Tratamiento de antibióticos antipseudomonas intravenosos simples versus combinados para personas con fibrosis quística
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | No withdrawals, symptomatic regimen. | |
| Participants | 28 participants randomised. PsA colonised, age not stated. | |
| Interventions | Carbenicillin 675 mg/kg/day vs sisomycin 10.5 mg/kg/day vs carbenicillin 590 mg/kg/day plus sisomycin. Variable duration of course. | |
| Outcomes | CXR and symptom scores, bacteriology, development of resistant strains. | |
| Notes | Abstract: no data. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, but no further details given. |
| Allocation concealment (selection bias) | Unclear risk | Not directly discussed, but referred to as a controlled clinical trial. |
| Blinding (performance bias and detection bias) | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not discussed, but appears to be no withdrawals. |
| Methods | Double‐blind trial, no withdrawals, symptomatic regimen. Parallel trial. | |
| Participants | 16 participants randomised. Mixed PsA and non‐PsA, age not stated. | |
| Interventions | Ticarcillin 300 mg/kg/day vs ticarcillin plus tobramycin 6 mg/kg/day. 10‐day course. | |
| Outcomes | Lung function, number readmitted within one month, CXR and symptom scores, bacteriology. | |
| Notes | Abstract: Lung function, CXR and symptom score data not given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentions randomisation code, but no details given of how it was generated. |
| Allocation concealment (selection bias) | Unclear risk | Mentions randomisation code, but no details of how this may have been concealed. |
| Blinding (performance bias and detection bias) | Low risk | Stated as double‐blind, but no further details given. |
| Incomplete outcome data (attrition bias) | Unclear risk | Not discussed, but appears to be no drop outs or withdrawals. |
| Methods | Double‐blind trial, symptomatic regimen. Parallel trial. | |
| Participants | 83 participants randomised. PsA colonised, age not stated. 51 participants randomized, of these, 21 in the tobramycin and ceftazidime group (51 admissions assessed) and 23 in the tobramycin group (47 admissions assessed). 12 participants in the tobramycin and ceftazidime group and 9 participants in the tobramycin group were eligible for long‐term assessment. Participants in both groups experienced an average of 3.1 and 3.0 admissions, respectively, for IV antibiotic treatment during the study period. Tobramycin and ceftazidime group: mean (SD) age 16 (7) years | |
| Interventions | Tobramycin 24‐hourly vs tobramycin and ceftazidime 8‐hourly. 10‐day course. | |
| Outcomes | Lung function, adverse events. | |
| Notes | Full paper. Exclusion criteria stated. The study was halted for a period of 3 months when | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was stratified for age and disease |
| Allocation concealment (selection bias) | Low risk | The treatment code was broken only at the |
| Blinding (performance bias and detection bias) | Low risk | Medical staff, nursing staff and participants were blinded to the treatment. |
| Incomplete outcome data (attrition bias) | Low risk | Flow chart showing numbers randomized and included/excluded (with reasons) at each stage in paper. |
| Methods | Not double blind, no withdrawals, symptomatic regimen. | |
| Participants | 17 participants treated (8 in piperacillin group, 9 in piperacillin plus tobramycin group); 3 participants treated on more than one occasion (2 initially in piperacillin group and several months later randomised to other group; 1 participant enrolled 2x in piperacillin group). 20 data sets. Mixed PsA and non‐PsA, aged 2 to 12 years. | |
| Interventions | Piperacillin 600 mg/kg/day vs piperacillin plus tobramycin 8 to 10 mg/kg/day, minimum duration 10 days. | |
| Outcomes | Lung function, weight, symptom scores, adverse events, bacteriology. | |
| Notes | No data for lung function, weight and symptom scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Treatment randomly assigned, no further details. |
| Allocation concealment (selection bias) | Low risk | Used sequentially numbered envelopes. |
| Blinding (performance bias and detection bias) | High risk | Not double‐blind. |
| Incomplete outcome data (attrition bias) | Low risk | Clear explanation of participants in groups, no drop outs occurred. |
| Methods | Double‐blind trial, symptomatic regimen. | |
| Participants | 41 participants randomised (11 years and older), 34 completed. No ITT analysis. Mean age 21 years. PsA in 98%. | |
| Interventions | Azlocillin 300 mg/kg/day, 4‐hourly plus placebo vs azlocillin plus tobramycin 6 mg/kg/day, 8‐hourly. 10‐day course. Group 1: azlocillin 300 mg/kg/day in 6 divided doses plus tobramycin (6 mg/kg per day) in 3 divided doses. | |
| Outcomes | Lung function, symptom scores, development of resistant strains, time to next course. | |
| Notes | Participants were white, of various socioeconomic backgrounds and lived in New England. Exclusion criteria stated. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly selected, but no further details given. |
| Allocation concealment (selection bias) | Low risk | Hospital pharmacist used consecutively numbered sealed envelopes. |
| Blinding (performance bias and detection bias) | Low risk | Neither participants or clinicians knew which regimen they were receiving. |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 participants in azlocillin plus placebo group withdrawn due to suspected drug‐related complications; 2 participants discharged improved before completion of antibiotic course; 3 withdrawn due to incomplete outcome data. |
| Methods | Not double blind, alternate allocation, no withdrawals, symptomatic regimen. Parallel trial. | |
| Participants | 21 male, 21 female, mean age 15.1 years. PsA colonised. 14 participants in each of 3 treatment groups. | |
| Interventions | Ticarcillin 300 mg/kg/day, 4‐hourly vs gentamicin 3 ‐ 4 mg/kg/day (adults), 4 ‐ 7 mg/kg/day (children) vs combination. Variable length of course. | |
| Outcomes | Lung function, bacteriology, adverse events, CBC, sedimentation rate, urinalysis, serum electrolytes, blood urea nitrogen, creatinine, liver function tests, chest radiographs, blood gas determinations, sputum cultures, change in cough, weight. | |
| Notes | No data available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No discussion of how first participant was assigned to which treatment group. |
| Allocation concealment (selection bias) | High risk | Alternation. |
| Blinding (performance bias and detection bias) | High risk | Drugs administered in different ways so clinicians and participants couldn't be blinded, no discussion of blinding of outcome assessors. |
| Incomplete outcome data (attrition bias) | Unclear risk | No drop outs or withdrawals. |
| Methods | Not double blind, elective regimen, | |
| Participants | 20 participants (10 male, 10 female), mean age 12.6 years. PsA colonised. 3 drop outs, 17 completed trial. | |
| Interventions | Ceftazidime 150 mg/kg/day, 8‐hourly vs ceftazidime plus tobramycin 10 mg/kg/day, 8‐hourly, 14‐day course. | |
| Outcomes | Lung function, inflammatory markers, development of resistant strains. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no details of method given. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) | Low risk | Both interventions given with same volume and in same way. |
| Incomplete outcome data (attrition bias) | Low risk | 3 participants excluded ‐ reasons given (bacteriological resistance developed between treatment arms in 2 participants and a 3rd withdrew on first day of 2nd treatment arm due to nausea). |
| Methods | Double‐blind, computer‐generated randomisation, symptomatic regimen. Parallel trial. | |
| Participants | 37 male, 39 female, mean age 16.3 years. Group 1 (azlocillin): 33 participants (19 male) mean (SD) age 16.07 (7.4) years | |
| Interventions | Azlocillin 450 mg/kg/day, 4‐hourly plus placebo vs azlocillin plus tobramycin 240 mg/m2/day, 6‐hourly, 14 days course. | |
| Outcomes | Lung function, time to next admission, symptom scores, adverse events, bacteriology, inflammatory markers, resistant strains. | |
| Notes | No data for symptom scores. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation balance by FVC and center. |
| Allocation concealment (selection bias) | Low risk | Code generated by research pharmacist at the core center. |
| Blinding (performance bias and detection bias) | Low risk | Participants and clinicians blinded, serum concentrations monitored by unblinded 3rd party (research pharmacist). |
| Incomplete outcome data (attrition bias) | Low risk | 35 participants withdrawn (21 from azlocillin group), reasons given in a table. |
CBC: complete blood count
CXR: chest x‐ray
ITT: intention‐to‐treat
PsA: Pseudomonas aeruginosa
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Comparison of twice daily vs three times daily antibiotics, not single vs combination. | |
| Comparison of once vs multiple daily dosing, not single vs combination. | |
| Comparison of single vs multiple daily dosing, not single vs combination. | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Not a comparison of single vs combination antibiotics; a comparison of a single intravenous dose of an antibiotic and multiple oral doses of the same antibiotic. | |
| Comparison of two combination regimens. | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Comparison of colistin with multiple antibiotic combinations. | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Home vs hospital therapy, not single vs combination. | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Not a comparison of a single vs combination antibiotics; evaluation of tool to assess treatment response in children. | |
| Non‐randomised study: first 7 participants allocated to single treatment; next 7 to combination treatment with marked differences in baseline characteristics. | |
| Comparison of intermittent vs continuous infusions, not single vs combination. | |
| Comparison of anti‐staphylococcal drug (oxacillin) vs oxacillin plus 2 anti‐pseudomonal drugs. | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Not a comparison of a single vs combination antibiotic; comparison of intravenous and oral versions of the same agent. | |
| Study of eradication of Pseudomonas aeruginosa, not a comparison of single vs combination. | |
| Pseudo‐randomised study. Treatment and comparison groups were not sufficiently similar at baseline. | |
| Not a comparison of single vs combination antibiotics. | |
| Comparison of 2 single agents. | |
| Not a comparison of single vs combination antibiotics; evaluation of a twice‐daily tobramycin regimen. | |
| Review article on single vs combination antibiotic treatment, i.e. not an RCT. | |
| Inhaled vs systemic antibiotics. | |
| Reported number of courses of treatment instead of number of people included. Some participants may have been counted twice or included in both treatment group therefore analysis unclear. | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Not a comparison of single vs combination antibiotics; comparison of morning vs evening intravenous tobramycin. | |
| Continuous vs intermittent infusions. | |
| Randomisation method unclear ‐ participants appeared to have been randomised to single or combination therapy each morning using a cross‐over method. | |
| Not a comparison of single vs combination antibiotics; trial of inhaled tobramycin therapy. | |
| Comparison of single agent compared with two other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Not a comparison of single vs combination antibiotics; study of continuous vs intermittent infusion piperacillin‐tazobactam. | |
| Comparison of single agent compared with 2 other antibiotics (i.e. drug A vs drug B plus drug C). | |
| Efficacy of once daily tobramycin, not a comparison of single vs combination agents. |
CXR: chest X‐ray
vs: versus
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean FEV1 at end of course (% pred) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 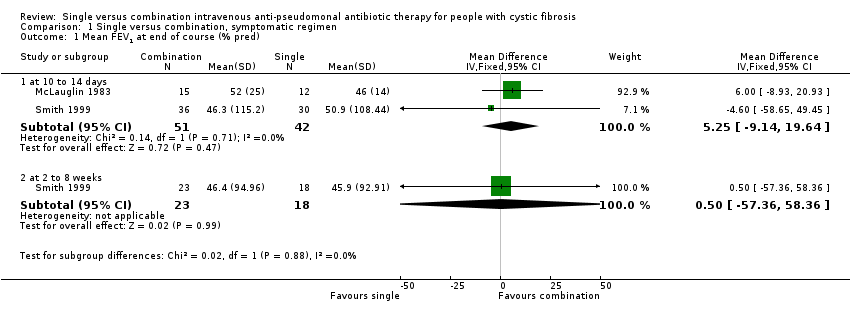 Comparison 1 Single versus combination, symptomatic regimen, Outcome 1 Mean FEV1 at end of course (% pred). | ||||
| 1.1 at 10 to 14 days | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [‐9.14, 19.64] |
| 1.2 at 2 to 8 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐57.36, 58.36] |
| 2 Mean FVC at end of course (% pred) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Single versus combination, symptomatic regimen, Outcome 2 Mean FVC at end of course (% pred). | ||||
| 2.1 at 10 to 14 days | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 1.84 [‐11.44, 15.12] |
| 2.2 at 2 to 8 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 6.90 [‐50.50, 64.30] |
| 3 Mean RV at end of course (% pred) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3 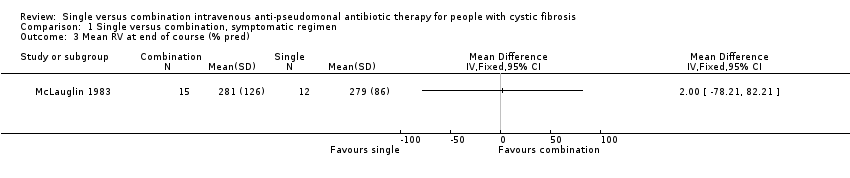 Comparison 1 Single versus combination, symptomatic regimen, Outcome 3 Mean RV at end of course (% pred). | ||||
| 4 Mean TLC at end of course (% pred) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4  Comparison 1 Single versus combination, symptomatic regimen, Outcome 4 Mean TLC at end of course (% pred). | ||||
| 5 Mean RV/TLC at end of course (% pred) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.5 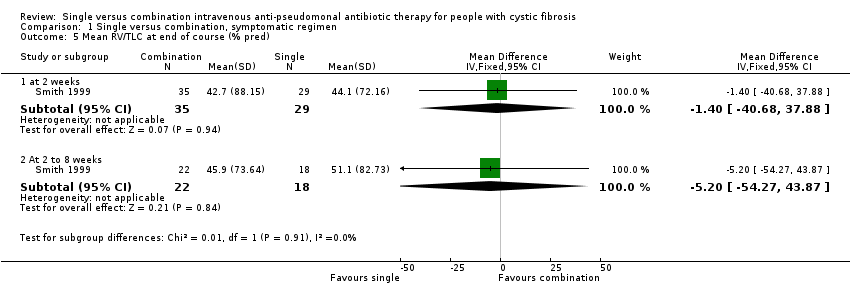 Comparison 1 Single versus combination, symptomatic regimen, Outcome 5 Mean RV/TLC at end of course (% pred). | ||||
| 5.1 at 2 weeks | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐40.68, 37.88] |
| 5.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐54.27, 43.87] |
| 6 Mean PFR at end of course (% pred) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Single versus combination, symptomatic regimen, Outcome 6 Mean PFR at end of course (% pred). | ||||
| 6.1 at 10 to 14 days | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 3.21 [‐11.49, 17.91] |
| 6.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐60.90, 65.50] |
| 7 Mean MMEF at end of course (% pred) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Single versus combination, symptomatic regimen, Outcome 7 Mean MMEF at end of course (% pred). | ||||
| 7.1 at 10 to 14 days | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 7.17 [‐8.22, 22.55] |
| 7.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐73.27, 69.47] |
| 8 Mean Schwachman score at end of course Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.8 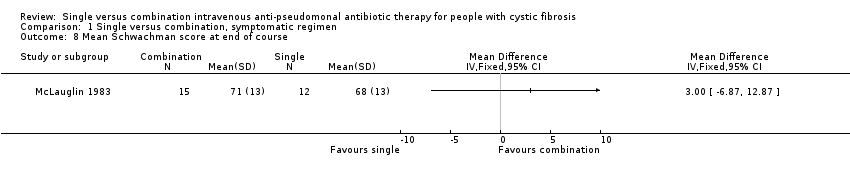 Comparison 1 Single versus combination, symptomatic regimen, Outcome 8 Mean Schwachman score at end of course. | ||||
| 9 Number of Pseudomonas isolates eradicated at end of course Show forest plot | 3 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.63 [2.12, 14.94] |
| Analysis 1.9  Comparison 1 Single versus combination, symptomatic regimen, Outcome 9 Number of Pseudomonas isolates eradicated at end of course. | ||||
| 10 Mean change Pseudomonas density in cfu/g at end of course Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.10  Comparison 1 Single versus combination, symptomatic regimen, Outcome 10 Mean change Pseudomonas density in cfu/g at end of course. | ||||
| 11 Number adverse events Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Single versus combination, symptomatic regimen, Outcome 11 Number adverse events. | ||||
| 11.1 local erythema / irritation | 2 | 131 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.09, 2.36] |
| 11.2 generalised rash | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.16 [0.12, 316.67] |
| 11.3 fever | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.05, 14.14] |
| 11.4 renal impairment (increased creatinine by 50%) | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.15, 15.56] |
| 11.5 auditory impairment | 1 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.86 [0.11, 305.44] |
| 11.6 proteinuria | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.62 [0.68, 19.30] |
| 12 Number readmitted Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.12  Comparison 1 Single versus combination, symptomatic regimen, Outcome 12 Number readmitted. | ||||
| 12.1 in 1 month | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 1.30] |
| 12.2 in 80 days | 1 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.12, 0.73] |
| 13 Mean time to next course of antibiotics (weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.13  Comparison 1 Single versus combination, symptomatic regimen, Outcome 13 Mean time to next course of antibiotics (weeks). | ||||
| 14 Mean WBC count at end of course Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.14 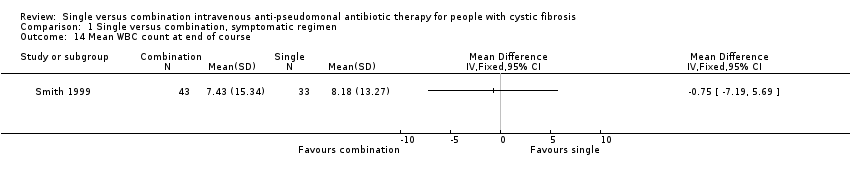 Comparison 1 Single versus combination, symptomatic regimen, Outcome 14 Mean WBC count at end of course. | ||||
| 15 Number resistant strains Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.15  Comparison 1 Single versus combination, symptomatic regimen, Outcome 15 Number resistant strains. | ||||
| 15.1 at baseline | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.38, 1.82] |
| 15.2 at end of course | 2 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.44 [0.94, 6.32] |
| 15.3 at 2 to 8 weeks | 2 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.17, 1.14] |
| 15.4 Difference between baseline and 2 to 8 weeks | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.06, 1.18] |

Comparison 1 Single versus combination, symptomatic regimen, Outcome 1 Mean FEV1 at end of course (% pred).

Comparison 1 Single versus combination, symptomatic regimen, Outcome 2 Mean FVC at end of course (% pred).

Comparison 1 Single versus combination, symptomatic regimen, Outcome 3 Mean RV at end of course (% pred).

Comparison 1 Single versus combination, symptomatic regimen, Outcome 4 Mean TLC at end of course (% pred).

Comparison 1 Single versus combination, symptomatic regimen, Outcome 5 Mean RV/TLC at end of course (% pred).

Comparison 1 Single versus combination, symptomatic regimen, Outcome 6 Mean PFR at end of course (% pred).

Comparison 1 Single versus combination, symptomatic regimen, Outcome 7 Mean MMEF at end of course (% pred).

Comparison 1 Single versus combination, symptomatic regimen, Outcome 8 Mean Schwachman score at end of course.

Comparison 1 Single versus combination, symptomatic regimen, Outcome 9 Number of Pseudomonas isolates eradicated at end of course.

Comparison 1 Single versus combination, symptomatic regimen, Outcome 10 Mean change Pseudomonas density in cfu/g at end of course.

Comparison 1 Single versus combination, symptomatic regimen, Outcome 11 Number adverse events.

Comparison 1 Single versus combination, symptomatic regimen, Outcome 12 Number readmitted.

Comparison 1 Single versus combination, symptomatic regimen, Outcome 13 Mean time to next course of antibiotics (weeks).

Comparison 1 Single versus combination, symptomatic regimen, Outcome 14 Mean WBC count at end of course.

Comparison 1 Single versus combination, symptomatic regimen, Outcome 15 Number resistant strains.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Mean FEV1 at end of course (% pred) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 at 10 to 14 days | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 5.25 [‐9.14, 19.64] |
| 1.2 at 2 to 8 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐57.36, 58.36] |
| 2 Mean FVC at end of course (% pred) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 at 10 to 14 days | 2 | 93 | Mean Difference (IV, Fixed, 95% CI) | 1.84 [‐11.44, 15.12] |
| 2.2 at 2 to 8 weeks | 1 | 41 | Mean Difference (IV, Fixed, 95% CI) | 6.90 [‐50.50, 64.30] |
| 3 Mean RV at end of course (% pred) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Mean TLC at end of course (% pred) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Mean RV/TLC at end of course (% pred) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 at 2 weeks | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐40.68, 37.88] |
| 5.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐5.20 [‐54.27, 43.87] |
| 6 Mean PFR at end of course (% pred) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 at 10 to 14 days | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 3.21 [‐11.49, 17.91] |
| 6.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [‐60.90, 65.50] |
| 7 Mean MMEF at end of course (% pred) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 at 10 to 14 days | 2 | 91 | Mean Difference (IV, Fixed, 95% CI) | 7.17 [‐8.22, 22.55] |
| 7.2 At 2 to 8 weeks | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | ‐1.90 [‐73.27, 69.47] |
| 8 Mean Schwachman score at end of course Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Number of Pseudomonas isolates eradicated at end of course Show forest plot | 3 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.63 [2.12, 14.94] |
| 10 Mean change Pseudomonas density in cfu/g at end of course Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 11 Number adverse events Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 11.1 local erythema / irritation | 2 | 131 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.46 [0.09, 2.36] |
| 11.2 generalised rash | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.16 [0.12, 316.67] |
| 11.3 fever | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.05, 14.14] |
| 11.4 renal impairment (increased creatinine by 50%) | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [0.15, 15.56] |
| 11.5 auditory impairment | 1 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.86 [0.11, 305.44] |
| 11.6 proteinuria | 1 | 63 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.62 [0.68, 19.30] |
| 12 Number readmitted Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 12.1 in 1 month | 1 | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 1.30] |
| 12.2 in 80 days | 1 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.30 [0.12, 0.73] |
| 13 Mean time to next course of antibiotics (weeks) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14 Mean WBC count at end of course Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 15 Number resistant strains Show forest plot | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 15.1 at baseline | 2 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.38, 1.82] |
| 15.2 at end of course | 2 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.44 [0.94, 6.32] |
| 15.3 at 2 to 8 weeks | 2 | 76 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.17, 1.14] |
| 15.4 Difference between baseline and 2 to 8 weeks | 1 | 29 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.06, 1.18] |

