Отсроченное введение энтеральных кормлений с постепенным увеличением объёма для предотвращения развития некротизирующего энтероколита у детей с очень низкой массой тела при рождении
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised controlled trial | |
| Participants | Preterm infants, 28‐36 weeks' gestation with birth weight < 10th centile, antenatal ultrasound showing intrauterine growth restriction, absent or reversed end diastolic flow on Doppler waveforms of the umbilical artery with evidence of cerebral redistribution, arterial cord pH ≥ 7.0 and base deficit ≥ ‐12 and 5‐minute Apgar score of > 5. Infants were excluded if there was any major congenital abnormality, twin‐twin transfusion, intrauterine transfusion, exchange transfusion, rhesus iso‐immunisation, significant multi‐organ failure, inotropic drug support or minimal enteral feeding had already started Setting: single centre: Women's Hospital, Hamad Medical Corporation, Qatar | |
| Interventions | Early introduction of progressive enteral feeds on day 3 (62 infants) versus late introduction of enteral feeds on day 6 (63 infants) | |
| Outcomes | Incidence of NEC (stage II/III), time to reach full enteral feeds (sustained for 72 hours), rates of feed intolerance, mortality and duration of hospital stay | |
| Notes | > 90% of participants were VLBW | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated tables |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Carers not blinded to intervention group |
| Blinding (performance bias and detection bias) | Unclear risk | No information on blinding of radiological assessors to intervention groups |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up assessment |
| Methods | Randomised controlled trial | |
| Participants | VLBW infants Infants were excluded if there was a congenital abnormality Setting: Isfahan Faculty of Medicine, Iran | |
| Interventions | Delayed introduction of progressive enteral feeds (only minimal volumes until day 7 (47 infants) versus early introduction on day 3 (35 infants) Infants received either unfortified breast milk or formula (no subgroup data available). Volumes and rates of advancement or progressive feeds were the same in both groups (20 mL/kg/day) | |
| Outcomes | Incidence of NEC, mortality, days to full enteral feeds, duration of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Carers not blinded to intervention group |
| Blinding (performance bias and detection bias) | Unclear risk | No information on blinding of radiological assessors to intervention groups |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up assessment |
| Methods | Randomised controlled trial | |
| Participants | Preterm infants, birth weight < 10th centile*, and antenatal evidence of absent or reversed end diastolic flow on Doppler waveforms of the umbilical artery Infants were excluded if there was a major congenital abnormality, receipt of mechanical ventilation or enteral feeding had already started Setting: single centre: Meir Medical Centre, Kfar Saba, Tel Aviv, Israel | |
| Interventions | Delayed progressive enteral feeding (day 4‐5 after birth, 30 infants) versus earlier enteral feeding (day 2 after birth, 30 infants) Infants received expressed breast or formula or both | |
| Outcomes | Incidence of NEC, mortality, nosocomial infection, days to reach full enteral feeds, duration of hospital stay | |
| Notes | *Most participants were VLBW (range 963‐1683 g) Original study published in Portuguese | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) | High risk | Carers not blinded to intervention group |
| Blinding (performance bias and detection bias) | Unclear risk | No information on blinding of radiological assessors to intervention groups |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up assessment |
| Methods | Randomised controlled trial | |
| Participants | 62 preterm infants with birth weight < 2000 g who were clinically stable and who had an umbilical artery catheter in place Infants were excluded if they had a lethal condition or had received a double‐volume exchange transfusion Setting: Division of Neonatology, Department of Pediatrics, University of Rochester Medical Center, US | |
| Interventions | Delayed introduction of enteral feeds (median 5 days; 31 infants) versus earlier introduction (median 2 days; 31 infants) Infants received either breast milk or diluted formula (no subgroup data available). Volumes and rates of advancement were the same in both groups | |
| Outcomes | Days to regain birth weight, days to full enteral feeding, duration of hospital stay, incidence of NEC and mortality | |
| Notes | The trial inclusion criterion for birth weight was < 2000 g. Since > 80% of infants were VLBW or very preterm, we decided to include the trial Infants in the delayed introduction group commenced enteral feeds when the umbilical artery catheter had been removed for 24 hours and the infant was clinically stable. Infants in the earlier introduction group commenced feeds with the umbilical artery catheter in situ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation method not reported |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes |
| Blinding (performance bias and detection bias) | High risk | Not stated but unlikely that carers were blinded to intervention groups |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Low risk | The trial excluded 2 infants in the early introduction group post‐randomisation due to protocol violation. All other participants were accounted for |
| Methods | Randomised controlled trial | |
| Participants | 84 singleton newborn infants of gestational age 27‐ 34 weeks' and birth weight < 10th percentile who also had antenatal Doppler ultrasound evidence within 7 days before birth of 'pathological fetal perfusion', defined as uterine or umbilical arterial pulsatility index > 90th percentile and middle cerebral arterial pulsatility index < 10th percentile for gestational age Infants were excluded with a major congenital anomaly or infection or had received exchange transfusion or inotrope support Setting: Neonatology Department, Aristotle University, Thessaloniki, Greece | |
| Interventions | Delayed (> 5 days after birth; 42 infants) versus early (≤ 5 days; 42 infants) introduction of enteral feeds (expressed breast milk or preterm formula milk) Minimal enteral feeding was continued until day 7 after birth and then feed volumes were advanced at daily targeted increments of 15 mL/kg | |
| Outcomes | Incidence of NEC, mortality*, days to full enteral feeds*, duration of hospital stay* | |
| Notes | *Unpublished data courtesy of Dr Karagianni Of the 84 infants enrolled, 81 completed the study. 3 infants died before 5 days after birth. We have included these infants in the intention‐to‐treat analysis of mortality > 90% of infants were VLBW | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Carers and clinical assessors not blinded to allocation groups |
| Blinding (performance bias and detection bias) | Unclear risk | No information available about blinding of radiological assessors |
| Incomplete outcome data (attrition bias) | Low risk | All data were included in the analyses |
| Methods | Randomised controlled trial | |
| Participants | 12 VLBW infants | |
| Interventions | Delayed introduction of enteral feeds (day 10 after birth; 7 infants) versus earlier introduction (< 4 days; 5 infants) All infants received standard calorie formula. Volumes and rates of advancement were the same in both groups | |
| Outcomes | Growth during the first 6 weeks after birth | |
| Notes | This trial was reported as an abstract only. Further (unpublished) methodological or outcome data were not available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Not described but unlikely to be blinded |
| Blinding (performance bias and detection bias) | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) | Unclear risk | Not described |
| Methods | Randomised controlled trial | |
| Participants | 404 preterm infants < 35 weeks' gestation and birth weight < 10th percentile and antenatal Doppler ultrasound evidence of: 1. absent or reversed end diastolic flow velocities on at least 50% of the Doppler waveforms from the umbilical artery on at least 1 occasion during pregnancy or 2. 'cerebral redistribution', defined as occurring when both the umbilical artery pulsatility index > 95th percentile and the middle cerebral artery pulsatility index < 5th percentile for gestational age (Hershkovitz 2000) Infants were excluded with a major congenital anomaly, receipt of in‐utero transfusion, multi‐organ failure or need for inotrope support Setting: 54 neonatal care centres in UK and Ireland | |
| Interventions | Delayed (day 5 after birth; 202 infants) versus early (day 2 after birth; 202 infants) introduction of milk feeds Protocol for advancing feed volumes was the same in both groups | |
| Outcomes | Days to full feeds (150 mL/kg/day) sustained for 3 days, incidence of NEC (all stages, and stage II/III), mortality, invasive infection, time to regain birth weight, duration of hospital stay | |
| Notes | > 90% of infants were VLBW | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | Central telephone randomisation |
| Blinding (performance bias and detection bias) | High risk | Carers were not blinded to the allocation groups |
| Blinding (performance bias and detection bias) | Low risk | All cases of NEC were reviewed independently by a committee that were blinded to the study groups |
| Incomplete outcome data (attrition bias) | Unclear risk | 2 infants (1 from each group) were excluded from the trial after randomisation occurred (error in recruitment and consent withdrawal) |
| Methods | Randomised controlled trial | |
| Participants | 38 VLBW infants assessed to be at 'high risk' of developing NEC based on a risk assessment score Setting: Perinatology Center, New York Hospital‐Cornell Medical Center, New York, USA | |
| Interventions | Delayed introduction of enteral feeds (day 7 after birth; 20 infants) versus earlier introduction (day 1; 18 infants) Infants received feeds by continuous intragastric infusion starting initially with sterile water, then progressing to 2.5% dextrose, diluted formula, then full‐strength standard calorie formula milk. Volumes and rates of advancement were the same in both groups: constant infusion at 1 mL/hour for 7 days then daily increments of 10 mL/kg/day | |
| Outcomes | Incidence of NEC and mortality | |
| Notes | Further details about exclusions after randomisation kindly provided by Dr La Gamma (March 2009) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Carers not blinded to allocation groups |
| Blinding (performance bias and detection bias) | Low risk | Radiologists reviewing abdominal films were blinded to the group assignments |
| Incomplete outcome data (attrition bias) | Low risk | 3 infants died before 7 days after birth. The investigators excluded 1 infant before day 14 because of a feeding protocol violation. We have included all of these infants in the relevant intention‐to‐treat analyses |
| Methods | Randomised controlled trial | |
| Participants | 239 very preterm or VLBW infants. Included infants had not received any previous enteral feeds Infants were excluded with congenital anomalies of the gastrointestinal tract, intrauterine growth restriction and respiratory or haemodynamic instability Setting: Ramón González Valencia de Bucaramanga University Hospital, Columbia | |
| Interventions | Delayed enteral feeding (day 5 after birth, 104 infants) versus earlier enteral feeding (day 1‐2 after birth, 135 infants) All infants received a combination of breast and formula milk. Feed volumes exceeded trophic volumes by the third day of enteral feeding | |
| Outcomes | Incidence of NEC, mortality, duration of hospital stay, growth, days to reach full feeds (150 mL/kg/day) | |
| Notes | Original study published in Spanish | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described (states "randomly assigned", and "controlled clinical trial") |
| Allocation concealment (selection bias) | Low risk | Not described |
| Blinding (performance bias and detection bias) | High risk | Carers or investigators not blinded to allocation groups |
| Blinding (performance bias and detection bias) | Low risk | Abdominal radiographs interpreted by radiologist who was independent from the study and blind to the allocation groups |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up assessment for primary outcomes |
NEC: necrotising enterocolitis; VLBW: very low birth weight.
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Both groups received early enteral feeds | |
| Infants were allocated alternately to either early (first day after birth) or delayed transpyloric enteral feeding. The delayed feeding group commenced enteral nutrition when assessed to be "clinically stable" but this included initiation within 4 days after birth | |
| Infants in the delayed progressive enteral feeds group received total parenteral nutrition as a co‐intervention | |
| This was not a randomised controlled trial | |
| Infants in the delayed progressive enteral feeding group received minimal enteral nutrition prior to feed advancement as a co‐intervention | |
| Both groups received early enteral feeds | |
| Infants in both groups received some enteral feeds before 4 days after birth | |
| Infants in the delayed progressive enteral feeds group also received delayed advancement of parenteral nutrition as a co‐intervention |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Necrotising enterocolitis Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 1 Necrotising enterocolitis. | ||||
| 1.1 All trials | 8 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.34] |
| 1.2 Trials of infants with intrauterine growth restriction or abnormal antenatal Doppler flow velocities | 4 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.54, 1.41] |
| 2 Mortality prior to discharge Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 2 Mortality prior to discharge. | ||||
| 2.1 All trials | 7 | 967 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.75, 1.88] |
| 2.2 Trials of infants with intrauterine growth restriction or abnormal antenatal Doppler flow velocities | 3 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.55, 2.05] |
| 3 Feed intolerance Show forest plot | 3 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.62, 1.15] |
| Analysis 1.3  Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 3 Feed intolerance. | ||||
| 4 Incidence of invasive infection Show forest plot | 2 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.95, 1.70] |
| Analysis 1.4 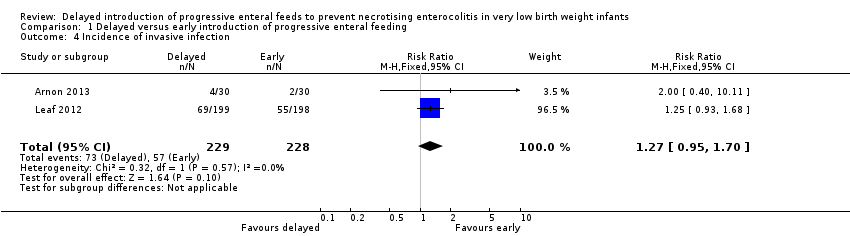 Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 4 Incidence of invasive infection. | ||||
| 5 Duration of hospital admission (days) Show forest plot | 3 | 346 | Mean Difference (IV, Fixed, 95% CI) | 2.11 [0.31, 3.90] |
| Analysis 1.5  Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 5 Duration of hospital admission (days). | ||||
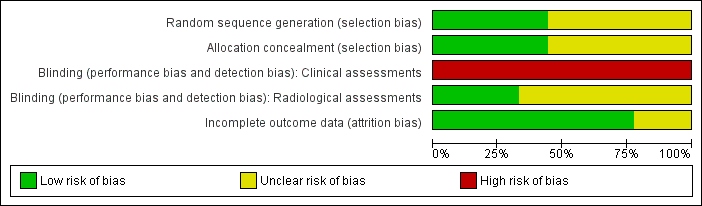
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
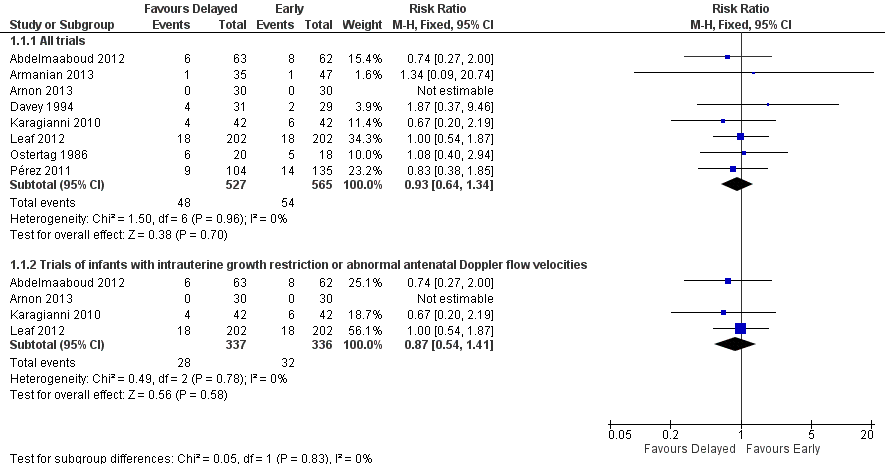
Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.1 Necrotising enterocolitis.
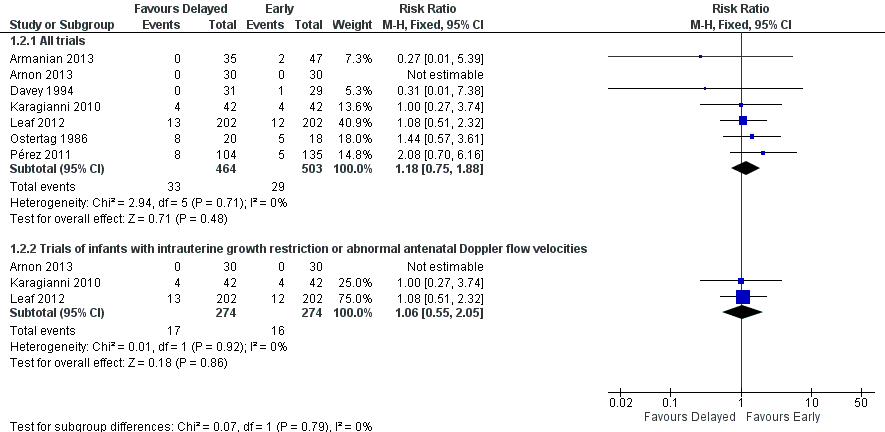
Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.2 Mortality prior to discharge.
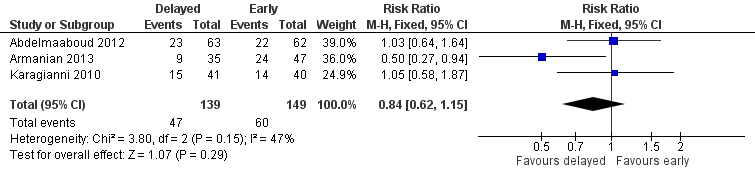
Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.3 Feed intolerance.
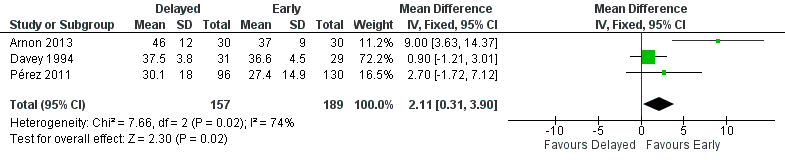
Forest plot of comparison: 1 Delayed versus early introduction of progressive enteral feeding, outcome: 1.5 Duration of hospital admission (days).

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 1 Necrotising enterocolitis.

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 2 Mortality prior to discharge.

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 3 Feed intolerance.

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 4 Incidence of invasive infection.

Comparison 1 Delayed versus early introduction of progressive enteral feeding, Outcome 5 Duration of hospital admission (days).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Necrotising enterocolitis Show forest plot | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 All trials | 8 | 1092 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.64, 1.34] |
| 1.2 Trials of infants with intrauterine growth restriction or abnormal antenatal Doppler flow velocities | 4 | 673 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.54, 1.41] |
| 2 Mortality prior to discharge Show forest plot | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 All trials | 7 | 967 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.75, 1.88] |
| 2.2 Trials of infants with intrauterine growth restriction or abnormal antenatal Doppler flow velocities | 3 | 548 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.55, 2.05] |
| 3 Feed intolerance Show forest plot | 3 | 288 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.62, 1.15] |
| 4 Incidence of invasive infection Show forest plot | 2 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.95, 1.70] |
| 5 Duration of hospital admission (days) Show forest plot | 3 | 346 | Mean Difference (IV, Fixed, 95% CI) | 2.11 [0.31, 3.90] |

