Interventions for treating oral leukoplakia to prevent oral cancer
Abstract
Background
Oral leukoplakia is a relatively common oral lesion that, in a small proportion of people, precedes the development of oral cancer. Most leukoplakias are asymptomatic; therefore, the primary objective of treatment should be to prevent onset of cancer. This review updates our previous review, published in 2006.
Objectives
To assess the effectiveness, safety and acceptability of treatments for leukoplakia in preventing oral cancer.
Search methods
We searched the following electronic databases: Cochrane Oral Health's Trials Register (to 16 May 2016), the Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 4), MEDLINE Ovid (1946 to 16 May 2016), Embase Ovid (1980 to 16 May 2016) and CancerLit via PubMed (1950 to 16 May 2016). We searched the metaRegister of Controlled Trials (to 10 February 2015), ClinicalTrials.gov (to 16 May 2016) and the World Health Organization (WHO) International Clinical Trials Registry Platform for ongoing trials (to 16 May 2016). We placed no restrictions on the language or date of publication when searching electronic databases.
Selection criteria
We included randomised controlled trials (RCTs) that enrolled people with a diagnosis of oral leukoplakia and compared any treatment versus placebo or no treatment.
Data collection and analysis
We collected data using a data extraction form. Oral cancer development, demonstrated by histopathological examination, was our primary outcome. Secondary outcomes were clinical resolution of the lesion, improvement of histological features and adverse events. We contacted trial authors for further details when information was unclear. When valid and relevant data were available, we conducted a meta‐analysis of the data using a fixed‐effect model when we identified fewer than four studies with no heterogeneity. For dichotomous outcomes, we calculated risk ratios (RRs) and 95% confidence intervals (CIs). We assessed risk of bias in studies by using the Cochrane tool. We assessed the overall quality of the evidence by using standardised criteria (Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE)).
Main results
We included 14 studies (909 participants) in this review. Surgical interventions, including laser therapy and cryotherapy, have never been studied by means of an RCT that included a no treatment or placebo arm. The included trials tested a range of medical and complementary treatments, in particular, vitamin A and retinoids (four studies); beta carotene or carotenoids (three studies); non‐steroidal anti‐inflammatory drugs (NSAIDs), specifically ketorolac and celecoxib (two studies); herbal extracts (four studies), including tea components, a Chinese herbal mixture and freeze‐dried black raspberry gel; bleomycin (one study); and Bowman‐Birk inhibitor (one study).
We judged one study to be at low risk of bias, seven at unclear risk and six at high risk. In general, we judged the overall quality of the evidence to be low or very low, so findings are uncertain and further research is needed.
Five studies recorded cancer incidence, only three of which provided useable data. None of the studies provided evidence that active treatment reduced the risk of oral cancer more than placebo: systemic vitamin A (RR 0.11, 95% CI 0.01 to 2.05; 85 participants, one study); systemic beta carotene (RR 0.71, 95% CI 0.24 to 2.09; 132 participants, two studies); and topical bleomycin (RR 3.00, 95% CI 0.32 to 27.83; 20 participants, one study). Follow‐up ranged between two and seven years.
Some individual studies suggested effectiveness of some proposed treatments, namely, systemic vitamin A, beta carotene and lycopene, for achieving clinical resolution of lesions more often than placebo. Similarly, single studies found that systemic retinoic acid and lycopene may provide some benefit in terms of improvement in histological features. Some studies also reported a high rate of relapse.
Side effects of varying severity were often described; however, it seems likely that interventions were well accepted by participants because drop‐out rates were similar between treatment and control groups.
Authors' conclusions
Surgical treatment for oral leukoplakia has not been assessed in an RCT that included a no treatment or placebo comparison. Nor has cessation of risk factors such as smoking been assessed. The available evidence on medical and complementary interventions for treating people with leukoplakia is very limited. We do not currently have evidence of a treatment that is effective for preventing the development of oral cancer. Treatments such as vitamin A and beta carotene may be effective in healing oral lesions, but relapses and adverse effects are common. Larger trials of longer duration are required to properly evaluate the effects of leukoplakia treatments on the risk of developing oral cancer. High‐quality research is particularly needed to assess surgical treatment and to assess the effects of risk factor cessation in people with leukoplakia.
PICOs
Plain language summary
Interventions for treating oral leukoplakia to prevent oral cancer
Review question
People with oral leukoplakia are at higher risk of developing oral cancer than those with normal oral mucosa. This review, produced through Cochrane Oral Health, seeks to evaluate whether people affected by leukoplakia can benefit from surgical, medical or complementary treatments, either local or systemic. In particular, we conducted this review to find out which, if any, treatment is able to prevent people with leukoplakia of the mouth from getting oral cancer. This review updates our previous review published in 2006.
Background
Oral leukoplakia is a white patch formed in the mouth lining that cannot be rubbed off. It often does not hurt and may go unnoticed for years. People with leukoplakia develop oral cancer more often than people without it. Preventing this is critical because rates of oral cancer survival longer than five years after diagnosis are low. Drugs, surgery and other therapies have been tried for treatment of oral leukoplakia.
Objectives
This review aimed to evaluate whether treatments for oral leukoplakia are effective in preventing oral cancer, and safe and acceptable to patients.
Study characteristics
The evidence on which this review is based is up‐to‐date as of May 2016. We found 14 randomised controlled trials (RCTs) of medical and complementary treatments, which involved 909 participants in total. Treatments included herbal extracts, anti‐inflammatory drugs, vitamin A, beta carotene supplements and others. Surgical treatment has not been compared with placebo or no treatment in an RCT.
Key results
Cancer development was measured in studies of three treatments: systemic vitamin A, systemic beta carotene and topical bleomycin. None of these treatments showed effectiveness in preventing cancer development, as measured up to two years for vitamin A and beta carotene, and seven years for bleomycin.
Some individual studies of vitamin A and beta carotene suggested that these treatments may be effective for improving or healing oral lesions. However, some studies observed a high rate of relapse in participants whose lesions were initially resolved by treatment.
Most treatments caused side effects of differing severity in a high proportion of participants.
It seems likely that interventions were well accepted by participants because drop‐out rates were similar between treatment and control groups.
Quality of the evidence
The available evidence is very limited. Most interventions were assessed by only one small study. Most studies had problems in the way they were conducted, making their results unreliable. We judged the quality of evidence for the outcome of cancer development to be very low.
Author conclusions
Larger, better studies of longer duration are required. As well as further studies of drug treatment and alternative treatments like vitamins, studies are needed to evaluate the effectiveness and safety of surgery, and of stopping risk factor habits such as smoking.
Authors' conclusions
Summary of findings
| Systemic or topical vitamin A vs placebo for treating leukoplakia | ||||||
| Patient or population: people with oral leukoplakia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with vitamin A or retinoids | |||||
| Cancer development at 24 months from start of treatment (treatment lasted 12 months) | 93 per 1000 | 10 per 1000 | RR 0.11 | 85 | ⊕⊝⊝⊝ | This study evaluated systemic treatment |
| Clinical resolution (not completely resolved) at 4 to 12 months | Studies could not be combined in meta‐analysis One study evaluated topical treatment and did not find evidence of benefit 3 studies of systemic vitamin A ‐ 2 showed benefit in terms of clinical resolution, and 1 did not | |||||
| Histological changes (not improved) at 3 months | 889 per 1000 | 382 per 1000 (213 to 676) | RR 0.43 (0.24 to 0.76) | 41 (1 RCT) | ⊕⊕⊕⊝ | This study evaluated systemic treatment |
| Safety of the intervention at 4 to 12 months | 3 studies (1 each evaluating topical acitretin, topical 13‐cis‐retinoic acid, 200,000 IU per week of vitamin A) found no adverse effects. Systemic 13‐cis‐retinoic acid (1 to 2 mg/kg/d) caused adverse effects of varying severity in 79% of participants | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| *From event rate in control group | ||||||
| Systemic beta carotene or carotenoids vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with beta carotene or carotenoids | |||||
| Cancer development at 24 months from start of treatment (treatment lasted 12 months) | 108 per 1000 | 79 per 1000 | RR 0.73 | 132 | ⊕⊕⊝⊝ | |
| Clinical resolution (not completely resolved) at 5 to 12 months | The 3 studies could not be combined in meta‐analysis. 2 found benefit for systemic beta carotene, and 1 did not | |||||
| Histological changes (not improved) at 5 months (treatment lasted 3 months) | 833 per 1000 | 200 per 1000 (100 to 383) | RR 0.24 (0.12 to 0.46) | 58 (1 RCT) | ⊕⊕⊝⊝ | |
| Safety of the intervention at 5 to 12 months | Systemic beta carotene did not cause any adverse effects in 1 study supplementing 10 mg/d, and caused adverse effects of varying severity in 9% of participants in another study supplementing 360 mg/wk No adverse effects were reported by participants treated with systemic lycopene in one study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| * From event rate in control group | ||||||
| NSAIDs vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: NSAIDs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with NSAIDs | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 3 months | 1 study evaluated systemic treatment and 1 evaluated topical treatment. Neither found benefit for NSAIDs | |||||
| Histological changes (not improved) | Not measured | |||||
| Safety of the intervention over 3 months | Systemic celecoxib (1 study) ‐ 32 intervention participants reported 56 adverse effects and 20 placebo participants in the placebo group reported 20 adverse effects. Minor adverse events included dizziness, diarrheoa and abdominal pain. 4 participants (2 from the placebo group and 2 from an intervention group) had grade 3 adverse events. 2 participants permanently discontinued treatment owing to an adverse event (grade 2 vision abnormality and hypertension in a participant receiving 400 mg twice daily of celecoxib and a grade 3 ischaemic cerebrovascular accident in a participant receiving 200 mg twice daily of celecoxib) Ketorolac oral rinse (1 study) caused adverse effects of varying severity in 29% of participants | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| Herbal extracts vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: herbal extracts ‐ tea components; a Chinese herbal mixture; curcumin chewing gum; freeze‐dried black raspberry gel Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with herbal extracts | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 3 to 6 months | 3 studies (1 of freeze‐dried black raspberry gel, 1 of green tea extract capsules and 1 of mixed tea treatment) did not find evidence of benefit for the intervention | |||||
| Histological changes (not improved) at 3 months | 2 studies (1 of green tea extract capsules and 1 of freeze‐dried raspberry gel) did not find evidence of benefit from these interventions | |||||
| Safety of the intervention up to 3 months | 3 studies measured adverse effects: green tea extract capsules caused high frequency (93%) of adverse effects of varying severity in 1 study; the mixed tea treatment study did not mention adverse effects; freeze‐dried black raspberry gel did not cause any adverse effects | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| Topical bleomycin vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with topical bleomycin | |||||
| Cancer development up to 7 years | 83 per 1000 | 250 per 1000 (27 to 1000) | RR 3.00 | 20 | ⊕⊝⊝⊝ | |
| Clinical resolution (not completely resolved) at 3 months | 917 per 1000 | 504 per 1000 (266 to 954) | RR 0.55 (0.29 to 1.04) | 22 (1 RCT) | ⊕⊝⊝⊝ | |
| Histological changes (not improved) at 3 months | 818 per 1000 | 401 per 1000 (180 to 900) | RR 0.49 (0.22 to 1.10) | 21 (1 RCT) | ⊕⊝⊝⊝ | |
| Safety of the intervention up to 3 months | "All patients in the bleomycin group developed erythema with erosion by the end of the applications, whereas erythema developed in the placebo group. Discomfort was reported by 60% of the bleomycin group, but analgesics were not required. Taste of the topical application as well‐tolerated. There was no observed systemic toxicity in the patient groups" | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| * From event rate in control group | ||||||
| Bowman‐Birk inhibitor vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: Bowman‐Birk inhibitor Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Bowman‐Birk inhibitor | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 6 months | 957 per 1000 | 957 per 1000 (871 to 1000) | RR 1.00 (0.91 to 1.09) | 21 (1 RCT) | ⊕⊕⊝⊝ | |
| Histological changes (not improved) at 6 months | Not measured | |||||
| Safety of the intervention up to 6 months | Trial authors reported that there were no significant adverse effects. 33 participants in the intervention group reported 75 adverse effects. 25 participants in the placebo group reported 63 adverse effects | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| * From event rate in control group | ||||||
Background
Description of the condition
"The term leukoplakia should be used to recognize white plaques of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer" (Warnakulasuriya 2007). Such a definition is the result of the efforts of an international working group comprising specialists in the fields of epidemiology, oral medicine, pathology and molecular biology with a special interest in cancer and precancer, who met in London in 2005. This meeting was co‐ordinated by the World Health Organization (WHO) Collaborating Centre for Oral Cancer and Precancer in the UK, to review definitions, classifications, natural history and management of potentially malignant disorders on the basis of previously published work (Axell 1984; Axell 1996; Kramer 1978) and new scientific acquisitions. Thus, 'leukoplakia' is a clinical term that is used when the clinician has excluded any other condition of the oral mucosa that can present as a white lesion (e.g. frictional keratosis, lichen planus, white sponge nevus, hairy leukoplakia). Such lesions warrant biopsy and histopathological examination to assess the possible presence of epithelial dysplasia or carcinoma. Leukoplakia is often associated with tobacco smoking or chewing, although idiopathic forms are not rare (Axell 1987). The role of alcohol, viruses and systemic conditions needs further investigation (Dietrich 2004; Syrjänen 2011).
Clinical variants of leukoplakia are often classified into two groups: (1) homogeneous leukoplakia, a lesion of uniform flat appearance that may exhibit superficial irregularities, but with consistent texture throughout; and (2) non‐homogeneous leukoplakia, a predominantly white or white and red lesion (erythroleukoplakia) with an irregular texture that may include ulceration and may be characterised by a speckled, nodular or verrucous topography. Histological features of both forms of leukoplakia are variable and may include ortho‐keratosis or para‐keratosis of various degrees, acanthosis or atrophy of the squamous epithelium, mild inflammation in the corium, dysplastic changes of various grades (i.e. mild, moderate or severe), carcinoma in situ or carcinoma. Some cases of predominantly white lesions that are difficult to diagnose, in spite of the availability of a biopsy.
Leukoplakia is not uncommon, and although it is highly variable among geographical areas and demographic groups, the prevalence of leukoplakia in the general population varies from less than 1% to more than 5% (Axell 1984; Axell 1987; Bouquot 1986; Ikeda 1991; Reichart 2000). In a systematic review that included studies with more than 1000 individuals, the pooled prevalence was estimated to be between 1.49% and 4.27% (Petti 2003). Incidence data are very scarce. A study from Japan reported an age‐adjusted incidence rate per 100,000 person‐years of 409.2 among males and 70 among females (Nagao 2005), and an Indian study, conducted in a population with distinctive risk factors for oral cancer, reported lower figures: 240 among males and 3 among females (Gupta 1980).
Leukoplakia is one of a group of conditions defined as potentially malignant disorders (i.e. "morphological alterations amongst which some may have an increased potential for malignant transformation, [they] are also indicators of risk of likely future malignancies elsewhere in (clinically normal appearing) oral mucosa and not only site specific predictors") (Warnakulasuriya 2007). The rate of malignant transformation into squamous cell carcinoma varies from almost 0% to 36.4% (Arduino 2013), and a study investigating the natural limit of malignant transformation on the basis of European epidemiological data concluded that the upper limit of the annual transformation rate of oral leukoplakia is unlikely to exceed 1% (Scheifele 2003).
Non‐homogeneous leukoplakias carry a higher degree of risk of transformation when compared with homogeneous variants. Among patients with a histopathological diagnosis of dysplasia, about 1/10 of the total may be at higher risk. Other reported risk factors of statistical significance for cancer development in people with leukoplakia include female gender, long duration of leukoplakia, non‐smoking status, location on the lateral tongue and/or floor of the mouth, size > 200 mm2 (Holmstrup 2006) and the presence of Candida albicans (Van der Waal 2009). Studies investigating biomarkers and histological features have suggested methods that can be used to identify which patients with leukoplakia will develop oral cancer, and which will not (Pitiyage 2009; Smith 2009); however, a definitive, evidence‐based and clinically useful predictor of malignant transformation for dysplastic and non‐dysplastic leukoplakias is not available at the moment. Aneuploid lesions (i.e. with abnormal DNA content) are more likely to transform to cancer compared with diploid lesions (i.e. with normal DNA content) (Sperandio 2013; Torres‐Rendon 2009).
Description of the intervention
Most leukoplakias are asymptomatic; therefore, the need for treatment is based primarily on the precancerous nature of the lesion, and the primary aim of management should be to avoid development of cancer. This is particularly important in view of the poor prognosis associated with oral squamous cell carcinoma, in which only about 50% of patients are still alive five years after diagnosis (Scully 2009), and of the morbidity associated with oral cancer and complications of oral cancer therapy (Epstein 2012).
Many approaches to the treatment of leukoplakia have been proposed in an attempt to prevent cancer development and to evaluate clinical/histological resolution of oral leukoplakias (Lodi 2008). These approaches include surgical excision with different techniques (scalpel, cryosurgery, photodynamic therapy, laser surgery and vaporisation), medical treatment (topical or systemic), cessation of risk activities (smoking and alcohol) and no intervention but strict surveillance.
How the intervention might work
The rationale of surgical excision is that removing the clinically altered tissue could prevent the onset of oral cancer. For medical treatments, the rationale depends on the mechanism of action of the agent employed: retinoids, vitamin A and carotenoids might influence epithelial turnover; non‐steroidal anti‐inflammatory drugs (NSAIDs) block cyclo‐oxygenase activity, thereby modulating specific prostaglandins possibly involved in carcinogenesis; and chemotherapeutic agents act directly on early neoplastic cells. As many of these treatments have potentially serious adverse effects, the “wait and see” approach, based on strict clinical and histological surveillance, is generally employed to identify early cancer onset and to initiate cancer treatment to render the best possible prognosis. Although surgery and medical treatments aim to remove or reduce the lesion, it must be stressed that no evidence has shown a relationship between changes in size or resolution and decreased risk of oral cancer.
Why it is important to do this review
Cochrane Oral Health undertook an extensive prioritisation exercise in 2014 to identify a core portfolio of titles that were the most clinically important reviews to maintain in The Cochrane Library (Worthington 2015). This review was identified as a priority title by the oral medicine expert panel (Cochrane OHG priority review portfolio). Treatment of leukoplakia continues to be based on expert opinion, and more research is needed. This review aims to provide evidence‐based support for clinicians and patients and a clinical research agenda for planning future studies.
Objectives
To assess the effectiveness, safety and acceptability of treatments for leukoplakia in preventing oral cancer.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing effects of surgery, medical or complementary treatments (local or systemic) or risk factor cessation versus placebo.
Types of participants
Anyone with a diagnosis of oral leukoplakia (without histopathological evidence of carcinoma) as defined, at the time of the studies, by consensus conferences held in 1978, 1983, 1994 and 2005 (Axell 1984; Axell 1996; Kramer 1978, Warnakulasuriya 2007).
Types of interventions
Active
-
Surgical removal of the lesion, including surgical excision, laser surgery, cryotherapy
-
Systemic medical treatment
-
Topical medical treatment, including anti‐inflammatory agents, antimycotic agents, carotenoids and retinoids, cytotoxic agents, etc.
-
Removal of predisposing habits (e.g. tobacco, alcohol)
-
Other treatment (e.g. photodynamic therapy)
-
Combined treatment
Control
-
Placebo
-
No treatment
Types of outcome measures
In light of the pre‐cancerous nature of leukoplakia, the primary objective of treatment is to prevent cancer development.
Primary outcomes
-
Oral cancer development, demonstrated by histopathological examination
Secondary outcomes
-
Clinical resolution, in terms of the proportion of lesions that did not resolve (with relapse data when provided)
-
Improvement of histological features, in terms of the proportion of lesions that did not show improvement in histological features
-
Safety of the intervention, as measured by the incidence of adverse effects
Search methods for identification of studies
To identify studies included in or considered for this review, we developed detailed search strategies for each database searched. These were based on the search strategy developed for MEDLINE Ovid but revised appropriately for each database. The search strategy used a combination of controlled vocabulary and free text terms and was linked with the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised controlled trials (RCTs) in MEDLINE: sensitivity‐maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in Box 6.4.c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011). We have provided details of the MEDLINE search in Appendix 1.
Electronic searches
We searched the following databases.
-
Cochrane Oral Health's Trials Register (to 16 May 2016) (see Appendix 2);
-
The Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library, 2016, Issue 4) (see Appendix 3);
-
MEDLINE Ovid (1946 to 16 May 2016) (see Appendix 1);
-
Embase Ovid (1980 to 16 May 2016) (see Appendix 4);
-
CancerLit via PubMed (1950 to 16 May 2016) (see Appendix 5).
We placed no restrictions on the language or date of publication when searching the electronic databases.
Searching other resources
We searched the following databases for ongoing trials (see Appendix 6 for details of the search strategy).
-
metaRegister of Controlled Trials (to 10 February 2015);
-
ClinicalTrials.gov (to 16 May 2016);
-
The WHO International Clinical Trials Registry Platform (to 16 May 2016).
We manually checked the reference lists of included studies and existing reviews. The metaRegister of Controlled Trials is no longer available and so was not searched in May 2016.
Data collection and analysis
Selection of studies
Two review authors (GL and RF) separately examined the title and abstract of each article identified by the different search strategies. When at least one review author considered the article relevant, it progressed in the review process and was included in a digital archive prepared by using dedicated software. We obtained full reports for all relevant studies.
Data extraction and management
All studies meeting the inclusion criteria underwent data extraction performed by at least two review authors, using a specially designed form. We present the characteristics of trial participants, interventions and outcomes for the included trials in the Characteristics of included studies tables.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of included trials and resolved disagreements through discussion and consensus. We used the recommended approach for assessing risk of bias in studies included in Cochrane reviews (Higgins 2011a). This approach addresses the following seven specific domains.
-
Random sequence generation (selection bias)
-
Allocation concealment (selection bias)
-
Blinding of participants and personnel (performance bias)
-
Blinding of outcome assessment (detection bias)
-
Incomplete outcome data (attrition bias)
-
Selective reporting (reporting bias)
-
Other bias
Each domain in the tool includes one or more specific entries in a ‘Risk of bias’ table. Within each entry, the first part of the tool describes what was reported to have happened in the study, in sufficient detail to support a judgement about risk of bias. The second part of the tool assigns a judgement related to the risk of bias for that entry ‐ ‘low risk’, ‘high risk’ or ‘unclear risk’. After taking into account the additional information provided by trial authors, we summarised the risk of bias in included studies as follows.
-
Low risk of bias: low risk of bias for all key domains
-
Unclear risk of bias: unclear risk of bias for one or more key domains
-
High risk of bias: high risk of bias for one or more key domains
We completed a 'Risk of bias' table for each included study (see Characteristics of included studies) and presented results graphically by study (Figure 1) and by domain over all studies (Figure 2).

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
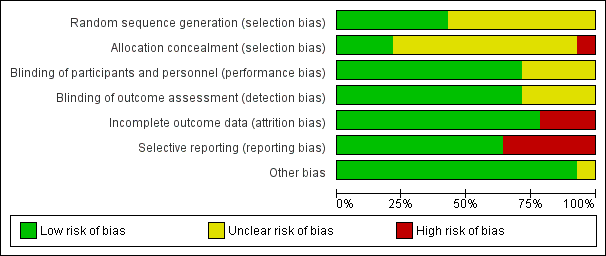
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Measures of treatment effect
The primary measure of intervention effect was onset of oral cancer. Dichotomous data were reported for this outcome measure: cancer development versus absence of cancer development.
Secondary outcomes, clinical resolution, histological changes and adverse effects were usually reported as ordinal measures. We dichotomised data: clinical resolution vs partial or no clinical response; decreased severity vs worsening of histology or no change in histological features.
For each intervention, we sought and summarised data on the number of participants from both intervention and control groups who experienced the event (outcome) and the total number of participants. We analysed dichotomous data by calculating risk ratios. As we anticipated pooling data from studies in which true treatment effects were likely to differ, we planned to use a random‐effects model in statistical analyses; however, we used a fixed‐effect model because of the very small number of studies combined.
Unit of analysis issues
The individual participant was the unit of analysis.
Dealing with missing data
Whenever possible, we obtained missing data from tables and graphs or through personal contact with study authors. When this was not possible, and we found no evidence that data were missing because of a specific bias, we analysed only available data (Higgins 2011). This represents a change from the previous version of the review, in which missing data were imputed with the assumption that all were poor outcomes.
Assessment of heterogeneity
We assessed the significance of discrepancies in the estimates of treatment effects provided by different trials by using Cochran’s test for heterogeneity and the I² statistic; the latter describes the percentage total variation across studies that is due to heterogeneity rather than to chance. Heterogeneity was considered statistically significant if the P value was less than 0.1. A rough guide to the interpretation of I² given in the Cochrane Handbook for Systematic Reviews of Interventions is as follows: 0 to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, 75% to 100% represents very substantial ('considerable') heterogeneity (Higgins 2011).
Assessment of reporting biases
We attempted to minimise reporting biases by conducting a thorough search of multiple sources including trial registries, and by making efforts to identify unpublished trials and non‐English language publications.
Data synthesis
When valid and relevant data were collected, we undertook a meta‐analysis of the data. We grouped and analysed studies on the basis of intervention category. We conducted meta‐analyses in Review Manager software, using the Mantel‐Haenszel method with a fixed‐effect model. We had planned to use a random‐effects model, but this would not have been appropriate because of the small number of studies included. We did not pool data when substantial heterogeneity was identified.
Subgroup analysis and investigation of heterogeneity
We had planned to conduct subgroup analyses for smoking and non‐smoking participants, and for lesions with or without dysplasia. Unfortunately, as such data were not available, we did not perform subgroup analyses.
Sensitivity analysis
We had planned to undertake sensitivity analysis excluding studies at high risk and at unclear risk of bias.
Summarising findings and assessing the quality of the evidence
We constructed 'Summary of findings' tables for each comparison to present the results for our review outcomes. We assessed the quality of the evidence using GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) criteria.
Results
Description of studies
See Characteristics of included studies, Characteristics of excluded studies and Characteristics of ongoing studies.
Results of the search
This review was originally published in 2001, and updates were published in 2004 and 2006. Since its first publication up until May 2016, we have identified a total of 3438 articles through the search strategy. We have examined titles and abstracts for eligibility and have eliminated those not matching the inclusion criteria. We identified 68 apparently eligible studies and rejected 30 because they were not pertinent. Nine of the studies are ongoing (see Characteristics of ongoing studies for details). After we obtained the full‐text version of the remaining 29 studies, we excluded 13 additional studies (Characteristics of excluded studies) ‐ one because it was quasi‐randomised, four because of inadequate allocation, four for problems in selection of participants and four for lack of an adequate control group. We categorised two studies as awaiting classification (Califano 2012; Chiba 2012; Characteristics of studies awaiting classification). Thus we included 14 studies in this review. See Figure 3.

Study flow diagram
Included studies
Characteristics of trial setting and design
Location
Of the 15 studies included, six were conducted in the USA (Armstrong 2013; Hong 1986; Mallery 2014; Mulshine 2004; Papadimitrakopoulou 2008; Tsao 2009), three in India (Sankaranarayan 1997; Singh 2004; Stich 1988), one in Italy (Piattelli 1999), two in China (Li 1999; Sun 2009), one in Canada (Epstein 1994) and one in Japan (Nagao 2015). The setting for all studies was a university hospital.
Design
Ten trials had a two‐arm parallel design (Armstrong 2013; Epstein 1994; Hong 1986; Li 1999; Mallery 2014; Mulshine 2004; Nagao 2015; Piattelli 1999; Stich 1988; Sun 2009); three, a three‐arm parallel design (Papadimitrakopoulou 2008; Sankaranarayan 1997; Singh 2004); and one, a four‐arm parallel design (Tsao 2009). In three of the four studies with more than two arms, we pooled together data from the active arms: interventions differed in dosage in Papadimitrakopoulou 2008; Singh 2004; and Tsao 2009.
Duration
The trials varied in length. Three studies used an open follow‐up (Epstein 1994; Nagao 2015; Tsao 2009); one study lasted two years (Sankaranarayan 1997); and all other studies lasted less than one year.
Funding
Two trials did not specify any funding source (Epstein 1994; Sankaranarayan 1997). In two trials, some study authors worked for the company that supplied the study drug (Mulshine 2004; Tsao 2009); in another, the first study author had ownership interest in the patent of the drug tested (Mallery 2014). One study was supported by Central Soya Company and NIH (National Institutes of Health) (Armstrong 2013), one by Hoffmann‐La Roche and the National Cancer Institute (Hong 1986), one by the Chinese National Natural Science Foundation (Li 1999), one by NIH (Mallery 2014), one by the National Cancer Institute Specialized Programs of Reasearch Excellence (SPORE) Program (Mulshine 2004), one by the Butterfield Award of the Sasakawa Foundation GB and DSM Nutrition Japan (Nagao 2015), one by Pfizer (Papadimitrakopoulou 2008), one by the Italian National Research Council (CNR) and the Italian Ministry of University, Research, Science and Technology (MURST) (Piattelli 1999), one by Jagsonpal Pharmaceuticals Ltd., New Delhi, India (Singh 2004), one by the National Cancer Institute of Canada (Stich 1988), one by the Beijing Natural Science Foundation, the National Natural Science Foundation of China and the Tenth 5‐Year Plan of National Key Technologies R&D Program in China and NIH (Sun 2009) and one by Ito En Ltd. (Tsao 2009).
Characteristics of participants
The total number of participants randomised in the trials was 909, with a mean of 64.9 participants per study (ranged from 10 to 131 participants).
The reported proportion of smoking and drinking participants (the two main risk factors for oral cancer) varied from 8% (Papadimitrakopoulou 2008) to 86% (Mulshine 2004), and from 9% (Sun 2009) to 71% (Mulshine 2004), respectively. Use of tobacco products (Mallery 2014) and smoking (Nagao 2015) were exclusion criteria in two studies. None of the study authors reported significant changes in these habits during the course of the trial. In two studies, all participants recruited were chewers of tobacco‐containing betel quid (another well‐known risk factor for oral cancer) from the same Indian village (Trivandrum, Kerala) (Sankaranarayan 1997; Stich 1988). All participants enrolled in the studies underwent a confirmatory biopsy; however, only four studies reported the histological criteria employed (Epstein 1994; Papadimitrakopoulou 2008; Stich 1988; Sun 2009). Seven studies reported the percentage of dysplastic lesions, which ranged from 18.75% (Sun 2009) to 73.2% (Tsao 2009) (see Table 1). One study excluded lesions with severe dysplasia (Li 1999), and another study included cases with at least one of the following features: at least mild dysplasia, high‐risk location, significant extent of tissue involvement and presence of symptoms (Tsao 2009).
| Study | Participants with dysplastic leukoplakia (any grade) |
| Not reported | |
| 22% | |
| 27% | |
| 20% | |
| 72.5% | |
| Not reported | |
| Not reported | |
| Not reported | |
| Not reported | |
| Not reported | |
| 59% | |
| Not reported | |
| 18.75% | |
| 73.2% |
Characteristics of interventions
We did not identify any RCTs that compared surgical treatments with placebo or no treatment, nor did we identify any RCTs of risk factor cessation. All included trials compared medical or complementary treatment versus placebo, usually a preparation similar to the treatment, without the active ingredient; in one case, the placebo contained vitamin C, which we considered an inactive ingredient (Nagao 2015).
Four RCTs compared topical treatment versus placebo (129 participants) (Epstein 1994; Mallery 2014; Mulshine 2004; Piattelli 1999), and nine RCTs compared systemic treatment versus placebo (716 participants) (Armstrong 2013; Hong 1986; Nagao 2015; Papadimitrakopoulou 2008; Sankaranarayan 1997; Singh 2004; Stich 1988; Sun 2009; Tsao 2009). One RCT compared a combination of topical and systemic treatments versus placebo (64 participants) (Li 1999).
Four studies tested vitamin A or retinoids (Hong 1986; Piattelli 1999; Sankaranarayan 1997; Stich 1988); three studies tested beta carotene or carotenoids (Nagao 2015; Sankaranarayan 1997; Singh 2004); two studies tested NSAIDs: ketorolac (Mulshine 2004) and celecoxib (Papadimitrakopoulou 2008); and four studies tested herbal extracts, in particular, tea components (Li 1999 ‐ mixed; Tsao 2009 ‐ green tea extract capsules), a Chinese herbal mixture (Sun 2009) and gel containing freeze‐dried black raspberries (Mallery 2014). The other interventions tested were bleomycin (Epstein 1994) and Bowman‐Birk inhibitor (Armstrong 2013).
Characteristics of outcomes
Five studies reported data on oral cancer development (Epstein 1994; Nagao 2015; Papadimitrakopoulou 2008; Sankaranarayan 1997; Tsao 2009). In Epstein's trial, although seven out of 12 participants in the control group received the active treatment at the end of the study period, we conducted an intention‐to‐treat (ITT) analysis for this review.
All studies used lesion measurement as the clinical parameter to assess change; five studies also used pictures of the lesions for clinical evaluation (Armstrong 2013; Epstein 1994; Hong 1986; Mallery 2014; Piattelli 1999). In 11 RCTs, a complete response was defined as complete disappearance of the lesion (Armstrong 2013; Epstein 1994; Hong 1986; Li 1999; Mulshine 2004; Nagao 2015; Papadimitrakopoulou 2008; Piattelli 1999; Sankaranarayan 1997; Singh 2004; Tsao 2009), lasting at least four weeks in four of them (Hong 1986; Mulshine 2004; Sankaranarayan 1997; Singh 2004). For partial response, nine studies used the definition 'greater than 50% reduction', and one used a slightly different criterion ‐ 'greater than 30% reduction' (Li 1999). Three studies defined “stable disease” as a reduction of less than 50% of the lesion (Hong 1986; Mulshine 2004; Singh 2004); three studies adopted an otherwise non‐specified "unchanged clinical aspect" (Li 1999; Stich 1988; Sun 2009); and two studies defined "stable disease" as lesions not satisfying any other category (Papadimitrakopoulou 2008; Tsao 2009). Eight studies gave similar definitions of "disease progression" as an increase in the size of the lesion or the appearance of new lesions (Armstrong 2013; Hong 1986; Li 1999; Mulshine 2004; Papadimitrakopoulou 2008; Singh 2004; Sun 2009; Tsao 2009). One study included the "no response" category, indicating stable and progressive lesions (Sankaranarayan 1997). Three studies adopted different categories for clinical evaluation. Stich 1988 used the following: remission, no change, new leukoplakia. Sun 2009 used positive response (including complete and partial response), stable disease and progressive disease. One study reported the change in lesion measurement, expressed in mm2 (Mallery 2014). Clinical response was recorded immediately at the end of treatment in 10 studies (Armstrong 2013; Li 1999; Mallery 2014; Nagao 2015; Papadimitrakopoulou 2008; Piattelli 1999; Sankaranarayan 1997; Singh 2004; Stich 1988; Tsao 2009), two weeks after the end of treatment in Epstein 1994 and three months after the end of treatment in Sun 2009. In two studies, it was not clear when the reported clinical assessment was recorded (Hong 1986; Mulshine 2004).
Six studies reported assessment of histological changes (Armstrong 2013; Epstein 1994; Hong 1986; Papadimitrakopoulou 2008; Singh 2004; Tsao 2009). These studies did not use a unique histological classification, and the comparison between pre‐treatment and post‐treatment histological features was highly variable. One study defined histological response as an otherwise non‐specified "reversal" or "improvement" of dysplastic features (Papadimitrakopoulou 2008); another adopted a graphical method for evaluating histological changes (Armstrong 2013). Stich 1988 reported histological changes in the treatment group only. In one study, a control biopsy was taken only if development of cancer was suspected (Sankaranarayan 1997).
Biomarkers evaluated included bcl‐2 immunostaining (Piattelli 1999), AgNOR (silver‐stained nucleolar organizer region) and PCNA (proliferation cell nuclear antigen) labelling indexes (Li 1999; Sun 2009), biomarkers of DNA damage in exfoliated cells and peripheral blood lymphocytes (Li 1999), Neu protein of exfoliated cells and serum (Armstrong 2013), epidermal growth factor receptors (EGFRs) (Li 1999) and p53 and Ki67 (protein; cellular marker of neoplasia) (Nagao 2015).
Most trials monitored safety of the intervention. Only Li 1999 and Sun 2009 did not appear to measure adverse effects.
Excluded studies
The primary reason for exclusion of each study is given in the Characteristics of excluded studies table. Many trials were ineligible for more than one reason; however, the more common reasons for exclusion were inappropriate selection of participants, lack of random allocation and absence of a proper control arm. In particular, although we found three randomised controlled trials evaluating surgical interventions (Chee 2013; López‐Jornet 2013; Schwarz 2005), we were unable to include them in the review as they did not include a no treatment or placebo group.
Risk of bias in included studies
On the basis of criteria used in the critical appraisal of studies, one study had an overall low risk of bias (Hong 1986). We judged seven studies as having unclear risk of bias (Epstein 1994; Mallery 2014; Papadimitrakopoulou 2008; Piattelli 1999; Sankaranarayan 1997; Singh 2004; Tsao 2009). We considered the remaining studies to be at high risk of bias (Armstrong 2013; Li 1999; Mulshine 2004; Nagao 2015; Stich 1988; Sun 2009). See Figure 1 and Figure 2.
Allocation
We assessed the generation of the randomisation sequence as having low risk of bias for six trials and unclear risk for eight trials.
We assessed the concealment of allocation as having low risk of bias for three trials, at unclear risk for 10 trials and at high risk for one trial (Armstrong 2013). In Armstrong 2013, the block size was two.
Blinding
We assessed blinding of participants and personnel, as well as blinding of outcome assessment, as low risk of bias for 11 trials and unclear risk for four studies in which not enough information was provided.
Incomplete outcome data
The reported drop‐out rate ranged from 0% (Epstein 1994; Mallery 2014; Singh 2004) to 32.5% (Armstrong 2013). We assessed 11 trials as having low risk of bias with regard to attrition bias because they reported there were no drop‐outs, or because drop‐out was not likely to influence findings. We assessed three studies with high drop‐out rates as having high risk of bias (Armstrong 2013; Nagao 2015; Stich 1988).
Selective reporting
Most trials reported important outcomes and were assessed as having low risk of bias. Five studies failed to report histological results (Li 1999; Mulshine 2004; Nagao 2015; Stich 1988; Sun 2009); we assessed these trials as having high risk of bias for this domain.
Other potential sources of bias
We assessed Epstein 1994, which had discrepant published and unpublished data, along with baseline imbalance, as having unclear risk of bias for this domain. See Figure 2.
Effects of interventions
See: Summary of findings for the main comparison Vitamin A or retinoids versus placebo for treating oral leukoplakia; Summary of findings 2 Systemic beta carotene or carotenoids vs placebo for treating oral leukoplakia; Summary of findings 3 Non‐steroidal anti‐inflammatory drugs (NSAIDs) vs placebo for treating oral leukoplakia; Summary of findings 4 Herbal extracts vs placebo for treating oral leukoplakia; Summary of findings 5 Topical bleomycin vs placebo for treating oral leukoplakia; Summary of findings 6 Bowman‐Birk inhibitor versus placebo for oral leukoplakia
Vitamin A and retinoids versus placebo
Four studies (one at high, two at unclear and one at low risk of bias) compared vitamin A and retinoids versus placebo. Three of these studies evaluated systemic treatment (Hong 1986; Sankaranarayan 1997; Stich 1988), and one evaluated topical treatment (Piattelli 1999).
Oral cancer development
One study reported effects of systemic vitamin A on cancer incidence (Sankaranarayan 1997). Investigators found no evidence of benefit compared with placebo (risk ratio (RR) 0.11, 95% confidence interval (CI) 0.01 to 2.05; 85 participants) (Analysis 1.1; Figure 4).

Forest plot of comparison: 1 Vitamin A or retinoids vs placebo, outcome: 1.1 Cancer development
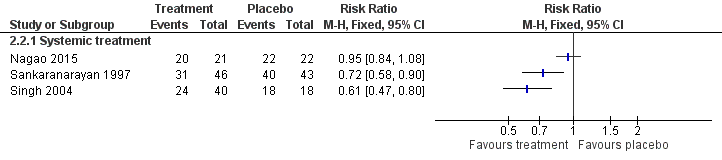
Clinical resolution
Five studies reported effects of vitamin A or retinoids on clinical features of leukoplakia, in particular, on its resolution (Analysis 1.2; Figure 5). In particular, three studies tested systemic treatment (Hong 1986; Sankaranarayan 1997; Stich 1988), but because heterogeneity was high (I2 = 94%), it was inappropriate to combine findings in a meta‐analysis. Two of the three studies at high or unclear risk of bias showed some benefit (Sankaranarayan 1997: RR 0.51, 95% 0.37 to 0.71; 85 participants; Stich 1988: RR 0.44, 95% CI 0.27 to 0.73; 54 participants), but Hong 1986, which was at low risk of bias, showed no clear evidence of benefit (RR 0.92, 95% CI 0.78 to 1.08; 40 participants).

Forest plot of comparison: 1 Vitamin A or retinoids vs placebo, outcome: 1.2 Oral lesion not completely resolved
Hong 1986 provided relapse data: nine out of 16 (56%) participants who responded to treatment (partially or completely) subsequently relapsed (no information was available regarding the two partial responders from the placebo group). Sankaranarayan 1997 reported that 14 out of 22 (64%) complete responders developed recurrent lesions (no information was available regarding the three complete responders in the placebo group).
One study at unclear risk of bias tested topical treatment (nine participants) and found treatment was not more likely to completely resolve the lesion than placebo: RR 0.83, 95% CI 0.48 to 1.44 (Piattelli 1999).
In Piattelli 1999, one out of five (20%) participants responding completely or partially to the experimental treatment relapsed, and one out of four (25%) participants responding to placebo relapsed.
Improvement of histological features
A single study recorded histological improvement and showed that improvement in histological features of lesions was more likely in participants treated with systemic retinoic acid than in those treated with placebo (RR 0.43, 95% CI 0.24 to 0.76; 39 participants; Analysis 1.3) (Hong 1986).
Safety
Topical 13‐cis‐retinoic acid (Piattelli 1999) and 200,000 IU per week of vitamin A (Stich 1988) produced no adverse effects. Systemic 13‐cis‐retinoic acid (1 to 2 mg/kg/d) (Hong 1986) caused adverse effects of varying severity in 79% of participants (see Table 2). Two participants withdrew from Hong 1986 because of severe conjunctivitis and hypertriglyceridaemia.
| Study | Arms | Active treatment | Placebo | Adverse effects |
| Bowman Birk inhibitor concentrate vs placebo | 75 reported from 33 of 67 participants | 63 reported from 25 of 65 participants | Minor adverse effects | |
| Topical bleomycin vs placebo | 10/10 | 0/12 | Bleomycin group ‐ erythema and erosion (100%), discomfort (60%) Placebo group ‐ erythema only | |
| Systemic 13‐cis‐retinoic acid (from 1 to 2 mg/kg per day) vs placebo | 19/24 | 4/20 | Cheilitis, facial erythema, dryness and peeling of skin, conjunctivitis, hypertriglyceridaemia | |
| Systemic and topical tea vs placebo | Not measured or reported | |||
| Freeze‐dried black raspberry gel vs placebo gel | 0/22 | 0/18 | "No participant experienced any treatment‐associated complications" | |
| Ketorolac oral rinse vs placebo | 11/38 | 3/19 | Pain, toxicity grade 1 and 2 | |
| Beta carotene and vitamin C vs placebo | 0/23 | 0/23 | No untoward side effects were noted | |
| Celecoxib vs placebo | 56 reported from 32 participants | 20 reported from 18 participants | 4 participants presented grade 3 adverse events: 2 in placebo arm and 2 in active treatment arm. 2 participants from intervention groups discontinued treatment owing to adverse effects (1 grade 2 and 1 grade 3). | |
| Topical 13‐cis‐retinoic acid vs placebo | 0/5 | 0/5 | "No side effects from the use of the gel | |
| Vitamin A (300,000 IU per week) vs placebo | 13/50 | 1/55 | Headache, muscular pain, dry mouth | |
| Beta carotene (360 mg per week) vs placebo | 5/55 | 1/55 | Headache, muscular pain | |
| Lycopene (8 mg or 4 mg) vs placebo | 0/40 | 0/18 | "No side effects, toxicity of any sort were encountered in the complete duration of the therapy" | |
| Vitamin A (200,000 IU per week) vs placebo | 0/30 | 0/35 | "The administered doses of vitamin A did not produce any detectable adverse | |
| Chinese herbal mixture vs placebo | Not measured or reported | |||
| Green tea extract at different doses (500, 750 or 1000 mg/m2 daily) vs placebo | 28/30 | 8/11 | Grade 1 to 2 adverse events including insomnia, headache, nausea and nervousness |
vs = versus
Beta carotene or carotenoids versus placebo
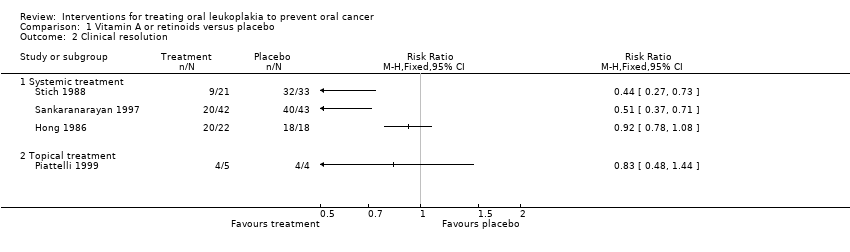
Three studies compared systemic beta carotene or carotenoids versus placebo (Nagao 2015; Sankaranarayan 1997; Singh 2004).
Oral cancer development
Two studies reported the effects of systemic beta carotene on cancer incidence (Nagao 2015; Sankaranarayan 1997). Investigators found no evidence of benefit when compared with placebo (RR 0.71, 95% CI 0.24 to 2.09; 132 participants; I2 = 0%; Analysis 2.1; Figure 6).

Forest plot of comparison: 2 Beta carotene or carotenoids vs placebo, outcome: 2.1 Cancer development
Clinical resolution
Three studies tested effects of systemic beta carotene and carotenoids on clinical resolution (Nagao 2015; Sankaranarayan 1997; Singh 2004) (Analysis 2.2; Figure 7). Owing to high heterogeneity (I2 = 87%), it was not appropriate to combine findings in a meta‐analysis. Two of the individual studies, which were at unclear risk of bias, found that systemic beta carotene was more effective than placebo for complete resolution of the lesion (Sankaranarayan 1997: RR 0.72, 95% CI 0.58 to 0.90; 89 participants; Singh 2004: RR 0.61, 95% CI 0.47 to 0.80; 58 participants). The other study, at high risk of bias, failed to show evidence of benefit (Nagao 2015: RR 0.95, 95% CI 0.84 to 1.08; 43 participants).
Forest plot of comparison: 2 Beta carotene or carotenoids vs placebo, outcome: 2.2 Oral lesion not completely resolved
Sankaranarayan 1997 reported that eight out of 15 (54%) complete responders developed recurrent lesions (no information was available regarding the three complete responders in the placebo group).
Improvement of histological features
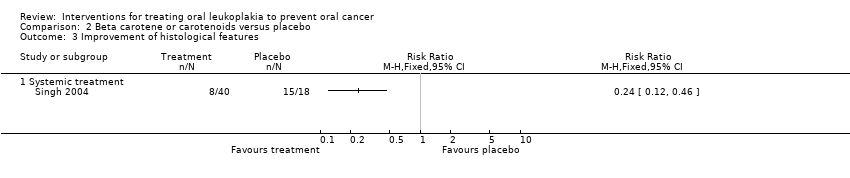
Evidence of histological improvement was recorded when lycopene (a carotenoid) was compared with placebo (RR 0.24, 95% CI 0.12 to 0.46; one study; 58 participants; Analysis 2.3) (Singh 2004).
Safety
Systemic treatment with beta‐carotene produced no adverse effects in one study supplementing 10 mg/d (Nagao 2015). It caused adverse effects of varying severity in 9% of participants in another study supplementing 360 mg/wk (Sankaranarayan 1997). Researchers reported no adverse effects among participants treated with systemic lycopene (Singh 2004). See Table 2.
Non‐steroidal anti‐inflammatory drugs (NSAIDs) versus placebo
Two studies at unclear risk of bias compared NSAIDs ‐ ketorolac in Mulshine 2004 and celecoxib in Papadimitrakopoulou 2008 ‐ versus placebo.
Oral cancer development
Cancer development was among the outcomes reported in Papadimitrakopoulou 2008. This did not occur in either arm, probably because of the extremely short duration of the study (12 weeks).
Clinical resolution
Investigators found no evidence of benefit for systemic celecoxib (Papadimitrakopoulou 2008: RR 0.94, 95% CI 0.83 to 1.08; 46 participants) nor topical ketorolac (Mulshine 2004: RR 0.94, 95% CI 0.81 to 1.10; 56 participants) compared with placebo in terms of clinical resolution of lesions (Analysis 3.1).
Improvement of histological features
Histological changes were not among the outcomes in the studies testing NSAIDs.
Safety
In Papadimitrakopoulou 2008, which tested systemic celecoxib, trialists reported that the treatment was "safe and well tolerated". Thirty‐two intervention participants reported 56 adverse effects, and 20 placebo participants reported 20 adverse effects. Minor adverse events included dizziness, diarrheoa and abdominal pain. No participants had grade 4 adverse events. Four participants (two from the placebo group and two from an intervention group) had grade 3 adverse events. Two people discontinued treatment due to an adverse event (grade 2 vision abnormality and hypertension in a participant receiving 400 mgtwice daily of celecoxib and a grade 3 ischemic cerebrovascular accident in a participant receiving 200 mg twice daily of celecoxib.
Ketorolac oral rinse caused adverse effects of varying severity in 29% of participants (see Table 2). One person withdrew from the trial after the first dose because of mouth pain.
Herbal extracts versus placebo
Four studies compared herbal extracts, in particular, tea components (Li 1999; Tsao 2009), a Chinese herbal mixture (Sun 2009) and freeze‐dried black raspberry gel (Mallery 2014), versus placebo.
Oral cancer development
Cancer development was among the outcomes in Tsao 2009; however, it was not possible to analyse data because trial authors reported the cumulative number of cases, without specifying the allocation arm.
Clinical resolution
The four studies testing herbal extracts included clinical resolution among outcomes (Analysis 4.1).
Systemic treatment with green tea extract showed no evidence of benefit in terms of clinical resolution of leukoplakia when compared with the control in one study at unclear risk of bias (Tsao 2009: RR 0.99, 95% CI 0.86 to 1.14; 39 participants). Li 1999, which was at high risk of bias, investigated a treatment integrating systemic (capsules containing 0.38 g of dried mixture of the whole water extract of green tea, green tea polyphenols and tea pigments) and topical preparations of mixed tea extract (mixed tea in glycerin at the concentration of 10%), but was not able to demonstrate benefit when compared with placebo in terms of clinical resolution (RR 1.00, 95% CI 0.94 to 1.07; 59 participants).
In one study investigating effects of a Chinese herbal mixture, it was not possible to extract data on clinical resolution (Sun 2009).
One topical herbal treatment (freeze‐dried black raspberries) showed no evidence of benefit when compared with placebo in a study that was at unclear risk of bias (Mallery 2014: RR 4.13, 95% CI 0.21 to 80.91; 40 participants). Among participants who did respond to such treatment, six of 22 (32%) in the treatment arm and seven of 17 (41%) in the placebo arm had visible evidence of lesion recurrence at former treatment sites at three months post trial follow‐up (Mallery 2014).
Improvement of histological features
In two studies reporting histological changes, neither active treatment (topical freeze‐dried black raspberries and systemic green tea extract) showed benefit when compared with placebo (Mallery 2014: RR 0.97, 95% CI 0.58 to 1.60; Tsao 2009: RR 0.86, 95% CI 0.66 to 1.13; Analysis 4.2).
Safety
People undergoing treatment with green tea reported very high frequency (93%) of adverse effects of varying severity in one study (Tsao 2009). Adverse effects were not mentioned in Li 1999. Freeze‐dried black raspberry gel caused no adverse effects (Mallery 2014). Sun 2009 stated, "drug toxicity was not monitored in the clinical trial". See Table 2.
Topical bleomycin versus placebo
Topical bleomycin was tested against placebo in a single small study at unclear risk of bias that included 22 participants (Epstein 1994). Following post‐treatment biopsy, seven participants in the placebo group were crossed over to receive the active intervention. An ITT analysis was conducted for outcomes measured after post‐treatment biopsy.
Mean follow‐up from the end of the study was 15 months for group A and 22 months for group B.
Oral cancer development
The trial found no evidence of benefit of topical bleomycin compared with placebo in reducing cancer development among participants affected by leukoplakia (RR 3.00, 95% CI 0.32 to 27.83; 20 participants; Analysis 5.1).
Clinical resolution
Topical bleomycin showed no benefit for clinical resolution when compared with placebo: RR 0.55, 95% CI 0.29 to 1.04. In addition, among participants for whom follow‐up information was available, two out of four (50%) participants with a complete response relapsed and one out of two (50%) participants with a partial response relapsed (Analysis 5.2).
Improvement of histological features
Epstein 1994 reported histological changes, showing no benefit of topical bleomycin when compared with placebo: RR 0.49, 95% CI 0.22 to 1.10 (Analysis 5.3).
Safety
Topical bleomycin caused adverse effects of varying severity in 100% of participants (see Table 2). Participants in the bleomycin group developed erythema with erosion. Erythema developed in the placebo group; 60% of the bleomycin group reported discomfort but did not require analgesics. The trial found no evidence of systemic toxicity.
Bowman‐Birk Inhibitor versus placebo
A single study tested the Bowman‐Birk inhibitor against placebo (Armstrong 2013).
Oral cancer development
Cancer development was not among the outcomes of the study testing the Bowman‐Birk inhibitor.
Clinical resolution
The topical Bowman‐Birk inhibitor showed no benefit for clinical resolution when compared with placebo: RR 1.00, 95% CI 0.91 to 1.09 (Analysis 6.1).
Improvement of histological features
Data on histological changes from Armstrong 2013 were not available for analysis, but study authors reported no statistically significant differences in histological changes between study arms.
Safety
The Bowman‐Birk inhibitor caused adverse effects of varying severity in 49% of participants (see Table 2).
Sensitivity analysis
We did not undertake sensitivity analysis excluding studies at high or unclear risk of bias, as the only study at low risk of bias was not included in a meta‐analysis (Hong 1986).
Discussion
Leukoplakia is the most common potentially malignant oral disorder. Although rates of oral ccancer development may vary among studies, probably as the result of differences in diagnostic criteria for leukoplakia and follow‐up intervals, the morbidity and mortality associated with oral cancer suggest that leukoplakia is a relevant health issue for affected individuals. Yet, of the 14 studies included in the present review, none evaluated a surgical intervention nor the effect of habit cessation, and only three studies provided data on the effects of a medical or complementary treatment on cancer incidence.
Summary of main results
At present, there is no evidence that any of the medical or complementary treatments studied for people with leukoplakia can reduce the likelihood of oral cancer development. It should be noted that this conclusion is based on only three studies, namely, those testing systemic vitamin A, systemic beta carotene and topical bleomycin. These studies, which were at high or unclear risk of bias, included relatively few participants and had limited follow‐up. Overall, the quality of the evidence was very low.
Clinical change, in terms of variation in lesion size, was an outcome reported by all studies, although esearchers used different methods of measurement. Some single studies suggested effectiveness of some proposed treatments, namely, vitamin A, beta carotene and lycopene, in achieving complete clinical resolution of lesions more often than placebo (Sankaranarayan 1997; Singh 2004; Stich 1988). Similarly, single studies showed that vitamin A and lycopene provided some benefit in terms of improvement in histological features (Hong 1986; Singh 2004).
Leukoplakias generally are not associated with significant signs and symptoms, and the risk of developing cancer is relatively low (i.e. many patients with leukoplakia receive treatment that is not necessary). Therefore, proposed treatments should have minimal propensity for adverse effects. The proportion of participants reporting adverse effects varied between 0 and 100% in the active arms of the included trials, and between 0 and 90% in the placebo arms; however adverse effects were always more common in the study group than in the control group (see Table 2). It seems likely that interventions were well accepted by participants because drop‐out rates were similar between treatment and control groups (see Table 3); however, follow‐up may not have been long enough to permit this assessment. Adverse effects caused participants to withdraw in three studies: when systemic 13‐cis‐retinoic acid induced severe conjunctivitis and hypertriglyceridaemia (Hong 1986); when intolerable mouth pain followed the initial ketorolac mouthrinse (Mulshine 2004); and in two participants treated with celecoxib, because of vision abnormality and hypertension in one, and ischaemic cerebrovascular accident in another (Papadimitrakopoulou 2008).
| Study | Arms | Active treatment | Placebo |
| Bowman Birk inhibitor concentrate vs placebo | 24/67 | 19/65 | |
| Topical bleomycin vs placebo | 0/10 | 1/12 | |
| Systemic 13‐cis‐retinoic acid (from 1 to 2 mg/kg per day) vs placebo | 2/24 | 2/20 | |
| Systemic and topical tea vs placebo | 3/32 | 2/32 | |
| Freeze‐dried black raspberry gel vs placebo gel | 0/22 | 0/18 | |
| Ketorolac oral rinse vs placebo | 1/38 | 0/19 | |
| Beta carotene and vitamin C vs placebo | 5/23 | 5/23 | |
| Celecoxib vs placebo | 3/32 | 1/18 | |
| Topical 13‐cis‐retinoic acid vs placebo | 0/5 | 1/5 | |
| Vitamin A (300,000 IU per week) vs placebo | 8/50 | 12/55 | |
| Beta carotene (360 mg per week) vs placebo | 9/55 | 12/55 | |
| Lycopene (8 mg or 4 mg) vs placebo | 0/40 | 0/18 | |
| Vitamin A (200,000 IU per week) vs placebo | 9/30 | 2/35 | |
| Chinese herbal mixture vs placebo | 1/60 | 7/60 |
vs = versus
Overall completeness and applicability of evidence
Less than half (33% to 42%) of people with leukoplakia who develop oral cancer do so within two years of diagnosis (Lind 1987; Silverman 1984), and the incidence of oral cancer increases with the duration of follow‐up (Shiu 2000). Therefore, to properly test the effects of treatments on cancer incidence, it would be necessary to plan studies with large groups of participants and a long follow‐up period ‐ ideally, multi‐centre randomised controlled trials (RCTs) assessing outcomes at 10 years. As the duration of the studies included in this review was less than 12 months in all but four studies (Epstein 1994; Nagao 2015; Sankaranarayan 1997; Tsao 2009), cancer incidence rates are likely to be underestimated. Indeed, most of the studies did not include cancer incidence as an endpoint, but rather employed outcomes that assessed clinical or histological markers or both. Although easier to perform, studies using such outcomes pose a double problem: first, there is little evidence of the predictive value of many of those outcomes; second, they are difficult to compare. In addition, widespread outcomes, such as dysplasia grade, may be affected by high inter‐observer and even intra‐observer variation (Abbey 1995; Karabulut 1995).
It is noteworthy that, although surgery is the first choice in leukoplakia management for many clinicians, there is an absence of RCTs comparing the effects of surgical excision versus no treatment or placebo (Marley 1998). The only data available are from observational studies comparing rates of cancer incidence in people who did or did not undergo surgical treatment for oral leukoplakias. Such studies have differences in diagnostic and inclusion criteria, follow‐up interval, participant characteristics and surgical techniques employed (scalpel, laser, cryotherapy). They show highly variable results and sometimes are conflicting in their conclusions (Saito 2001; Schepman 1998). In addition, on the basis of animal and clinical studies, it has been speculated that surgery itself might act to promote carcinogenesis in pre‐malignant oral lesions (Holmstrup 2009). Trials evaluating interventions directed against risk factors (e.g. smoking) are also missing.
The applicability of results of two of the included studies (Sankaranarayan 1997; Stich 1988) should be considered in the context of their different risk factor profile as the participants were all betel quid chewers, a risk factor uncommon in individuals from geographical areas outside South Asia.
Leukoplakias with different histological or molecular characteristics may have different risks of transforming into cancer. However, the value of predictive factors proposed so far in the literature requires sound confirmatory data. The presence of epithelial dysplasia may be predictive of a transformation to oral cancer and the risk of cancer may increase with the severity of dysplastic changes (Lumerman 1995; Schepman 1998; Warnakulasuriya 2011), although this hypothesis has been recently challenged (Holmstrup 2006). Unfortunately, the available data did not allow us to perform a subgroup analysis of lesions with and without dysplasia, thus it is not possible to establish whether any particular treatment may be more indicated in the presence of dysplasia of different severity. Many different molecular biomarkers have been proposed, but no single marker or battery of markers seems predictive enough to be implemented during clinical care.
Quality of the evidence
Of the studies included in the present review, we judged one as having low risk of bias, six asunclear risk of bias and seven ashigh risk of bias. Although these studies were randomised trials, information about randomisation methods was missing or incomplete in most studies. In particular, methods used for random sequence generation were unclear in eight out of 15 studies, and details on allocation concealment were missing in 10 out of 15 studies. The body of evidence for cancer incidence comprises three studies investigating three different treatments: vitamin A, beta carotene and topical bleomycin. We assessed these studies as having very low quality according to GRADE (Grades of Recommendation, Assessment, Development and Evaluation) assessment criteria (see summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 5). Thus, the quality and the number of included trials, often with short follow‐up times, suggest cautious interpretation of results.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies

Forest plot of comparison: 1 Vitamin A or retinoids vs placebo, outcome: 1.1 Cancer development

Forest plot of comparison: 1 Vitamin A or retinoids vs placebo, outcome: 1.2 Oral lesion not completely resolved

Forest plot of comparison: 2 Beta carotene or carotenoids vs placebo, outcome: 2.1 Cancer development

Forest plot of comparison: 2 Beta carotene or carotenoids vs placebo, outcome: 2.2 Oral lesion not completely resolved

Comparison 1 Vitamin A or retinoids versus placebo, Outcome 1 Oral cancer development.
Comparison 1 Vitamin A or retinoids versus placebo, Outcome 2 Clinical resolution.

Comparison 1 Vitamin A or retinoids versus placebo, Outcome 3 Improvement of histological features.

Comparison 2 Beta carotene or carotenoids versus placebo, Outcome 1 Oral cancer development.

Comparison 2 Beta carotene or carotenoids versus placebo, Outcome 2 Clinical resolution.
Comparison 2 Beta carotene or carotenoids versus placebo, Outcome 3 Improvement of histological features.

Comparison 3 NSAIDs versus placebo, Outcome 1 Clinical resolution.

Comparison 4 Herbal extracts versus placebo, Outcome 1 Clinical resolution.

Comparison 4 Herbal extracts versus placebo, Outcome 2 Improvement of histological features.

Comparison 5 Topical bleomycin versus placebo, Outcome 1 Oral cancer development.
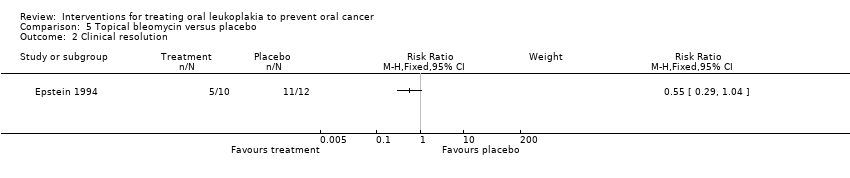
Comparison 5 Topical bleomycin versus placebo, Outcome 2 Clinical resolution.
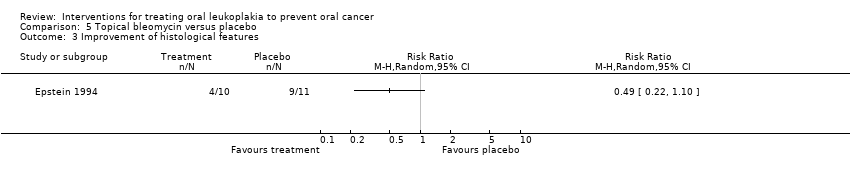
Comparison 5 Topical bleomycin versus placebo, Outcome 3 Improvement of histological features.

Comparison 6 Bowman‐Birk inhibitor versus placebo, Outcome 1 Clinical resolution.
| Systemic or topical vitamin A vs placebo for treating leukoplakia | ||||||
| Patient or population: people with oral leukoplakia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with vitamin A or retinoids | |||||
| Cancer development at 24 months from start of treatment (treatment lasted 12 months) | 93 per 1000 | 10 per 1000 | RR 0.11 | 85 | ⊕⊝⊝⊝ | This study evaluated systemic treatment |
| Clinical resolution (not completely resolved) at 4 to 12 months | Studies could not be combined in meta‐analysis One study evaluated topical treatment and did not find evidence of benefit 3 studies of systemic vitamin A ‐ 2 showed benefit in terms of clinical resolution, and 1 did not | |||||
| Histological changes (not improved) at 3 months | 889 per 1000 | 382 per 1000 (213 to 676) | RR 0.43 (0.24 to 0.76) | 41 (1 RCT) | ⊕⊕⊕⊝ | This study evaluated systemic treatment |
| Safety of the intervention at 4 to 12 months | 3 studies (1 each evaluating topical acitretin, topical 13‐cis‐retinoic acid, 200,000 IU per week of vitamin A) found no adverse effects. Systemic 13‐cis‐retinoic acid (1 to 2 mg/kg/d) caused adverse effects of varying severity in 79% of participants | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| *From event rate in control group | ||||||
| Systemic beta carotene or carotenoids vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with beta carotene or carotenoids | |||||
| Cancer development at 24 months from start of treatment (treatment lasted 12 months) | 108 per 1000 | 79 per 1000 | RR 0.73 | 132 | ⊕⊕⊝⊝ | |
| Clinical resolution (not completely resolved) at 5 to 12 months | The 3 studies could not be combined in meta‐analysis. 2 found benefit for systemic beta carotene, and 1 did not | |||||
| Histological changes (not improved) at 5 months (treatment lasted 3 months) | 833 per 1000 | 200 per 1000 (100 to 383) | RR 0.24 (0.12 to 0.46) | 58 (1 RCT) | ⊕⊕⊝⊝ | |
| Safety of the intervention at 5 to 12 months | Systemic beta carotene did not cause any adverse effects in 1 study supplementing 10 mg/d, and caused adverse effects of varying severity in 9% of participants in another study supplementing 360 mg/wk No adverse effects were reported by participants treated with systemic lycopene in one study | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| * From event rate in control group | ||||||
| NSAIDs vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: NSAIDs Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with NSAIDs | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 3 months | 1 study evaluated systemic treatment and 1 evaluated topical treatment. Neither found benefit for NSAIDs | |||||
| Histological changes (not improved) | Not measured | |||||
| Safety of the intervention over 3 months | Systemic celecoxib (1 study) ‐ 32 intervention participants reported 56 adverse effects and 20 placebo participants in the placebo group reported 20 adverse effects. Minor adverse events included dizziness, diarrheoa and abdominal pain. 4 participants (2 from the placebo group and 2 from an intervention group) had grade 3 adverse events. 2 participants permanently discontinued treatment owing to an adverse event (grade 2 vision abnormality and hypertension in a participant receiving 400 mg twice daily of celecoxib and a grade 3 ischaemic cerebrovascular accident in a participant receiving 200 mg twice daily of celecoxib) Ketorolac oral rinse (1 study) caused adverse effects of varying severity in 29% of participants | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| Herbal extracts vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: herbal extracts ‐ tea components; a Chinese herbal mixture; curcumin chewing gum; freeze‐dried black raspberry gel Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with herbal extracts | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 3 to 6 months | 3 studies (1 of freeze‐dried black raspberry gel, 1 of green tea extract capsules and 1 of mixed tea treatment) did not find evidence of benefit for the intervention | |||||
| Histological changes (not improved) at 3 months | 2 studies (1 of green tea extract capsules and 1 of freeze‐dried raspberry gel) did not find evidence of benefit from these interventions | |||||
| Safety of the intervention up to 3 months | 3 studies measured adverse effects: green tea extract capsules caused high frequency (93%) of adverse effects of varying severity in 1 study; the mixed tea treatment study did not mention adverse effects; freeze‐dried black raspberry gel did not cause any adverse effects | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| Topical bleomycin vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with topical bleomycin | |||||
| Cancer development up to 7 years | 83 per 1000 | 250 per 1000 (27 to 1000) | RR 3.00 | 20 | ⊕⊝⊝⊝ | |
| Clinical resolution (not completely resolved) at 3 months | 917 per 1000 | 504 per 1000 (266 to 954) | RR 0.55 (0.29 to 1.04) | 22 (1 RCT) | ⊕⊝⊝⊝ | |
| Histological changes (not improved) at 3 months | 818 per 1000 | 401 per 1000 (180 to 900) | RR 0.49 (0.22 to 1.10) | 21 (1 RCT) | ⊕⊝⊝⊝ | |
| Safety of the intervention up to 3 months | "All patients in the bleomycin group developed erythema with erosion by the end of the applications, whereas erythema developed in the placebo group. Discomfort was reported by 60% of the bleomycin group, but analgesics were not required. Taste of the topical application as well‐tolerated. There was no observed systemic toxicity in the patient groups" | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| * From event rate in control group | ||||||
| Bowman‐Birk inhibitor vs placebo for treating oral leukoplakia | ||||||
| Patient or population: people with oral leukoplakia Intervention: Bowman‐Birk inhibitor Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | Number of participants | Quality of the evidence | Comments | |
| Risk with placebo | Risk with Bowman‐Birk inhibitor | |||||
| Cancer development | Not measured | |||||
| Clinical resolution (not completely resolved) at 6 months | 957 per 1000 | 957 per 1000 (871 to 1000) | RR 1.00 (0.91 to 1.09) | 21 (1 RCT) | ⊕⊕⊝⊝ | |
| Histological changes (not improved) at 6 months | Not measured | |||||
| Safety of the intervention up to 6 months | Trial authors reported that there were no significant adverse effects. 33 participants in the intervention group reported 75 adverse effects. 25 participants in the placebo group reported 63 adverse effects | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) | ||||||
| GRADE Working Group grades of evidence | ||||||
| * From event rate in control group | ||||||
| Study | Participants with dysplastic leukoplakia (any grade) |
| Not reported | |
| 22% | |
| 27% | |
| 20% | |
| 72.5% | |
| Not reported | |
| Not reported | |
| Not reported | |
| Not reported | |
| Not reported | |
| 59% | |
| Not reported | |
| 18.75% | |
| 73.2% |
| Study | Arms | Active treatment | Placebo | Adverse effects |
| Bowman Birk inhibitor concentrate vs placebo | 75 reported from 33 of 67 participants | 63 reported from 25 of 65 participants | Minor adverse effects | |
| Topical bleomycin vs placebo | 10/10 | 0/12 | Bleomycin group ‐ erythema and erosion (100%), discomfort (60%) Placebo group ‐ erythema only | |
| Systemic 13‐cis‐retinoic acid (from 1 to 2 mg/kg per day) vs placebo | 19/24 | 4/20 | Cheilitis, facial erythema, dryness and peeling of skin, conjunctivitis, hypertriglyceridaemia | |
| Systemic and topical tea vs placebo | Not measured or reported | |||
| Freeze‐dried black raspberry gel vs placebo gel | 0/22 | 0/18 | "No participant experienced any treatment‐associated complications" | |
| Ketorolac oral rinse vs placebo | 11/38 | 3/19 | Pain, toxicity grade 1 and 2 | |
| Beta carotene and vitamin C vs placebo | 0/23 | 0/23 | No untoward side effects were noted | |
| Celecoxib vs placebo | 56 reported from 32 participants | 20 reported from 18 participants | 4 participants presented grade 3 adverse events: 2 in placebo arm and 2 in active treatment arm. 2 participants from intervention groups discontinued treatment owing to adverse effects (1 grade 2 and 1 grade 3). | |
| Topical 13‐cis‐retinoic acid vs placebo | 0/5 | 0/5 | "No side effects from the use of the gel | |
| Vitamin A (300,000 IU per week) vs placebo | 13/50 | 1/55 | Headache, muscular pain, dry mouth | |
| Beta carotene (360 mg per week) vs placebo | 5/55 | 1/55 | Headache, muscular pain | |
| Lycopene (8 mg or 4 mg) vs placebo | 0/40 | 0/18 | "No side effects, toxicity of any sort were encountered in the complete duration of the therapy" | |
| Vitamin A (200,000 IU per week) vs placebo | 0/30 | 0/35 | "The administered doses of vitamin A did not produce any detectable adverse | |
| Chinese herbal mixture vs placebo | Not measured or reported | |||
| Green tea extract at different doses (500, 750 or 1000 mg/m2 daily) vs placebo | 28/30 | 8/11 | Grade 1 to 2 adverse events including insomnia, headache, nausea and nervousness | |
| vs = versus | ||||
| Study | Arms | Active treatment | Placebo |
| Bowman Birk inhibitor concentrate vs placebo | 24/67 | 19/65 | |
| Topical bleomycin vs placebo | 0/10 | 1/12 | |
| Systemic 13‐cis‐retinoic acid (from 1 to 2 mg/kg per day) vs placebo | 2/24 | 2/20 | |
| Systemic and topical tea vs placebo | 3/32 | 2/32 | |
| Freeze‐dried black raspberry gel vs placebo gel | 0/22 | 0/18 | |
| Ketorolac oral rinse vs placebo | 1/38 | 0/19 | |
| Beta carotene and vitamin C vs placebo | 5/23 | 5/23 | |
| Celecoxib vs placebo | 3/32 | 1/18 | |
| Topical 13‐cis‐retinoic acid vs placebo | 0/5 | 1/5 | |
| Vitamin A (300,000 IU per week) vs placebo | 8/50 | 12/55 | |
| Beta carotene (360 mg per week) vs placebo | 9/55 | 12/55 | |
| Lycopene (8 mg or 4 mg) vs placebo | 0/40 | 0/18 | |
| Vitamin A (200,000 IU per week) vs placebo | 9/30 | 2/35 | |
| Chinese herbal mixture vs placebo | 1/60 | 7/60 | |
| vs = versus | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral cancer development Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Clinical resolution Show forest plot | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Systemic treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Topical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improvement of histological features Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral cancer development Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Systemic treatment | 2 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.09] |
| 2 Clinical resolution Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Systemic treatment | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Improvement of histological features Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical resolution Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Systemic treatment | 1 | 46 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.83, 1.08] |
| 1.2 Topical treatment | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.81, 1.10] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical resolution Show forest plot | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Topical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Systemic plus topical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Improvement of histological features Show forest plot | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Systemic treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Topical treatment | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Oral cancer development Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical resolution Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3 Improvement of histological features Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Clinical resolution Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

