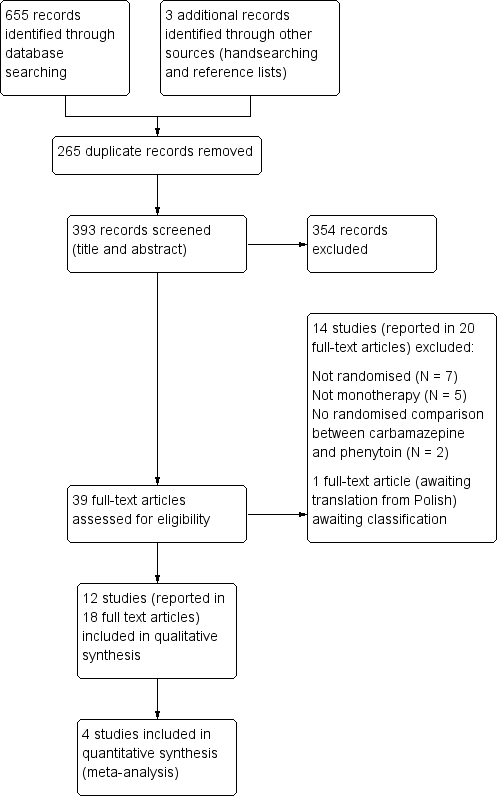
Carbamazepine versus phenytoin monotherapy for epilepsy: an individual participant data review
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Single‐centre, randomised, parallel‐group trial of people referred for assessment at Cork Regional Hospital, Ireland. Three treatment arms: carbamazepine, phenytoin, sodium valproate Dates conducted: Not stated | |
| Participants | Adults and children with a minimum of 2 untreated generalised or partial seizures in the 6 months preceding the study Number randomised: PHT = 58, CBZ = 59 52 participants (44%) with partial epilepsy. 61 (52%) men Age range: 4 to 75 years. Duration of treatment (range in months): 3 to 47 | |
| Interventions | Monotherapy with PHT or CBZ Mean daily dose achieved: PHT = 5.4 mg/kg, CBZ = 10.9 mg/kg | |
| Outcomes | Seizure control: excellent (complete freedom of seizures) good (> 50% reduction in seizure frequency) poor (< 50% reduction in seizure frequency or no response) Side effects | |
| Notes | Outcomes chosen for this review were not reported. IPD not available Funding: Grants provided by Labaz, Geigy, and Warner‐Lambert. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation based on 2 Latin squares without stratification. The first, second and third preference of drug for the participant appears to have been taken into account in the process. Unclear if assignment was completely random |
| Allocation concealment (selection bias) | High risk | An independent person (department secretary) selected the “drug of first preference” from randomisation list on a sequential basis. Allocation not adequately concealed |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported. Intention‐to‐treat approach taken, all randomised participants analysed |
| Selective reporting (reporting bias) | Low risk | Primary outcomes (seizure control) and secondary outcomes (side effects) reported sufficiently |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised, double‐blind cross‐over trial with three 21‐day treatment periods and 2‐week washout period (regular medications used) conducted in a single centre in Newcastle, Indiana, United States Three treatment arms: carbamazepine, phenytoin and phenobarbitone Dates conducted: Not stated | |
| Participants | Institutionalised adults with uncontrolled seizures on current medication Number randomised: PHT = 45, CBZ = 45 41 participants (91%) with partial epilepsy. 28 (62%) men. Age range: 18 to 51 years Study duration 13 weeks (3 x 21‐day treatment periods plus 2 x 2‐week washout periods) | |
| Interventions | Monotherapy with PHT or CBZ | |
| Outcomes | Behaviour outcomes Time to treatment withdrawal due to poor seizure control | |
| Notes | Outcomes chosen for this review were not reported due to cross‐over design Funding: Supported in part by an NIH research contract Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation of groups from random number tables (confirmed by author) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | Withdrawal rates reported, no further information provided |
| Selective reporting (reporting bias) | Low risk | All efficacy and tolerability outcomes specified in the Methods sections reported well in the Results section. No protocol available, outcomes for this review not available due to trial cross‐over design |
| Other bias | High risk | Cross‐over design may not be appropriate for monotherapy designs, likely carry‐over effects from 1 period to another, so the comparison may not be entirely monotherapy |
| Methods | 36‐month randomised, comparative study Four treatment arms: carbamazepine, sodium valproate, phenytoin, phenobarbitone Dates conducted and country: Not stated (assumed conducted in Poland due to author affiliations) | |
| Participants | Adults with newly‐diagnosed epilepsy Number randomised: CBZ = 30, PHT = 30 100% partial epilepsy, Age range: 18 to 40 years Percentage men and range of follow‐up not mentioned (outcome recorded at 3 years) | |
| Interventions | Monotherapy with PHT or CBZ Starting doses CBZ = 400 mg/day, PHT = 200 mg/day. Dose achieved not stated | |
| Outcomes | Proportion achieving 24‐month remission at 3 years and exclusions after randomisation due to adverse effects or no efficacy | |
| Notes | Abstract only. Outcomes chosen for this review were not reported, IPD pledged but not received Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study randomised but no further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) | Unclear risk | "Exclusion rates" reported for all treatment groups, no further information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol available, study available in abstract format only. Outcomes for this review not available |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised, parallel‐group, open‐label paediatric study conducted in 2 centres in the United Kingdom. Trial conducted between 1981 and 1987 Four treatment arms: carbamazepine, sodium valproate, phenytoin, phenobarbitone | |
| Participants | Children with newly‐diagnosed epilepsy (2 or more untreated partial or generalised tonic‐clonic seizures in the 12 months preceding the study) Number randomised: CBZ = 54, PHT = 54 64 children (59%) with partial epilepsy. 59 (55%) boys. Mean age (range): 9 (3 to 16) years Range of follow‐up: 3 to 88 (months) | |
| Interventions | Monotherapy with PHT or CBZ. Median daily dose achieved: PHT = 175 mg/day, CBZ = 400 mg/day | |
| Outcomes | Time to first seizure recurrence after start of therapy Time to 12‐month remission from all seizures Adverse effects and withdrawals due to adverse events | |
| Notes | IPD provided for all randomised participants. All outcomes in this review calculated from IPD Funding: support provided by the Medical Research Council, the Health Promotion Trust, Ciba‐Geigy, Parke‐Davis, and Sanofi Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via 4 batches of concealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unblinded; authors state masking of treatment would not be “practicable or ethical” and would “undermine compliance.” Unclear if lack of masking influenced outcome |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unblinded; authors state masking of treatment would not be “practicable or ethical” and would “undermine compliance.” Unclear if lack of masking influenced outcome |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided1 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided1 |
| Other bias | Low risk | No other bias detected |
| Methods | Single‐centre, randomised, parallel‐group trial. Three treatment arms: carbamazepine, phenytoin, sodium valproate Dates conducted and country: Not stated (assumed conducted in United Kingdom due to author affiliations) | |
| Participants | Children with at least 3 newly‐diagnosed generalised or partial seizures within a period of 6 months Number randomised: PHT = 20, CBZ = 23 No information on epilepsy type, sex or range of follow‐up Age range: 5 to 14 years. Study duration: 12 months | |
| Interventions | Monotherapy with PHT or CBZ Mean dose: PHT = 6.1 mg/day, CBZ = 17.9 mg/day | |
| Outcomes | Cognitive assessments Summary of withdrawals from randomised drug | |
| Notes | Outcomes chosen for this review were not reported IPD not available, but could be constructed from the publication for the outcome 'Time to withdrawal of allocated drug' Funding: A grant was obtained from the Yorkshire Regional Health Authority, support for measuring serum levels provided by Ciba‐Geigy PLC and Sanofi PLC. Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quota allocation by sex, age, seizure type and current treatment is an inadequate randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | High risk | Personnel and participants (and parents) unblinded |
| Blinding of outcome assessment (detection bias) | Low risk | Outcome assessors single‐blinded for cognitive testing |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, results reported and analysed for all participants randomised and all who completed various stages of follow‐up |
| Selective reporting (reporting bias) | Unclear risk | 1 of 4 outcomes for this review reported. Cognitive outcomes described in Methods section well reported in Results section. Adverse effects reported, no seizure outcomes reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised, parallel‐group, open‐label paediatric study conducted in 2 centres in the United Kingdom. Trial conducted between 1981 and 1987 Four treatment arms: carbamazepine, sodium valproate, phenytoin, phenobarbitone | |
| Participants | Adults with newly‐diagnosed epilepsy (2 or more untreated partial or generalised tonic‐clonic seizures in the 12 months preceding the study) Number randomised: CBZ = 61, PHT = 63 52 participants (42%) with partial epilepsy. 64 (52%) men. Mean age (range): 31 (13 to 72) years Range of follow‐up (months): 1 to 91 | |
| Interventions | Monotherapy with PHT or CBZ. Median daily dose achieved: PHT = 300 mg/day, CBZ = 600 mg/day | |
| Outcomes | Time to first seizure recurrence after start of therapy Time to 12‐month remission from all seizures Adverse effects and withdrawals due to adverse events | |
| Notes | IPD provided for all randomised participants. All outcomes in this review calculated from IPD Funding: support provided by the Medical Research Council, the Health Promotion Trust, Ciba‐Geigy, Parke‐Davis, and Sanofi Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list generated using permuted blocks of size 8 or 16 with stratification for centre, seizure type and presence of neurological signs |
| Allocation concealment (selection bias) | Low risk | Allocation concealed via 4 batches of concealed opaque envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Unblinded; authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large drop‐out rate.” Unclear if outcome was influenced |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unblinded; authors state masking of treatment would not be “practical” and would have “introduced bias due to a very large drop‐out rate.” Unclear if outcome was influenced |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided1 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided1 |
| Other bias | Low risk | No other bias detected |
| Methods | Multicentre, randomised, parallel‐group, double‐blinded study over 10 centres in the USA with separate randomisation schemes used for each seizure type. Four treatment arms: carbamazepine, phenytoin, phenobarbitone, primidone Dates conducted: Not stated | |
| Participants | Adults with previously untreated or under‐treated simple or complex partial or secondary generalised tonic‐clonic seizures Number randomised: PHT = 165, CBZ = 155 100% partial epilepsy. 278 (87%) men. Mean age (range): 41 (18 to 82) years Range of follow‐up: 0 to 66 months | |
| Interventions | Monotherapy with PHT or CBZ. Median daily dose achieved: PHT = 400 mg/day, CBZ = 800 mg/day | |
| Outcomes | Participant retention/time to drug failure (length of time participant continued to take randomised drug) Composite scores of seizure frequency (seizure rates and total seizure control) and toxicity Incidence of side effects | |
| Notes | IPD provided for all randomised participants. All outcomes in this review calculated from IPD Funding: supported by the Veterans Adminstration Medical Research Service Cooperative Studies Program (CS 118) Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomised with stratification for seizure type. Method of randomisation not stated and not provided by authors |
| Allocation concealment (selection bias) | Unclear risk | No information provided in the publication or by study authors |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind (participants and personnel) achieved using an additional blank tablet |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessment was blinded, no information provided |
| Incomplete outcome data (attrition bias) | Low risk | Attrition rates reported, all randomised participants analysed from IPD provided1 |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or calculated with IPD provided1 |
| Other bias | Low risk | No other bias detected |
| Methods | Prospective randomised study. Three treatment arms: carbamazepine, phenytoin and sodium valproate Dates conducted and country: Not stated (assumed conducted in Japan due to author affiliation) | |
| Participants | Children aged 1 to 14 with previously untreated partial seizures and/or generalised tonic‐clonic seizures Number randomised: PHT = 51, CBZ = 66. 84 (72%) with partial seizures. No information on gender Range of follow‐up: 6 to 66 months, mean follow‐up: 37 months in PHT group, 34 in CBZ group | |
| Interventions | Monotherapy with PHT or CBZ. Initial daily dose: PHT = 7.2 ± 1.4 mg/kg/day, CBZ = 13.0 ± 1.6 mg/kg/day | |
| Outcomes | Proportion of all randomised participants with seizure recurrence (by seizure type) Proportion of participants with optimum plasma levels with seizure recurrence (by seizure type) | |
| Notes | Very limited information available.The study is reported in a summary publication of 3 different studies (other 2 studies are not CBZ vs PHT). Outcomes chosen for this review were not reported, and IPD not available Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study is described as "randomised" but no further details are provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided; unclear if the study was blinded or not |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided; unclear if the study was blinded or not |
| Incomplete outcome data (attrition bias) | Low risk | Ranges of follow‐up given for both treatment groups. Results reported "at the end of follow up," no withdrawals or exclusions mentioned, all participants included in analysis |
| Selective reporting (reporting bias) | Unclear risk | Seizure recurrence outcomes described and well reported. No adverse events reported; no protocol available so unclear if adverse events were planned a priori. Outcomes for this review not available |
| Other bias | Low risk | No other bias detected |
| Methods | Double‐blinded, parallel‐group, randomised study conducted in a single centre in Nigeria between October 2000 and October 2002 Three treatment arms: carbamazepine, phenytoin, phenobarbitone | |
| Participants | Consecutive newly‐diagnosed people aged 14 or over presenting at the outpatient neurology clinic of the University Teaching Hopsital, Benin City, Nigeria with recurrent, untreated afebrile seizures Number randomised: PHT = 19, CBZ = 19 8 participants with partial seizures (22%), 23 men (62%). Mean age (range): 29.8 years (14 to 38 years) All participants followed up for 12 weeks | |
| Interventions | Monotherapy with PHT or CBZ. Median daily dose (range): PHT = 200 mg (100 to 300 mg), CBZ = 600 mg (400 to 1200 mg) | |
| Outcomes | Cognitive measures (reaction times, mental speed, memory, attention) | |
| Notes | IPD provided for all randomised participants. Study duration was 12 weeks; all participants completed the study without withdrawing, so outcomes 'Time to withdrawal of allocated drug', 'Time to six‐month remission' and 'Time to 12‐month remission' could not be calculated. 'Time to first seizure' calculated from IPD provided Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study randomised using simple randomisation. Each participant was asked to pick 1 from a table of numbers (1 ‐ 60), numbers corresponded to allocation of 1 of 3 drugs (information provided by author) |
| Allocation concealment (selection bias) | Low risk | Recruitment/randomisation of participants and allocation of treatments took place on different sites (information provided by author) |
| Blinding of participants and personnel (performance bias) | Low risk | Participants single‐blinded. Research assistant recruiting participants and counselling on medication adherence was not blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Investigators performing cognitive assessments were single‐blinded |
| Incomplete outcome data (attrition bias) | Low risk | All randomised participants completed the study. All randomised participants analysed from IPD provided1 |
| Selective reporting (reporting bias) | Low risk | 1 outcome for this review calculated from IPD provided1. Other outcomes for this review not available due to short study length. All cognitive outcomes from the study well reported |
| Other bias | Low risk | No other bias detected |
| Methods | Single‐centre, randomised, parallel‐group trial of participants, referrals to the outpatient department of neurology of the Central Hospital of Paijat‐Hame, Finland. Two treatment arms: carbamazepine and phenytoin Dates conducted: Not stated | |
| Participants | Adults (eligible age range 15 to 57) with newly‐diagnosed epilepsy Number randomised: PHT = 20, CBZ = 23* 10 (23%) participants with partial epilepsy. 20 (47%) men Mean age (SD) years: PHT = 31.5 (11.3), CBZ = 26.8 (13.2) | |
| Interventions | Monotherapy with PHT or CBZ. Dose information not reported | |
| Outcomes | Cognitive assessments (visual motor speed, co‐ordination, attention and concentration, verbal and visuospatial learning, visual and recognition memory, reasoning, mood, handedness) Harmful side effects | |
| Notes | *59 participants were randomised but 16 were subsequently excluded. Results were presented only for the 43 participants who completed the entire study. Outcomes chosen for this review were not reported. IPD not available Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly assigned to treatment groups, method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided; unclear if participants and personnel were blinded |
| Blinding of outcome assessment (detection bias) | Low risk | Cognitive outcome assessor was blinded |
| Incomplete outcome data (attrition bias) | High risk | 16/59 (27%) of participants excluded from analysis. Results presented only for 43 participants who completed the study |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes described in Methods section well reported in Results section. Adverse effects reported, no seizure outcomes reported and outcomes chosen for this review not reported. No protocol available so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | No other bias detected |
| Methods | Randomised, 'two compartment' parallel study, conducted in the United States. Two treatment arms: carbamazepine and phenytoin Dates conducted: Not stated | |
| Participants | Adults, previously untreated, with at least 2 seizures or at least 1 seizure and an EEG with paroxysmal features Number randomised: PHT = 45, CBZ = 42 55 participants (63%) with partial epilepsy. 60 (69%) men. Overall mean age (range) 37.4 (18 to 77) years. Study duration: 2 years. Range of follow‐up not reported | |
| Interventions | Monotherapy with PHT or CBZ Mean daily dose achieved (for the 54 participants with no major side effects): PHT = 5.35 mg/kg/day, CBZ = 9.32 mg/kg/day | |
| Outcomes | Laboratory measures Side effects (major and minor) Seizure control/treatment failure | |
| Notes | 7 participants on CBZ and 10 participants on PHT were “dropped for non‐compliance” and excluded from analysis Outcomes chosen for this review were not reported. IPD not available Funding: Supported in part by the Southern Foundation for Brain Research Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants randomly assigned to treatment groups; method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Low risk | Double‐blind (participants and personnel) achieved with additional blank tablet |
| Blinding of outcome assessment (detection bias) | Unclear risk | Unclear if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) | High risk | 17/87 (19.5%) of participants excluded from analysis for "non‐compliance". Results presented only for participants who completed the study |
| Selective reporting (reporting bias) | Low risk | All efficacy and tolerability outcomes specified in the Methods sections reported well in the Results section. No protocol available. Outcomes chosen for this review were not reported |
| Other bias | Unclear risk | No other bias detected |
| Methods | Single‐centre, randomised, parallel‐group study of participants referred to the Neurology Clinic of Nehru Hospital, Chandigarh, India. Two treatment arms: carbamazepine and phenytoin Dates conducted: Not stated | |
| Participants | Newly‐diagnosed and drug naïve adults over the age of 14 attending the Neurology Clinic of Nehru Hospital, Chandigarh, India Number randomised: PHT = 20, CBZ = 20 11 participants with partial epilepsy (27.5%), 28 men (70%) Mean age (range): PHT group 23.4 (14 to 44 years), CBZ 24.4 (14 to 45 years) Study duration 10 to 12 weeks. Range of follow‐up not reported | |
| Interventions | Monotherapy with PHT or CBZ. Initial daily dose: PHT = 5 mg/kg/day, CBZ = 10 mg/kg/day | |
| Outcomes | Cognitive measures before and after treatments (verbal, performance, memory, visuomotor, perceptomotor organisation, visual organisation, dysfunction) | |
| Notes | 6 participants on CBZ and 8 participants on PHT were excluded from final analysis of cognitive assessments who were lost to follow‐up or who had uncontrolled seizures Outcomes chosen for this review were not reported. IPD not available Funding: Not stated Conflicts of interest: None stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The subjects were randomised to one of the two study groups," no further information given on methods of randomisation |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | No information provided; unclear if study was blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | No information provided; unclear if study was blinded |
| Incomplete outcome data (attrition bias) | High risk | 14/40 (35%) of participants excluded from analysis who were lost to follow‐up or experienced uncontrolled seizures. Results presented only for participants who completed the study |
| Selective reporting (reporting bias) | Unclear risk | Cognitive outcomes described in Methods section well reported in Results section. No seizure outcomes or adverse events reported and outcomes chosen for this review not reported. No protocol available, so unclear if seizure outcomes were planned a priori |
| Other bias | Low risk | No other bias detected |
1For studies in which IPD were provided (De Silva 1996; Heller 1995; Mattson 1985; Ogunrin 2005) attrition and reporting bias are reduced as attrition rates and unpublished outcome data are requested.
CBZ: carbamazepine
EEG: electroencephalograph
IPD: individual participant data
PHT: phenytoin
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Unclear whether trial is randomised and unclear whether participants received either CBZ or PHT as monotherapy. Authors could not be contacted to clarify therefore trial excluded due to uncertainties. | |
| Comparison between CBZ monotherapy and PHT monotherapy cannot be made. Participants were given phenobarbital initially which was later withdrawn whilst either CBZ or PHT was also introduced | |
| Comparison between CBZ monotherapy and PHT monotherapy cannot be made. No randomised monotherapy comparison between CBZ and PHT. Participants were separated into 2 treatment arms (based on previous drug failure) and randomised to CBZ and clobazam in 1 arm and PHT or clobazam in the other arm | |
| Comparison between CBZ monotherapy and PHT monotherapy cannot be made. Participants who failed CBZ or PHT monotherapy were randomised to levetiracetam or VPS monotherapy | |
| Participants were randomised to lamotrigine or 'standard therapy' (PHT, CBZ or VPA at the choice of the investigator). No randomised comparison can be made of CBZ and PHT | |
| Comparison between CBZ monotherapy and PHT monotherapy cannot be made. All medication except phenobarbital and primidone were discontinued gradually, whilst dose of randomised drug CBZ or PHT was increased | |
| Study is not randomised; participants were already on CBZ or PHT monotherapy on entry into the study | |
| Unclear if the study was randomised. Comparison between CBZ monotherapy and PHT monotherapy cannot be made. The trial has a cross‐over design with a 2‐week washout period in which both drugs were taken to make a gradual transition | |
| Not fully randomised: “The treatment was chosen at random unless the individual diagnoses required a specific drug” | |
| Direct comparison between CBZ and PHT not available. The publication reports 2 separate randomised studies, the first compares VPS and PHT and the second compares VPS and CBZ | |
| Study is not randomised | |
| Randomised participants were slowly withdrawn from their previous treatment as part of the trial and therefore a comparison between CBZ and PHT monotherapy cannot be made | |
| All participants received PHT for 2 months prior to entering a randomised cross‐over period. It is unclear whether a comparison between CBZ and PHT monotherapy could be made | |
| The study is not randomised ‐ the investigator made the choice of treatment for each participant |
CBZ: carbamazepine
PHT: phenytoin
VPS: sodium valproate
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | 2‐arm trial of carbamazepine and phenytoin. Unclear from information provided in the abstract if the study is randomised |
| Participants | 64 participants with untreated partial (n = 9), partial complex (n = 27), partial secondary generalised (n = 22), or primary generalised seizures (n = 6) |
| Interventions | Monotherapy with carbamazepine or phenytoin. Unclear how many participants were allocated to each drug |
| Outcomes | Somatosensoric evoked potentials (mean wave amplitude, mean proximal conduction time, mean central conduction time) |
| Notes | Full‐text available only in Polish; abstract available in English. Full‐text is awaiting translation before eligibility can be judged |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to withdrawal of allocated treatment Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.75, 1.30] |
| Analysis 1.1  Comparison 1 Carbamazepine versus phenytoin, Outcome 1 Time to withdrawal of allocated treatment. | ||||
| 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.78, 1.39] |
| Analysis 1.2  Comparison 1 Carbamazepine versus phenytoin, Outcome 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type. | ||||
| 2.1 Partial onset | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 1.18 [0.87, 1.60] |
| 2.2 Generalised seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 0.42 [0.18, 0.96] |
| 3 Time to achieve 12‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.79, 1.25] |
| Analysis 1.3 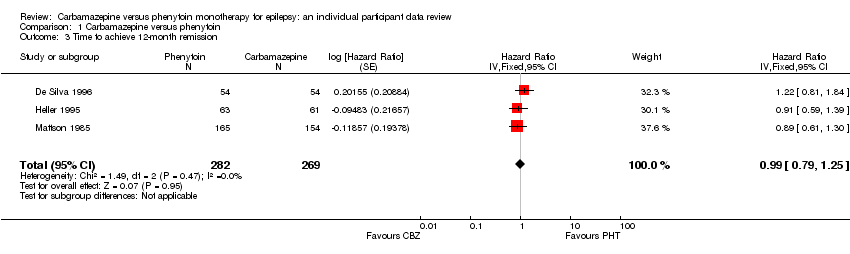 Comparison 1 Carbamazepine versus phenytoin, Outcome 3 Time to achieve 12‐month remission. | ||||
| 4 Time to achieve 12‐month remission ‐ stratified by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Random, 95% CI) | 1.01 [0.78, 1.31] |
| Analysis 1.4 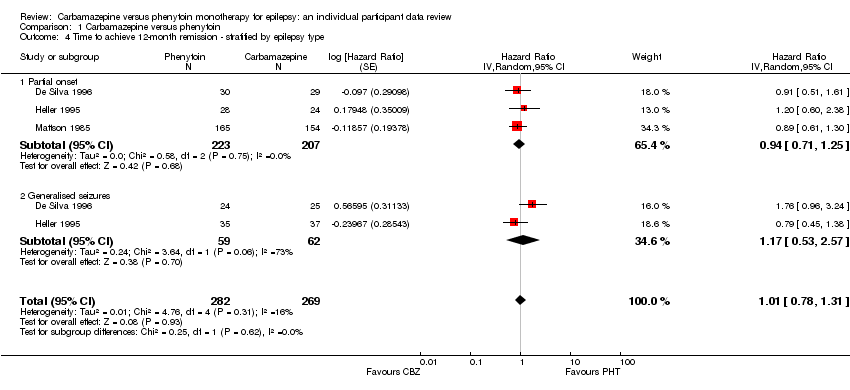 Comparison 1 Carbamazepine versus phenytoin, Outcome 4 Time to achieve 12‐month remission ‐ stratified by epilepsy type. | ||||
| 4.1 Partial onset | 3 | 430 | Hazard Ratio (Random, 95% CI) | 0.94 [0.71, 1.25] |
| 4.2 Generalised seizures | 2 | 121 | Hazard Ratio (Random, 95% CI) | 1.17 [0.53, 2.57] |
| 5 Time to achieve six‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| Analysis 1.5 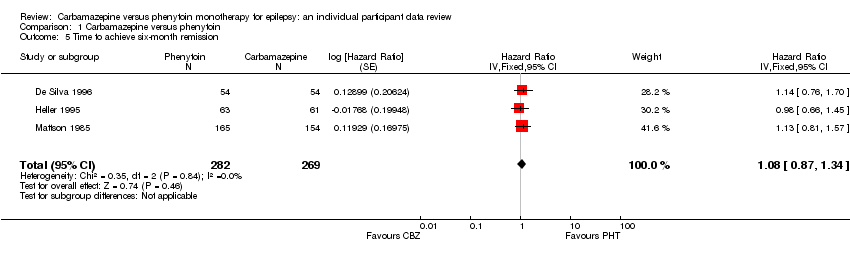 Comparison 1 Carbamazepine versus phenytoin, Outcome 5 Time to achieve six‐month remission. | ||||
| 6 Time to achieve six‐month remission ‐ stratified by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.11 [0.89, 1.37] |
| Analysis 1.6  Comparison 1 Carbamazepine versus phenytoin, Outcome 6 Time to achieve six‐month remission ‐ stratified by epilepsy type. | ||||
| 6.1 Partial onset | 3 | 430 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.79, 1.33] |
| 6.2 Generalised seizures | 2 | 121 | Hazard Ratio (Fixed, 95% CI) | 1.30 [0.89, 1.92] |
| 7 Time to first seizure post‐randomisation Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| Analysis 1.7 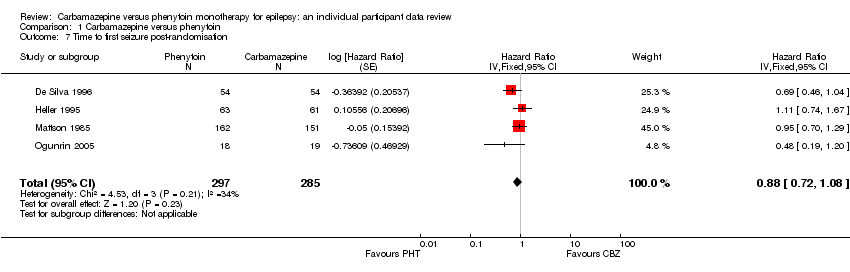 Comparison 1 Carbamazepine versus phenytoin, Outcome 7 Time to first seizure post‐randomisation. | ||||
| 8 Time to first seizure post‐randomisation ‐ stratified by epilepsy type Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.70, 1.04] |
| Analysis 1.8 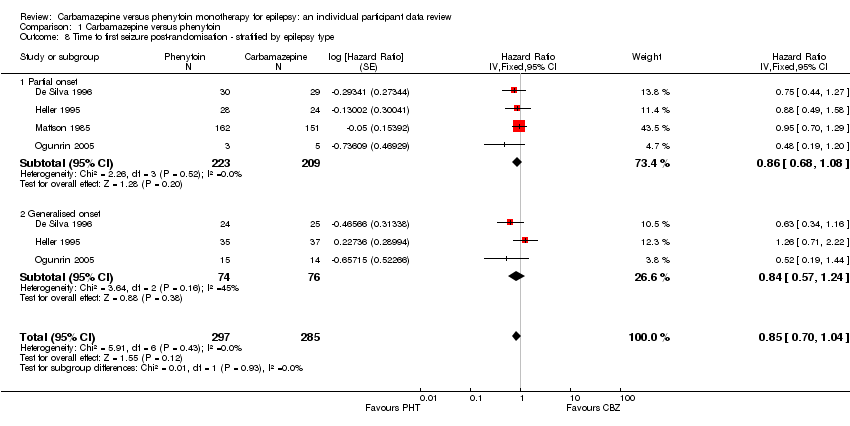 Comparison 1 Carbamazepine versus phenytoin, Outcome 8 Time to first seizure post‐randomisation ‐ stratified by epilepsy type. | ||||
| 8.1 Partial onset | 4 | 432 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 8.2 Generalised onset | 3 | 150 | Hazard Ratio (Fixed, 95% CI) | 0.84 [0.57, 1.24] |
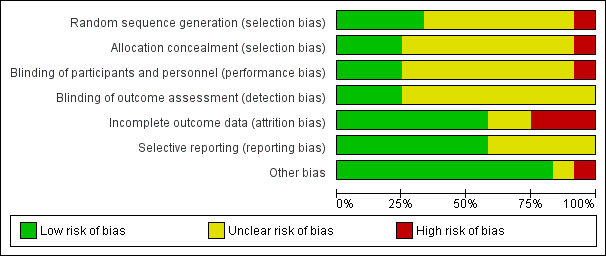
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Time to withdrawal of allocated treatment
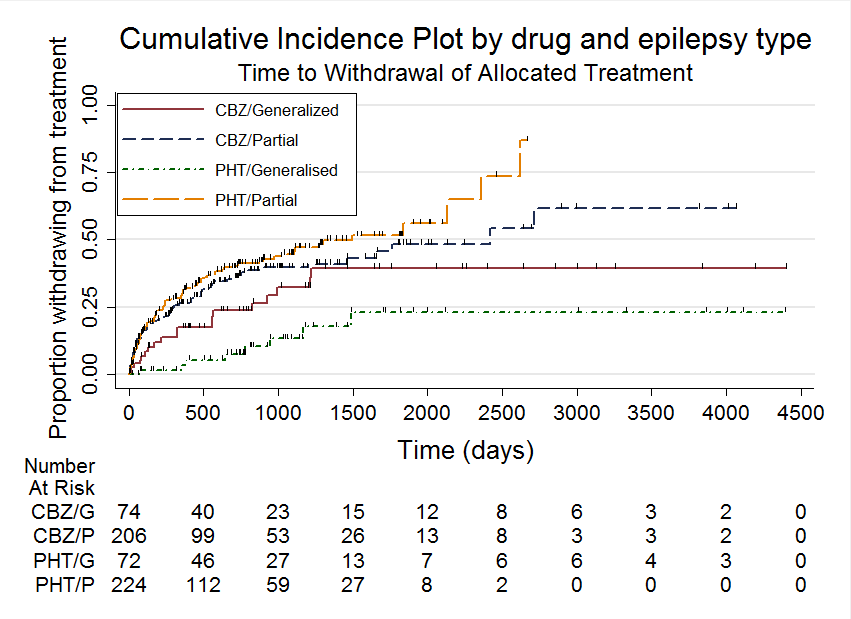
Time to withdrawal of allocated treatment, stratified by epilepsy type
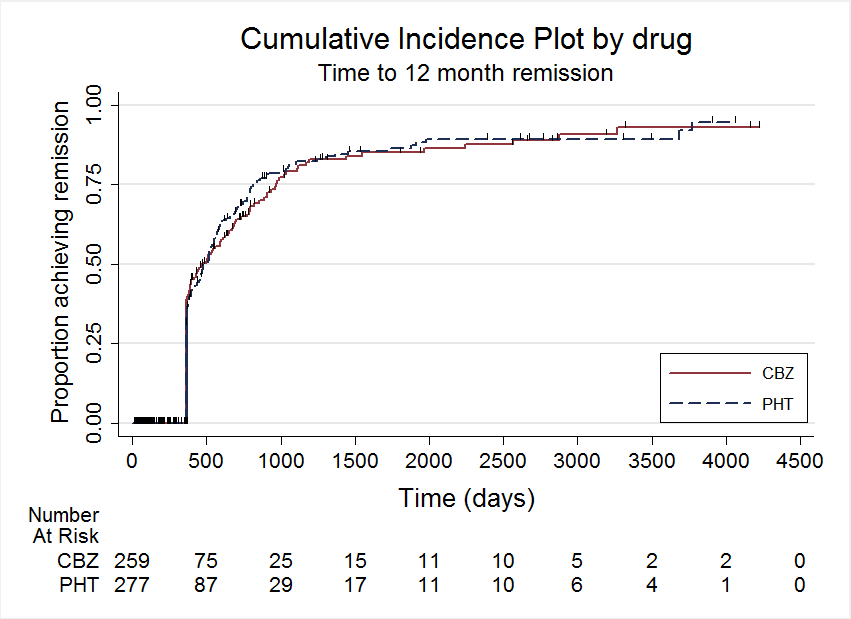
Time to 12 month remission

Time to 12 month remission, stratified by epilepsy type

Time to 6 month remission, stratified by epilepsy type

Time to first seizure, stratified by epilepsy type
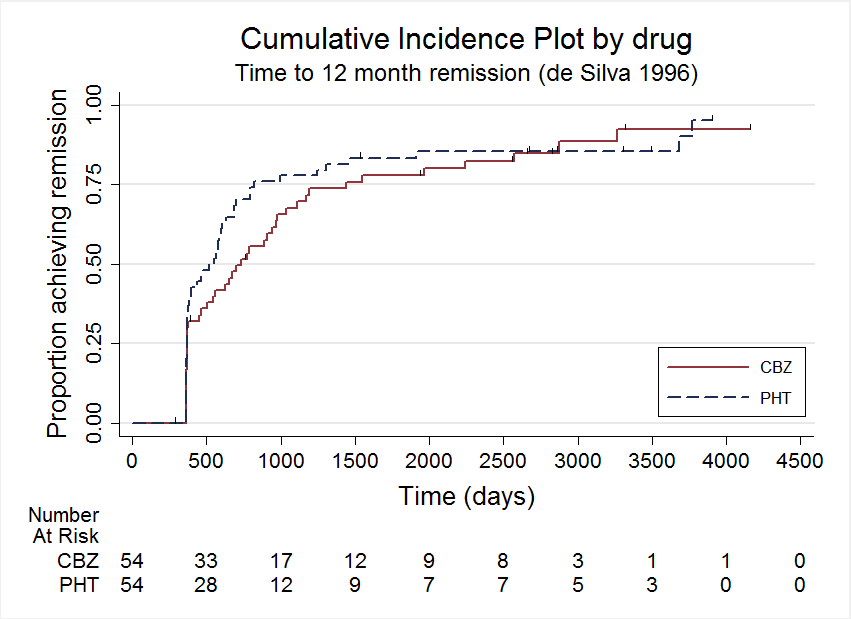
Time to 12 month remission, deSilva 1996

Time to 6 month remission, deSilva 1996
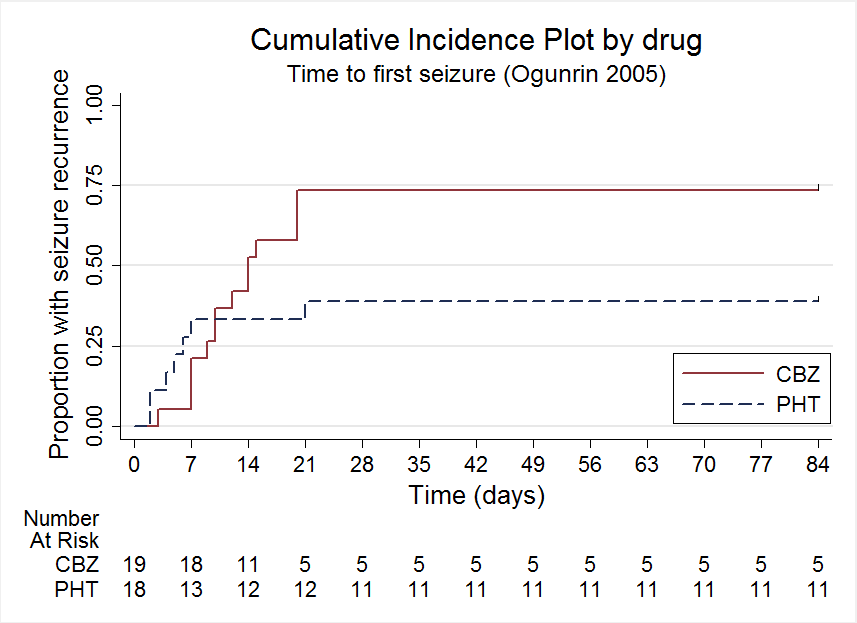
Time to first seizure, Ogunrin 2005

Comparison 1 Carbamazepine versus phenytoin, Outcome 1 Time to withdrawal of allocated treatment.

Comparison 1 Carbamazepine versus phenytoin, Outcome 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type.

Comparison 1 Carbamazepine versus phenytoin, Outcome 3 Time to achieve 12‐month remission.

Comparison 1 Carbamazepine versus phenytoin, Outcome 4 Time to achieve 12‐month remission ‐ stratified by epilepsy type.

Comparison 1 Carbamazepine versus phenytoin, Outcome 5 Time to achieve six‐month remission.

Comparison 1 Carbamazepine versus phenytoin, Outcome 6 Time to achieve six‐month remission ‐ stratified by epilepsy type.

Comparison 1 Carbamazepine versus phenytoin, Outcome 7 Time to first seizure post‐randomisation.

Comparison 1 Carbamazepine versus phenytoin, Outcome 8 Time to first seizure post‐randomisation ‐ stratified by epilepsy type.
| Carbamazepine compared with phenytoin for epilepsy | ||||||
| Patient or population: adults and children with new‐onset partial or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenytoin | Carbamazepine | |||||
| Time to withdrawal of allocated treatment Range of follow‐up (all participants): 1 day to 4403 days | 37 per 100 | 35 per 100 (28 to 44) | HR 1.04 (0.78 to 1.39) | 546 (3 studies) | ⊕⊕⊕⊝ moderate2,3 | HR > 1 indicates a clinical advantage for carbamazepine |
| Time to withdrawal of allocated treatment Range of follow‐up (all participants): 1 day to 4064 days | 42 per 100 | 37 per 100 (29 to 47) | HR 1.18 (0.87 to 1.60) | 428 (3 studies) | ⊕⊕⊕⊝ moderate2,3 | HR > 1 indicates a clinical |
| Time to withdrawal of allocated treatment Range of follow‐up (all participants): 1 day to 4403 days | 14 per 100 | 30 per 100 (15 to 57) | HR 0.42 (0.18 to 0.96) | 118 (2 studies) | ⊕⊕⊕⊝ moderate2,3 | HR > 1 indicates a clinical |
| Proportion of withdrawals due to adverse effects Range of follow‐up (all participants): 1 day to 4403 days | 4 per 100 | 6 per 100 (5 to 7) | RR 1.42 (1.13 to 1.80) | 546 (3 studies) | ⊕⊕⊕⊝ moderate2 | RR < 1 indicates a clinical advantage for carbamazepine |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The assumed risk is calculated as the event rate in the phenytoin treatment group. The corresponding risk in the carbamazepine treatment group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk is calculated as the assumed risk x the relative risk (RR) of the intervention where RR = (1 ‐ exp(HR x ln(1 ‐ assumed risk)) ) / assumed risk | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Pooled HR for all participants adjusted for seizure type. | ||||||
| Carbamazepine compared with phenytoin for epilepsy | ||||||
| Patient or population: adults and children with new‐onset partial or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No. of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenytoin | Carbamazepine | |||||
| Time to 12‐month remission Range of follow‐up (all participants): 0 days to 4222 days | 55 per 100 | 55 per 100 (46 to 65) | HR 1.01 (0.78 to 1.31) | 551 (3 studies) | ⊕⊕⊕⊝ moderate2,3 | HR > 1 indicates a clinical |
| Time to 12‐month remission Range of follow‐up (all participants):0 days to 4222 days | 47 per 100 | 45 per 100 (36 to 55) | HR 0.94 (0.71 to 1.25) | 430 (3 studies) | ⊕⊕⊕⊝ moderate2,3 | HR > 1 indicates a clinical |
| Time to 12‐month remission Range of follow‐up (all participants): 7 days to 4163 days | 85 per 100 | 88 per 100 (63 to 99) | HR 1.174 (0.53 to 2.57) | 121 (2 studies) | ⊕⊕⊝⊝ low2,3,4 | HR > 1 indicates a clinical |
| Time to 6‐month remission Range of follow‐up (all participants): 0 days to 4222 days | 63 per 100 | 67 per 100 (59 to 75) | HR 1.11 (0.89 to 1.37) | 551 (3 studies) | ⊕⊕⊕⊝ moderate2,3 | HR >1 indicates a clinical |
| Time to 6‐month remission Range of follow‐up (all participants): 0 days to 4222 days | 56 per 100 | 56 per 100 (47 to 66) | HR 1.02 (0.79 to 1.33) | 430 (3 studies) | ⊕⊕⊕⊝ moderate2,3 | HR > 1 indicates a clinical |
| Time to 6‐month remission Range of follow‐up (all participants): 7 days to 4163 days | 93 per 100 | 97 per 100 (91 to 99) | HR 1.30 (0.89 to 1.92) | 121 (2 studies) | ⊕⊕⊕⊝ moderate2,3 | HR > 1 indicates a clinical |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The assumed risk is calculated as the event rate in the Phenytoin treatment group The corresponding risk in the carbamazepine treatment group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk is calculated as the assumed risk x the relative risk (RR) of the intervention where RR = (1 ‐ exp(HR x ln(1 ‐ assumed risk)) ) / assumed risk | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Pooled HR for all participants adjusted for seizure type. | ||||||
| Carbamazepine compared with phenytoin for epilepsy | ||||||
| Patient or population: adults and children with new‐onset partial or generalised epilepsy Settings: outpatients Intervention: carbamazepine Comparison: phenytoin | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Phenytoin | Carbamazepine | |||||
| Time to first seizure Range of follow‐up (all participants): 0 days to 4589 days | 65 per 100 | 71 per 100 (63 to 77) | HR 0.85 (0.70 to 1.04) | 582 (4 studies) | ⊕⊕⊝⊝ low2,3,4 | HR > 1 indicates a clinical |
| Time to first seizure Range of follow‐up (all participants): 0 days to 4589 days | 63 per 100 | 68 per 100 (60 to 77) | HR 0.86 (0.68 to 1.08) | 432 (4 studies) | ⊕⊕⊝⊝ low2,3,4 | HR > 1 indicates a clinical |
| Time to first seizure Range of follow‐up (all participants): 2 days to 4070 days | 69 per 100 | 75 per 100 (61 to 87) | HR 0.84 (0.57 to 1.24) | 150 (3 studies) | ⊕⊕⊝⊝ low2,3,4 | HR > 1 indicates a clinical |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The assumed risk is calculated as the event rate in the Phenytoin treatment group The corresponding risk in the carbamazepine treatment group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk is calculated as the assumed risk x the relative risk (RR) of the intervention where RR = (1 ‐ exp(HR x ln(1 ‐ assumed risk)) ) / assumed risk | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Pooled HR for all participants adjusted for seizure type. 2Risk of bias unclear for one element of all of the three studies included in the analysis. De Silva 1996 and Heller 1995 are open‐label and it is unclear whether the lack of masking impacted upon the results; and we do not know how allocation was concealed in Mattson 1985. 348 adult participants in Heller 1995 and Ogunrin 2005 may have had their seizure type wrongly classified as generalised onset; sensitivity analyses show misclassification is unlikely to have had an impact on results and conclusions. 4Ogunrin 2005 is a short study (12 weeks) and has a small sample size of 37 compared to the other three studies of duration 3 ‐ 10 years and sample sizes of around 100 to 300 participants (De Silva 1996; Heller 1995; Mattson 1985). Ogunrin 2005 is less precise with wide CIs, and there is evidence that the treatment effect in this study changes over time. | ||||||
| Trial | Outcomes reported | Summary of results |
| 1. Seizure control: excellent (seizure‐free) | 1. PHT (n = 58); CBZ (n = 59) PHT: 39 (67%); CBZ: 22 (37%) | |
| 1. Behaviour measured with rating scale modified from the Ward Behaviour Rating Scale 2. Seizure control 3. Side effects 4. Withdrawals | 1. Behavioural scores were similar on both drugs 2. No difference between CBZ and PHT in terms of seizure control 3. Gastrointestinal and “impaired function” side effects were more common on CBZ than PHT in the first few study days. Side effects of both drugs were minimal in later stages of the study 4. PHT: 21 withdrawals out of 45 participants (47%); CBZ: 27 withdrawals out of 45 participants (60%) | |
| 1. Proportion achieving 24‐month remission at 3 years 2. Proportion excluded after randomisation due to adverse effects or no efficacy | 1. PHT: 59%; CBZ: 62% 2. PHT: 23%; CBZ: 30% | |
| 1. Cognitive assessments 2. Withdrawals from randomised drug | 1. No significant differences between the two treatment groups on any cognitive tests | |
| 1. Proportion of all randomised participants with seizure recurrence (by seizure type) 2. Proportion of participants with optimum plasma levels with seizure recurrence (by seizure type) | PHT (n = 51); CBZ (n = 66) 1. PHT (partial): 10/31 (32%); PHT (generalised): 7/20 (35%); 2. PHT (partial): 4/17 (24%); PHT (generalised): 1/8 (13%); | |
| 1. Cognitive assessments (visual motor speed, co‐ordination, attention and concentration, verbal and visuospatial learning, visual and recognition memory, reasoning, mood, handedness) 2. Harmful side effects | 1. Compared to CBZ, participants on PHT became slower (motor speed of the hand) and their visual memory decreased. There was an equal decrease in negative mood (helplessness, irritability, depression) on PHT and CBZ 2. Three participants taking PHT complained of tiredness, and 1 participant taking CBZ complained of facial skin problems, another tiredness and memory problems | |
| 1. Side effects (major and minor) 3. Laboratory results | 1. Incidence of:
2. Treatment failures among analysed participants: Seizure control (among analysed participants with no major side effects): PHT: 23/27 participants (86%); CBZ: 22/27 participants (82%) 3. Significantly lower mean LDH level at 24 weeks in CBZ participants than PHT participants (P < 0.01). Other laboratory results similar across treatment groups | |
| 1. Cognitive measures (verbal, performance, memory, visuomotor, perceptomotor organisation, visual organisation, dysfunction) | 1. No significant differences between any tests of cognitive function taken before treatment and after 10 ‐ 12 weeks for both treatment groups | |
| CBZ = carbamazepine, LDH = lactate dehydrogenase, PHT= phenytoin | ||
| Trial | Number randomised | Time to withdrawal of allocated treatment | Time to 12‐month remission | Time to 6‐month remission | Time to first seizure | ||||||||||
| PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | PHT | CBZ | Total | |
| 54 | 54 | 108 | 53 | 53 | 106 | 54 | 54 | 108 | 54 | 54 | 108 | 54 | 54 | 108 | |
| 63 | 61 | 124 | 61 | 60 | 121 | 63 | 61 | 124 | 63 | 61 | 124 | 63 | 61 | 124 | |
| 165 | 155 | 320 | 165 | 154 | 319 | 165 | 154 | 319 | 165 | 154 | 319 | 162 | 151 | 313 | |
| 20 | 23 | 43 | 20 | 23 | 43 | Information not available | Information not available | Information not available | |||||||
| 18 | 19 | 37 | Information not available | Information not available | Information not available | 18 | 19 | 37 | |||||||
| Total | 320 | 312 | 632 | 299 | 290 | 589 | 282 | 269 | 551 | 282 | 269 | 551 | 297 | 285 | 582 |
| CBZ = carbamazepine, PHT= phenytoin 1Individual participant data (IPD) supplied for 114 participants recruited in De Silva 1996; randomised drug not recorded in six participants. Reasons for treatment withdrawal not available for two participants (one randomised to CBZ and one to PHT); these participants are not included in analysis of Time to treatment withdrawal. | |||||||||||||||
| Reason for early termination | Classification | Heller 19952,3 | Total1 | ||||||||
| CBZ n = 53 | PHT n = 53 | CBZ n = 23 | PHT n = 20 | CBZ n = 60 | PHT n = 63 | CBZ n = 154 | PHT n = 165 | CBZ n = 290 | PHT n = 299 | ||
| Adverse events | Event | 3 | 2 | 4 | 1 | 8 | 1 | 11 | 8 | 26 | 12 |
| Seizure recurrence | Event | 12 | 10 | 2 | 1 | 5 | 8 | 3 | 6 | 22 | 25 |
| Both seizure recurrence and adverse events | Event | 6 | 5 | 0 | 0 | 4 | 2 | 31 | 33 | 31 | 40 |
| Non‐compliance/participant choice | Event | 0 | 0 | 3 | 4 | 0 | 0 | 11 | 26 | 14 | 30 |
| Participant went into remission | Censored | 18 | 24 | 0 | 0 | 6 | 14 | 0 | 0 | 24 | 38 |
| Lost to follow‐up | Censored | 0 | 0 | 0 | 0 | 0 | 0 | 26 | 19 | 26 | 19 |
| Death4 | Censored | 0 | 0 | 0 | 0 | 0 | 0 | 4 | 5 | 4 | 5 |
| Other5 | Censored | 0 | 0 | 0 | 0 | 0 | 0 | 16 | 11 | 16 | 11 |
| Completed the study (did not withdraw) | Censored | 14 | 12 | 14 | 14 | 37 | 38 | 53 | 57 | 118 | 121 |
| n = number of individuals contributing to the outcome 'Time to withdrawal of allocated treatment’ 1All participants in Ogunrin 2005 completed the study without withdrawing, so this study did not contribute to 'Time to withdrawal of allocated treatment'. | |||||||||||
| Analysis | Time to withdrawal | Time to six‐month remission | Time to 12‐month remission* | Time to first seizure |
| Original analysis | P: 1.18 (0.87, 1.60) G: 0.42 (0.18, 0.96) O: 1.04 (0.78, 1.39) | P: 1.02 (0.79, 1.33) G: 1.30 (0.89, 1.92) O: 1.11 (0.89, 1.37) | P: 0.94 (0.71, 1.25) G: 1.17 (0.53, 2.57) O: 1.01 (0.78, 1.31) | P: 0.86 (0.68, 1.08) G: 0.84 (0.57, 1.24) O: 0.85 (0.70, 1.04) |
| Test for interaction | Chi2 = 5.18; df = 1 P = 0.02; I2 = 80.7% | Chi2 = 1.03; df = 1 P = 0.31; I2 = 3.4% | Chi2 = 0.25; df = 1 P = 0.62; I2 = 0% | Chi2 = 0.01; df = 1 P = 0.93; I2 = 0% |
| Generalised and age at onset > 30 (classified as uncertain epilepsy type) | P: 1.18 (0.87, 1.60) G: 0.51 (0.21, 1.24) U: 0.19 (0.02, 2.14) O: 1.05 (0.79, 1.40) | P: 1.02 (0.79, 1.33) G: 1.69 (1.07, 2.27) U: 0.84 (0.35, 1.98) O: 1.13 (0.91, 1.41) | P: 0.94 (0.71, 1.25) G: 1.44 (0.90, 2.31) U: 0.52 (0.20, 1.34) O: 1.01 (0.80, 1.28) | P: 0.86 (0.68, 1.08) G: 0.91 (0.57, 1.46) U: 0.97 (0.43, 2.18) O: 0.88 (0.72, 1.07) |
| Test for interaction | Chi2 = 4.99; df = 2 P = 0.08; I2 = 59.9% | Chi2 = 4.01; df = 2 P = 0.13; I2 = 50.2% | Chi2 = 4.32; df = 2 P = 0.12; I2 = 53.7% | Chi2 = 0.12; df = 2 P = 0.94; I2 = 0% |
| Generalised and age at onset > 30 (reclassified as partial epilepsy) | P: 1.11 (0.82, 1.50) G: 0.51 (0.21, 1.24) O: 1.02 (0.77, 1.36) | P: 1.02 (0.80, 1.31) G: 1.69 (1.07, 2.27) O: 1.15 (0.92, 1.42) | P: 0.91 (0.69, 1.19) G: 1.44 (0.90, 2.31) O: 1.02 (0.81, 1.29) | P: 0.86 (0.69, 1.08) G: 0.91 (0.57, 1.46) O: 0.87 (0.71, 1.07) |
| Test for interaction | Chi2 = 2.65; df = 1 P = 0.10; I2 = 62.3% | Chi2 = 3.63; df = 1 P = 0.06; I2 = 72.5% | Chi2 = 2.79; df = 1 P = 0.09; I2 = 64.2% | Chi2 = 0.04; df = 1 P = 0.83; I2 = 0% |
| df = degrees of freedom of Chi² distribution, G = generalised epilepsy, O = overall (all participants), P = partial epilepsy, U = uncertain seizure type Results are presented as pooled hazard ratio (HR) (95% confidence interval (CI)) with fixed‐effect. See Analysis 1.2; Analysis 1.4; Analysis 1.6; and Analysis 1.8 for original analyses of 'Time to treatment withdrawal', 'Time to 12‐month remission', 'Time to 6‐month remission' and 'Time to first seizure', all stratified by epilepsy respectively. * Original analysis calculated with random‐effects model due to substantial heterogeneity (see Analysis 1.4). Sensitivity analyses calculated with fixed‐effect model as no heterogeneity is present following reclassification of 29 participants in Heller 1995. | ||||
| Trial | Adverse event data1 | Summary of reported results | |
| Carbamazepine (CBZ) | Phenytoin (PHT) | ||
| All adverse events according to drug (note: no participants withdrew due to adverse events) | CBZ (n = 59): drowsiness (n = 2), rash (n = 3) | PHT (n = 58): gum hypertrophy (n = 2), rash (n = 2), ataxia (n = 2) | |
| Most frequently observed side effects | Gastrointestinal side effects and “impaired function” (general malaise). Frequency not clearly stated | Gastrointestinal side effects and “impaired function” (general malaise). Frequency not clearly stated | |
| “Exclusions” due to adverse events or no efficacy” | Proportion “excluded”: CBZ: 30% (out of 30 randomised to CBZ) | Proportion “excluded”: PHT: 23.3% (out of 30 randomised to PHT) | |
| “Unacceptable” adverse events leading to drug withdrawal5 | CBZ (n = 54): drowsiness (n = 1), blood dyscrasia (n = 1) | PHT (n = 54): drowsiness (n = 2), skin rash (n = 1), blood dyscrasia (n = 1), hirsutism (n = 1) | |
| Withdrawal due to adverse events (no other adverse event data reported) | 4 participants out of 23 randomised to CBZ withdrew for the following reasons (some withdrew for more than adverse event): slowing of mental function, headache, anorexia, nausea, abdominal pain, fatigue and drowsiness2 | 1 participant out of 20 randomised to PHT withdrew from the study due to depression and anorexia | |
| “Unacceptable” adverse events leading to drug withdrawal5 | CBZ (n = 61): drowsiness (n = 3), rash (n = 2), headache (n = 1), depression (n = 1) | PHT (n = 63): myalgia (n = 1), irritability (n = 1) | |
| Narrative report of ‘Adverse effects’ and ‘Serious side effects’ | CBZ (n = 155): motor disturbance (ataxia, incoordination, nystagmus, tremor: 33%); dysmorphic and idiosyncratic side effects (gum hypertrophy, hirsutism, acne and rash: 14%); gastrointestinal problems (27%); decreased libido or impotence (13%); No serious side effects | PHT (n = 165); motor disturbance (ataxia, incoordination, nystagmus, tremor: 28%); dysmorphic and idiosyncratic side effects (gum hypertrophy, hirsutism, acne and rash: 22 %); gastrointestinal problems (24%); decreased libido or impotence (11%) 1 serious side effect – 1 participant has confirmed lymphoma, rash improved rapidly following discontinuation of PHT | |
| No adverse events reported | N/A | N/A | |
| Participant reported symptomatic complaints (provided as IPD) | CBZ (n = 19): memory impairment (n = 9) psychomotor retardation (n = 1) inattention (n = 1) transient rash (n = 1) CBZ‐induced cough (n = 1) | PHT (n = 18): memory impairment (n = 7) psychomotor retardation (n = 1) inattention (n = 2) transient rash (n = 1) | |
| Participant‐reported adverse events | 1 participant on CBZ complained of facial skin problems; 1 participant on CBZ complained of tiredness and memory problems | 3 participants on PHT complained of tiredness | |
| Major and minor side effects | CBZ (n = 35): Major side effects: rash (n = 1), pruritus (n = 1), impotence (n = 2), dizziness (n = 1), headaches (n = 1), impaired cognition (n = 1), elevated liver enzymes (n = 1) Mild side effects: nausea (33%), headaches (24%), cognitive impairment (33%), nystagmus (52%), sedation (33%), fine tremor (20%) | PHT (n = 35): Major side effects: rash (n = 4), exfoliative dermatitis (n = 1), impotence (n = 1), dizziness (n = 1), nausea/vomiting (n = 1) Mild side effects: nausea (38%), gingival hyperplasia (12%), headaches (32%), cognitive impairment (15%), nystagmus (40%), sedation (15%), fine tremor (28%) | |
| No adverse events reported | N/A | N/A | |
| CBZ = carbamazepine, N/A = not available, PHT= phenytoin 1Adverse event data are recorded as reported narratively in the publications, so exact definition of a symptom may vary. Adverse event data supplied as IPD for Ogunrin 2005. Adverse event data were not requested in original IPD requests (De Silva 1996; Heller 1995; Mattson 1985) but will be for all future IPD requests. For numbers of withdrawals due to adverse events in studies for which IPD were provided (De Silva 1996; Heller 1995; Mattson 1985) see Table 3. | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Time to withdrawal of allocated treatment Show forest plot | 4 | 589 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.75, 1.30] |
| 2 Time to withdrawal of allocated treatment ‐ stratified by epilepsy type Show forest plot | 3 | 546 | Hazard Ratio (Fixed, 95% CI) | 1.04 [0.78, 1.39] |
| 2.1 Partial onset | 3 | 428 | Hazard Ratio (Fixed, 95% CI) | 1.18 [0.87, 1.60] |
| 2.2 Generalised seizures | 2 | 118 | Hazard Ratio (Fixed, 95% CI) | 0.42 [0.18, 0.96] |
| 3 Time to achieve 12‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 0.99 [0.79, 1.25] |
| 4 Time to achieve 12‐month remission ‐ stratified by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Random, 95% CI) | 1.01 [0.78, 1.31] |
| 4.1 Partial onset | 3 | 430 | Hazard Ratio (Random, 95% CI) | 0.94 [0.71, 1.25] |
| 4.2 Generalised seizures | 2 | 121 | Hazard Ratio (Random, 95% CI) | 1.17 [0.53, 2.57] |
| 5 Time to achieve six‐month remission Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.08 [0.87, 1.34] |
| 6 Time to achieve six‐month remission ‐ stratified by epilepsy type Show forest plot | 3 | 551 | Hazard Ratio (Fixed, 95% CI) | 1.11 [0.89, 1.37] |
| 6.1 Partial onset | 3 | 430 | Hazard Ratio (Fixed, 95% CI) | 1.02 [0.79, 1.33] |
| 6.2 Generalised seizures | 2 | 121 | Hazard Ratio (Fixed, 95% CI) | 1.30 [0.89, 1.92] |
| 7 Time to first seizure post‐randomisation Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 8 Time to first seizure post‐randomisation ‐ stratified by epilepsy type Show forest plot | 4 | 582 | Hazard Ratio (Fixed, 95% CI) | 0.85 [0.70, 1.04] |
| 8.1 Partial onset | 4 | 432 | Hazard Ratio (Fixed, 95% CI) | 0.86 [0.68, 1.08] |
| 8.2 Generalised onset | 3 | 150 | Hazard Ratio (Fixed, 95% CI) | 0.84 [0.57, 1.24] |