitone | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] | ||||||||||||||||||||||||||||||||||||
| 4 Number failing or relapsing after treatment stopped Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||||||||||||||||||||||||||
| Analysis 2.4  Comparison 2 DRUG ‐ DRUG COMPARISONS, Outcome 4 Number failing or relapsing after treatment stopped. | ||||||||||||||||||||||||||||||||||||||||
| 4.1 meprobamate + hydroxyzine vs imipramine | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.90, 1.59] | ||||||||||||||||||||||||||||||||||||
| 4.2 oxybutinin vs imipramine | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.76, 1.50] | ||||||||||||||||||||||||||||||||||||
| 4.3 oxybutinin vs imipramine + oxybutinin | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.17, 3.27] | ||||||||||||||||||||||||||||||||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size | ||||||||||||
| 1 Number of wet nights per week | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |||||||||||||
| 2 Number of wet nights per week (no SDs) Show forest plot | Other data | No numeric data | ||||||||||||||
| Analysis 3.2
Comparison 3 DRUG vs BEHAVIOURAL INTERVENTIONS, Outcome 2 Number of wet nights per week (no SDs). | ||||||||||||||||
| 2.1 amphetamine sulphate/ephedrine + atropine vs alarm | Other data | No numeric data | ||||||||||||||
| 3 Number not achieving 14 consecutive dry nights Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |||||||||||||
| Analysis 3.3  Comparison 3 DRUG vs BEHAVIOURAL INTERVENTIONS, Outcome 3 Number not achieving 14 consecutive dry nights. | ||||||||||||||||
| 3.1 amphetamine vs alarm | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.12, 4.29] | ||||||||||||
| 3.2 methedrine + alarm vs alarm | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.20] | ||||||||||||
| 3.3 piracetam vs play + supportive therapy | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.01, 2.75] | ||||||||||||
| 3.4 piracetam vs piracetam + play + supportive therapy | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.18, 6.03] | ||||||||||||

Comparison 1 DRUGS vs PLACEBO, Outcome 1 Number of wet nights per week.
| Study | Drug | Placebo |
| amphetamine sulphate/ephedrine + atropine vs placebo | ||
| Wright 1974 | Mean = 4.1, N=8 | Mean = 3.5, N=10 |
| furosemide vs placebo | ||
| Moltke 1979 | Mean = 5.11, N=41 | Mean = 4.69, N=44 |
| meprobamate vs placebo | ||
| Breger 1961 | Mean = 3.26, N=34 | Mean = 3.21, N=33 |
| propantheline bromide vs placebo | ||
| Leys 1956 | Mean = 4.7, N=33 | mean = 5.36, N=32 |
| emepronium vs placebo | ||
| Petersen 1974# | Mean = 4.36, N=61 | Mean = 4.37, N=61 |
| indoramin 20 mg vs placebo | ||
| Shaffer 1978# | Mean = 5.2, N=14 | Mean = 5.35, N=14 |
| indoramin 10 mg vs placebo | ||
| Shaffer 1978# | Mean = 4.99, N=14 | Mean = 5.35, N=14 |
| propantheline vs placebo | ||
| Mayon‐White 1956 | Mean = 2.11, n=9 | Mean = 1.75, n=6 |
Comparison 1 DRUGS vs PLACEBO, Outcome 2 Number of wet nights per week (no SDs).

Comparison 1 DRUGS vs PLACEBO, Outcome 3 Number not achieving 14 consecutive dry nights.

Comparison 1 DRUGS vs PLACEBO, Outcome 4 Number failing or relapsing after end of treatment.
| Study | Drug | Placebo |
| propantheline bromide vs placebo | ||
| Leys 1956 | Mean = 4.96, N=33 | Mean = 5.33, N=32 |
Comparison 1 DRUGS vs PLACEBO, Outcome 5 Number of wet nights per week after treatment stopped (no SDs).

Comparison 2 DRUG ‐ DRUG COMPARISONS, Outcome 1 Number of wet nights per week.
| Study | Drug 1 | Drug 2 |
| furosemide vs imipramine | ||
| Moltke 1979 | Mean = 5.11, N=41 | Mean = 3.43, N=43 |
| triclofos vs ephedrine | ||
| GP Research Gp 1970# | Mean = 4.1, N=28 | Mean = 4.5, N=27 |
| emepronium vs imipramine | ||
| Petersen 1974# | Mean = 4.36, N=61 | Mean = 2.4, N=61 |
| emepronium vs imipramine‐N‐oxide | ||
| Petersen 1974# | Mean = 4.36, N=61 | Mean = 3.24, N=61 |
| indoramin 20 mg vs indoramin 10 mg | ||
| Shaffer 1978# | Mean = 5.2, N=14 | Mean = 4.99, N=14 |
Comparison 2 DRUG ‐ DRUG COMPARISONS, Outcome 2 Number of wet nights per week (no SDs).

Comparison 2 DRUG ‐ DRUG COMPARISONS, Outcome 3 Number not achieving 14 consecutive dry nights.

Comparison 2 DRUG ‐ DRUG COMPARISONS, Outcome 4 Number failing or relapsing after treatment stopped.
| Study | Drug | Behavioural method |
| amphetamine sulphate/ephedrine + atropine vs alarm | ||
| Wright 1974 | Mean = 4.1, N=8 | Mean = 1.7, N=10 |
Comparison 3 DRUG vs BEHAVIOURAL INTERVENTIONS, Outcome 2 Number of wet nights per week (no SDs).

Comparison 3 DRUG vs BEHAVIOURAL INTERVENTIONS, Outcome 3 Number not achieving 14 consecutive dry nights.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of wet nights per week Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 phenmetrazine vs placebo | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐2.52, 0.52] |
| 1.2 indomethacin suppository vs placebo | 2 | 92 | Mean Difference (IV, Fixed, 95% CI) | ‐3.06 [‐3.89, ‐2.23] |
| 1.3 diclofenac sodium vs placebo | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐4.21 [‐5.76, ‐2.66] |
| 1.4 diazepam vs placebo | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐4.87 [‐6.25, ‐3.49] |
| 2 Number of wet nights per week (no SDs) Show forest plot | Other data | No numeric data | ||
| 2.1 amphetamine sulphate/ephedrine + atropine vs placebo | Other data | No numeric data | ||
| 2.2 furosemide vs placebo | Other data | No numeric data | ||
| 2.3 meprobamate vs placebo | Other data | No numeric data | ||
| 2.4 propantheline bromide vs placebo | Other data | No numeric data | ||
| 2.5 emepronium vs placebo | Other data | No numeric data | ||
| 2.6 indoramin 20 mg vs placebo | Other data | No numeric data | ||
| 2.7 indoramin 10 mg vs placebo | Other data | No numeric data | ||
| 2.8 propantheline vs placebo | Other data | No numeric data | ||
| 3 Number not achieving 14 consecutive dry nights Show forest plot | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 chlorprotixine vs placebo | 1 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.85, 1.07] |
| 3.2 indomethacin suppository vs placebo | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.16, 0.79] |
| 3.3 diclofenac vs placebo | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.38, 0.70] |
| 3.4 meprobamate vs placebo | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.92, 1.35] |
| 3.5 hydroxyzine chloride vs placebo | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.70, 0.97] |
| 3.6 methylphenidate hydrochloride vs placebo | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.67, 0.94] |
| 3.7 atropine sulphate + ephedrine sulphate vs placebo | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.71, 1.41] |
| 3.8 chlordiazepoxide + amitriptyline vs placebo | 1 | 215 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.95, 1.01] |
| 3.9 piracetam vs placebo | 1 | 32 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.91, 2.11] |
| 3.10 propantheline vs placebo | 2 | 226 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.89, 1.08] |
| 3.11 oxybutinin vs placebo | 1 | 39 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.24] |
| 3.12 diazepam vs placebo | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.11, 0.46] |
| 3.13 propantheline vs placebo | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.15, 11.64] |
| 3.14 atomoxetine vs placebo | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.70, 0.95] |
| 4 Number failing or relapsing after end of treatment Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 atropine sulphate + ephedrine sulphate vs placebo | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.80, 1.06] |
| 4.2 chlordiazepoxide + amitriptyline vs placebo | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.03] |
| 4.3 oxybutinin vs placebo | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.79, 1.62] |
| 5 Number of wet nights per week after treatment stopped (no SDs) Show forest plot | Other data | No numeric data | ||
| 5.1 propantheline bromide vs placebo | Other data | No numeric data | ||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of wet nights per week Show forest plot | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 meprobamate + hydroxyzine vs imipramine | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | 4.6 [3.24, 5.96] |
| 1.2 ephedrine sulphate vs imipramine | 1 | 36 | Mean Difference (IV, Fixed, 95% CI) | 2.05 [1.02, 3.08] |
| 1.3 indomethacin suppository vs desmopressin (nasal drops) | 1 | 60 | Mean Difference (IV, Fixed, 95% CI) | 1.45 [0.53, 2.37] |
| 1.4 oxybutinin vs pseudoephedrine | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.75 [‐0.95, 2.45] |
| 1.5 oxybutinin vs indomethacin | 1 | 18 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐2.02, 1.62] |
| 1.6 oxybutinin vs dicyclomine | 1 | 58 | Mean Difference (IV, Fixed, 95% CI) | ‐5.70 [‐6.34, ‐5.06] |
| 1.7 pseudoephedrine vs indomethacin | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐2.67, 0.77] |
| 2 Number of wet nights per week (no SDs) Show forest plot | Other data | No numeric data | ||
| 2.1 furosemide vs imipramine | Other data | No numeric data | ||
| 2.2 triclofos vs ephedrine | Other data | No numeric data | ||
| 2.3 emepronium vs imipramine | Other data | No numeric data | ||
| 2.4 emepronium vs imipramine‐N‐oxide | Other data | No numeric data | ||
| 2.5 indoramin 20 mg vs indoramin 10 mg | Other data | No numeric data | ||
| 3 Number not achieving 14 consecutive dry nights Show forest plot | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 meprobamate + hydroxyzine vs imipramine | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.04, 2.69] |
| 3.2 hydroxyzine chloride vs methylphenidate hydrochloride | 1 | 85 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.84, 1.30] |
| 3.3 triclofos vs ephedrine | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.80, 1.07] |
| 3.4 diclofenac vs desmopressin (nose drops) | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.13, 3.33] |
| 3.5 oxybutinin vs imipramine | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.65, 2.39] |
| 3.6 oxybutinin vs imipramine + oxybutinin | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.95, 3.71] |
| 3.7 propantheline vs propantheline + phenobarbitone | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.96, 1.24] |
| 4 Number failing or relapsing after treatment stopped Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 meprobamate + hydroxyzine vs imipramine | 1 | 25 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.90, 1.59] |
| 4.2 oxybutinin vs imipramine | 1 | 21 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.76, 1.50] |
| 4.3 oxybutinin vs imipramine + oxybutinin | 1 | 31 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.17, 3.27] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of wet nights per week | 0 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Number of wet nights per week (no SDs) Show forest plot | Other data | No numeric data | ||
| 2.1 amphetamine sulphate/ephedrine + atropine vs alarm | Other data | No numeric data | ||
| 3 Number not achieving 14 consecutive dry nights Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 amphetamine vs alarm | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [1.12, 4.29] |
| 3.2 methedrine + alarm vs alarm | 1 | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.14 [0.01, 2.20] |
| 3.3 piracetam vs play + supportive therapy | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [1.01, 2.75] |
| 3.4 piracetam vs piracetam + play + supportive therapy | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.67 [1.18, 6.03] |

