Administración temprana de corticosteroides inhalados para la prevención de la enfermedad pulmonar crónica en neonatos prematuros de muy bajo peso al nacer
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Multi‐national randomised placebo‐controlled clinical trial. Study setting: 40 centres in 9 European countries. Study period: 1 April 1 2010 to 3 August 2013. Blinding of randomisation: yes | |
| Participants | Infants with PMA 230/7 weeks to 276/7 weeks and chronological age of 12 hours or less, who required any form of positive pressure support. Exclusion criteria: palliative care; dysmorphic feature or congenital abnormalities likely to affect life expectancy or neurologic development; strongly suspected cyanotic heart disease; were from a multiple‐birth pregnancy (other than the second infant in birth order). Demographic data: values are presented as mean (SD) or percentage or median (IQR) Budesonide group: n = 437; followed to first discharge home Birth weight (g): 798 (193) Gestational age (weeks): 26.1 (1.3) Sex (% male): 50.8 Age at randomisation (hours): 6.7 (4.0 to 10.3) Placebo group: n = 419; followed to first discharge home Birth weight (g): 803 (189) Gestational age (weeks): 26.1 (1.2) Sex (% male): 50.8 Age at randomisation (hours): 6.6 (3.8 to 10.6) | |
| Interventions | The Budesonide group (n = 441 were assigned to budesonide; 437 were followed to first discharge home) received two puffs of budesonide (200µg/puff) administered every 12 hours for the first 14 days of life and one puff administered every 12 hours from day 15 until the last dose of study drug had been administered. Study drugs were administered until infants no longer needed supplemental oxygen and positive pressure support or reached a PMA of 320/7 weeks, regardless of ventilator status. The control group (n = 422; 419 were followed to first discharge home) received placebo containing hydrofluoroalkane propellant | |
| Outcomes | Primary outcome: a composite of death or BPD at 36 weeks' PMA. BPD defined as the requirement for positive pressure support, the requirement for supplemental oxygen at FiO₂ > 0.30, or, if infants receiving low amounts of oxygen, an inability to maintain an oxygen saturation value above 90% during a structured, short period of saturation monitoring coupled with gradual weaning from oxygen to ambient air (oxygen reduction test). Secondary outcomes: death by any cause at 36 weeks' PMA, BPD at 36 weeks' PMA (defined as per above), duration of positive pressure respiratory support or supplemental oxygen, ventriculomegaly with or without IVH on ultrasound at or before 36 weeks' PMA, PDA requiring medical or surgical treatment, intestinal perforation or NEC (we included this outcome under NEC), ROP (≥ stage 2), culture‐proven infections, increase in body weight and head circumference from birth to day 28, length of hospital stay, need for reintubation after the last dose of drug had been administered, occurrence of oral candidiasis requiring treatment, hyperglycaemia requiring insulin treatment, hypertension requiring treatment. Neurodevelopmental disability testing to be conducted at 18 to 22 months (results not reported in this publication). | |
| Notes | ClinicalTrials.gov: NCT01035190 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | The manufacturer of the study drug received the sequence of study drug assignments from a statistician at the coordinating centre and prepared drug packages, each of which contained 8 sequentially numbered metered dose inhalers that were identical in appearance. Packages of coded inhalers containing the study drugs were delivered to each participating centre to ensure concealment of randomisation. |
| Blinding of participants and personnel (performance bias) | Low risk | See comments above. |
| Blinding of outcome assessment (detection bias) | Low risk | No one involved in patient care or in the assessment and analysis of outcomes was aware of the individual study group assignments before completion of the analysis. |
| Incomplete outcome data (attrition bias) | Low risk | 4 in the budesonide group and 3 in the control group had unknown outcome because of withdrawal of consent or right to use data. |
| Selective reporting (reporting bias) | Low risk | The study was registered as ClinicalTrials.gov number NCT01035190. There does not seem to be any major deviations from the protocol. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Multicentre randomised, double‐blind, placebo‐controlled trial. Infants were stratified for randomisation according to: study site, sex, birth weight (≤ 900 g or > 900 g), and severity of pulmonary disease (oxygenation index ≤ 5). Blinding of randomisation: yes | |
| Participants | Preterm infants < 33 weeks gestational age and birth weight ≤ 1250 g who required assisted ventilation between 3 and 14 days of life were eligible. 256 infants were enrolled in the study, 3 excluded due to sepsis (n = 2) and one infant had received systemic glucocorticoid therapy prior to enrolment. Demographic data: values presented as mean (SD) Beclomethasone dipropionate group: n = 123 Birth weight (g): 800 (193) Placebo group: n = 130 Exclusion criteria: | |
| Interventions | Beclomethasone dipropionate (n = 123) | |
| Outcomes | Primary outcome: Secondary outcomes: The incidence of adverse events: | |
| Notes | Randomisation schedule was provided by the data coordinating centre to the pharmacy at each study centre. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Multicentre randomised, double‐blind, placebo‐controlled trial. Infants were stratified for randomisation according to: study site, sex, birth weight (≤ 900 grams or > 900 grams), and severity of pulmonary disease (oxygenation index ≥ 5 or < 5). Method of sequence allocation unknown. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Low risk | Blinding of outcome measurement: yes |
| Incomplete outcome data (attrition bias) | Unclear risk | Complete follow‐up: no |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Multi‐centre randomised placebo‐controlled clinical trial. Study setting: 6 neonatal intensive care units in France. Study period: April 1993 to April 1995 Blinding of randomisation: no information provided | |
| Participants | Infants with respiratory distress syndrome and PMA < 31 weeks were eligible for the study if they required ventilator support on the 10th postnatal day. 178 infants were randomised, 5 were withdrawn leaving 173 infants in the trial who were assigned to 4 groups of which 2 are included in our review. Beclomethasone: n = 43 Birth weight (g): 1082 (260) Placebo group: n = 43 | |
| Interventions | Beclomethasone (n = 43): 250 µg was given 4 times a day (1000 µg daily) starting on the 10th or 11th postnatal day and given for 28 days, with dose tapering over a period of 8 days. Placebo (n = 43): 250 µg was given 4 times a day (1000 µg daily) starting on the 10th or 11th postnatal day and given for 28 days, with dose tapering over a period of 8 days. | |
| Outcomes | The main outcome criterion was CLD. The diagnosis of CLD was made at 28 days of age on the basis of clinical (oxygen dependence) and radiographic criteria. CLD was categorized in 3 grades of severity: severe: ventilation with ET > 3 months or oxygen supplementation > 4 months; moderate: ventilation with ET > 1 month or oxygen supplementation > 2 months; and mild: ventilation with ET < month and oxygen supplementation < 2 months Survival without CLD Death (during hospital stay) Ventilatory support (nasal IMV, CPAP or IMV) (days) Need for supplementary oxygen (days) Need for systemic dexamethasone (IV) Sepsis (positive blood culture) (no mention of meningitis) | |
| Notes | The definition of CLD is unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | The trial is described as a prospective, randomised, double‐blind trial, but no supporting evidence is provided by the authors |
| Blinding of outcome assessment (detection bias) | Unclear risk | The trial is described as a prospective, randomised, double‐blind trial, but no supporting evidence is provided by the authors |
| Incomplete outcome data (attrition bias) | Low risk | Initially 178 infants were randomised, but informed consent was either not obtained or withdrawn for five infants leaving 173 infants in the trial. Data reported for those 173 infants who were randomised into 4 groups. Data reported for 43 infants in each of the beclomethasone and placebo groups, which are included in this review. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot judge if there were any deviations from the protocol. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Randomised controlled trial. Blinding of randomisation: yes | |
| Participants | Preterm infants < 32 weeks gestational age, birth weight < 1.5 kg and requiring mechanical ventilation were eligible if 6 to 10 hours after the second dose of surfactant the arterial PO₂:alveolar PO₂ was < 0.25. Demographic data: values are presented as mean (SD) Placebo group: n = 26 Exclusion criteria: | |
| Interventions | Fluticasone propionate group (n = 27) | |
| Outcomes | Primary outcomes: Secondary outcomes: Hyperglycemia was defined as a blood glucose reading > 7 mmol/L. Static respiratory system compliance (Crs) and resistance (Rrs) were measured immediately before the start of aerosol treatment, and repeated on days 3, 7, and 14 in infants who remained intubated and ventilated. Both Crs and Rrs were measured using a SensorMedics Pulmonary Cart (SensorMedics Inc., Yorba Linda, CA, USA) using the passive flow‐volume technique. The measurement were carried out using a pneumotachograph (Hans Rudolph Inc., USA) with a small dead space (1.8 ml) connected to the endotracheal tube. | |
| Notes | Infants were randomised using computer‐generated random numbers into treatment and control groups and allocation to the groups was performed using opaque, sealed envelopes. Extubation was considered when the FiO₂ and ventilator rate decreased to < 0.4 and < 10 breaths/minute, respectively. The decision to extubate was made by the attending neonatologists who were blinded to the study protocol and the nature of the aerosol given to the infants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Randomised controlled trial Blinding of randomisation: yes | |
| Participants | Preterm infants < 1250 grams diagnosed with RDS and requiring ventilatory support at 72 hours Demographic data: values presented as mean (SD) Placebo group: n = 30 Exclusion criteria: Study centre: Halifax, Canada | |
| Interventions | Beclomethasone dipropionate (n = 30) | |
| Outcomes | Primary outcome: Secondary outcomes: | |
| Notes | Randomisation was performed for 3 weight strata in blocks of 4 using sealed envelopes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Randomised double‐blind placebo‐controlled trial Blinding of randomisation: yes | |
| Participants | Very low birth weight infants who were mechanically ventilated on day 6 of life or if extubated, nasal continuous positive airway pressure with FiO₂ of ≥ to 0.3 were included. Exclusion criteria: Demographic data: values are presented as median (range) or number (%) Budesonide group: n = 15 Placebo group: n = 15 | |
| Interventions | Budesonide (Pulmicort) (Astra Draco, Lund, Sweden) or placebo aerosol was used. The drug was delivered using an electronic dosimetric jet nebulizer (Spira Electro 4, Respiratory Centre, Hameenlinna, Finland) | |
| Outcomes | Primary outcome: reduction in the FiO₂ levels after 14 days of treatment Secondary outcomes include: | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias |
| Methods | Randomised double‐blind placebo controlled trial Blinding of randomisation: yes | |
| Participants | Preterm infants with birth weight of 750 to 1500 grams, gestational age of 25 to 32 weeks, ventilator dependency on day 3 of life with a ventilator rate ≥ 15 breaths/min and FiO₂ of > 0.25 to maintain an oxygen saturation of > 90%. 24 infants were enrolled in the study, one infant in the placebo group withdrawn due to severe sepsis 1 day after starting inhalation therapy. Demographic data: values are presented as median (range) Budesonide group: n = 12 Placebo group: n = 11 Exclusion criteria: Study centre: Aachen, Germany | |
| Interventions | Budesonide (Astra Draco, Lund, Sweden) or placebo aerosol were used. Two puffs of budesonide (200 µg/puff) or placebo was administered 4 times a day for a total of 10 days or until the infants were extubated. | |
| Outcomes | Primary outcome: Secondary outcomes: CLD was defined as requirement of supplemental oxygen at 28 days of life and at 36 weeks' PMA. | |
| Notes | Infants were ventilated with Stephan respirator HF 300 (Fa. Stephan, Gackenbach, Germany) in the IPPV or IMV mode. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Randomised double‐blind placebo controlled trial Blinding of randomisation: yes | |
| Participants | Infants (n = 211) with birth weight < 1000 grams who needed endotracheal intubation and respiratory support due to respiratory failure. Demographic data: values are presented as mean (SD) or mean (range) or percentage Fluticasone propionate group: n = 107 Birthweight (g): 784 (135) Gestational age (weeks): 26.1 (25.1 to 27.3) Sex (% male): 58.9 Placebo group: n = 104 Birth weight (g): 784 (127) Gestational age (weeks): 26.2 (25.1 to 27.3) Sex (% male): 48.1 | |
| Interventions | Prophylactic inhaled steroids starting within 24 h of birth and continuing until 6 weeks of age or extubation. Two doses of 50 µg fluticasone propionate (FP) were administered every 24 h (n = 107). The placebo contained only hydrofluoroalkane propellant (n = 104). | |
| Outcomes | The primary outcome measure used to indicate the morbidity of severe BPD was death or oxygen dependence at discharge. The secondary outcomes were death, severe BPD and neurodevelopmental outcomes at 18 months' PMA and 3 years of age | |
| Notes | Because of financial constraints the study was stopped early. The authors reported all outcomes as a combination of death and a clinical complication of preterm birth. We wrote to the first author on 20 May 2016, for clarifications regarding outcomes, but as of 20th July 2016 we have not received a response. We chose to report the data as per the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An independent Internet‐based patient registration and randomisation robot. Central randomisation. |
| Allocation concealment (selection bias) | Low risk | Fluticasone propionate (FP) and placebo metered‐dose inhalers providing 50 g per actuation were obtained from the drug manufacturer. Fluticasone propionate and placebo were delivered from the metered‐dose inhaler with a valve space chamber interposed between the neonatal anaesthesia bag and the endotracheal tube. Double‐blind placebo‐controlled trial. |
| Blinding of participants and personnel (performance bias) | Low risk | Staff was blinded to FP and placebo. |
| Blinding of outcome assessment (detection bias) | Low risk | All outcomes were assessed blinded to the groups except for the neuromotor exams. |
| Incomplete outcome data (attrition bias) | Low risk | All infants enrolled are accounted for. |
| Selective reporting (reporting bias) | Unclear risk | The protocol for the study was not available to us so we cannot ascertain if there were any deviations from the protocol. Because of financial constraints the study was stopped early. |
| Other bias | Low risk | Appears free of other bias. |
| Methods | Randomised double‐blind placebo‐controlled trial Blinding of randomisation: yes | |
| Participants | Preterm infants < 28 weeks and ≤ 1100 grams at birth who were ventilator dependent due to RDS were enrolled at 48 to 96 hours of age. Demographic values: Placebo group: n = 17 | |
| Interventions | Flunisolide or placebo 500 µg 3 times a day via spacer chamber connected to the ventilator | |
| Outcomes | Outcome assessed: | |
| Notes | The authors do not state whether the demographic data are presented as means or medians | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial Method of sequence generation unknown |
| Allocation concealment (selection bias) | Low risk | Randomised double‐blind placebo controlled trial Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Unclear risk | Study published in abstract form and not enough information was provided to judge if there were other bias or not. |
| Methods | Randomised double‐blind placebo controlled trial. Blinding of randomisation: yes | |
| Participants | Preterm infants < 32 weeks and requiring mechanical ventilation from birth were recruited within 18 hours of birth. 40 infants enrolled in the study. Demographic data: values are presented as mean (SD) Fluticasone propionate group: n = 20 Placebo group: n = 20 Exclusion criteria: Study centre: Jessop Hospital for Women, Sheffield, UK. | |
| Interventions | Fluticasone propionate or placebo (expient without active ingredient) Mode of delivery: MDI and Aerochamber if ventilated, Babyhaler if extubated | |
| Outcomes | Data were collected on survival, duration of mechanical ventilation and oxygen supplementation and other measures of morbidity (BP, glucose and IVH). Weight gain and skeletal growth was assessed by knemometry. | |
| Notes | Additional data from the investigators were available for this trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised double‐blind placebo controlled trial. Method of sequence generation unknown. |
| Allocation concealment (selection bias) | Low risk | Blinding of randomisation: yes |
| Blinding of participants and personnel (performance bias) | Low risk | Blinding of intervention: yes |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome measurement: can't tell |
| Incomplete outcome data (attrition bias) | Low risk | Complete follow‐up: yes |
| Selective reporting (reporting bias) | Unclear risk | The trial was not registered so we cannot tell if there was selective reporting or not. |
| Other bias | Low risk | The principal investigator provided additional data. |
BPD = bronchopulmonary dysplasia
CLD = chronic lung disease
CPAP = continuous positive airway pressure
ET = endotracheal tube
FiO2 = fraction of inspired oxygen
g = grams
IMV = intermittent mandatory ventilation
IV = intravenous
IVH = intraventricular haemorrhage
IQR = inter‐quartile range
µg = micrograms
n = number
NEC = necrotizing enterocolitis
PDA = patent ductus arteriosus
PMA = postmenstrual age
PO2 = partial pressure of oxygen
ROP = retinopathy of prematurity
SD = standard deviation
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Excluded as infants who required supplemental oxygen at 36 weeks' PMA were included. | |
| Excluded as infants were randomised between 28 and 60 days of age. | |
| Excluded because a combination of systemic (dexamethasone) and inhaled corticosteroid (budesonide) was used. | |
| The study compared the effect of intratracheal administration of surfactant/budesonide with that of surfactant alone on the incidence of death or BPD. This study design did not meet our review objectives of "To determine the impact of inhaled corticosteroids administered to ventilated preterm infants with birth weight of ≤ 1500 grams beginning in the first two weeks of life for the prevention of CLD as reflected by the requirement for supplemental oxygen at 36 weeks' PMA". |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CLD at 36 weeks PMA Show forest plot | 5 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.52] |
| Analysis 1.1  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 1 CLD at 36 weeks PMA. | ||||
| 2 CLD at 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.14] |
| Analysis 1.2  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 2 CLD at 28 days of age. | ||||
| 3 Death by 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| Analysis 1.3  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 3 Death by 28 days of age. | ||||
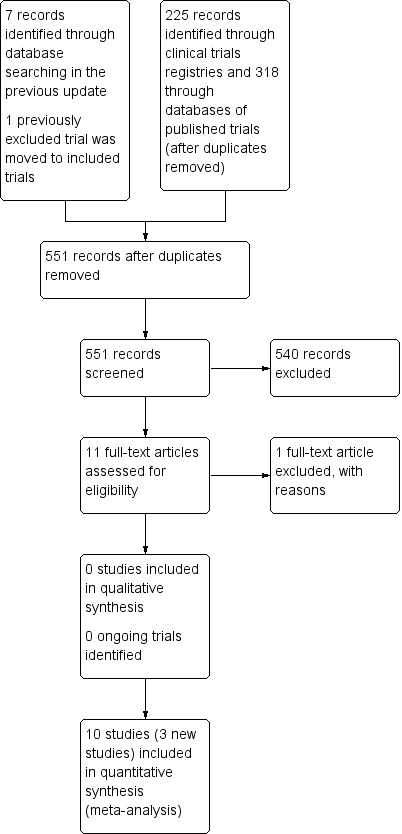
| 4 Death by 36 weeks PMA Show forest plot | 6 | 1285 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| Analysis 1.4 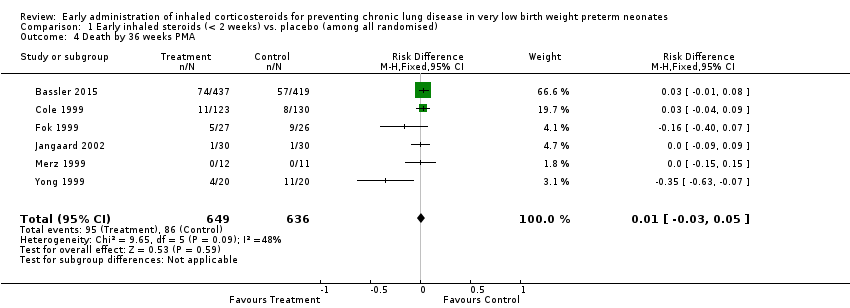 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 4 Death by 36 weeks PMA. | ||||
| 5 Death by or CLD at 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| Analysis 1.5  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 5 Death by or CLD at 28 days of age. | ||||
| 6 Death by or CLD at 36 weeks PMA Show forest plot | 6 | 1285 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.86 [0.75, 0.99] |
| Analysis 1.6  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 6 Death by or CLD at 36 weeks PMA. | ||||
| 7 Survival to hospital discharge without CLD Show forest plot | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [‐0.06, 0.34] |
| Analysis 1.7  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 7 Survival to hospital discharge without CLD. | ||||
| 8 Death during hospital stay Show forest plot | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| Analysis 1.8 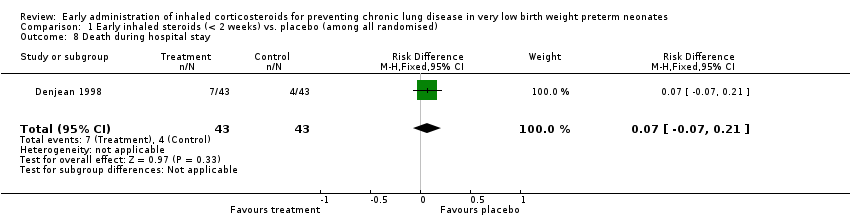 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 8 Death during hospital stay. | ||||
| 9 Culture proven infection during hospital stay Show forest plot | 6 | 1121 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.00, 0.11] |
| Analysis 1.9 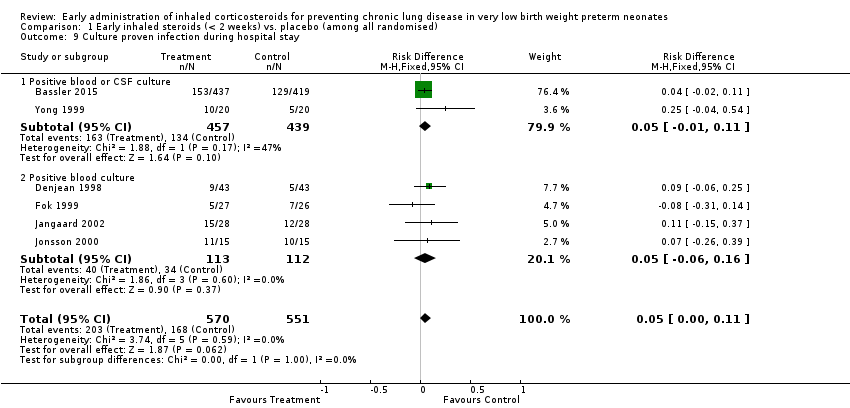 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 9 Culture proven infection during hospital stay. | ||||
| 9.1 Positive blood or CSF culture | 2 | 896 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 9.2 Positive blood culture | 4 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.06, 0.16] |
| 10 Hyperglycaemia Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.10  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 10 Hyperglycaemia. | ||||
| 10.1 Hyperglycaemia | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.44] |
| 10.2 Hyperglycaemia requiring insulin treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 11 Hypertension Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 11 Hypertension. | ||||
| 11.1 Hypertension | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.36, 3.99] |
| 11.2 Hypertension requiring treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.57] |
| 12 Gastrointesinal bleeding Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.34] |
| Analysis 1.12  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 12 Gastrointesinal bleeding. | ||||
| 13 Cataract Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.56] |
| Analysis 1.13  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 13 Cataract. | ||||
| 14 Intraventricular haemorrhage Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.41] |
| Analysis 1.14  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 14 Intraventricular haemorrhage. | ||||
| 15 Periventricular leukomalacia Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.59, 3.46] |
| Analysis 1.15  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 15 Periventricular leukomalacia. | ||||
| 16 Brain injury Show forest plot | 1 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.65] |
| Analysis 1.16  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 16 Brain injury. | ||||
| 17 Necrotizing enterocolitis Show forest plot | 3 | 1162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.24] |
| Analysis 1.17 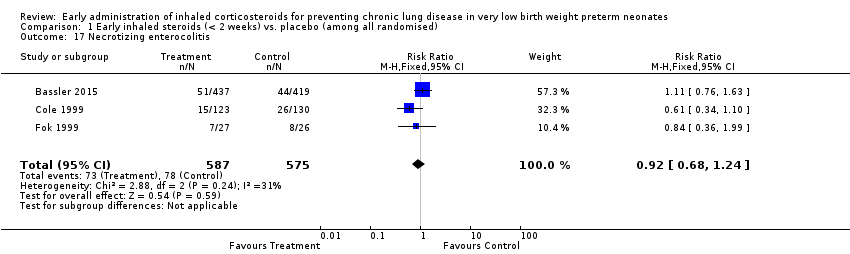 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 17 Necrotizing enterocolitis. | ||||
| 18 Retinopathy of prematurity (any stage) Show forest plot | 3 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| Analysis 1.18 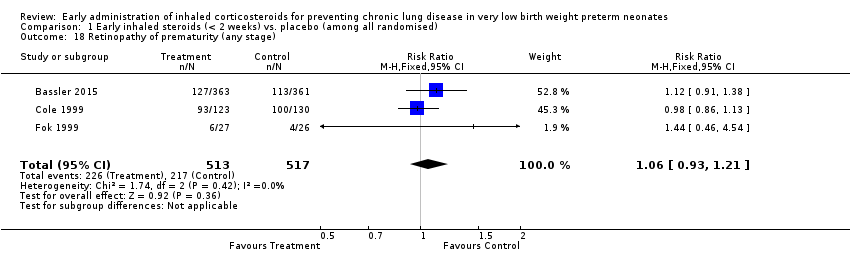 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 18 Retinopathy of prematurity (any stage). | ||||
| 19 Patent ductus arteriosus Show forest plot | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.17] |
| Analysis 1.19 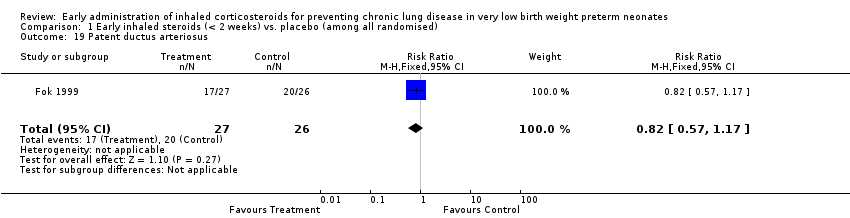 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 19 Patent ductus arteriosus. | ||||
| 20 Reintubation Show forest plot | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.96] |
| Analysis 1.20 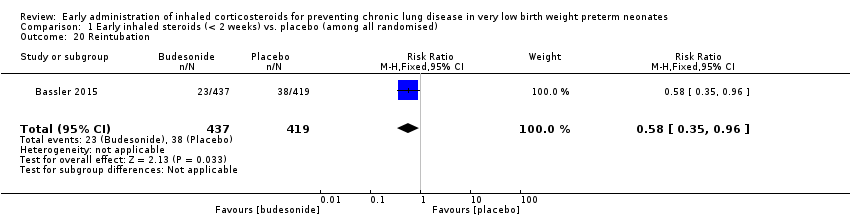 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 20 Reintubation. | ||||
| 21 Requirement for systemic steroids Show forest plot | 8 | 1403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| Analysis 1.21 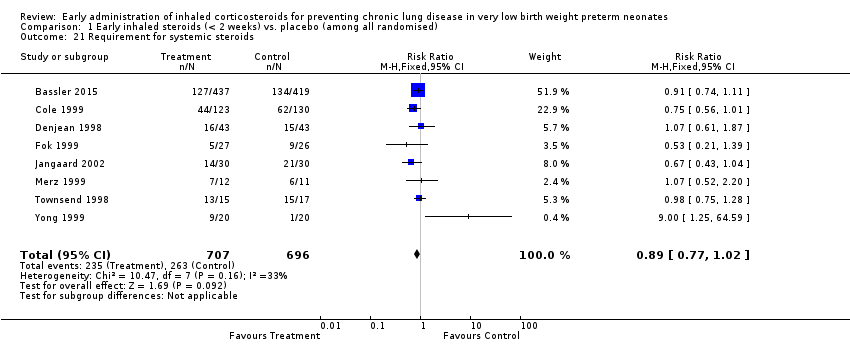 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 21 Requirement for systemic steroids. | ||||
| 22 Failure to extubate within 14 days Show forest plot | 5 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| Analysis 1.22  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 22 Failure to extubate within 14 days. | ||||
| 23 Death or oxygen dependency at discharge Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.15] |
| Analysis 1.23  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 23 Death or oxygen dependency at discharge. | ||||
| 24 Death or severe BPD Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.25] |
| Analysis 1.24 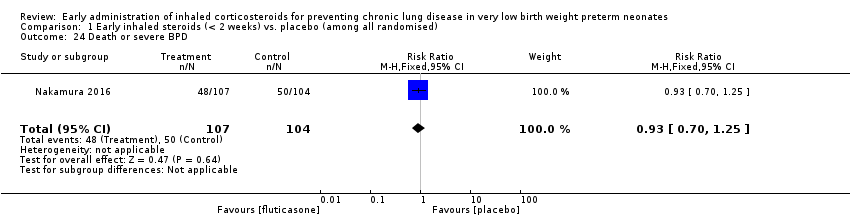 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 24 Death or severe BPD. | ||||
| 25 Death or grade 3 or 4 IVH Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.68] |
| Analysis 1.25 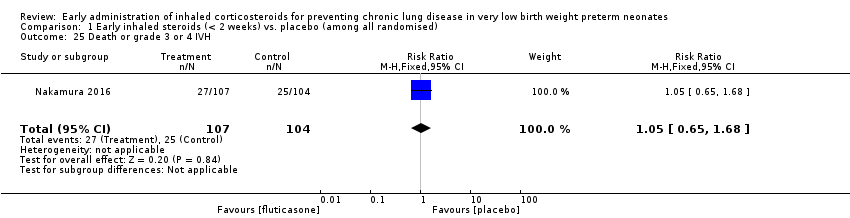 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 25 Death or grade 3 or 4 IVH. | ||||
| 26 Death or PVL Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| Analysis 1.26  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 26 Death or PVL. | ||||
| 27 Death or NEC Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.06] |
| Analysis 1.27 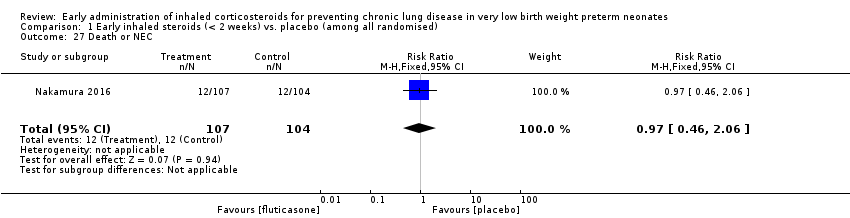 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 27 Death or NEC. | ||||
| 28 Death or sepsis Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.40] |
| Analysis 1.28 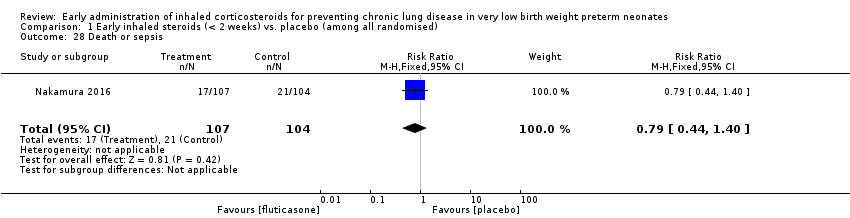 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 28 Death or sepsis. | ||||
| 29 Death or ROP (stage not stated) Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| Analysis 1.29 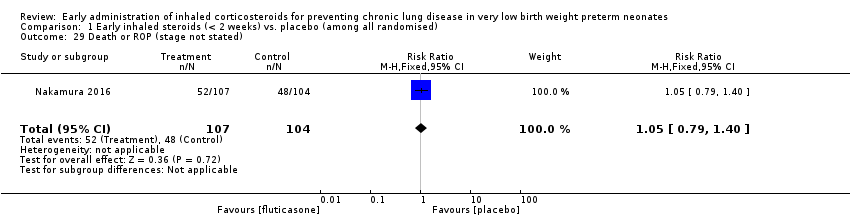 Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 29 Death or ROP (stage not stated). | ||||
| 30 Death or neurodevelopmental impairment at 18 months PMA Show forest plot | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.70, 1.70] |
| Analysis 1.30  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 30 Death or neurodevelopmental impairment at 18 months PMA. | ||||
| 31 Death or neurodevelopmental impairment at 3 years of age Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.56] |
| Analysis 1.31  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 31 Death or neurodevelopmental impairment at 3 years of age. | ||||
| 32 Death or cerebral palsy at 3 years of age Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.64, 1.96] |
| Analysis 1.32  Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 32 Death or cerebral palsy at 3 years of age. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CLD at 36 weeks PMA Show forest plot | 6 | 1088 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.02] |
| Analysis 2.1 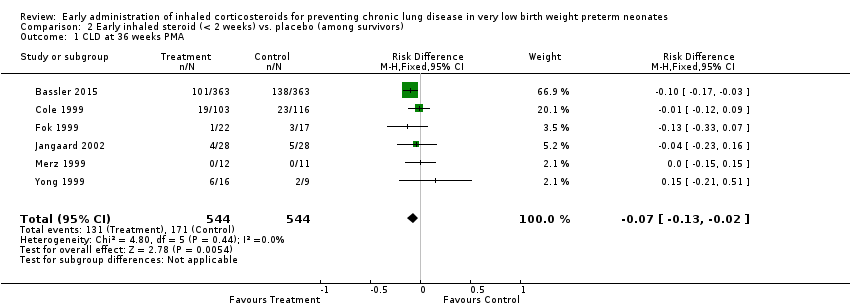 Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 1 CLD at 36 weeks PMA. | ||||
| 2 CLD at 28 days of age Show forest plot | 5 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| Analysis 2.2  Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 2 CLD at 28 days of age. | ||||
| 3 Cerebral palsy Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.42] |
| Analysis 2.3 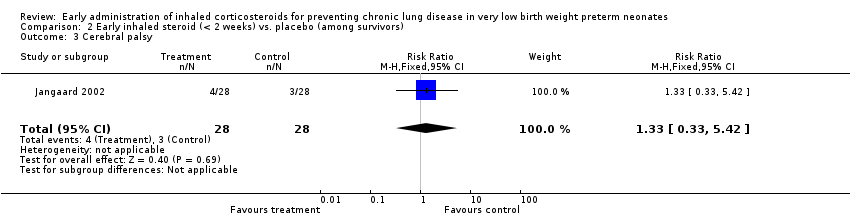 Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 3 Cerebral palsy. | ||||
| 4 Mean developmental index on BSID‐II < 2 SD of the mean Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.17] |
| Analysis 2.4  Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 4 Mean developmental index on BSID‐II < 2 SD of the mean. | ||||
| 5 Respiratory readmission Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.29] |
| Analysis 2.5  Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 5 Respiratory readmission. | ||||
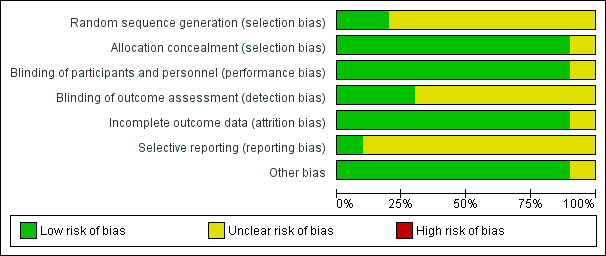
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
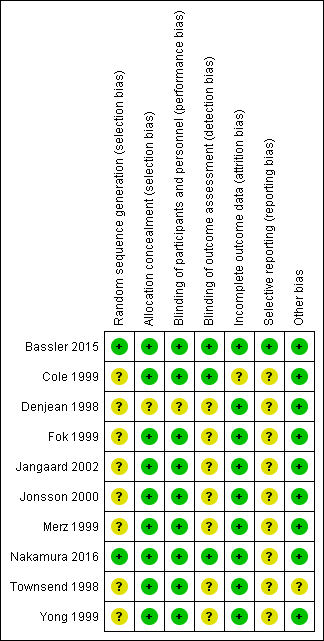
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), outcome: 1.1 CLD at 36 weeks' PMA.

Forest plot of comparison: 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), outcome: 1.6 Death by or CLD at 36 weeks' PMA.
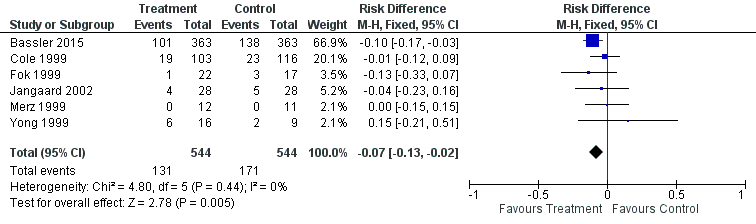
Forest plot of comparison: 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), outcome: 2.1 CLD at 36 weeks' PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 1 CLD at 36 weeks PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 2 CLD at 28 days of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 3 Death by 28 days of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 4 Death by 36 weeks PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 5 Death by or CLD at 28 days of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 6 Death by or CLD at 36 weeks PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 7 Survival to hospital discharge without CLD.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 8 Death during hospital stay.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 9 Culture proven infection during hospital stay.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 10 Hyperglycaemia.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 11 Hypertension.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 12 Gastrointesinal bleeding.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 13 Cataract.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 14 Intraventricular haemorrhage.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 15 Periventricular leukomalacia.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 16 Brain injury.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 17 Necrotizing enterocolitis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 18 Retinopathy of prematurity (any stage).

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 19 Patent ductus arteriosus.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 20 Reintubation.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 21 Requirement for systemic steroids.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 22 Failure to extubate within 14 days.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 23 Death or oxygen dependency at discharge.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 24 Death or severe BPD.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 25 Death or grade 3 or 4 IVH.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 26 Death or PVL.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 27 Death or NEC.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 28 Death or sepsis.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 29 Death or ROP (stage not stated).

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 30 Death or neurodevelopmental impairment at 18 months PMA.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 31 Death or neurodevelopmental impairment at 3 years of age.

Comparison 1 Early inhaled steroids (< 2 weeks) vs. placebo (among all randomised), Outcome 32 Death or cerebral palsy at 3 years of age.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 1 CLD at 36 weeks PMA.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 2 CLD at 28 days of age.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 3 Cerebral palsy.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 4 Mean developmental index on BSID‐II < 2 SD of the mean.

Comparison 2 Early inhaled steroid (< 2 weeks) vs. placebo (among survivors), Outcome 5 Respiratory readmission.
| Early inhaled steroids (< 2 weeks) compared to placebo (among all randomised) for preventing chronic lung disease in very low birth weight preterm neonates | ||||||
| Patient or population: very low birth weight preterm neonates | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (among all randomised) | Early inhaled steroids (< 2 weeks) | |||||
| CLD at 36 weeks' PMA | Study population | RR 0.97 | 429 | ⊕⊕⊕⊝ | ||
| 152 per 1000 | 148 per 1000 | |||||
| Moderate | ||||||
| 115 per 1000 | 112 per 1000 | |||||
| Death by, or CLD at, 36 weeks' PMA | Study population | RR 0.86 | 1285 | ⊕⊕⊕⊝ | ||
| 403 per 1000 | 346 per 1000 | |||||
| Moderate | ||||||
| 350 per 1000 | 301 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Method of sequence generation was unclear in all included studies except for the study by Bassler 2015. In the studies by Fok 1999, Jangaard 2002, Merz 1999 and Yong 1999 blinding of outcome assessment was unclear. Except for the study by Bassler 2015, none of the included studies were registered and we were unable to identify whether there was selective reporting or not. | ||||||
| Early inhaled steroid (< 2 weeks) compared to placebo (among survivors) for preventing chronic lung disease in very low birth weight preterm neonates | ||||||
| Patient or population: Very low birth weight preterm neonates | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| placebo (among survivors) | Early inhaled steroid (< 2 weeks) | |||||
| CLD at 36 weeks' PMA | Study population | RR 0.76 | 1088 | ⊕⊕⊕⊝ | ||
| 314 per 1000 | 239 per 1000 | |||||
| Moderate | ||||||
| 188 per 1000 | 143 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CLD at 36 weeks PMA Show forest plot | 5 | 429 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.62, 1.52] |
| 2 CLD at 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.08, 0.14] |
| 3 Death by 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.04 [‐0.09, 0.01] |
| 4 Death by 36 weeks PMA Show forest plot | 6 | 1285 | Risk Difference (M‐H, Fixed, 95% CI) | 0.01 [‐0.03, 0.05] |
| 5 Death by or CLD at 28 days of age Show forest plot | 5 | 429 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.02 [‐0.11, 0.07] |
| 6 Death by or CLD at 36 weeks PMA Show forest plot | 6 | 1285 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.86 [0.75, 0.99] |
| 7 Survival to hospital discharge without CLD Show forest plot | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.14 [‐0.06, 0.34] |
| 8 Death during hospital stay Show forest plot | 1 | 86 | Risk Difference (M‐H, Fixed, 95% CI) | 0.07 [‐0.07, 0.21] |
| 9 Culture proven infection during hospital stay Show forest plot | 6 | 1121 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.00, 0.11] |
| 9.1 Positive blood or CSF culture | 2 | 896 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.01, 0.11] |
| 9.2 Positive blood culture | 4 | 225 | Risk Difference (M‐H, Fixed, 95% CI) | 0.05 [‐0.06, 0.16] |
| 10 Hyperglycaemia Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Hyperglycaemia | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.49, 1.44] |
| 10.2 Hyperglycaemia requiring insulin treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.74, 1.27] |
| 11 Hypertension Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Hypertension | 3 | 116 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.36, 3.99] |
| 11.2 Hypertension requiring treatment | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.21, 1.57] |
| 12 Gastrointesinal bleeding Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.04, 3.34] |
| 13 Cataract Show forest plot | 1 | 253 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.56] |
| 14 Intraventricular haemorrhage Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.77, 1.41] |
| 15 Periventricular leukomalacia Show forest plot | 2 | 306 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.59, 3.46] |
| 16 Brain injury Show forest plot | 1 | 838 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.94, 1.65] |
| 17 Necrotizing enterocolitis Show forest plot | 3 | 1162 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.24] |
| 18 Retinopathy of prematurity (any stage) Show forest plot | 3 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.93, 1.21] |
| 19 Patent ductus arteriosus Show forest plot | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.17] |
| 20 Reintubation Show forest plot | 1 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.96] |
| 21 Requirement for systemic steroids Show forest plot | 8 | 1403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 22 Failure to extubate within 14 days Show forest plot | 5 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.76, 1.24] |
| 23 Death or oxygen dependency at discharge Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.35, 1.15] |
| 24 Death or severe BPD Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.70, 1.25] |
| 25 Death or grade 3 or 4 IVH Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.65, 1.68] |
| 26 Death or PVL Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.41, 1.93] |
| 27 Death or NEC Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.46, 2.06] |
| 28 Death or sepsis Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.44, 1.40] |
| 29 Death or ROP (stage not stated) Show forest plot | 1 | 211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.79, 1.40] |
| 30 Death or neurodevelopmental impairment at 18 months PMA Show forest plot | 1 | 187 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.70, 1.70] |
| 31 Death or neurodevelopmental impairment at 3 years of age Show forest plot | 1 | 179 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.68, 1.56] |
| 32 Death or cerebral palsy at 3 years of age Show forest plot | 1 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.64, 1.96] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 CLD at 36 weeks PMA Show forest plot | 6 | 1088 | Risk Difference (M‐H, Fixed, 95% CI) | ‐0.07 [‐0.13, ‐0.02] |
| 2 CLD at 28 days of age Show forest plot | 5 | 380 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.21] |
| 3 Cerebral palsy Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.33, 5.42] |
| 4 Mean developmental index on BSID‐II < 2 SD of the mean Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.37, 4.17] |
| 5 Respiratory readmission Show forest plot | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.44, 2.29] |