Antagonistas de la hormona liberadora de gonadotrofina en la tecnología de reproducción asistida
References
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en espera de evaluación
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | RCT, parallel design | |
| Participants | 124 poor responders undergoing IVF | |
| Interventions | GnRH antagonist (n = 62): 450 IU HP‐FSH (Urofollitropin) + cetrorelix 0.25 mg daily added to the ovarian stimulation when the largest follicle measures ≥ 14 mm (flexible protocol) GnRH agonist (n = 62): 450 IU HP‐FSH (Urofollitropin) + triptoreline 0.05 mg daily (half the standard dose) initiated in the mid‐luteal phase prior to the treatment cycle (minidose long protocol) Mean number of embryos transferred: antagonist: 2.1 ± 1.2 versus agonist: 2.3 ± 1.3 Follow‐up: Up to clinical pregnancy | |
| Outcomes | Clinical pregnancy rate, cycle cancellation rate, duration of stimulation, total gonadotrophins requirement, estradiol level on day of hCG, mean numbers of mature oocytes retrieved, mean numbers of embryos formed, mean numbers of embryos transferred | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not reported but unlikely to influence measurement of outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient data provided regarding withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available to identify outcomes of interest. Live birth rate not reported |
| Other bias | Low risk | None identified |
| Methods | RCT, open‐label parallel design, multi‐centre (7 centres), multi‐national | |
| Participants | 293 Infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI Inclusion criteria: with no more than three previous IVF‐ET attempts with all causes of infertility (except polycystic ovary and moderate or severe endometriosis) Baseline characteristics: age 31.9 ± 3.7 versus 31.6 ± 3.8. Duration of infertility: not stated. FSH: not stated. BMI not stated | |
| Interventions | Group I (n = 198): hMG (menogon, humegon, pergonal) was started at 2 or 3 ampoules for four days and the dose was adjusted according to response + multiple‐dose regimen of 0.25 mg of GnRH antagonist (cetrorelix) was administered SC starting from day 6 of hMG treatment to 115 participants up to and including day of hCG administration (Fixed) Group II, GnRH agonist (n = 95): mid‐luteal GnRH analogue (Buserlin 150 µg four times daily intranasally) + hMG (menogon, humegon,pergonal) was started at 2 or 3 ampoules for four days and the dose was adjusted according to response Luteal phase support: daily vaginal progesterone or hCG injections | |
| Outcomes | Premature LH surge defined as (LH > 10 IU/L) and progesterone level > 1 ng/L. Stimulation length, no. of hMG ampoules. E2 on hCG, no of oocytes retrieved, clinical pregnancy/OPU, clinical pregnancy/ET. Miscarriage | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Only reported as a randomised trial with no further details of how randomisation was performed |
| Allocation concealment (selection bias) | Low risk | Concealed; central telephone, 2:1 randomisation ratio |
| Blinding (performance bias and detection bias) | High risk | Open |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports include most expected outcomes |
| Other bias | Unclear risk | Supported by pharmaceutical company, the study appears to be free from other sources of bias |
| Methods | RCT | |
| Participants | 41 women Inclusion criteria: 21 to 40 years, anti‐Mullerian hormone > 1 ng/ml Exclusion criteria: no details Setting and timing: no details of setting. USA. No details of timing Baseline characteristics: no details | |
| Interventions | GnRH antagonist (no details) (n = 21) when follicle size reached 12 mm during the COS cycle Agonist (Leuprolide acetate) (n = 20) starting on day 18 of the oral contraceptive pill cycle Fixed protocol of human derived gonadotrophins at a 3:1 ratio (225/75 IU) for the first four days of stimulation followed by a flexible protocol to improve response hCG given when at least three follicles reached 17 mm diameter All women underwent a cycle using an oral contraceptive pill before starting the controlled ovarian stimulation cycle for IVF | |
| Outcomes | E2, LH, oocytes retrieved, mature oocytes, fertilisation rate, implantation rate, pregnancy rate | |
| Notes | The table included in the abstract does not match the abstract. No data could be included in the meta‐analyses Sample size calculation ‐ unclear ITT analysis ‐ unclear Funding ‐ Ferring Pharmaceuticals | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomised' no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | No details, unlikely to be blinded but this should not influence fertility outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Conference abstract, unclear if this is the full sample size or reporting preliminary data |
| Selective reporting (reporting bias) | High risk | No raw data reported. The table does not match the abstract. No full paper identified |
| Other bias | Unclear risk | Conference abstract only. No details of demographics |
| Methods | RCT | |
| Participants | 413 women (564 cycles) Inclusion criteria: women < 40 years undergoing COH but no other details Exclusion criteria: not stated Baseline characteristics: not stated Setting and timing: no details | |
| Interventions | Agonist long protocol ‐ no details Agonist short protocol ‐ no details Antagonist protocol ‐ no details | |
| Outcomes | Pregnancy rate, miscarriage rate | |
| Notes | Data are only reported as the number of antral follicles and not by the allocated treatment. The denominator for the pregnancy rate does not match either the total number of women or the number of cycles. Data could not be included in a meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘randomly received’ no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | No details |
| Incomplete outcome data (attrition bias) | Unclear risk | No details |
| Selective reporting (reporting bias) | High risk | Conference abstract only. No details as to number allocated to each group. Pregnancy and miscarriage rates reported only |
| Other bias | Unclear risk | Conference abstract only |
| Methods | RCT, two‐centre trial | |
| Participants | 111 infertile women undergoing IVF/ICSI Inclusion criteria: were not at an a priori increased risk for chromosomally abnormal embryos. < 38 years of age, regular indication for IVF and with a partner with a sperm count > 5 million progressively motile sperm per millilitre, regular menstrual cycles (ranging from 25 to 35 days), BMI 19 ‐ 29 kg m2, no Baseline characteristics:female age (years) 34.1 (28 – 37) vs 33.2 (22 – 37), basal FSH 8.1 (4.4 – 13.8) vs 7.6 (5.5 – 18.4), Inhibin B level on cycle day 3 or 4: 86 (2 – 1056) vs 88 (15 – 593) | |
| Interventions | Group I (n = 67):150 IU FSH on 2nd day of the cycles (fixed) + 0.25 mg SC of GnRH antagonist co‐treatment (ganirelix (Orgalutran)) administered when at least one follicle measuring > 14 mm (flexible protocol) GnRH agonist (n = 44): 225 IU rFSH (fixed) + long GnRH agonist, 0.1 mg triptorelin(Long GnRH agonist protocol) Oocyte maturation triggering: 10,000 IU SC hCG (Pregnyl) when one follicle > 18 mm plus 2 follicles > 15 mm Maximum embryos transferred: 2 Follow up: OPR was confirmed by vaginal ultrasound scan at 12 weeks of gestation | |
| Outcomes | Primary outcome measures: ovarian response, as assessed by the number of oocytes obtained and the proportion of chromosomally abnormal embryos per participant. This was expressed as the ratio of abnormal embryos on the number of embryos diagnosed per participant. | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule in a ratio of 4:6 (conventional group: mild group) |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes. After the participant agreed to participate, the next available numbered envelope on entry into the study was opened by the treating physician during the preparatory IVF consultation |
| Blinding (performance bias and detection bias) | Low risk | Embryologist |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | High risk | Funding was provided by university and non‐governmental organisation Early stopping due to benefit occurred. "The proportion of chromosomally abnormal embryos per patient was found to be significantly reduced after mild ovarian stimulation (P ¼ 0.02, which is below the Pocock critical bound of 0.0354 for a single interim analysis after 61% (111 of 181) of women had been included (Pocock, 1977)) and the study was terminated." |
| Methods | RCT, single‐centre, open‐label, parallel design | |
| Participants | 100 infertile women undergoing ICSI Inclusion criteria: primary infertility patients, 18 to 39 years old, with regular menstrual cycle and FSH levels < 10 IU/L done at cycle day 3 and ultrasound examination showed normal uterus Exclusion criteria:women with severe endometriosis (American Fertility Society stage III and IV), and azoospermic males were excluded from the study Baseline characteristics: age 30.8 ± 4.8 vs 30.28 ± 5.9 years | |
| Interventions | GnRH antagonist (n = 50): 225 IU Menogon hMG (menogon, humegon, pergonal) was started at 2 or 3 ampoules for four days (adjusted) + 0.25 mg of GnRH antagonist SC ganirelix started from day 6 of hMG treatment/lead follicle measures 14 mm (Flexible multiple‐dose GnRH antagonist) Oocyte maturation triggering: hCG 10,000 IU (Choriomon) was administered deeply IM when the leading follicle reached 20 mm in mean diameter with at least three follicles > 18 mm Oocyte retrieval: 34 ‐ 36 hours Embryo transfer: 2 ‐ 3 days after OPU | |
| Outcomes | Female partner age Infertility duration years Baseline FSH mIU/ml Day 3 LH mIU/ml Day 14 E2 pg/ml E2 pg/ml on day of hCG hMG ampoule Stimulation duration Number of follicles Size of follicles (mm) Endometrial thickness Number of oocytes retrieved Number of MII oocytes Number of oocytes fertilised Fertilisation rate Embryos No of transferred embryos Pregnancy rate/ET Abortion rate OHSS | |
| Notes | Number of participants at randomisation: 100 (ganirelix 50/Superfact 50) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation stated to be using sealed envelopes without any further details |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | No blinding |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data, LBR was not reported |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias. |
| Methods | RCT, single‐centre, open‐label, parallel design, randomisation: 1:1 (cetrorelix: leuprolide acetate) ratio | |
| Participants | 148 women with PCOS, no previous ART or had hyperprolactinaemia or thyroid abnormalities | |
| Interventions | GnRH antagonist (n = 75): antagonist protocol: cetrorelix 0.25 mg/d subcutaneously started when the leading follicle reached 14 mm. hCG 10,000 IU administered when at least two follicles reached 18 mm (Flexible) GnRH agonist (n = 70): agonist protocol: L.A. 0.5 mg, on day 14 of the cycle. Daily administration of gonadotrophins, 2 or 3 ampoules initiated on the third day of the anteceding menstrual period (Long GnRH agonist protocol) Oocyte maturation triggering: hCG 10,000 IU administered when at least two follicles reached 18 mm | |
| Outcomes | Days of analogue treatment | |
| Notes | Number of participants at randomisation: 148 (cetrorelix: 75/leuprolide acetate: 73) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data, LBR not reported |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, multi‐centre (4 US centres), open‐label, parallel design | |
| Participants | 80 women undergoing IVF/ICSI Inclusion criteria: < 39 years of age, day 3 FSH level of < 10, E2 level of < 60 pg/mL, AFC > 5 with a menstrual cycle range of 26 ‐ 34 days, and no more than one previous failed IVF or IVF/ICSI cycle. BMI 19 ‐ 32 kg/m2 no hydrosalpinx present by hysterosalpingogram, laparoscopy, or ultrasound within the past year Exclusion criteria: history of previous poor response (< 4 follicles and/or an E2 level of < 500 pg/mL on the day of hCG), had taken infertility medications (clomiphene and/or gonadotrophins) within the past month, or had failed to consent to taking OCs, GnRH‐analogues, or gonadotrophins. | |
| Interventions | GnRH antagonist (n = 40): OC (Desogen; Organon USA) on cycle days 2 to 4 for 14 to 28 days + 300 IU/day rFSH SC (adjusted) + 250 µg ganirelix was initiated when a lead follicle obtained a mean diameter of 12 to 14 mm (flexible) GnRH agonist (n= 40): leuprolide (GnRH‐agonist group), 0.5 mg per day during the mid‐luteal phase with approximately a 5‐day overlap with the OCs. Once adequate pituitary desensitisation was achieved the dose of GnRH agonist was reduced to 0.25 mg per day + 300 IU/day rFSH SC (adjusted) Oocyte retrieval: 35 to 36 hours later Embryo transfer: at 3 or 5 days Luteal phase support: progesterone, one centre treated women with P, 25 mg IM, on the day of retrieval, followed by P, 50 mg IM daily, with some women being supplemented with hCG 2,500 IU on days 3 and 6 after retrieval. The other centres prescribed luteal support with a daily dose of P (50 mg IM). | |
| Outcomes | Participants to oocyte retrieval (n = 77) | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Dark sealed envelopes (true), randomisation: 1:1 (ganirelix acetate: leuprolide acetate) ratio |
| Allocation concealment (selection bias) | Low risk | Dark sealed envelopes (true) |
| Blinding (performance bias and detection bias) | High risk | Not reported clearly |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre, parallel design, randomisation: 1:1 (cetrorelix: triptorelin) ratio | |
| Participants | 120 infertile women undergoing IVF/ICSI Inclusion/Exclusion criteria: | |
| Interventions | GnRH antagonist (n = 57): in the antagonist arm, rFSH from day 2 of the cycle, and when the leading follicle reached 13 mm cetrorelix 0.25 mg (Cetrotide; Serono) was started. The LH levels were checked on the day, when the leading follicle reached 13 mm (LH1) and 21 mm (LH2) (Flexible) GnRH agonist (n = 63): in the agonist arm, triptorelin 3,75 (Diphereline SR 3,75mg; Beaufour Ipsen) was administered on day 20 of the preceding cycle and 14 days later if E2 < 30 pg/mL, stimulation with rFSH (Gonal‐F; Serono) was initiated | |
| Outcomes | Number of FSH ampoules used | |
| Notes | Number of participants at randomisation: 120 (cetrorelix: 57/ triptorelin: 63) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported clearly |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported clearly |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | Two‐arm parallel RCT | |
| Participants | 60 infertile women undergoing IVF at IVF centre; no further details were reported about participants | |
| Interventions | GnRH antagonist: no details were reported GnRH agonist: no details were given | |
| Outcomes | Only pregnancy rate was reported but type of pregnancy was not defined | |
| Notes | Unclear definition of pregnancy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was reported on random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No information was reported on blinding of participants and/or personnel; however, non blinding of outcome assessment may not affect some outcomes of interest as they are objectively assessed |
| Incomplete outcome data (attrition bias) | Unclear risk | No information was reported on incomplete outcome data/attrition |
| Selective reporting (reporting bias) | Unclear risk | Methods section not detailed enough to make conclusive judgement |
| Other bias | Low risk | None identified |
| Methods | RCT, single‐centre, open‐label, parallel design, randomisation: 1:1 (ganirelix: leuprolide) ratio | |
| Participants | Couples requiring IVF or intracytoplasmic sperm injection (ICSI) | |
| Interventions | Agonist regimen: leuprolide acetate 0.5 mg qd for 10 days from mid‐luteal phase | |
| Outcomes | Clinical pregnancy, viable pregnancy, implantation rate | |
| Notes | Number of participants at randomisation: 60 (ganirelix: 30/leuprolide: 30) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | High risk | Intention‐to‐treat analysis was not used and drop‐out rate was above 10% with reasons provided |
| Selective reporting (reporting bias) | Low risk | Protocol not available but materials match results |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | RCT, single‐centre, parallel design | |
| Participants | 66 infertile women undergoing IVF/ICSI Inclusion/ Exclusion criteria: Women with polycystic ovaries were excluded from the study | |
| Interventions | GnRH antagonist (n = 33): OCP (Nordette) 30 µg of ethinyl estradiol and 150 µg of levonorgestrel for 21 days + 300 IU daily rFSH (Gonal‐F) + 0.25 mg SC cetrorelix (Cetrotide) (fixed), multi‐dose GnRH antagonist protocol starting on day 6 of the stimulation (fixed) Oocyte retrieval: 36 hrs later. ICSI was performed only in cases with severe male factor or previous fertilisation failure Maximum number of embryo transfer: depending on the number of embryos available, up to three embryos were transferred on day 3 after oocyte retrieval Luteal phase support: IM hCG (Profasi) 2000 IU given every 3 days for four doses starting on the day of oocyte retrieval A clinical pregnancy was established when there was a gestational sac seen on ultrasonography | |
| Outcomes | The main outcome measures were duration of stimulation, consumption of gonadotrophins, cycle cancellation rate, and the number of mature follicles recruited and total oocytes retrieved. The hormone levels throughout the cycle, laboratory outcomes and clinical pregnancy rates were also reviewed | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random‐number table (true), randomisation: 1:1 (cetrorelix: buserelin acetate) ratio |
| Allocation concealment (selection bias) | Low risk | Performed |
| Blinding (performance bias and detection bias) | Low risk | Blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | Three‐arm randomised controlled trial. Single centre | |
| Participants | 61 infertile women (67 cycles) Inclusion criteria: PCOS (ESHRE/ASRM criteria). No other details Exclusion criteria: no details Setting and timing ‐ Seoul, Korea. April 2009 to November 2010 Baseline characteristics: age ‐ antagonist 32.9 ± 2.9 years versus agonist 34.4 ± 3.8 years | |
| Interventions | IVM/IVF with FSH and hCG priming protocol (n = 11 women; 14 cycles) Antagonist multidose flexible protocol (n = 36 women; 39 cycles). Stimulation started on day 2 or 3 and 0.25 mg cetrorelix acetate or 0.25 mg ganirelix acetate administered when follicles > 12 mm or serum E2 > 200 pg/ml Agonist long protocol (n = 14 women; 14 cycles) 0.5 mg buserelin acetate or 0.1 mg leuprolide acetate given from mid‐luteal phase of previous cycle to day of hCG administration 250 micrograms hCG given when lead follicle > 18 mm diameter Oocyte retrieval 36 hours after hCG Dose of gonadotrophin antagonist 1656.7 ± 669.77 versus agonist 2700.0 ± 824.3 | |
| Outcomes | OHSS, clinical pregnancy, miscarriage, live birth rate: all reported as 'per cycle' and thus could not be pooled | |
| Notes | Sample size calculation ‐ no ITT analysis ‐ yes Funding ‐ not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomized' no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | No details. Unlikely that participants and researchers were blinded but unlikely to affect fertility outcome. Blinding of outcome assessors is unclear |
| Incomplete outcome data (attrition bias) | Low risk | Data reported on all women analysed |
| Selective reporting (reporting bias) | Low risk | Outcomes reported including live birth rate |
| Other bias | Unclear risk | Groups appeared balanced at baseline. Data is per cycle and not per woman randomised and could not be included in the meta‐analysis |
| Methods | Randomised controlled trial | |
| Participants | 64 women undergoing ICSI (first cycle) Inclusion criteria: 37 years or less, first IVF/ICSI cycle, BMI < 30 kg/m2, regular menses, both ovaries present Exclusion criteria: PCOS, severe endometriosis, ovarian cysts assessed by transvaginal ultrasound, basal FSH 10 IU/ml or greater Baseline characteristics: age ‐ antagonist group 32.5 ± 3.0 years versus agonist group 33.2 ± 3.0 years Setting and timing: Center for Human Reproduction, Brazil | |
| Interventions | GnRH antagonist (n = 32) cetrorelix. Ovarian stimulation using 150 to 225 IU recombinant FSH and 75 IU/day recombinant LH for five days starting on day 3. Follicular development monitored on Day 8. rFSH adapted according to ovarian response and rLH increased to 150 IU/day when one or more follicles reached 10 mm or more in diameter. Cetrorelix 0.25 mg/day SC started when at least one follicle was 14 mm or greater in diameter. Administered until day of hCG injection GnRH agonist (n = 32) leuprolide acetate 1 mg/day starting in luteal phase of previous cycle for 14 days. Ovarian stimulation using 150 to 225 IU rFSH and 75 IU/day rLH for 7 days. rFSH adapted according to ovarian response and rLH increased to 150 IU/day when one or more follicles reached 10 mm or more in diameter. Administered until day of hCG injection Oocyte maturation: 250 micrograms recombinant hCG given SC when at least two follicles were 17 mm or greater in diameter Oocyte retrieval: 34 ‐ 36 hours after hCG injection Total dose gonadotrophins: antagonist group 1877.3 ± 817 IU versus agonist group 2185.5 IU | |
| Outcomes | Oocyte morphology. Implantation rate, pregnancy rate (not defined) | |
| Notes | Sample size calculation: yes ITT analysis ‐ yes Funding: no details Data cannot be included in a meta‐analysis as it is unclear if the pregnancy rate refers to a biochemical or clinical or ongoing pregnancy. Another trial Lavorato 2012 reports on 32 women from the same authoring group and centre with the outcomes of DNA fragmentation and apoptosis in granulosa cells. No pregnancy data reported in this paper either | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Double randomisation using computer‐generated number table then lots |
| Allocation concealment (selection bias) | Low risk | One nurse, blinded to subject identities, performed all the lot draws |
| Blinding (performance bias and detection bias) | Unclear risk | No evidence of blinding of participants. Blinding of outcome assessors unclear |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised appear to be analysed |
| Selective reporting (reporting bias) | Unclear risk | Authors report pregnancy rate but it is not defined, therefore unclear if it is biochemical, clinical or ongoing and cannot be included in a meta‐analysis. Other data is reported per oocyte of which there were 300 per group |
| Other bias | Low risk | Groups appeared balanced at baseline |
| Methods | RCT, single‐centre study | |
| Participants | 136 consecutive patients undergoing ICSI Inclusion criteria: age 24 ‐ 42 years, baseline FSH level < 10 IU/ml, absence of uterine or ovarian abnormalities or severe endometriosis or polycystic ovary syndrome, no more than three previous IVF attempts and, no oral contraceptive pills taken before the stimulation cycle. Male factor infertility cases, such as number of spermatozoa < 5.0 million/ml and < 30% motility were included. Criteria for cycle cancellation were as follows: 53 follicles with diameter 14 mm after 8– 10 days of stimulation Baseline characteristics: age 34.4 ± 4 vs 34 ± 3.9 yrs. Basal FSH (mIU/ml) 6.4 ± 2.4 vs 5.7 ± 2. Basal E2 (pg/ml) 21.71 ± 3 vs 16.7 ± 9.4. BMI (kg/m2) 23.7 ± 4.1 vs 22.7 ± 3.4 | |
| Interventions | GnRH antagonist (n = 67): a daily dose of cetrorelix 0.25 mg (Cetrotide) was administered when a leading follicle reached a diameter of 12 – 14 mm + rFSH (Gonal F) starting on cycle day 2 – 3 at a dose of 225 UI/daily, for the first five days (adjusted) (Flexible protocol) GnRH agonist (n= 69): 0.1 mg triptorelin (Decapeptyl 0.1 mg) were administered subcutaneously daily, starting in the late luteal phase (day 21) of the previous cycle + rFSH starting on cycle day 2 – 3 at a dose of 225 UI/daily, for the first five days (adjusted) Final oocyte maturation: was achieved with 6500 UI of hCG (Ovitrelle) when two or more follicles reached a diameter 18 mm Embryo transfer: 3 embryos on day 2 Luteal phase support: was supplemented with progesterone in oil, 50 mg/ day (Prontogest) starting the day after oocyte retrieval and continuing until 12 weeks' gestation if pregnancy was achieved | |
| Outcomes | Oocyte and embryo grading, implantation rate, clinical pregnancy and ongoing rates | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list at initiation of stimulation, to receive GnRH antagonist or agonist, by using a free internet software for randomisation (Graphpad Software QuickCalcs) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Low risk | The embryologist scoring the embryos was blinded to the study groups |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | Outcomes were reported in a pre‐specified manner |
| Other bias | Low risk | Baseline characteristics balanced in both groups |
| Methods | RCT, single‐centre, parallel design, randomisation: 1:1 | |
| Participants | 160 patients scheduled for ICSI | |
| Interventions | Agonist group were treated with buserelin/hMG stimulation (long luteal protocol) while the antagonist group were treated with cetrorelix/hMG stimulation (flexible protocol). | |
| Outcomes | The treatment period | |
| Notes | Number of participants at randomisation: 160 (cetrorelix: 80/buserelin: 80) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Reported as randomisation using opaque envelopes without any further details |
| Allocation concealment (selection bias) | Low risk | Opaque envelopes |
| Blinding (performance bias and detection bias) | Low risk | Blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, LBR/OPR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre | |
| Participants | 60 women (high responders), aged 20 – 39 years , normal basal FSH concentration ≤ 10.0 IU/L), and undergoing their first cycle of IVF with either PCOS or PCOM (defined according to the Rotterdam consensus guidelines (Rotterdam 2004)) or undergoing a subsequent cycle with a history of high response in a previous IVF cycle. Participants were recruited for the trial for only one cycle Exclusion criteria: women with hypogonadotrophic hypogonadism were excluded Baseline characteristics: age (yrs) 32.0 ± 3.7 vs 33.1 ± 3.6, BMI (kg/m2) 28.3 ± 7.1 vs 30.7 ± 6.4. Baseline serum FSH (IU/L) 5.4 ± 1.8 vs 5.3 ± 1.2 | |
| Interventions | Study group: OCPs for 21 days + 112 ‐ 225 IU/day rFSH + ganirelix acetate when the dominant follicle ≥ 14 mm (Flexible multiple‐dose protocol) Control group: OCP for 25 days overlapping with 1 mg leuprolide acetate (Lupron), then reduced to 0.5 mg once down‐regulation was achieved (Low‐dose long GnRH agonist protocol) + 112 ‐ 225 IU/day rFSH Oocyte maturation triggering: when 2 ‐ 3 leading follicles were > 18 mm in diameter Study group: GnRH agonist, 1 mg approximately 12 hours after the last dose of ganirelix Control group: 3300 to 10,000 IU hCG (Profasi) Oocyte retrieval: 35 hours later, followed by IVF/ ICSI Maximum number of embryos transferred: 3 Luteal phase support: study group: 50 mg IM P in oil daily + 0.1 mg transdermal E2 patches every other day; control group: 50 mg IM P in oil daily | |
| Outcomes | OHSS, implantation rate, number of oocytes retrieved, proportion of mature oocytes retrieved, fertilisation rate, mid‐luteal phase mean ovarian volume (MOV), clinical and ongoing pregnancy rates, and luteal phase serum E2 and P levels | |
| Notes | The diagnosis of OHSS was based on the criteria by Golan et al | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. To ensure similar distribution of previous high response in the two groups, separate randomisation schedules were drawn up for women undergoing their first cycle and for women with a previous high response by the use of stratified randomised blocks |
| Allocation concealment (selection bias) | Low risk | Research nurse used a series of consecutively numbered sealed opaque envelopes |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, multi‐centre (12 centres, 9 countries), Europe and Middle East, multi‐national, open‐label, parallel | |
| Participants | 321 infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI with all causes of infertility Inclusion criteria: healthy female partners of infertile couples, age at time of screening ≥ 18 but < 39 years, a body mass index (BMI) between 18 and 29 kg/m², a regular menstrual cycle, and willing to give written informed consent Age: not stated Duration of infertility: ganirelix 4.3 years, triptorelin 4.1 years FSH: ganirelix 5.8 IU/ml, triptorelin 2.8 IU/ml BMI: not stated | |
| Interventions | GnRH antagonist (n = 215): 150 IU rFSH (Puregon) (adjusted) + multiple‐dose regimen of 0.25 mg of GnRH antagonist (ganirelix) was administered SC starting from day 6 of stimulation (fixed) GnRH agonist (n=106): mid‐luteal GnRH analogue (triptorelin 0.1 mg SC ) + 150 IU rFSH (Puregon) (adjusted) Oocyte maturation triggering: hCG (Pregnyl) 10,000 IU in 1 ml saline when at least three follicles ≥ 17 mm Oocyte retrieval: About 30 ‐ 36 hours later followed by IVF or ICSI Maximum embryos transferred: 3 Luteal phase support: done according to the centre routine practice Follow up: until ongoing pregnancy | |
| Outcomes | Premature LH surge: (defined as (LH > 10 IU/L) and progesterone level > 1 ng/L) ganirelix group 1 versus triptorelin group 0 Stimulation length: ganirelix group 9 versus triptorelin group 26 E2 on hCG: ganirelix group 1090 pg/ml versus triptorelin group 1370 pg/ml Number of oocytes retrieved: ganirelix group 7.9 ± 5.1 versus triptorelin group 9.6 ± 6.8 Number of embryos obtained: ganirelix group 4.0 ± 3.0 versus triptorelin group 4.7 ± 3.0 Number of embryos transferred: not mentioned Clinical preg/cycle: ganirelix group 32.3 versus triptorelin group 37.8 Clinical preg/ET: ganirelix group 35.8 versus triptorelin group 41.7 Ongoing pregnancy rate: ganirelix group 31.4 versus triptorelin group 33.9 Cancellation: ganirelix group 22 versus triptorelin group 15 Miscarriage: ganirelix group 10.3 versus triptorelin group 11.4 Ectopic: ganirelix group 2 versus triptorelin group 0 OHSS: ganirelix group 4 versus triptorelin group 1 Severe OHSS: only one case ganirelix group Local reaction: ganirelix group 11.9 versus triptorelin group 24.1 | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive response voice system, stratified randomisation, 2:1 randomisation ratio |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | RCT, multi‐centre (20 centres), open‐label, parallel design | |
| Participants | 730 Infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI with all causes of infertility Baseline characteristics: age ganirelix 31.9 ± 3.6, buserelin 31.9 ± 3.8, duration of infertility: ganirelix 4.5 ± 2.7, buserelin 4.4 ± 2.7, FSH: ganirelix 7.7, buserelin 8.4, BMI ganirelix 23 ± 2.9, buserelin 23 ± 2.7 | |
| Interventions | GnRH antagonist (n= 486): rFSH (Puregon) was started at fixed daily dose of 150 IU for five days and the dose was adjusted according to response + multiple‐dose regimen of 0.25 mg of GnRH antagonist (ganirelix) was administered SC starting from day 6 of hMG treatment (fixed) GnRH agonist (n= 244): mid‐luteal GnRH analogue (buserelin 0.6 or 1.2 mg four times daily intranasally) + rFSH (Puregon) was started at fixed daily dose of 150 IU for 5 days and the dose was adjusted according to response IVF was done in 357 cases, ICSI was done in 291 cases and 10 cases had both IVF and ICSI Luteal phase support: was done according to the centre's routine practice | |
| Outcomes | Premature LH surge defined as (LH > 10 IU/L) and progesterone level > 1 ng/L | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive response voice system (true), 2:1 randomisation ratio |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, parallel design | |
| Participants | 60 women undergoing IVF treatment Inclusion criteria: healthy female partners of infertile couples, regular menstrual cycles of 26 ‐ 34 days, BMI between 20 and 25 kg/m2, age between 20 and 39 years at the time of screening, baseline serum FSH level < 10 IU/l, and baseline serum E2 level ≤ 45 pg/ml on cycle day 3 Baseline characteristics: Age (years) 34.0 ± 4.3 vs. 34.6 ± 4.3, BMI (kg/m2) 22.9 ± 0.8 vs. 23.4 ± 0.7, baseline serum FSH (IU/l) 6.63 ± 3.25 vs. 6.73 ± 2.09, baseline serum LH (IU/l) 4.60 ± 2.21 vs. 4.53 ± 1.92 | |
| Interventions | GnRH antagonist (n = 30): 225 IU daily SC rFSH (Gonal F) from cycle day 3 (adjusted) + 0.25 mg SC daily cetrorelix acetate (Cetrotide) when there was a 14 mm dominant follicle until and including the day of hCG administration GnRH agonist (n = 30): 0.5 mg/day SC leuprorelin acetate (Enantone die) beginning at the mid‐luteal phase + 225 IU SC rFSH (Gonal‐F) starting 14 days after pituitary down‐regulation (adjusted). (long protocol) Oocyte maturation triggering: 10,000 IU of hCG (Gonasi HP) Oocyte retrieval: 36 hours after hCG administration Mean number of embryos transferred: antagonist: 2.10 ± 1.50 vs. agonist: 2.67 ± 0.96 Luteal phase support: 100 mg intravaginal micronised progesterone (Esolut) twice a day starting one day before embryo transfer Follow‐up: Clinical monitoring was carried out daily by transvaginal pelvic ultrasound (6.5 MHz) and E2 assay up till clinical pregnancy | |
| Outcomes | Clinical pregnancy rate, serum E2 levels on day 8 of follicular stimulation, serum E2 levels on day of hCG administration, serum LH levels on hCG day, number of mature follicles, number of retrieved oocytes, number of transferred embryos, follicular fluid (FF) insulin‐like growth factor‐I (IGF‐I) level, FF vascular endothelial growth factor (VEGF) level, FF E2 and androstenedione levels | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The patients were assigned randomly (computer‐generated randomization list; SPSS Inc., Chicago, IL, USA) to two different GnRH analogue regimens GnRH‐a (Group A, 30 patients) and Gn‐RH‐ant (Group B, 30 patients)” |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported but unlikely to affect measurement of outcome |
| Incomplete outcome data (attrition bias) | Low risk | Number randomised = 60, number analysed = 60 |
| Selective reporting (reporting bias) | Unclear risk | All expected outcomes reported as pre‐specified however live birth rate not reported |
| Other bias | Low risk | None identified |
| Methods | Randomised controlled trial, single centre | |
| Participants | 144 women Inclusion criteria: no specific details but included women who had had moderate or severe OHSS or who had been at risk of OHSS during their first IVF/ICSI cycle with a mid‐luteal long agonist protocol Exclusion criteria: no details Setting and timing: Italy; no details of timing Baseline characteristics: not stated | |
| Interventions | Antagonist ‐ cetrorelix 0.25 mg/day starting on day 3 of the menstrual cycle Agonist ‐ triptorelin 0.1 mg/day starting on day 21 of the menstrual cycle Ovarian stimulation was achieved with rFSH initiated on day 3 of the cycle at a maximum dose of 150 IU and dose adjusted depending on ovarian response Luteal phase support ‐ with micronised progesterone vaginal gel (no other details) Follow‐up ‐ followed‐up to live birth | |
| Outcomes | Live birth, clinical pregnancy, cancellation rate, oocytes retrieved | |
| Notes | As no denominators for groups are given, the data cannot be included in a meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | Open label but blinding unlikely to effect fertility outcome. No details of blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Although the abstract states that there were 144 women randomised, there are no other denominators to indicate how many women were allocated to each group. No details on withdrawals or losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract only. Outcomes are not pre‐specified. OHSS not reported |
| Other bias | Unclear risk | Conference abstract only. Groups were reported as being similar at baseline |
| Methods | RCT, single‐centre | |
| Participants | 235 infertile women undergoing IVF/ICSI Inclusion criteria: first cycle of the ART, age < 35 years, and basal FSH < 10 IU/L Baseline characteristics:age (years) 28.71 ± 2.8 vs 28.36 ± 3.1, BMI (kg/m2) 28.1 ± 3.4 vs 27.54 ± 4.3, basal FSH (IU/L) 5.77 ±1.2 vs 5.54 ± 1.1 | |
| Interventions | GnRH antagonist (n =118): 225 IU rFSH on 2nd day of the cycles (adjusted) + HMG + 0.25 mg SC of ganirelix took place on the 6th day of the stimulation (fixed protocol) GnRH agonist (n = 117): 150‐225 IU rFSH (adjusted) + long GnRH agonist, 500 μg buserelin per day (Suprefact) (SC), during menstrual cycle 21 and onwards, once down‐regulation was achieved, the dose of buserelin was reduced to 250 µg (low‐dose GnRH agonist protocol) Oocyte maturation triggering: hCG 10,000 IU (Profasi) was administered intramuscularly (IM) when at least two follicles were 18 mm Maximum of embryo transferred: 3 Luteal phase support: 800 mg daily cyclogest suppository (Aburaihan, Iran) was started on the day of oocyte collection to provide luteal phase support, and it continued until the fetal heart activity was documented by ultrasonography | |
| Outcomes | Primary outcome measures: clinical pregnancy rate per cycle and ongoing pregnancy, which later were defined as pregnancy proceeding beyond the 12th gestational week Secondary outcome measures: OHSS, defined by ≥ 15 follicles with a mean diameter ≥ 14 mm per each ovary at the end of the follicular phase of stimulation and/or E2 levels on the day of hCG administration > 3000 pg/mL and/or presence of ascites after hCG administration in ultrasonography | |
| Notes | Drop out: GnRH antagonist group: 6, GnRH agonist: 9 8 cycles of ET cancelled (due to poor quality of embryos in GnRH antagonist group) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedules |
| Allocation concealment (selection bias) | Low risk | Sealed in envelopes and handed to participants |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, multi‐centre (11 centres, United States and Canada), open‐label, parallel design | |
| Participants | 313 infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI with all causes of infertility Baseline characteristics: age: ganirelix 33.0 ± 3.4 vs leuprolide 32.8 ± 4.0 Duration of infertility: ganirelix 4.1 ± 3.0 vs leuprolide 3.8 ± 2.6. FSH: ganirelix 7.9 IU/ml vs leuprolide 3.3 IU/ml. BMI: ganirelix 23.0 ± 3.0 vs leuprolide 23.0 ± 3.0 | |
| Interventions | GnRH antagonist (n = 205): A multiple‐dose regimen of 0.25 mg of GnRH antagonist (ganirelix) was administered SC starting from day 6 of rFSH treatment up to and including day of hCG administration (Fixed) + rFSH (Follistim) was started at fixed daily dose of 150 IU for five days and the dose was adjusted according to response GnRH agonist (n= 108): mid‐luteal GnRH analogue (leuprolide 1.0 mg SC) to 99 participants of the control group + rFSH (Follistim) was started at fixed daily dose of 150 IU for five days and the dose was adjusted according to response Luteal phase support was done according to the centre's routine practice | |
| Outcomes | Premature LH surge defined as (LH > 10 IU/L) and progesterone level > 1 ng/L Stimulation length ganirelix group versus leuprolide group rFSH: ganirelix group IU versus leuprolide group IU E2 on hCG ganirelix group pg/ml versus leuprolide group pg/ml Number of oocytes retrieved ganirelix group ± versus leuprolide group ± Number of embryos obtained ganirelix group ± versus leuprolide group ± Number of embryos transferred Implantation rate ganirelix group versus leuprolide group Clinical pregnancy/cycle ganirelix group versus leuprolide group Clinical pregnancy/ET ganirelix group versus leuprolide group Ongoing pregnancy rate ganirelix group versus leuprolide group Cancellation ganirelix group versus leuprolide group Miscarriage ganirelix group versus leuprolide group Ectopic ganirelix group versus leuprolide group OHSS ganirelix group 4 versus leuprolide group 1 Severe OHSS: ganirelix 3 cases, leuprolide 2 Local reaction ganirelix group 11.9 versus leuprolide group 24.1 | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Interactive response voice system (true), 2:1 randomisation ratio, stratified randomisation |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, open‐label, parallel design, single‐centre | |
| Participants | 20 participants without specific ovulatory dysfunction aged ≤ 37 who would be submitted to ovarian stimulation | |
| Interventions | Group A (n = 14): 4 mg/day of estradiol valerate was started and continued for 14 days + rFSH (Puregon) was started one day after the end of estradiol valerate in a fixed dose of 150 ‐ 300 UI + ganirelix (Organolutran, Organon) was taken in a dose of 0.25 mg/day when the follicular diameter was ≥ 15 mm, and continued until the day of the hCG administration (Flexible) Group B (n = 6): In the 21st day of the menstrual cycle, a dose of 200 µg of nafarelin acetate was taken through nasal twice a day. After 14 days of administration of the agonist, with the blockage established (menstruation), the administration of recombinant FSH was started in a fixed dose of 150 ‐ 300 IU for a period of five days. Oocyte maturation triggering: 5 –10.000 IU uhCG at least two follicles ≥ 17 mm Maximum number of embryos transferred: 2 ‐ 3 Luteal phase support: not reported | |
| Outcomes | No of oocytes retrieved | |
| Notes | Number of participants at randomisation: 20 (ganirelix: 14/ nafarelin: 6) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation list, randomisation: 2:1 (ganirelix:nafarelin) ratio |
| Allocation concealment (selection bias) | Unclear risk | Unclear |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data, LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, open‐label, parallel design, single‐centre | |
| Participants | < 40 years old undergoing IVF due to male or tubal infertility | |
| Interventions | All participants received vaginal micronised progesterone (300 mg/day) as luteal supplementation. LP characteristics were compared between the two groups and between the conception and non conception cycles. Estradiol (E2), progesterone and LH levels were measured on the day of hCG administration (day 0), days +5, +8, +11 and +16. (Unclear) | |
| Outcomes | E2 and progesterone levels | |
| Notes | Power calculation: no power calculation Type of antagonist protocol: unclear | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Participants not blinded |
| Incomplete outcome data (attrition bias) | Low risk | Protocol is not available but the methods and results match |
| Selective reporting (reporting bias) | Low risk | Intention‐to‐treat analysis was not used but the drop‐out rate was less than 10% |
| Other bias | Low risk | Baseline characteristics are similar |
| Methods | Superiority, randomised open‐label, single centre RCT | |
| Participants | 360 idiopathic subfertile participants Inclusion criteria: subfertile women aged between 18 and 50 years with BMI between 18 and 30 Exclusion criteria: women with previous ovarian surgery, women with a previous diagnosis of endometriosis, women treated for benign endouterine disease (such as endometrial polyps, submucous myomas, intrauterine synechiae and/or uterine septus) in the six months before IVF cycle, history of abnormalities in thyroid pattern or alteration in basal serum prolactin value and E2 peak at ovulation induction < 13 nmol/l, history of smoking; untreated uterine myomas; absence of major systemic disease such as diabetes, multiple sclerosis, adrenal diseases, karyotype abnormalities, mutations of the cystic fibrosis gene, acquired or inherited thrombophilia and immunological disorders; previous or actual neoplasia; previous chemo and/or radio treatment for neoplasia; severe qualitative and quantitative alteration in partner's semen (according to World Health Organization guidelines); absence of retrieved oocytes at pick‐up and absence of at least one fertilised oocyte Baseline characteristics: age (years) 37.97 ± 3.73 vs. 35.80 ± 4.35 | |
| Interventions | GnRH antagonist (n = 90): rFSH (Gonal‐F) with a starting dose (maintained for the first five days) of 100, 225 and 300 UI per day in estimated high, normo and poor responders, respectively, administered in third day after spontaneous menstruation (pending the basal E2 < 0.3 nmol/l) (adjusted) + daily dose of GnRH agonist (n =180): rFSH (Gonal‐F) with a starting dose (maintained for the first five days) of 100, 225 and 300 UI per day in estimated high, normo and poor responders, respectively, administered at achievement of hypothalamic suppression + daily dose (0.1 mg) of triptorelin acetate (long protocol) Oocyte maturation triggering: 250 mg rhCG SC Luteal phase support: low‐dose PG (200 mg vaginal capsule twice daily) or high‐dose PG (200 mg vaginal capsule three times daily plus 100 mg intramuscular daily) or high‐dose PG (200 mg vaginal capsule three times daily plus 100 mg intramuscular daily) in association with valerate E2 (2 mg vaginal tablet twice daily) Follow‐up: Up to ongoing pregnancy | |
| Outcomes | Ongoing pregnancy, clinical pregnancy, mean value of E₂max at ovulation induction, mean value of endometrial thickness at pick‐up day, quality of embryos | |
| Notes | Three‐arm study, excluded short GnRH agonist protocol (n = 90) "After oocyte fertilization, according to the casual envelopes produced by software randomization, in each Group (A, B and C) patients were randomly assigned (with a randomization of 1:1) to one of three different subgroups (A1, A2 and A3; B1, B2 and B3 and C1, C2 and C3) in relation to the different pharmacological LPS starting from the first day after pick‐up
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study randomised participants into subgroups using appropriate random sequence generation but randomisation into large groups A, B, C not clearly stated |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation not reported for the randomisation of participants into large groups A, B, C |
| Blinding (performance bias and detection bias) | Unclear risk | Study is an open label trial. “As limitations we report:… the impossibility to blind patients and clinicians to the treatment…” “All serum sample were analysed by a single laboratory as well all the endometrial thickness measurements were performed by two skilled sonographers blinded to patients’ treatment” |
| Incomplete outcome data (attrition bias) | Unclear risk | Number randomised = 360, number analysed = unclear |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in a pre‐specified manner. However, live birth rate not reported |
| Other bias | Low risk | None identified |
| Methods | Single centre RCT | |
| Participants | 300 women with PCOS Inclusion criteria: women with PCOS younger than 35 years old and older than 23 years old: where diagnosis of PCOS was based on the Rotterdam 2004 Criteria, so patients with oligomenorrhoea (an irregular cycle duration greater than 45 days or less than 6 menstrual periods per year) and/or anovulation who also had at least one of the characteristics of hyperandrogenism (a hirsutism score of greater than 7 according to Ferriman and Gallwey (Ferriman 1961), and/or an elevated serum testosterone level which is over 0.8 ng/dl and measured in an Immulite One autoanalyser (Bio Diagnostic Products Corp., USA) using the chemiluminescent method) were diagnosed with PCOS after all the other causes of hyperandrogenism were excluded. Couples in their first IVF/ICSI cycles, women with PCOS whose body mass index was lower than 30 kg/m² and higher than 20 kg/m2 Exclusion criteria: women with PCOS whose ovaries did not appear polycystic (where having polycystic ovaries identified by ultrasonography was defined as the presence of 12 or more follicles in each ovary measuring 2 – 9 mm in diameter, and/or increased ovarian volume (> 10 ml)) Patients treated with hormonal medications and other oral anti‐diabetics within the previous three months Baseline characteristics: age (years) 27.57 ± 3.54 vs. 27.70 ± 3.59, BMI (kg/m2) 25.74 ± 4.37 vs. 24.97 ± 4.36, duration of infertility (years) 6.24 ± 3.64 vs. 5.85 ± 3.42, FSH (IU/ml) 4.77 ± 1.80 vs. 5.03 ± 1.36, LH (IU/ml) 5.94 ± 4.17 vs. 5.60 ± 3.49, E2 (pg/ml) 42.82 ± 33.62 vs. 38.83 ± 25.02, IVF/ICSI indications: PCOS + male factor (%) 40.7 (61/150) vs. 38.7 (58/150), PCOS only (%) 54.7 (82/150) vs. 53.3 (80/150), PCOS tubal factor (%) 4.7 (7/150) vs. 8 (12/150). | |
| Interventions | GnRH antagonist (n = 150): OCPs (30 μg of ethinyl estradiol (E2) and 3 mg of drosprinone (Yasmin)) for 21 days starting on cycle day 3 prior to the treatment cycle + 150 IU rFSH (Puregon) initiated on day 3 of menstruation after discontinuation of OCPs + 0.25 mg daily SC ganirelix initiated on day 6 of gonadotrophin stimulation until day of hCG administration. (OCP + GnRH fixed antagonist protocol) GnRH agonist (n = 150): OCPs (30 μg of ethinyl estradiol (E2) and 3 mg of drosprinone (Yasmin)) for 21 days starting on cycle day 3 prior to the treatment cycle + 1 mg daily leuprolide acetate (Lucrin) beginning on day 21 of the preceding menstruation (last three tablets of OCP). Dose reduced to 0.5 mg after ovarian suppression was achieved, until the day of hCG + 150 IU rFSH (Puregon) if there were no cysts ≥ 2 cm and the E2 was < 50 pg/ml. (OCP + long GnRH agonist protocol) Oocyte maturation triggering: 10,000 IU hCG when at least three follicles had a maximum diameter of > 17 mm Oocyte retrieval: 35 – 36 h after hCG injection, ICSI was performed after two hours of incubation and embryos were transferred on day 3 Maximum embryos transferred: 3 Luteal phase support: 90 mg/day intravaginal progesterone (Crinone 8% gel) starting after embryo transfer until the 8th gestational week Follow‐up: Up to ongoing pregnancy | |
| Outcomes | Ongoing pregnancy rate, miscarriage rate, OHSS rate, cycle cancellation rate, day 5 E2 level, E2 level at the day of hCG, progesterone level at the day of hCG, endometrium at the day of hCG, duration of COH, total gonadotrophin used, total cost of COH, MII oocyte number, germinal vesicle number, fertilisation rate, grade 1 embryo number, grade 2 embryo number, total transferred embryos, positive hCG rate, biochemical pregnancy rate, implantation rate, cryopreservation rate, multiple pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The 300 eligible patients were randomized into two groups by an allocation sequence generated from a random numbers table and assigned using consecutively numbered opaque, sealed envelopes on the day of initiation of OCP. Study subjects were randomized in blocks of 30; i.e. of every 30 subjects randomized, Fifteen were allocated to the GnRH agonist and Fifteen were allocated to the GnRH antagonist arm in a random manner.” |
| Allocation concealment (selection bias) | Low risk | “…assigned using consecutively numbered opaque, sealed envelopes on the day of initiation of OCP.” |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported but unlikely to affect measurement of outcome |
| Incomplete outcome data (attrition bias) | Low risk | Number randomised = 300, number analysed = 300 |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the pre‐specified manner, except that clinical pregnancy listed as outcome measure at protocol but not reported in study. Live birth rate not reported |
| Other bias | Low risk | None identified |
| Methods | RCT, two centres, parallel‐group randomised, open‐label, non‐inferiority effectiveness trial | |
| Participants | 404 infertile women undergoing IVF/ICSI Inclusion criteria: no previous IVF treatment or had borne a healthy child after previous IVF treatment, were aged younger than 38 years, and had a menstrual cycle length of 25–35 days and a body‐mass index of 18–28 kg/m² Baseline characteristics: age of women (years) 32.9 (3.1) vs 32.8 (3.2), BMI (kg/m²) 23.0 (2.6) vs 23.2 (2.5), Duration of infertility (years) 3.6 (1.9) vs 3.6 (2.1) | |
| Interventions | GnRH antagonist (n = 205): mild ovarian stimulation with gonadotrophin‐releasing hormone [GnRH] antagonist co treatment combined with single embryo transfer (mild protocol) GnRH agonist (n = 199): (stimulation with a GnRH agonist long protocol and transfer of two embryos (long GnRH agonist protocol) | |
| Outcomes | Primary outcome measures: pregnancy and term live‐birth within one year of randomisation; total costs per couple and child up to six weeks after expected delivery; and participant’s discomfort | |
| Notes | Supernumerary high‐quality embryos: cryopreserved and thawed for transfer in a subsequent unstimulated cycle before the start of a new IVF treatment cycle. These frozen‐thawed embryo‐transfer cycles were treated as a part of the previous IVF cycle. In both groups either one or two cryopreserved embryos were transferred, according to the participant’s preference Fund: ZonMw (Netherlands), programme Doelmatigheidsonderzoek | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random blocks of size four and six were stratified by centre |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes and made available at each centre; envelopes were sequentially allocated to consecutive participants and opened by treating physicians at IVF planning consultations |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free of other source of bias |
| Methods | Pilot randomised controlled trial, single centre | |
| Participants | 60 women Inclusion criteria: indication for IVF/ICSI Exclusion criteria: ovulatory factor (WHO class I‐III), age ≥ 37 years, more than three previous unsuccessful IVF cycles, TESA/TESE cycles, surrogacy cycles and refusal to participate Setting and timing: IVF unit, Israel. August 2012 to July 2013 Baseline characteristics: age ‐ agonist group 28.6 ± 4.3; antagonist group 29.8 ± 4.3 | |
| Interventions | Antagonist ‐ programmed to start on a Friday, antagonist (Cetrotide) ‐ no details of dose) was used in a flexible way. Programming achieved with oral estradiol valerate 2 mg twice daily during early follicular phase from day 2 of a spontaneous menstrual cycle until the first Friday following Agonist ‐ Long luteal agonist protocol (triptorelin‐ no details of dose) Mean number of embryos transferred: antagonist: 1.4 ± 0.7 vs agonist 1.2 ± 0.6 Number of ampoules used: antagonist 24.9 ± 8.1 vs agonist 25.5 ± 13.3 Controlled ovarian hyperstimulation initiated with rFSH alone or in combination with hMG. Adjustments made dependant on individual response | |
| Outcomes | Clinical pregnancy is the only prespecified outcome. Also reported on fertilisation rate, embryo quality, number of embryos transferred, amount of FSH, follicular size | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised but no other details |
| Allocation concealment (selection bias) | Low risk | Allocation using sealed envelopes handled by an administrator not involved in the study |
| Blinding (performance bias and detection bias) | Unclear risk | Open label study but blinding unlikely to effect fertility outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | 60 women were randomised and nine did not complete treatment (15%). Three chose another fertility unit, three decided to stop fertility treatment, two were screened out during routine laboratory testing and one had a spontaneous extrauterine pregnancy that required surgical intervention and then postponed fertility treatment. The groups that these women were allocated to was not specified 27/31 women in the antagonist group were analysed and 24/29 in the agonist group |
| Selective reporting (reporting bias) | Unclear risk | Clinical pregnancy rate was the only prespecified outcome although there are more outcomes reported in the results section. OHSS was not reported |
| Other bias | Low risk | Groups were balanced at baseline |
| Methods | RCT, university‐affiliated IVF centre, open‐label, parallel design, randomisation: 2:1 (cetrorelix:triptoreline) ratio | |
| Participants | 142 infertile women undergoing IVF/ICSI Inclusion criteria: age between 20 to 38 yrs; BMI 19 to 29 kg/m2; history of regular menstrual cycles, ranging from 25 to 35 days; no relevant systemic disease, severe endometriosis, or uterine and ovarian abnormalities; no more than three previous IVF cycles; and no previous IVF cycle with a poor response or ovarian hyperstimulation syndrome | |
| Interventions | Group A: GnRH agonist triptoreline (Decapeptyl) 1 mg/d, SC starting one week before the expected menses (usually cycle day 21) + fixed daily dose of 150 IU rFSH SC (Gonal‐F) Groups B and C: GnRH antagonist cetrorelix (Cetrotide) 0.25 mg/d, SC commencing when the largest follicle had reached a diameter of 14 mm + rFSH was initiated on cycle day 2 (group B) or 5 (group C). (Flexiblle) Oocyte maturation triggering: when the leading follicle had reached a diameter of 18 mm or more and at least three follicles had reached a diameter of 15 mm or more, 10,000 IU hCG (Pregnyl) was administered Embryo transfer: 35 hrs before the planned time of oocyte retrieval followed by IVF with or without ICSI Maximum number of embryos transferred: 2 embryos were transferred at 3 ‐ 5 days Luteal support: intravaginal progesterone (P; Progestan, Organon; 200 mg, three times daily) was given from the day of oocyte retrieval until a urine pregnancy test was performed 17 days later | |
| Outcomes | Included patients (n = 142) | |
| Notes | Number of participants at randomisation: 169 (cetrorelix: NA/ triptoreline: NA) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Schedule assigned via numbered sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | Randomised controlled trial | |
| Participants | 50 infertile couples undergoing IVF/ICSI Inclusion criteria: male factor, tubal factor or unexplained subfertility, no ovulatory dysfunction, age 40 years or less, normal basal FSH and LH (< 10 mIU/ml) Exclusion criteria: no details Baseline characteristics: age ‐ antagonist 33.8 ± 5.6 years versus agonist 30.4 ± 5.5 years Setting and timing: infertility centre, Iran June 2012 to November 2013 | |
| Interventions | Antagonist Fixed multi‐dose protocol (n = 24) cetrorelix acetate 0.25 mg/day on day 6 until day of hCG injection Agonist long protocol (n = 26) with OCP pre‐treatment starting on day 2 or day 3 of previous cycle. Buserelin acetate 500 micrograms started on day 21 until pituitary down‐regulation. Reduced to 250 micrograms per day when follicle > 10 mm diameter, E2 > 50 pg/ml until the day of hCG injection Ovarian stimulation started on day 3 using rFSH 150 to 225 IU hCG 5000 IU given IM when at least three follicles had a mean diameter of 17 mm Ooycte retrieval 34 to 36 hours after hCG injection | |
| Outcomes | Gene expression. No pregnancy outcomes reported for inclusion in a meta‐analysis | |
| Notes | Sample size calculation ‐ no ITT analysis ‐ no Funding ‐ Deputy Ministry for Research, Tehran | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | No details for participants or outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient details were reported to make a conclusive judgement |
| Selective reporting (reporting bias) | High risk | No pregnancy outcomes reported. Unable to include any data in a meta‐analysis |
| Other bias | Low risk | Groups appear balanced at baseline |
| Methods | Randomised controlled trial. Single centre | |
| Participants | 112 infertile women with PCOS (Rotterdam criteria) Inclusion criteria: PCOS, < 35 years, normal BMI (< 27 kg/m2), normal prolactin, normal thyroid levels, normal semen analysis for male partner Setting and Timing: Department of Infertility, Tehran, Iran. During 2006 Baseline characteristics: age‐ antagonist 27.8 ± 3.4 years versus agonist 29.3 ± 4.2 years | |
| Interventions | All participants had folic acid 1 mg/day before the induction cycle, low‐dose OCP on day 3 of previous cycle and doxycycline 100 mg twice daily for the first 10 days of the previous cycle Antagonist (n = 57) ovarian stimulation with Gonal F 150 IU commenced on day 3. When follicular diameter ≥ 14 mm cetrorelix 0.25 mg/day SC given for three days. HMG given after seventh day of stimulation. Agonist (n = 55) buserelin 0.5 mg SC started on day 21 of previous cycle. Ovarian stimulation with Gonal F 150 IU commenced on day 3 of next cycle and replaced with HMG after the seventh day of stimulation Oocycte maturation triggered when at least two follicles ≥ 17 mm and hCG 10,000 IU given IM Oocyte retrieval 36 to 38 hours after hCG. Luteal phase support ‐ progesterone suppository cyclogest 800 mg daily | |
| Outcomes | Clinical pregnancy, chemical pregnancy, number of oocytes retrieved, number and days of gonadotrophins, miscarriage, multiple pregnancy, OHSS and severe OHSS | |
| Notes | Sample size calculation ‐ no ITT analysis ‐ yes Funding ‐ none stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomized' |
| Allocation concealment (selection bias) | Unclear risk | 'Sequential' no details |
| Blinding (performance bias and detection bias) | Unclear risk | No details. Unlikely to have been blinded but blinding unlikely to affect fertility outcomes. Blinding of outcome assessors was not reported |
| Incomplete outcome data (attrition bias) | Low risk | Women randomised were analysed. No losses reported |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported. However, did not report on live birth or ongoing pregnancy as an outcome |
| Other bias | High risk | Women in the agonist group were slightly older at baseline |
| Methods | RCT phase III, open‐label, single‐centre study | |
| Participants | 251 infertile women undergoing IVF/ICSI Inclusion criteria: age at least 18 years but not older than 39 years; and body weight of 40 – 70 kg Baseline characteristics: age (yr) 33.9 ± 4.4 vs 32.3 ± 2.1 vs 31.6 ± 2.4 vs 30.9 ± 2.5 vs 32.1 ± 2.7; BMI (kg/m2) 20.6 ± 1.4 vs 19.0 ± 1.0 vs 19.5 ± 1.1 vs 20.7 ± 2.1 vs 21.1 ± 1.8; Baseline FSH (IU/L) 4.0 ± 1.8 vs 3.7 ± 1.6 vs 3.9 ± 1.3 vs 3.8 ± 1.4 vs 3.6 ± 1.8 | |
| Interventions | Down‐regulation Group 1 (n = 86): cetrorelix 0.25 mg/day, cetrorelix was administered from menstrual day 8 until the day of hCG administration. (Fixed) Group 2 (n = 28): cetrorelix 0.2 mg/day Group 3 (n = 30): cetrorelix 0.15 mg/day Group 4 (n = 58): leuprolide acetate 0.5 mg/day, administered on days 21–23 of the previous menstrual cycle Group 5 (n = 49): leuprolide acetate depot 1.88 mg. Single dose leuprolide acetate depot subcutaneous COH: 150 – 225 IU/day rFSH (Gonal‐F) in women < 34 years old, 225 ‐ 300 IU rFSH in women > 34 years Final oocyte maturation triggering: 5000 IU hCG were given when at least three mature ≥ 18 mm follicles were obtained Oocytes retrievals: 36 hrs later Maximum embryo transfer: six embryos were transferred at 72 hrs after IVF/ICSI injection Follow up: clinical pregnancy was determined by visualisation of a gestational sac, and fetal viability by ultrasound four weeks post‐ET | |
| Outcomes | Gn dosage, and serum concentration of LH and E2 on the day of hCG administration, retrieved oocyte and embryo numbers, development of OHSS, embryo quality, and pregnancy rate (PR), implantation rate (IR) and abortion rate (AR) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Unclear risk | No source of other bias identified |
| Methods | RCT, multi‐centre (eight European IVF centres), phase IIIb study | |
| Participants | 182 infertile women undergoing IVF/ICSI Inclusion criteria: participants needed to have a regular IVF/ICSI indication, a male partner with viable sperm in the ejaculate (testicular biopsy or epididymal sperm was not allowed), aged between 18 and 39 years Exclusion criteria: people with any previous assisted reproduction treatment cycles with less than three oocytes or three or more consecutive ART cycles without a clinical pregnancy or with any contraindication to ART, gonadotrophins or OC pills. People with a significant systemic disease Baseline characteristics: age (years) 32.8 ± 3.8 vs 32.2 ± 4.2. BMI (kg/m2) 23.7 ± 4.0 vs 22.6 ± 3.5. FSH (IU/l) on cycle day 2 or 3 7.2 ± 2.2 vs 7.4 ± 3.3 0. Estradiol (pmol/l) on cycle day 2 or 3 138 ± 55, 148 ± 103 | |
| Interventions | GnRH antagonist (n = 91 ): daily OCPs (30 μg ethinyl E2 and 150 μg levonorgestrel) for 21 – 28 days + r‐hFSH 150 – 225 IU (Gonal‐F) according to the study centre’s standard practice (adjusted)+ daily cetrorelix 0.25 mg subcutaneously started on stimulation day 6 and continued up to and including the day of r‐hCG administration. (Fixed) Group 2 (n = 91): daily buserelin, 500 μg, subcutaneously at the mid‐luteal phase of a natural cycle for at least 10 days until down‐regulation was achieved, after which the dose was reduced to 200 μg/day + r‐hFSH 150 – 225 IU (Gonal‐F), according to the study centre’s standard practice (adjusted) Final oocyte triggering: r‐hCG 250 μg (= 6500 IU) (Ovitrelle) was injected as soon as the largest follicle reached a mean diameter ≥ 18 mm and at least two other follicles of a mean diameter ≥ 16 mm Oocyte retrieval: 34 – 38 hrs after r‐hCG administration under ultrasound guidance, followed by a standard IVF or ICSI procedure Maximum number of embryo transfer: no more than two to three embryos were replaced either 2 – 3 days or 5 – 6 (blastocyst transfer) days Luteal phase support: intravaginal natural progesterone (three times daily 200 mg Progestan®, Organon, Oss, The Netherlands) was started as luteal support. This was continued up to a negative pregnancy test or during the first three weeks of pregnancy | |
| Outcomes | Number of oocytes retrieved in IVF/ICSI patients Pregnancy was defined as continuing increase in serum hCG. In that case, four and 10 weeks after embryo transfer, ultrasound was performed to assess the number of fetal sacs and heart activity. Clinical pregnancy was defined as the presence of a fetal sac, with or without heart activity. Ongoing pregnancy as a positive heart activity at a gestational age of 12 weeks | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated concealed randomisation list. Randomisation was performed by centre |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre Part II trial | |
| Participants | 60 PCOS infertile women undergoing IVF/ICSI Inclusion criteria: PCOS included: (i) chronic anovulation manifested by the symptoms of oligomenorrhoea (0.40 days per cycle), amenorrhoea or irregular menstrual cycle and confirmed by a basal body temperature chart or serum progesterone determination; (ii) ultrasonographic evidence of polycystic ovaries an enlarged ovary with > .10 peripherally located follicles of 3 – 8 mm diameter around a dense central stroma; and (iii) at least one of the two hormonal abnormalities (a) normal FSH concentration (3 – 10 mIU/ml) and elevated LH concentration ( .10 mIU/ml) or LH /FSH ratio .2; and (b) hyperandrogaenemia (serum testosterone concentrations .0.8 ng/ml). A diagnosis of congenital adrenal hyperplasia, Cushing’s syndrome, androgen‐producing tumours, hyperprolactinaemia and thyroid dysfunction were all excluded Exclusion criteria: Women older than 38 years or with serum FSH levels .12 mIU/ml. Baseline characteristics: age (years) 31.4 ± 3.5 vs 31.7 ± 3.7. Duration of infertility (years) 4.4 ± 1.9 vs 4.4 ± 1.6. BMI (kg/m2) 23.2 ± 2.8 vs 23.4 ± 2.9. Baseline FSH 5.8 ± 1.2 vs 5.4 ± 1.7 | |
| Interventions | GnRH antagonist: Diane‐35/day from day 5 of the cycle for 21 days + cetrorelix acetate was then initiated with a single dose of 0.25 mg administered SC + from day 4 to day 9, cetrorelix acetate was reduced to 0.125 mg/day + 150 IU of hMG (Pergonal) every day. The dose of cetrorelix acetate was increased to 0.25 mg/day from day 10 until the day before hCG (Pregnyl; NY Organon) injection, and the dose of HMG (Fixed) Oocyte maturation triggering: hCG, 10,000 IU, was administered IM when at least two follicles reached 18 mm in diameter with adequate E2 response Oocyte retrieval: was performed 36 hrs later Embryo transfer: was performed three days after oocyte recovery Luteal phase support: 600 mg of vaginally administered micronised progesterone (Utrogestan) daily starting from the day after oocyte retrieval Follow up: clinical pregnancy was defined as a visible fetal heart beat on ultrasonography at seven weeks of gestation | |
| Outcomes | The primary outcome measures: fertilisation, pregnancy and implantation rates The secondary outcome measures: serum LH and testosterone status upon starting and during HMG administration, and the total days of injection | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated block randomisation numbers with a block size of 10 |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) | Low risk | The laboratory staff were blinded to the stimulation protocol |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias. |
| Methods | RCT, single‐centre, parallel design | |
| Participants | Patients < 40, with FSH levels on day 3 < 12 IU/ml | |
| Interventions | GnRH agonist long protocol versus GnRH antagonist protocol (type of antagonist protocol: N/A) (unknown) | |
| Outcomes | Number and quality of retrieved oocytes | |
| Notes | Number of participants at randomisation: 45 (antagonist: 23/agonist: 22) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | No reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre trial | |
| Participants | 243 women who were candidates for ART Inclusion criteria: age 18 – 35 years, presence of a regular and proven ovulatory menstruation cycle with a length of 26 to 35 days, basal FSH < 10 IU/L, BMI of 18 – 30 kg/m2 and first IVF attempt. Indication for IVF were unexplained infertility, male factor, tubal factor, early stage endometriosis and cervical factor Baseline characteristics: age (years) 30.0 ± 2.3 vs 29.4 ± 2.4, BMI (kg/m2) 25.9 ± 2.3 vs 25.3 ± 1.9. Basal FSH (IU/L) 6.5 ± 1.2 vs 5.9 ± 1 | |
| Interventions | GnRH antagonist (n = 121): clomiphene citrate 100 mg from cycle day three through seven + rFSH 75 IU daily from cycle day 5 + 0.25 mg GnRH antagonist (ganirelix) daily was started with dominant follicle ≥ 12 mm and in this day 75 IU human menopausal gonadotrophin (hMG) (Menogon) increased to the initial gonadotrophin. (mild Flexible GnRH antagonist protocol) GnRH agonist (n = 122): buserelin (Suprefact) 500 µg SC everyday for menstrual cycle 21, once down‐regulation had been achieved, then the dose of buserelin would be reduced to 250 lg + 150 – 225 IU rFSH (Gonal F) SC Oocyte retrieval: 34 to 36 hrs after hCG and IVF or ICSI was performed ET: on day 2 or 3, under ultrasound guidance Luteal support: progesterone in oil 100 mg daily IM was started on the day of oocyte retrieval and continued until the documentation of fetal heart activity on ultrasound | |
| Outcomes | Primary outcome measures: clinical pregnancy rate per cycle and ongoing pregnancy; later were defined as pregnancy proceeding beyond the 12th gestational week Secondary outcome: OHSS, defined by ≥ 15 follicles with a mean diameter ≥ 14 mm per each ovary at the end of the follicular phase of stimulation, and/or E2 levels on the day of hCG administration 3,000 pg/mL and/or presence of ascites after hCG administration in ultrasonography | |
| Notes | In control group (GnRH agonist/gonadotrophin) six participants were excluded, 13 participants did not come back, and follow up in three participants lost. In study group (CC/gonadotrophin/antagonist) two participants were excluded, 12 participants did not come back, and follow up in seven participants lost | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data, however the study did not address live birth rate |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | Randomised controlled trial. Single centre | |
| Participants | 50 women Inclusion criteria: ≤ 35 years, regular menstrual cycles, normal basal FSH (≤ 10 IU/L), LH (≤ 10 IU/L) and estradiol ≤ 50 pg/ml), BMI 18 to 24 kg/m2 Exclusion criteria: clinical evidence of endometriosis, PCOS or OHSS during stimulation Setting and timing: Assisted Reproduction Unit, Caen, France. Timing not specified Baseline characteristics: age ‐ antagonist 31 ± 0.7 years versus agonist 31 ± 0.8 years | |
| Interventions | Antagonist ‐ (n = 22) cetrorelix 0.25 mg daily (multiple dose protocol) starting day when dominant follicle ≥ 14 mm and continued up to day of hCG administration Agonist ‐ (n = 28) triptorelin 0.1 mg/day from first day of menstrual cycle up to day of hCG administration rFSH 225 IU/day on second day of menstrual cycle hCG (5000 IU) when at least three follicles 18 mm diameter | |
| Outcomes | Aromatase activity in granulosa lutein cells | |
| Notes | Sample size calculation ‐ no ITT analysis ‐ yes Funding ‐ Prgramme Hospitalier Recherche Clinique | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'randomly assigned' 'ballot'; no further details reported |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | 'without blinding'. Lack of blinding is unlikely to affect fertility outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised were analysed |
| Selective reporting (reporting bias) | High risk | There were no pregnancy outcomes pre‐specified |
| Other bias | Low risk | Groups appear balanced at baseline |
| Methods | RCT, single‐centre, parallel design | |
| Participants | 41 women undergoing IVF/ICSI Inclusion/exclusion criteria: not stated | |
| Interventions | GnRH antagonist: OCP+ cetrorelix 0.125 mg/day, was administered on days 1 and 2 of COH with rFSH, and cetrorelix 0.25 mg/day was restarted when the leading follicle reached a mean diameter of 13 mm and continued to the day of hCG injection. (Flexible) GnRH agonist: no details were available for the agonist group, except that they were down‐regulated with triptorelin (triptorelin 0.1 mg/day) | |
| Outcomes | Number of retrieved oocytes | |
| Notes | Number of participants at randomisation: 41 (cetrorelix: 21/ triptorelin: 20) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, however LBR did not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | Parallel group study | |
| Participants | Country: authors are from South Korea 120 poor responders (repeated day 3 levels of FHS > 8,5 mIU/mL, and/or antral follicle count ≤ 5) Inclusion criteria: not clearly stated Exclusion criteria: PCOS (Rotterdam criteria) Mean age: Group 1: 36.7 ± 3.1 years, Group 2: 35.9 ± 2.8 years, Group 3: 36.4 ± 3.3 years Setting: University‐based infertility clinic, Seoul, South Korea | |
| Interventions | Pretreatment was ethinyl estradiol 0.03 mg and levonorgestrel 0.15 mg for 21 days in the cycle preceding controlled ovarian stimulation Group 1: GnRH antagonist multiple dose protocol after OCP pretreatment (n = 40) ovarian stimulation commenced five days after OCP discontinued using rFSH 225 IU/day (dose‐adjusted every three to four days). Cetrotide 0.25 mg started when lead follicle was 14 mm diameter and continued until day of hCG injection versus Group 2 GnRH antagonist multiple‐dose protocol without OCP pretreatment (n = 40) ovarian stimulation commenced on cycle day three using rFSH 225 IU/day (dose adjusted every three to four days). Cerotide 0.25 mg started when lead follicle was 14 mm diameter and continued until day of hCG injection. versus Group 3 GnRH agonist luteal low‐dose long protocol without OCP pretreatment (n = 40). Daily injection of decapeptyl 0.1 mg started from mid‐luteal phase and continued until menses followed by a dose reduction to 0.05 mg daily and continued until day of hCG injection Dose of rFSH: multidose protocol with OCP 2925.0 ± 423.9 IU versus multidose protocol without OCP 2905.0 ± 421.8 IU versus agonist 3273.6 ± 438.3 IU | |
| Outcomes | Primary ‐ number of mature oocytes retrieved Secondary ‐ total amount and days of rFSH, number of fertilised oocytes and grade I and II embryos, implantation rate, clinical pregnancy rate per cycle and live birth rate per cycle, miscarriage rate | |
| Notes | Power calculation ‐ yes ITT analysis ‐ yes Funding ‐ not reported Earlier publications were Kim 2005 and Kim 2009 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'computer‐generated lists' |
| Allocation concealment (selection bias) | Unclear risk | 'The sequence of allocation to the three groups was provided to the investigating physicians...' |
| Blinding (performance bias and detection bias) | Unclear risk | Trial not blinded but unlikely to affect outcome of pregnancy |
| Incomplete outcome data (attrition bias) | Low risk | In groups 1 and 3 there were no losses, withdrawals or cancellations. In group 2, one cycle was cancelled before embryo transfer |
| Selective reporting (reporting bias) | Low risk | All outcomes listed were reported: although multiple pregnancy is reported it is not listed a priori |
| Other bias | Low risk | Groups were balanced at baseline |
| Methods | RCT, single centre trial | |
| Participants | 211 infertile women with PCOS undergoing IVF Inclusion criteria: infertile women with PCOS (PCOS diagnosis was based on the revised PCOS diagnostic criteria of the 2003 Rotterdam consensus). Good health with normal cardiac, hepatic and renal functions, and had experienced spontaneous onset of puberty and normal sexual development Exclusion criteria: women who has taken any hormonal therapy within the preceding three months Baseline characteristics: age (years) 32.5 ± 4.5 vs. 32.2 ± 4.2, BMI (kg/m2) 22.9 ± 3.1 vs. 22.7 ± 2.9, infertility duration (years) 3.3 ± 1.6 vs. 3.1 ± 1.3, number of nullipara 64 (60.4) vs. 66 (62.9), AFC 27.7 ± 4.1 vs. 26.5 ± 3.9, fasting glucose (mg/dL) 97.4 ± 20.1 vs. 96.4 ± 18.4, two‐hour glucose after 75 g glucose load (mg/dL) 132.5 ± 27.8 vs. 128.5 ± 24.6, basal FSH (IU/L) 4.2 ± 1.3 vs. 4.3 ± 1.0, basal LH (IU/L) 7.5 ± 1.7 vs. 7.2 ± 1.6 | |
| Interventions | GnRH antagonist (n = 106): OCP + 50 to 150 IU of rhFSH (Gonal‐F) five days after discontinuation of OCP (adjusted) + 0.125 mg/day cetrorelix (Cetrotide) in the morning of stimulation days 1 and 2. When the mean diameter of lead follicle reached 13 mm, cetrorelix at a dose of 0.25 mg/day was started again and continued daily up to the day of r‐hCG injection. (Multiple dose protocol) GnRH agonist (n = 105): OCP + 50 to 150 IU of r‐hFSH (Gonal‐F) (adjusted) + 0.1 mg/day triptorelin (Decapeptyl) from day 18 of OCP pretreatment cycle. When pituitary desensitisation was achieved, ovarian stimulation was started and the dose of triptorelin was reduced to 0.05 mg daily and continued up to day of r‐hCG administration. (Long protocol) Oocyte maturation triggering: 250 μg r‐hCG SC when one or more follicles reached a mean diameter of 17 mm Oocyte retrieval: 36 hours after r‐hCG injection, followed by IVF or ICSI on the third day after oocyte retrieval Maximum embryos transferred: 4 Luteal phase support: 90 mg vaginal progesterone gel (Crinone gel 8%) once daily from the day of oocyte retrieval Follow‐up: Pregnancies were confirmed by rising serum β‐hCG concentrations and transvaginal ultrasonographic evidence of a gestational sac. The serum level of β‐hCG was measured 11 days after ET | |
| Outcomes | Live birth rate, miscarriage rate, clinical pregnancy rate, incidence of severe OHSS, cycle cancellation rate, progesterone levels, estradiol levels and endometrial thickness on the day of hCG injection, total amount and days of r‐hFSH administered, the numbers of retrieved, mature, fertilised oocytes and good quality embryos, numbers of embryos transferred and cryopreserved, embryo implantation rate, multiple pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The subjects, aged 25 to 39 years, were randomized into either the GnRH antagonist MDP‐EL (antagonist group, n = 106) or the GnRH agonist LP (agonist group, n = 105) by the use of sealed envelopes and a computer‐generated list." |
| Allocation concealment (selection bias) | Unclear risk | Study did not report whether envelope was sequentially‐numbered, opaque and safe‐guarded. “…by the use of sealed envelopes. The sequence of allocation to the two groups was provided to the investigating physicians and randomization was performed as planned according to the randomization list order.” |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not reported but it is unlikely to influence measurement of outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Number randomised = 211, number analysed = 208 (missing data balanced across the groups, and reasons similar) “One cycle (0.9%) in the antagonist group and 2 cycles (1.9%) in the agonist group were cancelled after oocyte retrieval due to a high risk of OHSS. There was no significant difference in cycle cancellation rate between the two groups” |
| Selective reporting (reporting bias) | Low risk | All outcomes reported as it is in the pre‐specified manner |
| Other bias | Low risk | None detected |
| Methods | RCT, single‐centre trial | |
| Participants | 74 PCOS meeting Rotterdam criteria undergoing ICSI Inclusion criteria: male factor subfertility, several unsuccessful intrauterine inseminations, previous ineffective IVF (none or < 30% of fertilisations), age ≤ 35 years, BMI < 26 kg/m2, basal FSH < 12 mIU/ml, negative HBV and HCV virus infection and HIV Baseline characteristics:age (years) 31.33 ± 3.91 vs 30.36 ± 3.40, BMI (kg/m2) 23.1 ± 1.3 vs 22.3 ± 1.6 | |
| Interventions | GnRH antagonist (n = 37): OCP (Cilest) + 150 IU rFSH on 2nd day of the cycle (adjusted) + 0.25 mg SC of cetrorelix acetate (Cetrotide) administered when follicles reached a diameter of 14 mm (flexible protocol) GnRH agonist (n = 37): OCP (Cilest) + 150 IU rFSH (adjusted) + long GnRH agonist triptorelin (Diphereline SR 3.75 ) (DepotGnRH agonist protocol) Oocyte maturation triggering: 10,000 IU hCG or SC injection of 250 μg hCG when the dominant follicle reached ≥ 18 mm with the following two ≥ 16 mm and estradiol level between 1000 and 4000 pg/mL Maximum of embryo transferred: 3 Luteal phase support: oral 30 mg/day of dydrogesterone (Duphaston), and intravaginal 150 mg/day of progesterone | |
| Outcomes | Primary endpoints Embryological: | |
| Notes | Financial support—grant number KBN 2 P05E 034 28 from State Committee for Scientific Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random letters (A for GnRH antagonists protocol or B for GnRH agonists |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes |
| Blinding (performance bias and detection bias) | High risk | Not blinded |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, open‐label, parallel design, single‐centre | |
| Participants | Women under the age of 40 yrs with previous cycles < 3 times, and BMI < 27 kg/m2 underwent COH for ART | |
| Interventions | Participants were treated with GnRH agonist long protocol, GnRH antagonist protocol, and GnRH antagonist with hCG 200 IU protocol following contraceptive pills for two to three weeks as pretreatment. (Unknown) | |
| Outcomes | Total amount of FSH dosage, Blood E2 level at hCG injection, the number of oocytes, small follicle (< 10 mm) counts at OPU, day 3 embryo high quality rate, clinical pregnancy rate, and severe OHSS rate | |
| Notes | Number of participants at randomisation: 192 (cetrorelix: 126/ buserelin: 66) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details. |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre | |
| Participants | 78 infertile women with PCOS undergoing IVF/ICSI Inclusion criteria: PCOS (presence of oligo‐ovulation/anovulation and polycystic ovaries), age 18 – 39 years, less than three previous IVF/ICSI attempts, no endometriotic cyst present as assessed by transvaginal, ultrasound examination, and basal hormonal levels of FSH in the early follicular phase of 10 IU l21 Baseline characteristics: age (years) 32.0 (14) vs 30.5 (16), BMI (kg m2) 23.2 (20.9) vs 23.6 (18.9), FSH (IU l21) 6.3 (1.7) vs 5.8 (2.6) | |
| Interventions | GnRH antagonist (n = 26): OCP + 150 IU rFSH on 2nd day of the cycle (adjusted) + 0.25 mg SC of ganirelix (Orgalutran) administered on day 2 of menses/day 1 of stimulation (flexible protocol) GnRH agonist (n = 52): OCP + 150 IU rFSH (adjusted) + long GnRH agonist, 0.1 mg triptorelin three days before discontinuation of the OCP, once down‐regulation was achieved, the dose of GnRH agonist was decreased on that day to 0.05 mg/day (low‐dose GnRH agonist protocol) Oocyte maturation triggering: 3 follicles > 17 mm, 10,000 IU of hCG was administered Maximum embryos transferred: 3 Luteal phase support: 600 mg of micronised progesterone was initiated two days after oocyte retrieval | |
| Outcomes | Primary outcome measure: E2 levels on day 5 of stimulation | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list in a 1:2 ratio |
| Allocation concealment (selection bias) | Low risk | By a study nurse, the responsible physicians (investigators) were not involved in the randomisation process |
| Blinding (performance bias and detection bias) | High risk | Neither participants nor doctors were blinded to the treatment assigned |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre | |
| Participants | 220 PCOS women undergoing ICSI Inclusion criteria: PCOS (presence of oligo‐ovulation/anovulation) and polycystic ovaries, age 18 – 39 years, no endometriotic cyst present, as assessed by transvaginal ultrasound examination, basal FSH 10 IU/ml Baseline characteristics:age (years) 32 (29 – 35) vs 31 (28 – 35), BMI (kg/m2) 23.2 (20.9 – 25.8) vs 24.6 (20.9 – 29.3), FSH (IU/l) 6.0 (4.3 – 6.9) vs 6.2 (4.8 – 7.5), LH (IU/l) vs 5.9 (3.4 – 7.6) 5.3 (4.0 – 7.5) | |
| Interventions | GnRH antagonist (n = 110): OCP + 150 IU FSH on 2nd day of the cycle (adjusted) + 0.25 mg SC of cetrorelix acetate (Cetrotide) administered when at least one of the following criteria were fulfilled, the presence of at least one follicle measuring > 14 mm, serum E2 levels > 600 pg/ml; and serum LH levels > 10 IU/l (flexible protocol) GnRH agonist (n = 110): OCP (Cilest) + 150 IU rFSH (adjusted) + long GnRH agonist, 0.1 mg triptorelin three days before discontinuation of the OCP, once down‐regulation was achieved, the dose of GnRH agonist was decreased on that day to 0.05 mg/day (low‐dose GnRH agonist protocol) Oocyte maturation triggering: 3 follicles > 17 mm, 5000 IU of hCG was administered Maximum of embryo transferred: 3 Luteal phase support: 600 mg of micronised progesterone was initiated two days after oocyte retrieval | |
| Outcomes | The primary outcome measure: ongoing pregnancy rate per participant randomised. Ongoing pregnancy and clinical pregnancy were defined as the presence of gestational sac with fetal heart beat detection at 12 weeks and at 6 – 7 weeks of gestation, respectively | |
| Notes | OHSS classification: a modified classification system based on combined criteria previously reported (Golan 1989; Navot 1992; Rizk 1999) was used in the current study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list, in a 1:1 ratio |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Neither participants nor doctors were blinded to the treatment assigned |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | Two‐arm parallel RCT | |
| Participants | 32 infertile women undergoing ICSI cycle Inclusion criteria: women aged 37 years or less and in their first IVF/ICSI cycle, BMI < 30 kg/m2, regular menses and the presence of two normal ovaries Baseline characteristics: age (years) antagonist: 32.1 ± 3.1; agonist: 33.7 ± 2.7, P = 0.12; BMI: GnRH antagonist 23.4 ± 2.7, GnRH agonist 23.7 ± 3.1, P = 0.75 | |
| Interventions | GnRH antagonist (n = 16): on the third day of the menstrual cycle, ovarian stimulation was started with a fixed dose of 150 – 225 IU rFSH and 75 IU/day rLH for five days. On the eighth day of the menstrual cycle (sixth day of ovarian stimulation), follicular development was monitored by transvaginal ultrasound. The dose of rFSH was adapted according to the ovarian response, and supplementation with rLH was increased to 150 IU/day when one or more follicles measuring 10 mm in diameter were found. The GnRH antagonist, at a dose of 0.25 mg/day SC was started when at least one follicle greater or equal to 14 mm was observed on ultrasound GnRH agonist (n = 16) First, pituitary down‐regulation was started during the luteal phase of the previous menstrual cycle with the GnRH agonist at a dose of 1 mg/day for 14 days. Then, ovarian stimulation was started with a fixed dose of 150–225 IU recombinant FSH (rFSH/Gonal F1; Serono, SP, Brazil) with 75 IU/day rLH (Luveris1; Serono, SP, Brazil) for seven days. On the eighth day of ovarian stimulation, follicular development was monitored by transvaginal ultrasound. The dose of rFSH was adapted according to the ovarian response, and rLH supplementation was increased to 150 IU/day when one or more follicles measuring greater than or equal to 10 mm in diameter were found Additional support: for both groups, 250 mg r‐hCG (Ovidrel1; Serono, SP, Brazil) was administered SC when at least two follicles reached a diameter of 17 mm during final oocyte maturation. Oocyte retrieval was performed by transvaginal aspiration under ultrasound guidance 34 – 36 h after r‐hCG injection | |
| Outcomes | None of the reported outcomes (DNA fragmentation, apoptosis) were relevant to the review | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Unclear risk | The method used in allocation concealment was not reported |
| Blinding (performance bias and detection bias) | Unclear risk | No information was reported on blinding of participants and personnel, including outcome assessors. However, none of the reported outcomes were relevant to the review |
| Incomplete outcome data (attrition bias) | Unclear risk | None of the reported outcomes were relevant to the review |
| Selective reporting (reporting bias) | Unclear risk | None of the reported outcomes were relevant to the review |
| Other bias | Low risk | Groups were balanced at baseline |
| Methods | RCT, single‐centre, university hospital, tertiary medical centre, parallel design | |
| Participants | 61 infertile women undergoing IVF/ICSI Inclusion criteria: were no more than 39 years of age, a history of regular menstruation cycles (menstrual cycle length 26 – 33 days), BMI 18 – 29 kg/m2, no history of poor ovarian response or reserve (less than three oocytes in a previous IVF cycle), baseline FSH levels < 11 IU/L, normal results for serum liver and renal function testing, presence of two ovaries, and no pill or hormone pretreatment within three months prior to stimulation cycle Exclusion criteria: ovarian‐factor or uterine‐factor infertility, or those suffering ovarian cysts, as determined by the use of ultrasound at the commencement of a stimulation cycle | |
| Interventions | COS with either a multiple‐dose (MD) or a single‐dose (SD) protocol for GnRH antagonist (cetrorelix) administration (Flexible), or with a long protocol (LP) for GnRH agonist (buserelin) administration, followed by oocyte retrieval, IVF/ICSI, and embryo transfer | |
| Outcomes | Amount of hMG administered (ampoules) | |
| Notes | Number of participants at randomisation: 61 (cetrorelix: 41/ buserelin: 20) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported clearly |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported clearly |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | University grant; the study appears to be free from other sources of bias |
| Methods | RCT, single‐centre trial | |
| Participants | 120 infertile women undergoing ICSI Inclusion criteria: age 20 – 38 years with regular cycles, normal basal FSH < 10 mIU/ml, LH < 10 mIU/ml and E2 < 60 pg/ml), BMI 18.5 ‐ 24.9 kg/m2, male‐factor infertility Exclusion criteria: endometriosis, anovulation, PCOS and hydrosalpinx Baseline characteristics: age (years) 31.3 ± 4.4 vs 30.7 ± 4.4, weight (kg) 53.5 ± 8.2 vs 55.2 ± 8.2, day 3 FSH level (mIU/ml) 5.12 ± 1.76 vs 5.28 ± 1.44, Day 3 LH level (mIU/ml) 4.75 ± 2.19 vs 4.31 ± 2.39 | |
| Interventions | GnRH antagonist (n = 60): 100 mg/day CC cd 3 to 7 + 2 ‐ 4 ampoules hMG was given on days 4, 6, 8 and 9 + 2.5 mg cetrorelix acetate (Cetrotide 1) when the leading follicle had reached 14 mm (Flexible) GnRH agonist (n = 60): 0.5 mg/day buserelin GnRH agonist long protocol + 2 – 4 ampoules of hMG (Pergonal) or FSH (Metrodin) Oocyte maturation triggering: 10,000 IU hCG (Pregnyl 1), when at least two follicles had reached 18 mm Oocyte retrieval: 34 – 36 hrs, followed by ICSI Maximum embryo transfer: not stated Luteal phase support: 600 mg/day vaginally of micronised progesterone (Utrogestan) starting from the day after oocyte retrieval Follow up: up to live birth | |
| Outcomes | Primary outcome measure: amount of gonadotrophin used Secondary outcome measures: endometrial thickness, number of oocytes and MII oocytes recovered, as well as rates of fertilisation and pregnancy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation |
| Allocation concealment (selection bias) | Low risk | Sealed in envelopes and the physicians were not aware of the allocation |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, university‐affiliated IVF centre, open‐label, parallel design | |
| Participants | Aged between 20 and 38 years, no low response in a previous treatment cycle, no uterine or ovarian anomalies, and history of regular menstrual cycles ranging from 25 to 35 days | |
| Interventions | GnRH antagonist: started ovarian stimulation on day 3 of the cycle with the administration of 225 IU of rFSH. Group B was treated with the GnRH antagonist cetrorelix (0.25mg/day, SC; Cetrotide) (Flexible), commencing when the largest follicle had reached a diameter of 14 mm, and simultaneous augmentation of 75 IU of FSH was initiated up to and including the day of hCG administration GnRH agonist: was treated with the GnRH agonist triptoreline (1 mg/day SC; Decapeptyl) starting one week before the expected menses. After down‐regulation was achieved (serum E2 < 50 pg), ovarian stimulation was commenced with a fixed daily dose of 225 IU of rFSH. | |
| Outcomes | Peak E2 (pg/mL) | |
| Notes | Number of participants at randomisation: 116 (cetrorelix: 58/ triptoreline: 58) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table, randomisation: 1:1 (cetrorelix:triptoreline) ratio |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre, open‐label, parallel design | |
| Participants | 60 infertile women (poor responders) undergoing IVF/ICSI Inclusion criteria: estradiol concentrations < 600 pg/ml concentration on the day of hCG administration and a poor response (number of oocytes retrieved < 3) after a previous standard long protocol using analogues for down‐regulation and recombinant gonadotrophin at a dose of 225 IU for stimulation (rFSH, Gonal‐F) | |
| Interventions | GnRH antagonist (n = 30): 375 IU rFSH (Gonal‐F) from cycle day 2 + GnRH antagonist cetrorelix 0.25 mg per day was then administered from when the two lead follicles had reached 14 mm diameter, irrespective of the day of the cycle until the day of hCG injection. (Flexible) GnRH agonist (n = 30): by analogues from day 23 of the cycle (Enantone 3.75 mg) + 375 IU daily , SC, rFSH, (Gonal‐F) from day 3 of the next cycle at a dose of 375 IU. In group B (n = 30), ovarian stimulation started at day 2 with rFSH at a dose of 375 IU (Gonal‐F) Oocyte maturation triggering: hCG (Profasi; Serono) 10,000 IU was administered IM 24 hrs after the last rFSH injection when at least two follicles had reached a diameter of 17 mm Oocyte retrieval: 36 hrs after hCG administration followed by IVF/ICSI Embryo transfers: were performed 48 hrs after oocyte retrieval Luteal phase: 2 × 200 mg/day of micronised vaginal progesterone (Prometrium) Follow up: serum hCG concentrations were measured 14 days after embryo transfer. Clinical pregnancies were confirmed 28 ‐ 35 days after embryo transfer by the presence of a gestational sac under ultrasound | |
| Outcomes | Age (years) Initiated cycles Stopped cycles Cycles with oocyte retrieval Stimulation duration (days) Number of ampoules Follicles > 15 mm Oocytes retrieved Oocytes fertilised Cycles with transfers Embryos transferred Endometrial thickness (mm) Clinical pregnancies | |
| Notes | Number of participants at randomisation: 60 (cetrorelix: 30/ enantone: 30) Number of participants at stimulation: 60 (cetrorelix: 30/ enantone: 30) Number of participants at OPU: 55 (cetrorelix: 29/ enantone: 26) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcomes data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre, donor‐recipient cycle | |
| Participants | 323 Donors: < 35 years, baseline FSH < 10 U, BMI < 30 kg/m2, no history of hereditary disease Baseline characteristics: Age (years) 27.2 + 4.7 vs 26.5 + 4.7, BMI (kg/m2) 23.0 + 3.5 vs 23.1 + 3.0, baseline FSH (IU/ml) 7.0 + 2.3 vs 6.5 + 2.1 Setting and timing: private hospital, Spain. January 2005 to November 2006 | |
| Interventions | GnRH antagonist: vaginal contraceptive (Nuvaring) + rFSH 150–200 U/day + ganirelix (Orgalutran) 0.25 mg/day, from day 6 of stimulation (Fixed) GnRH agonist: vaginal contraceptive (Nuvaring) + leuprorelin acetate, 3.75 mg + 2 – 3 ampoules per day of hMG Oocyte maturation triggering: 10,000 U of hCG when at least three follicles > 20 mm in diameter Follow up: 10 – 14 days after puncture | |
| Outcomes | Clinical pregnancy rate (confirmed by the presence of a gestational sac in the ultrasound examination carried out 4 – 5 weeks after transfer) The implantation rate was calculated by dividing the number of gestational sacs by the number of embryos transferred Other secondary results were the total number of OCCs retrieved, the number of days and total dose of gonadotrophin stimulation, and plasma estradiol levels on the day of hCG administration | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation table |
| Allocation concealment (selection bias) | Low risk | Telephone call |
| Blinding (performance bias and detection bias) | Low risk | Researchers |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias Study funding not reported |
| Methods | RCT | |
| Participants | 30 women Inclusion criteria: known to be low responders (developed fewer than six follicles > 12 mm in previous IVF cycles under the standard mid‐luteal phase down‐regulation protocol) Exclusion criteria: abnormally high FSH > 13 IU/l Setting and timing: centre for assisted conception, UK. timing not specified Baseline characteristics: age agonist group 37 (95% CI 35 to 39) vs antagonist group 36 (95% CI 33.5 to 38) | |
| Interventions | Agonist group ‐ 500 micrograms buserelin SC daily starting day 1 of menstrual cycle Antagonist group ‐ cetrorelix 0.25 mg SC daily started on cycle day 8 and continued until day of hCG injection Ovarian stimulation started on cycle day 3 with 225 to 375 IU gonadotrophin daily based on highest dose reached in previous IVF cycle Both buserelin and gonadotrophin continued until day of hCG 10,000 IU injection Gonadotrophin increased by 75 IU/day if fewer than three follicles measuring 12 mm or more were found on cycle day 9 Luteal support ‐ progesterone used for luteal support 400 mg twice daily Number of ampoules ‐ agonist 44.5 (95% CI 38.5 to 54) vs antagonist group 35.5 (95% CI 33 to 41) | |
| Outcomes | Serum LH and E2 levels, cycle cancellation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomised" but no other details |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used but no details as to whether opaque or given out sequentially |
| Blinding (performance bias and detection bias) | Unclear risk | No details but blinding unlikely. The lack of blinding is unlikely to effect the fertility outcomes |
| Incomplete outcome data (attrition bias) | Unclear risk | Two women not analysed from 30 women randomised due to cycle cancellation. Both were in the antagonist group |
| Selective reporting (reporting bias) | Unclear risk | No pregnancy outcomes of relevance were reported. The primary outcomes relate to serum LH and E2 levels Cycle cancellation was reported but was not pre‐specified as an outcome |
| Other bias | Low risk | No other bias identified, groups were balanced at baseline |
| Methods | RCT, single‐centre | |
| Participants | 93 women undergoing IVF/ICSI between May 2005 and August 2006, Age: 25 – 38 years Baseline characteristics: age: 30.91 ± 5.52 vs 30.25 ± 4.94. BMI: 29.36 ± 4.45 vs 26.58 ± 3.32. Basal E2: 6.63 ± 1.33 vs 6.32 ± 1.77. AFC: 5.02 ± 2.56 vs 8.02 ± 2.95 | |
| Interventions | GnRH antagonist (n = 45): OCP (Desogesteral + 225 IU FSH + 0.25 mg SC of cetrorelix acetate (Cetrotide) administered when follicles reached a diameter of ≥ 14 mm (flexible protocol) GnRH agonist (n = 48): OCP (Desogesteral + 225 IU FSH + long GnRH agonist leuprolide acetate 1 mg/day SC (Lupron) one week before the expected menses with approximately a five‐day overlap with the OCP. The dose of GnRH agonist was then reduced to 0.5 mg/day, (Low‐dose GnRH agonist protocol) Oocyte maturation triggering: hCG 5000 IU (Profai) SC > 3 follicles of 18 mm in diameter Maximum embryos transferred: 3 Luteal phase support: intravaginal progesterone gel (Crinone 8%) and was started no later than the day of ET until a urine pregnancy test was performed 12 days later | |
| Outcomes | Antral follicle numbers | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Numbered sealed envelopes |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias. Not reported |
| Methods | RCT, single‐centre trial | |
| Participants | 49 PCOS infertile women undergoing IVF/ICSI | |
| Interventions | GnRH antagonist (n = 25): 225 IU FSH on 2nd day of the cycle (fixed) + 0.25 mg SC of GnRH antagonist cetrorelix (Cetrotide, Serono International, Switzerland) started on the sixth day of stimulation (fixed protocol) GnRH agonist (n = 24): 225 IU rFSH (fixed) + long GnRH agonist, 3.75 mg of triptorelin (Dipherelin) in the mild‐luteal phase of the preceding cycle(long depot GnRH agonist protocol) Oocyte maturation triggering: 10,000 IU SC hCG (Pregnyl) when one follicle 18 ‐ 20 mm | |
| Outcomes | Duration of stimulation, number of ampoules of FSH, fertilisation rate, implantation rate, ongoing pregnancy rate, OHSS incidence | |
| Notes | Abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement |
| Methods | RCT, multi‐centre (9 centres ), open‐label, parallel design | |
| Participants | 169 infertile couples undergoing ovarian stimulation for IVF‐ET with or without ICSI Inclusion criteria: with no more than three previous IVF‐ET attempts with all causes of infertility (except polycystic ovary and moderate or severe endometriosis) Baseline characteristics: age cetrorelix 31.4 ± 3.7, decapeptyl 31.8 ± 3.8. Duration of infertility: cetrorelix 59.3 ± 35, decapeptyl 55.3 ± 38.1. FSH: cetrorelix 6.3 ± 2, decapeptyl 6.3 ±1.9. BMI cetrorelix 22.4, decapeptyl 22.8 | |
| Interventions | A single dose of 3 mg of GnRH antagonist (cetrorelix) (Depot) was administered SC to 115 participants On day 7 of hMG mid‐luteal GnRH analogue (decapeptyl 3.75) Ovarian suppression was confirmed by E2 > 50 pg/ml / FSH and LH < 10 IU/L, P < 1 µg/ml Luteal phase support using daily vaginal progesterone ICSI was done in 12 women in the cetrorelix group and five women in the decapeptyl group. | |
| Outcomes | Premature LH surge defined as (LH > 10 IU/L) and progesterone level > 1 ng/L Clinical pregnancy was defined as fetal heart beat on ultrasonography Ongoing pregnancy was defined as pregnancy ongoing after 12 weeks of amenorrhoea | |
| Notes |
| |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single centre | |
| Participants | 190 women Inclusion criteria: < 39 years, FSH < 12 mIU/mL, could start gonadotrophin at a dose fixed for five days and with a range of 100 to 300 IU, < 3 previous IVF cycles Exclusion criteria: known endocrine disorders, stage III/IV endometriosis, cases where blood was drawn and analysed in another laboratory, ovarian simulation cancellation if progesterone was > 1.5 ng/ml and estradiol was > 80 pg/ml on the day of initiation of gonadotrophins Setting and Timing: setting unclear, Greece. August 2007 to December 2009 Baseline characteristics: age agonist 32.8 ± 0.3 SE vs antagonist 32.2 ± 0.3 SE | |
| Interventions | Antagonist ‐ gonadotrophins administered from day 2 of the cycle if the hormone levels were basal and co‐treatment with ganirelix 0.25 mg or cetrorelix 0.25 mg started on day 6 of stimulation Agonist ‐ long protocol started on day 21 of preceding cycle with intranasal buserelin (600 mg /day). Gonadotrophins were administered after two to three weeks of down‐regulation and once hormonal levels were basal Gonadotrophin dose 150 ‐ 300 IU for all participants and remained fixed for five days. After this period the dose could be adjusted and individualised based on follicular growth, and serum estradiol levels Oocycte maturation induced with 250 micrograms of re hCG when at least 3 follicles of 17‐18 mm were present Oocycte retrieval 36 hours after hCG | |
| Outcomes | Live birth, ongoing pregnancy, miscarriage, clinical pregnancy rates. OHSS | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "computer generated list" |
| Allocation concealment (selection bias) | Low risk | "allocation to treatment arms was performed by a consulting nurse who had no intervention in the patients’ treatment" |
| Blinding (performance bias and detection bias) | Unclear risk | Both participants and treating physicians were aware of the exact protocol followed. Lack of blinding is unlikely to affect the fertility outcomes |
| Incomplete outcome data (attrition bias) | Low risk | All women randomised were analysed using ITT principle for those with available data. There were no losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All reported outcomes were pre‐specified |
| Other bias | Low risk | No other evidence of bias |
| Methods | Randomised controlled trial. Single centre | |
| Participants | 364 women Inclusion criteria: poor responders (exhibited a poor ovarian response with zero to four oocytes retrieved at a previous IVF cycle) Exclusion criteria: known severe endometriosis (stage III/IV), FSH ≥ 14 mIU/mL Setting and timing: IVF centre, Thessaloniki, Greece. January 2007 to December 2011 Baseline characteristics: age antagonist 36.2 ± 4.4 years, agonist 36.2 ± 4.5 years | |
| Interventions | Antagonist ‐ rFSH (puregon, NV Organon) 450 IU/day was commenced on day 2 of menstruation and the antagonist ganirelix (Orgalutran) 0.25 mg was added in the afternoon of the sixth day of stimulation Agonist ‐ triptorelin SC 0.1 mg/day commencing on the afternoon of the 21st day of the cycle prior to stimulation. Triptorelin SC 0.05 mg/day plus rFSH (puregon, NV Organon) 450 IU/day was commenced on day 2 of menstruation or later when estradiol was ≤ 50 pg/mL All women were given a contraceptive pill (Gynofen; Shering Hellas) for the cycle prior to stimulation (day 2 of the cycle to day 21). All women monitored by TVS and serum estradiol on day 2 and after four days of stimulation Daily dose of rFSH adjusted according to ovarian response based on estradiol levels and the number and size of follicles hCG 10,000 IU administered when one or more follicles present with a mean diameter on ultrasound ≥ 17 mm and serum estradiol ≥ 500 pg/mL Oocyte retrieval 34 to 36 hrs after hCG Luteal phase support with progesterone 200 mg three times daily Number of embryos transferred: antagonist 1.92 ± 0.8 vs agonist 2.08 ± 0.8 | |
| Outcomes | Oocyte retrieval rate, fertilisation rate, number of embryos transferred, cancellation rate, implantation rate, clinical pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "assigned at random" . ‘No further details were reported |
| Allocation concealment (selection bias) | Low risk | "using sealed envelopes" |
| Blinding (performance bias and detection bias) | Unclear risk | Woman was blinded to allocation, no further details were reported on other personnel. However non blinding of outcome assessors not likely to affect some of the outcome measures as they were objectively assessed |
| Incomplete outcome data (attrition bias) | Unclear risk | Antagonist group 168/182 completed. Agonist group 162/182 completed. No reasons given for withdrawals or losses to follow up |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes listed were not pre‐specified. No live birth or OHSS data were reported in this trial |
| Other bias | Low risk | No other source of bias identified. Groups balanced at baseline |
| Methods | RCT. Multi‐centre (number of sites not specified) | |
| Participants | 233 women Inclusion criteria: Chinese women, aged ≥ 18 and ≤ 35 years, BMI 18 to 29 kg/m2, normal menstrual cycle, access to ejaculatory sperm and for whom COS and IVF/ICSI were indicated Exclusion criteria: not specified. Setting and timing: reproductive centres, China. July 2007 to December 2008 Baseline characteristics: age ‐ antagonist 29.3 ± 2.8 years vs agonist 29.1 ± 3.0 years | |
| Interventions | Antagonist ‐ ganirelix 0.25 mg/day administered from stimulation day 6 up to and including the day of hCG 10,000 IU injection when three follicles ≥ 17 mm Agonist ‐ long protocol triptorelin 0.05 mg/day started during the luteal phase (days 21 to 24) up to and including the day of hCG 10,000 IU injection when three follicles ≥ 17 mm Starting dose of rFSH was 150 IU and dose could be adjusted from day 6 onwards depending on the ovarian response Luteal phase support IM progesterone (min. dose 60 mg) | |
| Outcomes | rFSH required, number of oocytes retrieved, embryo quality, ongoing pregnancy rate, fertilisation rate In addition data reported for cancellation rate, OHSS and multiple pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned, no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | Open label, no blinding of participants or researchers. Lack of blinding is unlikely to affect fertility outcomes |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up reported. States that intention‐to‐treat analysis was used |
| Selective reporting (reporting bias) | Unclear risk | Only ongoing pregnancy was specified as a secondary outcome. The authors reported on multiple pregnancy and clinical pregnancy rate and OHSS in the results. Live birth was not reported at all |
| Other bias | Low risk | No other source of bias identified. Groups were balanced at baseline |
| Methods | RCT | |
| Participants | 136 women undergoing ART Inclusion criteria: undergoing ARTs for the first time, serum FSH level ≤ 10 IU/ litre on day 3 of menstrual cycle, male or female factor of infertility Exclusion criteria: previous history of IVF or ICSI, hyperprolactinaemia, thyroid dysfunction, uterine abnormality, severe endometriosis (diagnosed by laparoscopy), secondary infertility Setting and timing: infertility centre, Iran. Timing not specified Baseline characteristics: age ‐antagonist 28.36 ± 3.4 years vs agonist 28.65 ± 3.9 years | |
| Interventions | Antagonist ‐ ovarian stimulation commenced on day 2 of the cycle with rFSH (Gonal F, Serono, Switzerland) 75 IU daily SC On day 6 of stimulation 0.25 mg cetrorelix when the follicle reached 14 mm diameter Agonist ‐ 500 μg SC buserelin daily (Suprefact, Aventis, Germany) commenced on 21st day of previous menstrual cycle and continued until baseline evaluation of serum level of E2 on day 2 of menstruation. If serum E2 < 50 pg/ml then buserelin reduced to 250 μg daily and ovarian stimulation commenced with SC rFSH (Gonal F, Serono, Switzerland) 75 IU daily Based on ovarian response detected by ultrasonography every two to three days, gonadotrophin dose was adjusted Administration of buserelin or cetrorelix continued until the time of the hCG (10,000 IU) injection (IM) when there were at least three follicles with a mean diameter of 18 mm Oocyte retrieval after 36 hours Luteal support with cyclogest suppository 800 mg Number of ampoules: antagonist 17.04 ± 6.04 vs agonist 20.14 ± 9.51 Number of embryos transferred: antagonist 2.66 ± 0.9 vs agonist 2.71 ± 0.86 | |
| Outcomes | Clinical pregnancy rate, ongoing pregnancy rate, moderate or severe OHSS | |
| Notes | Sample size calculation: not reported ITT analysis: Yes Funder: Isfahan University of Medical Sciences | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised", no other details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not reported but unlikely to be blinding as protocols varied and no placebo was used. Lack of blinding unlikely to affect fertility outcomes |
| Incomplete outcome data (attrition bias) | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes pre‐specified were reported. Live birth was not reported |
| Other bias | Low risk | No evidence of other bias. Groups were balanced at baseline |
| Methods | RCT, parallel design. | |
| Participants | 695 women with clinical, endocrine and ultrasound characteristics suggesting a low ovarian reserve and a poor responsiveness to COH Inclusion criteria: women undergoing IVF classified as “expected poor responders”: circulating day 3 FSH between 10 and 20 IU/l in the presence of estradiol (E2) serum level < 80 pg/ml; circulating AMH between 0.14 and 1.0 ng/ml; AFC between 4 and 10 Exclusion criteria: Women with basal FSH > 20 IU/l, undetectable AMH levels, AFC < 3 and aged more than 43 years Baseline characteristics: age (years) 38.5 ± 3.4 vs. 37.5 ± 3.6, BMI (kg/m2) 22.8 ± 3.8 vs. 23.1 ± 4.3, basal FSH (IU/L) 12.4 ± 4.4 vs. 13.7 ± 2.9, AMH (ng/ml) 0.71 ± 0.44 vs. 0.68 ± 0.35, AFC 5.3 ± 2.7 vs. 6.2 ± 2.8 | |
| Interventions | GnRH antagonist (n = 355): 100 mg/day CC (Serophene) orally for five days (from cycle day 2 to 6) + 150 IU/day SC Gn (Meropur) from cycle day 5 + 0.25 mg/d SC. GnRH‐antagonist cetrorelix (Cetrotide) from cycle day 8 until the day of hCG administration. (“mild” CC/Gn/GnRH‐antagonist protocol) GnRH agonist (n = 340): 0.8 mg/d intranasal GnRH agonist (Suprefact) starting from run‐in cycle day 21 for 14 days, at the beginning of Gn administration, the dose was reduced to 0.4 mg/d and continued during ovarian stimulation + 300 IU exogenous Gn (Meropur) after confirmation of pituitary block, dosage increased up to a maximum of 450 IU/d after one week. (Long protocol with GnRH‐agonist plus Gn at high doses) Oocyte maturation triggering: 10,000 IU SC hCG (Gonasi HP) when the leading follicle reached 18 mm, with appropriate serum E2 levels Oocyte retrieval: 36 hours after hCG injection, either IVF or ICSI was performed according to the clinical indication. After two days of in vitro culture, embryos transferred Maximum embryos transferred: 2 Luteal phase support: 180 mg/d natural progesterone (Crinone 8) for 15 days from the day of ET Follow‐up: pregnancy was assessed by serum hCG assay after 15 days from ET and then confirmed by transvaginal ultrasound after two further weeks | |
| Outcomes | Ongoing pregnancy rate, miscarriage rate, clinical pregnancy rate, cycle cancellation rate, endometrial thickness at OPU, cycles without retrieved oocytes, MII oocytes/OPU, number of oocytes retrieved, totally administered Gn dose, length of the ovarian stimulation, fertilisation rate (FR), implantation rate (IR), number of transferred embryos, hCG positive tests, number of gestational sacs | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed using a computerized algorithm without any restriction” |
| Allocation concealment (selection bias) | Low risk | “Allocation concealment was obtained using sequentially numbered dark envelopes: until they were opened at the time of allocation” |
| Blinding (performance bias and detection bias) | Unclear risk | “…both physicians and patients were blinded to the study” however blinding of outcome assessment not described but unlikely to influence measurement of outcomes |
| Incomplete outcome data (attrition bias) | High risk | Number randomised = 695, number analysed = 640 (55 Lost to follow‐up due to cancelled cycle (OPU not performed)); analysis was not based on ITT |
| Selective reporting (reporting bias) | Unclear risk | All outcomes reported in the pre‐specified manner. Live birth rate not reported |
| Other bias | Low risk | None identified |
| Methods | Prospective RCT, two centres | |
| Participants | 349 women Inclusion criteria: age 27 to 38 years, undergoing IVF, infertility due to tubal factor, moderate endometriosis, male factor or idiopathic subfertility. Basal FSH and LH < 12 mIU/mL, E2 < 50 pg/mL, prolactin < 30 ng/mL, regular menstrual and ovulatory cycles, normal uterine cavity, BMI 20 to 26 kg/m2. Previous cycle cancelled due to high risk of OHSS Exclusion criteria: no details Baseline characteristics: age antagonist 35.2 ± 4.7 years versus agonist 35.1 ± 4.9 years Setting and timing: assisted reproduction centre. January 2008 to December 2012 | |
| Interventions | Antagonist: mild/minimal stimulation protocol 75 IU/day of rFSH and 0.25 mg per day cetrorelix administered when the lead follicle was 14 mm diameter (n = 148) Agonist: triptorelin 0.1 mg per day SC from mid‐luteal phase of the previous cycle for a minimal of 14 days followed by 150 IU FSH and adjusted as necessary based on follicular size and E2 level Oocyte maturation triggered by 10,000 IU hCG Luteal phase support commenced on day of oocyte retrieval using progesterone 50 mg/ml IM daily | |
| Outcomes | Live birth, clinical pregnancy, and miscarriage. Also reported on oocytes retrieved, fertilisation rate, embryo cleavage rate, embryos transferred, clinical pregnancy, implantation rate, live birth rate, miscarriage rate, OHSS | |
| Notes | Sample size calculation ‐ yes ITT analysis ‐ yes Funding ‐ no details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "randomized" "using computer generated random assignment" |
| Allocation concealment (selection bias) | Low risk | "sealed and numbered envelopes" |
| Blinding (performance bias and detection bias) | Unclear risk | "open label" unlikely to influence fertility outcomes. No details as to whether outcome assessors were blinded; however, non‐blinding of outcome assessors not likely to affect some of the outcome measures as they were objectively assessed |
| Incomplete outcome data (attrition bias) | High risk | The proportion of women who did not undergo embryo transfer differed between the two treatment groups; reasons for not having embryo transfer were not reported and not all participants randomised were included in the final data analysis |
| Selective reporting (reporting bias) | Unclear risk | Only prespecified clinical pregnancy as an outcome but reported on other pregnancy outcomes including live birth and miscarriage rate. Data are reported per embryo transferred and not per woman randomised. However, numbers of embryos transferred were same as numbers of women |
| Other bias | Low risk | Groups balanced at baseline |
| Methods | RCT, 10 IVF centres, open‐label, parallel design | |
| Participants | 351 infertile women undergoing IVF/ICSI Inclusion criteria: healthy females of infertile couples, age at time of screening between 18 and 39 years, BMI between 18 and 29 kg/m2, body weight < 90 kg, a normal menstrual cycle with a range of 24 ‐ 35 days and an intra‐individual variation of ± 3 days, and willingness to give written informed consent Exclusion criteria: included contraindications for the use of gonadotrophins, endocrine abnormalities (e.g. polycystic ovary syndrome), more than three unsuccessful controlled ovarian stimulation cycles, a history of low or no ovarian response during FSH/HMG treatment, and clinically relevant abnormal laboratory values (including hormones) or medical examination findings | |
| Interventions | GnRH antagonist: treatment with the GnRH antagonist ganirelix (0.25 mg, Orgalutran) was started on day 5/6 of rFSH treatment. If no follicles 14 mm were observed by ultrasonography on that day, the start of ganirelix was delayed. Injections containing 0.25 mg ganirelix per 0.5 ml were administered SC in the thigh, once daily in the morning, until and including the day of hCG administration. (Flexible) GnRH agonist: in the OC‐scheduled group, women started taking a combined OC pill (30 µg ethinyl oestradiol/150 µg desogestrel) Marvelon® (NV Organon) on day 1 of the menstrual cycle. They took it daily for between 14 and 28 days, depending on the planned start of rFSH treatment Women in the nafarelin group started pretreatment with the GnRH agonist nafarelin (Synarel®; Pharmacia, Australia) on day 21 ‐ 24 of the preceding cycle. Nafarelin was administered intranasally at a daily dose of 0.8 mg until and including the day of hCG administration In all three groups ovarian stimulation was performed with rFSH (follitropin beta, Puregon®; NV Organon, The Netherlands), which was administered SC once daily in the morning at a fixed dose of 200 IU during the first 5 ‐ 6 days. After this period the dosage of rFSH could be adjusted depending on the ovarian response as assessed by ultrasound. Treatment was continued until (and including) the day of hCG administration. In the OC‐scheduled ganirelix group, stimulation with rFSH was started two days after discontinuation of the OC (irrespective of whether or not menses had started), in the non‐scheduled group on day 2 ‐ 3 of the menstrual cycle and in the nafarelin group after 2 ‐ 4 weeks of nafarelin treatment [as soon as pituitary down‐regulation had been achieved (i.e. serum estradiol 50 pg/ml or 200 pmol/l); if this stage was not achieved after four weeks of nafarelin treatment, the subject discontinued]. hCG, 10,000 IU in 1 ml saline (Pregnyl®, NV Organon, the Netherlands), was administered, either SC or IM, when at least three follicles 17 mm or at least one follicle 20 mm were observed on ultrasound. In case of risk of OHSS, the hCG dose was reduced to 5000 IU. Oocyte retrieval was performed 30 ‐ 36 hrs after hCG administration, followed by IVF or ICSI. No more than three embryos were transferred 2 ‐ 3 days after oocyte retrieval. Progesterone for luteal support was given daily (doses and administration form as per usual protocol of the participating centre), starting at the latest on the day of embryo transfer, for two weeks or up to menses The study was approved by the Ethics Committee of each participating centre. All women gave written informed consent. The study was performed according to the principles of the Declaration of Helsinki, and the ICH/Good Clinical Practice guidelines. The study was monitored by uniformly trained Clinical Research Associates of Organon with assistance of a contract research organisation for the clinics in Perth and Adelaide | |
| Outcomes | Prior to the start of treatment, a physical and gynaecological examination was performed to exclude any abnormality. Blood samples were taken for routine biochemistry, haematology, and hormonal parameters. A pregnancy test (urinary hCG) was performed. Blood samples for hormone assessments were taken just before the first rFSH injection (treatment day 1) and at least once every two days from day 5/6 of rFSH treatment (in the antagonist groups just before ganirelix injection) up to and including the day of hCG. Serum FSH, LH, estradiol, and progesterone values were determined by means of the automated Wallac AutoDelfia Fluoroimmunoassay system (PerkinElmer Inc., Wellesley MA, USA) at a central laboratory (ABL BV, Assen, The Netherlands). The maximum intra‐assay and inter‐assay coefficients of variation were 3.3% for FSH, 3.4% for LH, 4.9% for estradiol, and 4.3% for progesterone. To measure follicular development, ultrasonography was performed at least once every two days from day 5/6 of rFSH treatment up to and including the day of hCG. Other parameters assessed were treatment failure (defined as the number of women who did not have an hCG injection or who received an hCG injection because of premature luteinisation), number of LH rises (LH = 10 IU/l), number of oocytes retrieved, number of good quality embryos (grade 1 (defined as excellent: no fragmentation) and grade 2 (defined as good: 1 ‐ 20% fragmentation)), fertilisation rate, implantation rate, and ongoing pregnancy rate (assessed by ultrasound = 12 ‐ 16 weeks after embryo transfer) | |
| Notes | Number of participants at randomisation: 351 (ganirelix: 234/ nafarelin: 117) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as a randomised trial without any further details |
| Allocation concealment (selection bias) | Low risk | Interactive voice response system |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, open‐label, multi‐centre study. Phase III trial | |
| Participants | 74 infertile women (aged 18 ‐ 39 years) undergoing ICSI Baseline characteristics: mean age (± SD) of the ITT population was 32.6 ± 4.0 years. The age range was broad (22 ‐ 39 years) and there were no significant differences between the three treatment groups. Mean BMI was 24.2 ± 4.5 g/m2. again with no significant differences between groups. Fifty‐one of the 73 women in the ITT population (69.99%) were White and the proportion of White women did not differ between treatment groups | |
| Interventions | GnRH antagonist: OCP pretreatment for 14 – 18 days, followed by cetrorelix (3 mg), starting on day 7 + rFSH 225 IU, starting on day 5 after OCP/dose adjustments after day 6 GnRH antagonist II: OCP + cetrorelix and r‐FSH together with mid‐cycle r‐LH Embryo transfer: no more than three embryos were to be replaced: two if transferred at blastocyst stage Luteal phase support: Micronised progesterone according to centres’ practice | |
| Outcomes | The primary efficacy end‐point: the number of metaphase II oocytes retrieved per patient Secondary efficacy: end‐points were the duration and total dose of r‐hFSH therapy, the total number of follicles > 14 mm on the day of r‐hCG administration, oocyte and embryo quality and development, the number of participants with at least one embryo considered viable for cryopreservation, oestradiol concentration per follicle > 10 mm, total number of oocytes, implantation rates per‐embryos transferred and pregnancy rates (biochemical and clinical) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, internet‐based system. Randomisation 1:1:1 |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre | |
| Participants | 564 low responders, undergoing their first IVF cycle were eligible for the study Inclusion criteria: age 40 years or older and no previous IVF cycle Exclusion criteria: PCOS, FSH > 10 IU/ mL, a previous IVF cycle, and age 45 years or older Baseline characteristics: maternal age, years 42.3 1.4 vs 42.1 1.5, BMI 25.1 2.6 vs 24.8 2.4, basal FSH levels, IU/L 7.0 2.5 vs 6.9 2.4 | |
| Interventions | Group A (n= 285): 300 IU/day r‐hFSH (Gonal‐F) + 0.25 GnRH antagonist cetrorelix (Cetrotide) when the leading follicle ≃ 14 mm or the E2 plasma levels were 600 pg/mL (flexible multiple‐dose protocol) Group C (n= 285): buserelin 0.4 mg/day long GnRH agonist + 225 IU/day rhFSH (Gonal‐F) (GnRH agonist protocol) Oocyte maturation triggering: 10,000 IU of IM hCG when plasma E2 between 800 and 3500 pg/mL and at least three follicles > 16 mm in mean diameter Maximum number of embryos transferred: 3 Luteal phase support: 50 mg daily of P (Prontogest) IM from the day of replacement | |
| Outcomes | Primary outcomes: clinical pregnancy rate per cycle started and per transfer | |
| Notes | Drop out: four women in the cetrorelix group and two in the control group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation number sequence at the time that their cycle was scheduled |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data, however, LBR, OPR were not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | Groups balanced at baseline |
| Methods | RCT, single‐centre, parallel design | |
| Participants | 323 women of reproductive age Inclusion criteria: indication for IVF/ICSI, age 21 to 39 years, presence of two functional ovaries, normal uterus based on hysterosalpingography or hysteroscopic evaluation, fewer than three previous IVF/ICSI attempts, early follicular phase serum FSH 15 IU/L or less and E2 60 pg/ml or less, no history of low response, BMI 25 kg/m2 or less, no untreated endocrinological disease, no treatment with gonadotrophin for three or more months before study, male partner sperm 1% or greater strict morphology Exclusion criteria: not stated Baseline characteristics: age Group A 33.5 ± 0.4 years versus Group B 34.4 ± 0.4 years versus Group C 33.4 ± 0.3 years Setting and timing: Centre for Reproductive Medicine, Brazil. July 2002 to August 2005 | |
| Interventions | All protocols included an initial r‐ hFSH (Gonal‐F) dose ranging from 150 to 300 IU daily dependant on age, and the participants were grouped as follows: Group A (antagonist): r‐hFSH beginning on day 2 or 3 was continued in the full dose until follicles reached 13 to 14 mm or reached day 6 of stimulation, when the r‐hFSH dose was lowered to 75 IU and the participants began with 200 IU hCG along with cetrorelix 0.25 mg (flexible) continued until day of hCG injection 10,000 IU Group B (antagonist II): r‐hFSH beginning on day 2 or 3 was continued in the full dose until two or more codominant follicles reached 18 mm diameter. 0.25 mg cetrorelix daily SC began either when two codominant follicles reached 13 to 14 mm diameter or participant reached day 6 of stimulation. Continued until day of hCG injection Group C (agonist): Leuprolide 0.5 mg SC daily administered in mid‐luteal phase of previous menstrual cycle after which rFSH was administered. Continued until day of hCG injection 10,000 IU rFSH was adjusted based on number and size of follicles Oocyte retrieval ‐ 35 to 36 hours after hCG injection Luteal phase support ‐ daily IM progesterone in oil 25 mg and vaginal administration of one full applicator of 8% Crinone gel at bedtime beginning on the day after oocyte retrieval Dose of hFSH ‐ Group A 1674.7 ± 59.4 IU versus Group B 2197.9 ± 83.1 IU versus Group C 21,567 ± 80.8 IU | |
| Outcomes | Number of mature oocytes retrieved Dose of hFSH E2 Number and quality of embryos | |
| Notes | Sample size calculation ‐ yes ITT analysis ‐ yes Funding ‐ no details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | "A research nurse handed each patient a unique identification envelope in sequential chronological order". Unclear if sealed and opaque |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding unlikely. No details on blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | 23 subjects withdrew consent. 4/110 from group A; 11/107 from group B, 8/106 from group C. No reasons given. Group A had four cancellations (three due to poor ovarian response and one for no embryo transfer), group B had 10 cancellations (two conceived, four poor ovarian response, one stopped ovarian stimulation and three had no embryo transfer); Group C had six cancellations (four poor ovarian response and two had no embryo transfer) Group A had 102/110 analysed, Group B 86/107 and Group C 92/106 |
| Selective reporting (reporting bias) | High risk | Live birth rate was not addressed by the study |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT | |
| Participants | 83 women undergoing IVF/ICSI Inclusion criteria: not reported Exclusion criteria: prior IVF, uterine anomalies, testicular sperm aspiration needed, allergy to medication, reduced liver or kidney function, aged over 40 years, current or prior anti‐depressant medication Baseline characteristics: median age antagonist 31.2 years versus agonist 36.4 years Setting and timing: Copenhagen, Denmark. 2010 to 2012 | |
| Interventions | Antagonist (n = 42) rSH given for ovarian stimulation (150 to 225 IU depending on age) starting on day 2 to 3 of cycle. After five days women received ganirelix 0.25 mg daily Agonist (n = 41) nasal nafarelin acetate 200 mg 3 times daily starting on cycle day 21. After 14 days rFSH (150 to 225 IU depending on age). Nafarelin continued until day of oocyte pickup Ovulation induction hCG 6500 IU SC when three largest follicles were 17 mm or larger Oocyte retrieval 36 to 38 hours after hCG injection | |
| Outcomes | Personality inventory, profile of mood states, perceived stress scale, symptom checklist (revised), major depression inventory, E2. No pregnancy outcomes reported and therefore no data that could be included in a meta‐analysis | |
| Notes | Sample size calculation: No ITT analysis: unclear Funding: Danish Research Council for Independent Research and MSD Add on to large Danish trial | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised; no further details were reported |
| Allocation concealment (selection bias) | Unclear risk | No information was reported on allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No details for participants, researchers or outcome assessors although non‐blinding of outcome assessors not likely to affect some of the outcome measures as they were objectively assessed |
| Incomplete outcome data (attrition bias) | Unclear risk | Unclear if there were any losses or if all women were analysed |
| Selective reporting (reporting bias) | Unclear risk | No pregnancy outcomes were reported and therefore no data could be included in a meta‐analysis |
| Other bias | High risk | Women in agonist group were significantly older than women in antagonist group at baseline |
| Methods | RCT. Two centres | |
| Participants | 111 women Inclusion criteria: poor responder (previous IVF cycle with stimulation using daily gonadotrophin ≥ 300 IU and who had ≤ 3 oocytes retrieved or had cycle cancelled due to ≤ 3 mature follicles developing) Exclusion criteria: > 40 years, single ovary Setting and timing: assisted conception unit, London, UK. March 2007 to May 2012 Baseline characteristics: age ‐ antagonist 37.4 ± 3.4 years versus agonist 36.7 ± 2.6 years | |
| Interventions | Antagonist ‐ Gonadotrophin injections 450 IU/day after ultrasound confirmation of quiescence of the ovaries, presence of thin endometrium (≤ 5 mm) and recording of antral follicles on day 2 or 3 cetrorelix (Cetrotide; Merck‐Serono) 0.25 mg daily when the lead follicle reached a diameter of 14 mm. Both gonadotrophin and cetrorelix injections continued until administration of hCG (n = 37). Agonist ‐ Pituitary down‐regulation with nafarelin nasal spray 400 µg twice daily (Synarel; Pharmacia) commenced in the mid‐luteal phase and continued for two weeks. After confirmation of down‐regulation by ultrasound and recording of antral follicles, ovarian stimulation was commenced with gonadotrophin injections 450 IU/day and reduced dose of nafarelin 200 µg twice daily until hCG injection. hCG administered when three antral follicles reached ≥ 17 mm diameter (n = 37). There was a third group that received a short agonist protocol. This arm is not described further here as it is not a comparison for this review (n = 37) Oocyte retrieval performed 34 to 38 hours after hCG Luteal phase support with progesterone pessaries 400 mg once or twice daily commencing on the day of oocyte retrieval and continued to negative pregnancy test or 8 weeks' gestation Gonadotrophin dose: antagonist 4740.0 ± 1131.9 versus antagonist 5540.32 ± 1216.1 Embryos transferred: antagonist 1.8 ± 0.6 versus agonist 1.7 ± 0.5 | |
| Outcomes | Oocytes retrieved, dose of gonadotrophin, cycle cancellation, fertilisation rate, embryo transferred, clinical pregnancy rate, ongoing pregnancy rate | |
| Notes | Sample size calculation ‐ yes ITT analysis ‐ yes Funding ‐ assisted conception unit | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "internet based block randomization"; no further details were reported |
| Allocation concealment (selection bias) | Unclear risk | "allocated by a third party" "distant"; no further details were reported |
| Blinding (performance bias and detection bias) | Unclear risk | "doctor performing oocyte retrieval and the embryologist involved were blinded to the treatment allocation"; no information was available on blinding of participants |
| Incomplete outcome data (attrition bias) | Low risk | For the long agonist regimen 31/37 women received the allocated intervention (five decided not to have further IVF treatment and there was one spontaneous pregnancy). For the antagonist regimen 30/37 women received the allocated intervention (six decided not to have further IVF treatment and there was one spontaneous pregnancy) ITT analysis was used and 37 women were analysed in each group |
| Selective reporting (reporting bias) | High risk | Clinical pregnancy rate is not given per group and live birth and OHSS are not reported at all |
| Other bias | Low risk | No other source of bias identified. Groups balanced at baseline |
| Methods | RCT, single‐centre | |
| Participants | 96 poor responders who underwent ICSI‐ET cycles Inclusion criteria: baseline follicle stimulating hormone (FSH) < 13 m IU/ml, estradiol level on the day of human chorionic gonadotrophin (hCG) injection < 500 pg/ ml and a poor response (failure in obtaining of at least three follicles > 16 mm in diameter and the number of mature oocytes retrieved less than four) after a previous ovarian stimulation cycle Baseline characteristics: Age (years) 38.3 ± 4.23 vs 37.9 ± 74.87. Baseline FSH (IU/mL) 6.31 ± 2.19 vs 6.27 ± 2.82 | |
| Interventions | GnRH antagonist (n= 48): 300 IU r‐FSH and hMG starting on the second day of menstruation for 6 days (adjusted) + 0.25 mg of cetrorelix (Cetrotide) or 0.25 mg ganirelix (Orgalutran) were administered subcutaneously per day when the leading follicle reached 14 mm in diameter until the hCG injection. (Flexible) GnRH agonist (n= 48): 1 mg/ day leuprolide acetate (Lucrin) started on the 21st day prior to menstruation for pituitary desensitization. When exogenous gonadotrophins were started on day 2 of menstruation, the dose of leuprolide acetate was decreased to 0.5 mg/day + 300 IU rFSH and hMG starting on the second day of menstruation for 6 days (adjusted) Oocyte maturation triggering: When the leading follicle reached 18 mm in diameter or at least two follicles were >17 mm in diameter, a total of 10,000 units of hCG were administered intramuscularly. Oocyte retrieval: was performed 35–37 hrs later Embryos transfer: day 2–3 Follow up: clinical pregnancy was confirmed 28–35 days after embryo transfer by a gestational sac under ultrasound. Ongoing pregnancy was defined as fetal heart beat at 10–12 weeks of gestation. Early pregnancy loss was defined as the proportion of patients with initially positive hCG in whom pregnancy failed to develop before 12 weeks of gestation. | |
| Outcomes | Clinical and ongoing pregnancy per randomised patient, the duration of stimulation, consumption of gonadotrophins, cycle cancellation rate, the number of oocytes retrieved and embryos transferred | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐based program. |
| Allocation concealment (selection bias) | Unclear risk | No details were reported to make a conclusive judgement |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcomes data, however LBR did not addressed by the study |
| Selective reporting (reporting bias) | Unclear risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre trial | |
| Participants | 95 PCOs infertile women undergoing IVF/ICSI treatment Inclusion criteria: age < 35 years basal FSH < 10 IU/L and undergoing their first cycle of ART Exclusion criteria: secondary infertility, previous IVF or ICSI, thyroid dysfunction, hyper prolactinemia, uterine abnormality and solitary ovary Baseline characteristics: age (years) 28.99 ± 6.1 vs 30.43 ± 5/08. Duration of infertility (years) 7.82 ± 4.70 vs 8.6 ± 4.61, BMI (kg/m2) 28.99 ± 6.12 vs 30.43 ± 5/08. Baseline FSH (IU/L) 5.4 ± 1.80 vs 5.3 ± 1.22 | |
| Interventions | GnRH antagonist (n= 45): OCP for 21 days in the previous cycles + 150 – 225 IU hMG (Merional) IM based on the patient's age and BMI + 0.25 GnRH antagonist cetrorelix (Cetrotide) when the leading follicle ≃ 14 mm (flexible multiple‐dose protocol) GnRH agonist (n= 47): OCP (30 g ethinyl estradiol plus 0.3 mg levonorgestrel) for 21 days + 500 µg buserelin per day (Superfact) SC, commenced on day 19 – 20 of OCP cycle. Once the down‐regulation was achieved, the dose of buserelin was reduced to 250 µg daily + 150 – 225 IU hMG (Merional) IM once daily depending on patient's age and BMI (GnRH agonist protocol) Oocyte maturation triggering: when at least two leading follicles were 18 mm in diameter, serum E2 levels were measured. If E2 level was measured to be less than 3000 pg/ml, participants in both groups would receive 10,000 IU hCG (Profasi) IM. In the control group, if E2 level was > 3000 pg/ml, hMG administration was stopped while Superfact injection was continued. Daily measurement of E2 level was performed and hCG was administered when E2 level fell below 3000 pg/ml (Coasting). In the study group, if E2 > 3000 pg/ml, Superfact 500 mg SQ was administered for final oocyte maturation Oocyte retrieval: 34 ‐ 36 hours later, followed by IVF/ICSI Maximum number of embryos transferred: 3 Luteal phase support: 800 mg vaginal micronised progesterone (Cyclogest) and 4 mg oral estradiol valerate daily started the evening after oocyte retrieval and continued until a negative pregnancy test or a 10‐week gestation Follow up: the serum hCG level on day 16 after oocyte recovery was tested to determine chemical pregnancy, if any, vaginal ultra sonography would be carried out on day 35 of oocyte retrieval for documentation of fetal heart activity and confirming a clinical pregnancy | |
| Outcomes | The primary outcome measures: incidence of moderate and severe OHSS The secondary endpoints: fertilisation and pregnancy rate | |
| Notes | The diagnosis of OHSS was based on the criteria by Golan 1989 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomised schedules |
| Allocation concealment (selection bias) | Low risk | Sealed in envelopes |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported clearly |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | Two‐arm parallel trial | |
| Participants | 300 normo‐responder women undergoing IVF in infertility clinic | |
| Interventions | GnRH antagonist: no details reported GnRH agonist: no further details reported | |
| Outcomes | Ongoing pregnancy rate and clinical pregnancy rate | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were allocated to two groups according to a sequence of computer generated random numbers (0 or 1).” |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) | Unclear risk | Blinding not reported but unlikely to influence measurement of outcomes |
| Incomplete outcome data (attrition bias) | Low risk | Number randomised = 300, number analysed = 300 |
| Selective reporting (reporting bias) | Unclear risk | Methods section not detailed enough to make conclusive judgement on reporting bias |
| Other bias | Unclear risk | Insufficient details to make a conclusive judgement |
| Methods | Two‐arm parallel RCT | |
| Participants | 1099 women undergoing first IVF/ICSI cycles, less than 40 years of age including both low and high responders | |
| Interventions | GnRH‐antagonist (550 women, mean age 32.1, BMI 23.1); no further details were given about treatment GnRH‐agonist (549 women, mean age 32.0, BMI 22.7); no further details were reported about treatment Fixed rFSH dose of 150 IU or 225 IU depending on the age (less than or equal to 36 years or greater than 36 years) with dose adjustment at stimulation day 6 | |
| Outcomes | Ongoing pregnancy rates OHSS rates (mild, moderate and severe) | |
| Notes | This is a conference abstract with limited information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information was provided on sequence generation; it was only stated that randomisation was done in ratio 1:1 |
| Allocation concealment (selection bias) | Unclear risk | No information was given on allocation concealment |
| Blinding (performance bias and detection bias) | Unclear risk | No information was provided on blinding of participants and/or personnel, including outcome assessors; however, non‐blinding of outcome assessors not likely to affect some of the outcome measures as they were objectively assessed |
| Incomplete outcome data (attrition bias) | Unclear risk | No information was reported on attrition, withdrawals or exclusions and number of women analysed in each treatment group at the end of study was not reported |
| Selective reporting (reporting bias) | Low risk | All outcome measures were pre‐specified |
| Other bias | Unclear risk | It was unclear if the numbers of participants were balanced at randomisation as the numbers of participants in the treatment groups were not reported |
| Methods | RCT, single‐centre, open‐label design | |
| Participants | 131 infertile women undergoing IVF/ICSI Inclusion/Exclusion criteria: women were considered eligible if they were scheduled for controlled ovarian stimulation and IVF with or without ICSI. Women older than 40 years or with day 3 FSH = 10 IU/L, or with more than three previous IVF/ICSI cycles were excluded from the study | |
| Interventions | GnRH antagonist protocol: rFSH treatment was begun on day 3 of the menstrual cycle. The starting dose for the first five days varied between 150 and 450 IU, depending on age and previous experience and was administered daily by SC injection. Thereafter, the dose was adjusted on the basis of ultrasonographic and analytic findings. On day 6 of rFSH treatment, cetrorelix was started if the ovarian response was adequate (at least one follicle = 13 mm or serum estradiol levels = 400 pg/mL). If the ovarian response was not adequate, cetrorelix administration was postponed until ultrasonographic or analytic criteria were achieved (Flexible). 250 microgram was administered daily by SC injection. When at least three follicles = 17 mm were observed, rFSH and cetrorelix administration was interrupted and hCG (10,000 IU IM) was administered for the timed oocyte retrieval 35 h later. Vaginal micronised progesterone was started 24 h after oocyte retrieval for luteal support in a standard dose of 600 mg daily for 14 days. Serum hCG was to be measured approximately two weeks after embryo transfer. Any pregnancy was confirmed by vaginal ultrasound scan at six weeks' gestation | |
| Outcomes | The main outcome measures for assessing efficacy and safety of both protocols were: the clinical pregnancy rate per cycle and per transfer (gestational sac visualised on ultrasound at six weeks' gestation), number of oocytes collected, number of days of stimulation, number of days of analogue administration and the number of detected cases of moderate and severe OHSS. Other variables assessed were the total amount of rFSH used, serum estradiol level on the day of hCG administration, number of follicles = 15 mm on the day of the oocyte retrieval, endometrial thickness on the day of the oocyte retrieval, fertilisation rate, quality of the embryos transferred and the number of cancelled cycles. | |
| Notes | Number of participants at randomisation: 131 (cetrorelix: 66/buserelin: 65) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number table, randomisation: 1:1 (Cetrorelix:Buserelin) ratio |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data, however LBR not addressed by the study |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
| Methods | RCT, single‐centre | |
| Participants | 220 IVF/ICSI cycles were included, age 25 to 35 years old, BMI 18 – 25 kg/m2; the number of previous IVF cycles < 3, and no previous poor response to ovarian stimulation (poor ovarian response was characterised by cancellation of the cycle due to either poor follicular development or ≤ 4 cumulus oocyte‐ complexes collected at oocyte retrieval); normal ovulatory cycles (25 to 35 days), both ovaries present and normal uterus; no hormone therapy within the past three months; and no current or past diseases affecting ovaries, gonadotrophin, sex steroid secretion, clearance or excretion | |
| Interventions | Study group: E2 pre‐treatment oral estradiol valerate 4 mg preceding the IVF cycle from day 21 until day 2 of next cycle + 225 IU of rFSH (Gonal‐F, Serono) from day 3 + 0.25 GnRH antagonist cetrorelix (Cetrotide) was injected daily when the leading follicles reached 12 – 14 mm in diameter (flexible) Final oocyte maturation triggering: 10,000 IU hCG (Profasi) were given when at least three mature ≥ 18 mm follicles were obtained Oocytes retrievals: 36 hrs later Embryo transfer: 2 to 3 embryos were transferred at 72 hrs after IVF/ ICSI injection | |
| Outcomes | Number of oocytes collected, MII oocytes, fertilisation, implantation, live birth and early pregnancy rate, and hormone profiles (LH, P, E2) | |
| Notes | The early pregnancy loss was defined as spontaneous abortion before 12 weeks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) | High risk | Not blind |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it is clear that the published reports included most expected outcomes |
| Other bias | Low risk | The study appears to be free from other sources of bias |
AFC: Antral Follicle Count
AMH: anti‐mullerian hormone
CC: clomiphene citrate
COS: controlled ovarian stimulation
ET: embryo transfer
FSH: follicle‐stimulating hormone
hCG: human chorionic gonadotrophin
IM: intramuscularly
IVF‐ET: in vitro fertilisation embryo transfer
MII = metaphase II
SC: subcutaneously
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| No available data for inclusion | |
| Retrospective analysis | |
| No available data for inclusion | |
| Quasi‐randomised study | |
| RCT in IUI cycles | |
| Quasi‐randomised trials, were randomly assigned to all women on the basis of the day of the week of their first appointment | |
| Microdose GnRH agonist protocol used and number of women in each group not specified | |
| Overlap with Eijkemans 2006; Heijnen 2007; Polinder 2008 | |
| Cross‐over study. Data not provided separately before and after cross‐over | |
| Overlap with Heijnen 2007 | |
| RCT, participant randomised on the day of hCG to receive, evaluate the effect of using vaginal micronised E2 administration, in addition to progesterone supplementation as luteal support, on clinical pregnancy rates in patients undergoing their first cycle of IVF/intracytoplasmic sperm injection (ICSI) treatment | |
| Irrelevant interventions. Study objective was “to determine whether the addition of recombinant LH in women with low response predictors, improved response to ovarian stimulation and clinical outcome in cycles of IVF/ICSI with a GnRH antagonist protocol.” Study did not compare with long GnRH agonist protocol arm | |
| Administration of GnRH analogues in the luteal phase of ART index cycle. Type GnRH agonist protocol not reported. No data/numbers provided to extract although the outcome reported fulfils the outcomes of interest | |
| Retrospective study | |
| No available data for inclusion | |
| Marked heterogeneity between the two study groups | |
| Study used a GnRH agonist short protocol for pituitary suppression | |
| RCT, quasi‐randomised, as odd or even days of the consultation delivery of treatment | |
| Study used microdose flare‐up GnRH agonist protocol for pituitary suppression | |
| Numbers of participants randomised at baseline to each treatment group were not reported | |
| Study used microdose GnRH agonist flare‐up (microdose protocol) for pituitary suppression | |
| Retrospective study | |
| Retrospective study | |
| Prospective observational/comparative study | |
| No available data for inclusion. Surrogate outcome. Failure to contact authors | |
| Not reported to be an RCT | |
| Study used short GnRH agonist (triptoreline) protocol in alternate days for pituitary suppression | |
| Study used GnRH agonist short regimen for pituitary suppression | |
| Study used microdose flare‐up GnRH agonist protocol for pituitary suppression. No data (numbers) given for cycle cancellation rate although it was mentioned as being "similar in both groups". Clinical pregnancy rates were expressed as ‘per cycle’ in percentage | |
| Protocol used microdose flare‐up | |
| Prospective observational study | |
| Retrospective trial | |
| Study used GnRH agonist microdose flare‐up protocol for pituitary suppression | |
| No available data for inclusion | |
| No available data for inclusion. Failure to contact authors | |
| Prospective observational study | |
| Overlap with Heijnen 2007 | |
| RCT, some women were used twice as donors | |
| Study did not specify the number of women in each group. It is impossible to separate out, from each group, the number of women from the number of cycles to obtain the right unit of measurement (per woman) for the outcomes of interest | |
| Donor oocyte cycles | |
| Numbers of women randomised to each treatment group was not reported | |
| Probably not RCT, described as controlled clinical study. Clinical pregnancy rate expressed as ‘per embryo transfer’. Unit of measurement for miscarriage rate not specified. Other outcomes reported do not fulfil the review’s outcomes of interest | |
| The number of women randomised in each group was not stated | |
| Inadequate randomisation (quasi‐randomised trial) | |
| Study used microdose GnRH agonist protocol for pituitary suppression | |
| No available data for inclusion | |
| IUI treatment cycles instead of IVF/ICSI |
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | open‐label, RCT |
| Participants | 1099 infertile women referred for their first IVF/ICSI at two public fertility clinics All less than 40 years of age and with no uterine malformation including women with poor ovarian reserve, polycystic ovary syndrome and irregular cycles. |
| Interventions | women allocated to either short GnRH antagonist |
| Outcomes | difference in severe OHSS, rates of mild and moderate OHSS, positive plasma (p)‐hCG, on‐going pregnancy and live birth |
| Notes | A total of 49 women withdrewtheir consent, thus 1050 subjects were |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman randomised Show forest plot | 12 | 2303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| Analysis 1.1 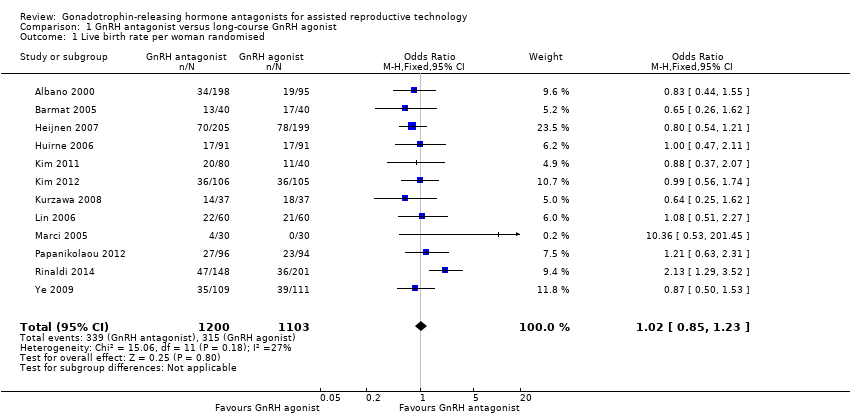 Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 1 Live birth rate per woman randomised. | ||||
| 2 Live birth rate per woman randomised ‐ minimal stimulation Show forest plot | 2 | 524 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.26] |
| Analysis 1.2  Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 2 Live birth rate per woman randomised ‐ minimal stimulation. | ||||
| 3 Live birth rate per woman randomised ‐ grouped by trigger Show forest plot | 12 | 2303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| Analysis 1.3  Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 3 Live birth rate per woman randomised ‐ grouped by trigger. | ||||
| 3.1 hCG trigger | 11 | 1899 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 3.2 Unknown trigger | 1 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.21] |
| 4 Ovarian hyperstimulation per woman randomised ‐ all women Show forest plot | 36 | 7944 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.51, 0.72] |
| Analysis 1.4 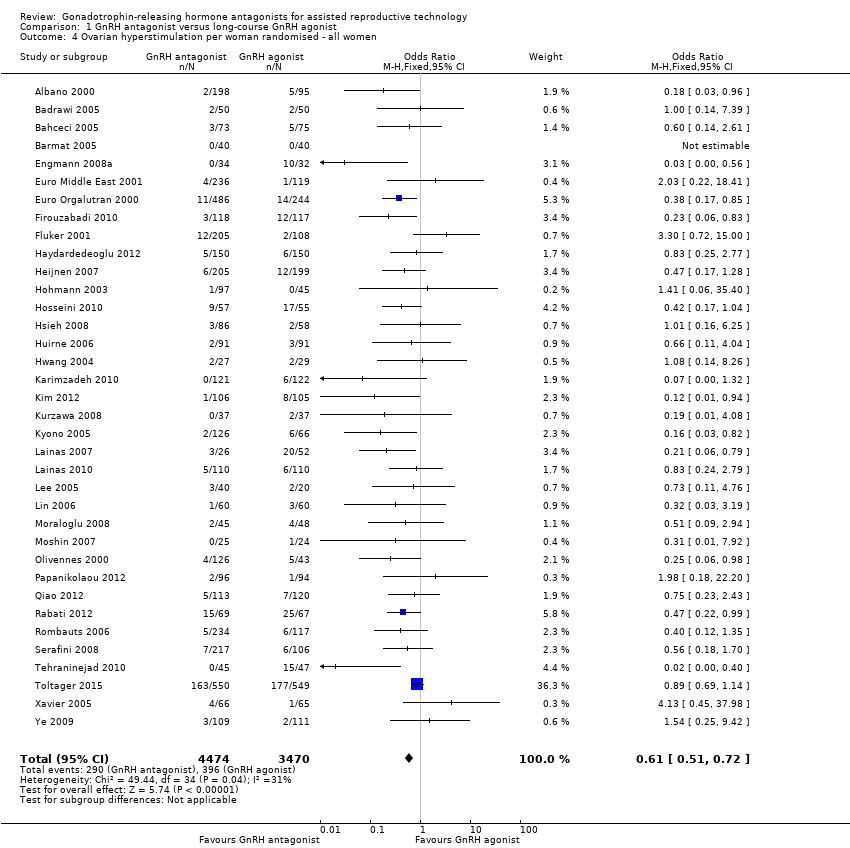 Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 4 Ovarian hyperstimulation per woman randomised ‐ all women. | ||||
| 5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe Show forest plot | 20 | 5141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.40, 0.69] |
| Analysis 1.5 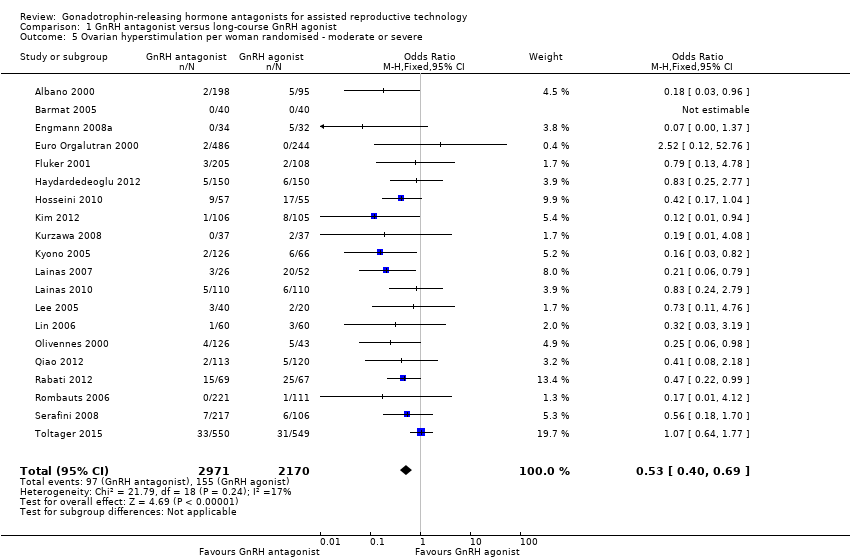 Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe. | ||||
| 6 Ongoing pregnancy rate per woman randomised ‐ all women Show forest plot | 37 | 8311 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.01] |
| Analysis 1.6 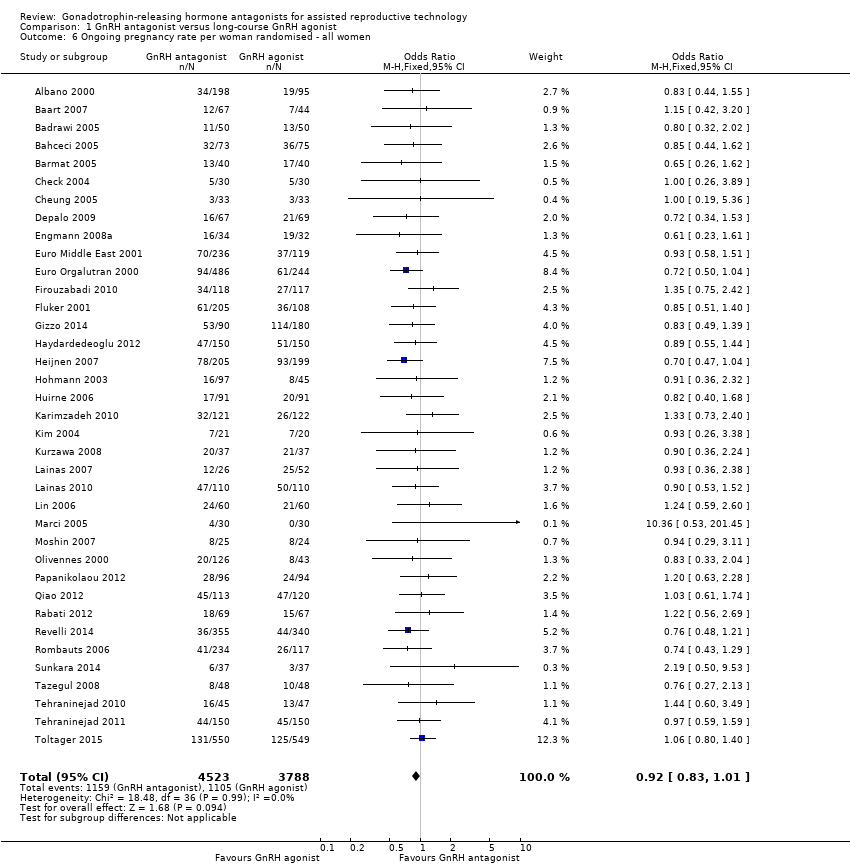 Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 6 Ongoing pregnancy rate per woman randomised ‐ all women. | ||||
| 7 Ongoing pregnancy rate per woman randomised ‐ minimal stimulation Show forest plot | 7 | 1456 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.18] |
| Analysis 1.7 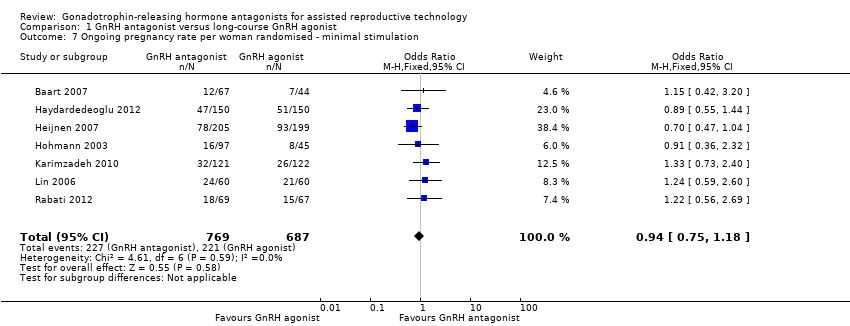 Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 7 Ongoing pregnancy rate per woman randomised ‐ minimal stimulation. | ||||
| 8 Ongoing pregnancy rate per women randomised ‐ grouped by trigger Show forest plot | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.8 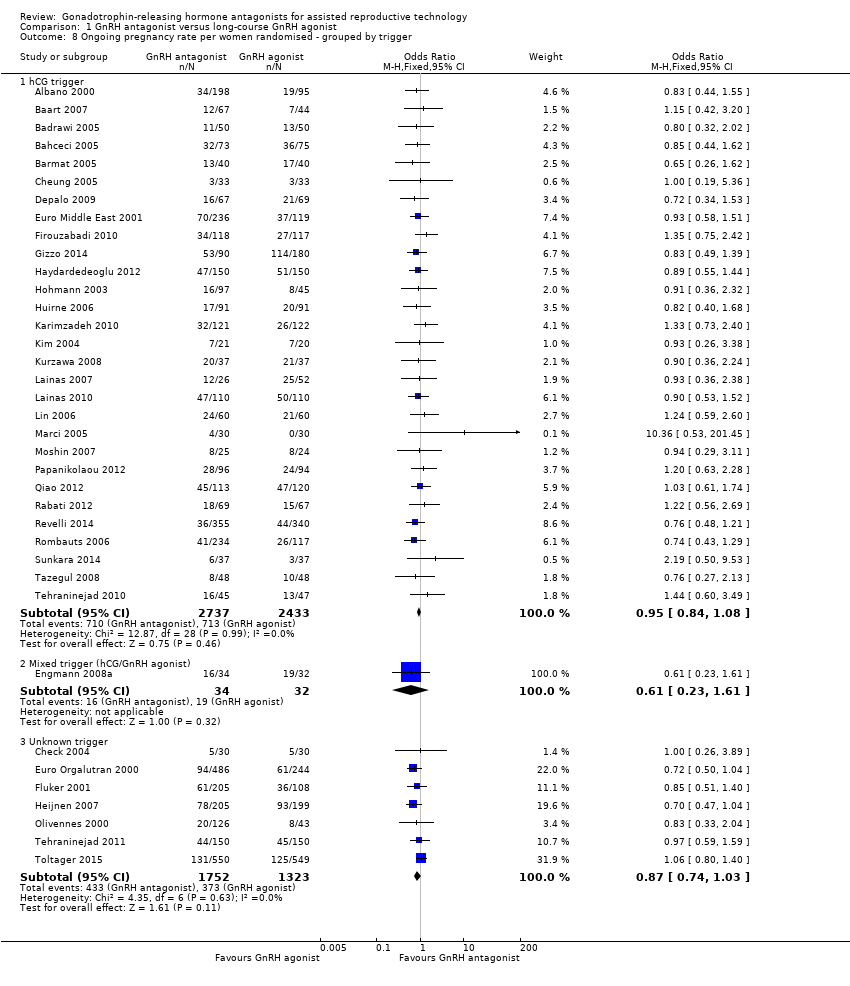 Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 8 Ongoing pregnancy rate per women randomised ‐ grouped by trigger. | ||||
| 8.1 hCG trigger | 29 | 5170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.08] |
| 8.2 Mixed trigger (hCG/GnRH agonist) | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.61] |
| 8.3 Unknown trigger | 7 | 3075 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 9 Clinical pregnancy rate per woman randomised ‐ all women Show forest plot | 54 | 9959 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| Analysis 1.9 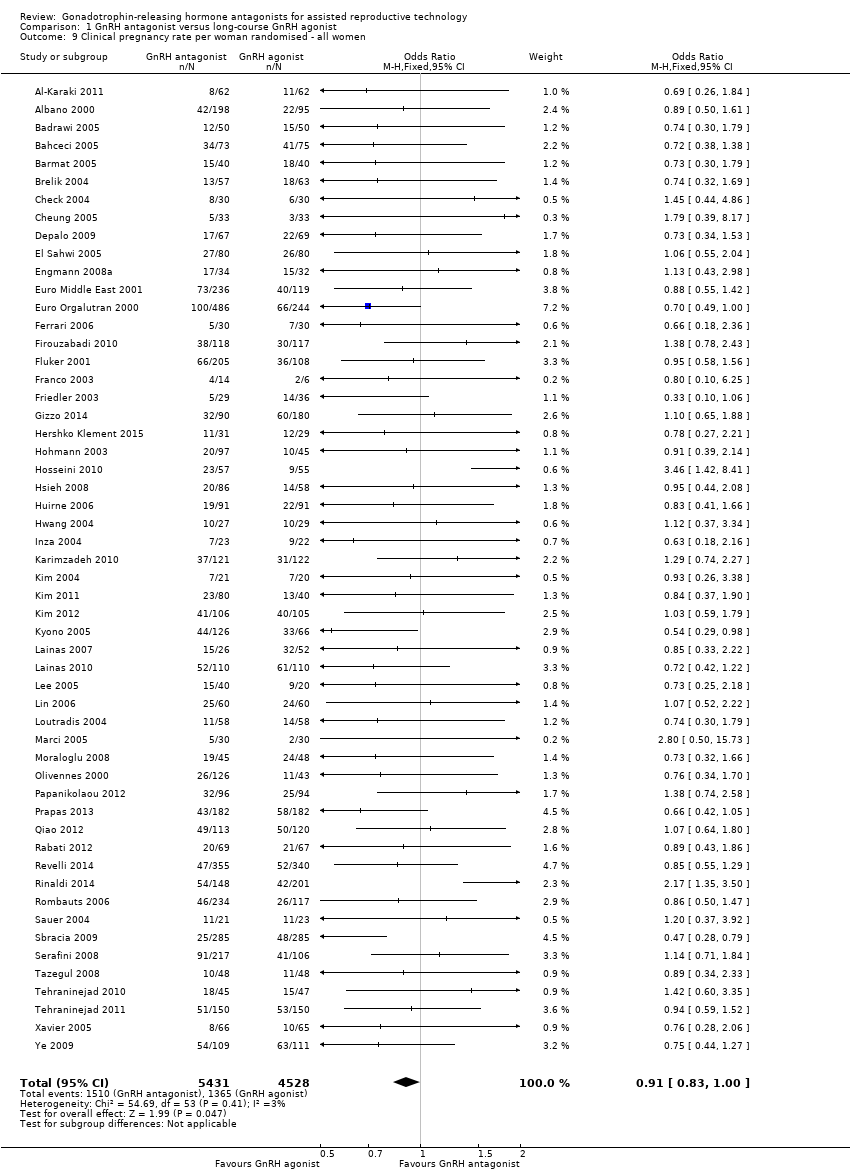 Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 9 Clinical pregnancy rate per woman randomised ‐ all women. | ||||
| 10 Clinical pregnancy rate per woman randomised ‐ minimal stimulation Show forest plot | 6 | 1102 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.15, 1.96] |
| Analysis 1.10 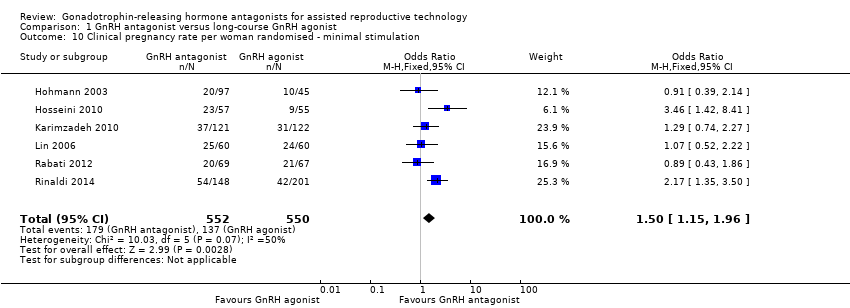 Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 10 Clinical pregnancy rate per woman randomised ‐ minimal stimulation. | ||||
| 11 Miscarriage rate per woman randomised Show forest plot | 34 | 7082 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| Analysis 1.11  Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 11 Miscarriage rate per woman randomised. | ||||
| 12 Miscarriage rate per clinical pregnancy Show forest plot | 34 | 2308 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.37] |
| Analysis 1.12  Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 12 Miscarriage rate per clinical pregnancy. | ||||
| 13 Cycle cancellation rate per woman randomised Show forest plot | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.13  Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 13 Cycle cancellation rate per woman randomised. | ||||
| 13.1 Cancellation due to high risk of OHSS | 19 | 4256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.69] |
| 13.2 Cancellation due to poor ovarian response | 25 | 5230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.65] |
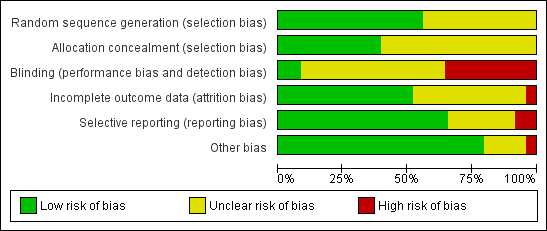
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.1 Live birth rate per woman randomised.
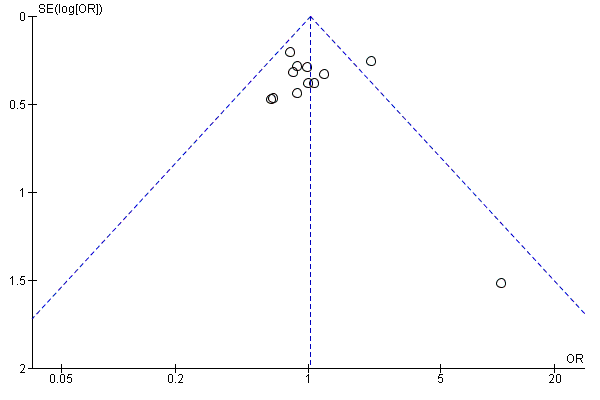
Funnel plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.1 Live birth rate per woman randomised.
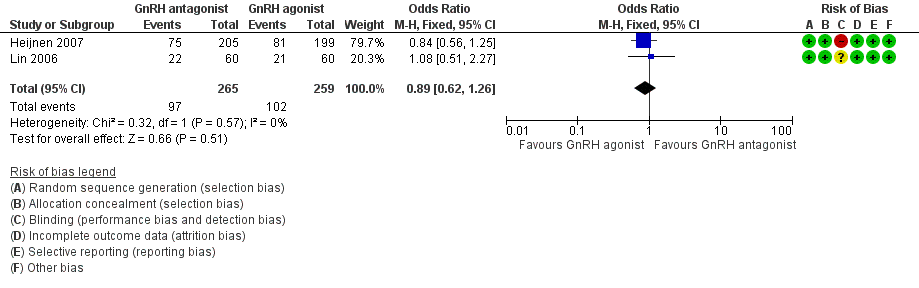
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.2 Live birth rate per woman randomised ‐ minimal stimulation.
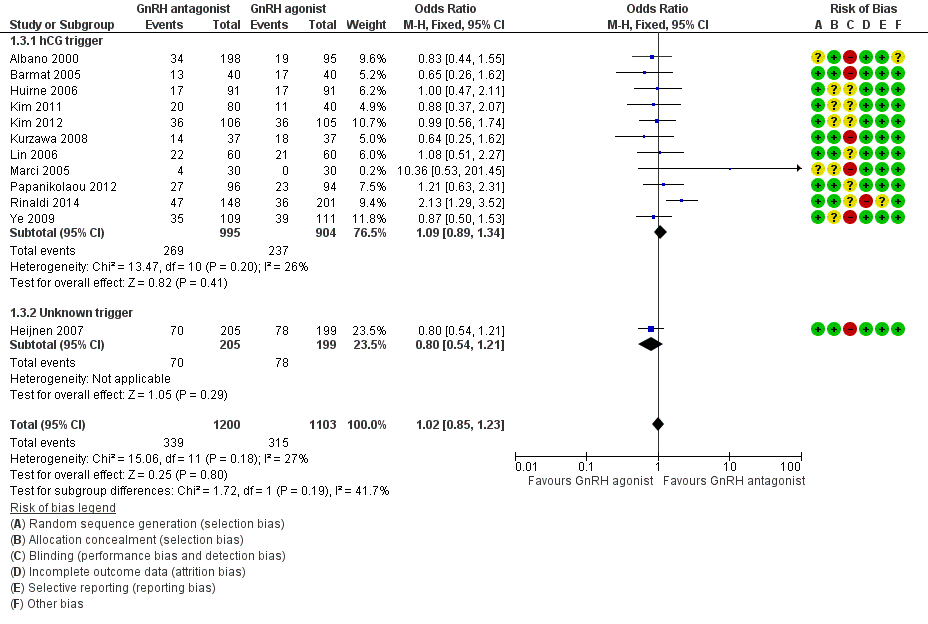
Forest plot of comparison: 1 GnRH antagonist versus long‐course GnRH agonist, outcome: 1.3 Live birth rate per woman randomised ‐ grouped by trigger.
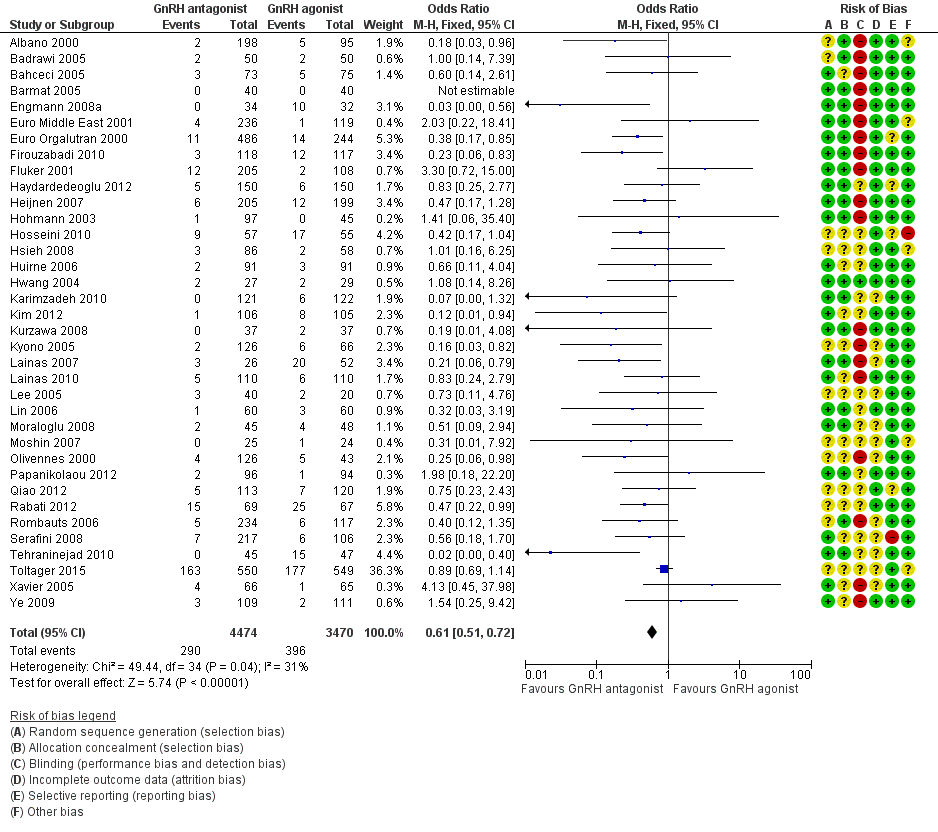
Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.4 Ovarian hyperstimulation per woman randomised ‐ all women.

Forest plot of comparison: 1 GnRH antagonist versus long course GnRH agonist, outcome: 1.5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 1 Live birth rate per woman randomised.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 2 Live birth rate per woman randomised ‐ minimal stimulation.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 3 Live birth rate per woman randomised ‐ grouped by trigger.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 4 Ovarian hyperstimulation per woman randomised ‐ all women.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 6 Ongoing pregnancy rate per woman randomised ‐ all women.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 7 Ongoing pregnancy rate per woman randomised ‐ minimal stimulation.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 8 Ongoing pregnancy rate per women randomised ‐ grouped by trigger.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 9 Clinical pregnancy rate per woman randomised ‐ all women.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 10 Clinical pregnancy rate per woman randomised ‐ minimal stimulation.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 11 Miscarriage rate per woman randomised.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 12 Miscarriage rate per clinical pregnancy.

Comparison 1 GnRH antagonist versus long‐course GnRH agonist, Outcome 13 Cycle cancellation rate per woman randomised.
| GnRH antagonist compared to long‐course GnRH agonist for assisted reproductive technology (ART) | ||||||
| Population: women undergoing assisted reproductive technology (ART) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Long course GnRH agonist | GnRH antagonist | |||||
| Live birth rate per woman randomised | 286 per 1000 | 290 per 1000 | OR 1.02 | 2303 | ⊕⊕⊕⊝ | |
| OHSS per woman randomised (any grade) | 114 per 1000 | 73 per 1000 | OR 0.61 | 7944 | ⊕⊕⊕⊝ | |
| Ongoing pregnancy rate per woman randomised | 293 per 1000 | 276 per 1000 | OR 0.92 | 8311 | ⊕⊕⊕⊝ | |
| Clinical pregnancy rate per woman randomised | 303 per 1000 | 283 per 1000 | OR 0.91 | 9959 | ⊕⊕⊕⊝ | |
| Miscarriage rate per woman randomised | 48 per 1000 | 49 per 1000 | OR 1.03 | 7082 | ⊕⊕⊕⊝ | |
| Cycle cancellation due to poor ovarian response | 64 per 1000 | 83 per 1000 | OR 1.32 | 5230 | ⊕⊝⊝⊝ | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Asymmetry of the funnel plot with small study effects in favour of GnRH antagonist | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Live birth rate per woman randomised Show forest plot | 12 | 2303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 2 Live birth rate per woman randomised ‐ minimal stimulation Show forest plot | 2 | 524 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.62, 1.26] |
| 3 Live birth rate per woman randomised ‐ grouped by trigger Show forest plot | 12 | 2303 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.85, 1.23] |
| 3.1 hCG trigger | 11 | 1899 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.89, 1.34] |
| 3.2 Unknown trigger | 1 | 404 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.54, 1.21] |
| 4 Ovarian hyperstimulation per woman randomised ‐ all women Show forest plot | 36 | 7944 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.51, 0.72] |
| 5 Ovarian hyperstimulation per woman randomised ‐ moderate or severe Show forest plot | 20 | 5141 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.40, 0.69] |
| 6 Ongoing pregnancy rate per woman randomised ‐ all women Show forest plot | 37 | 8311 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.83, 1.01] |
| 7 Ongoing pregnancy rate per woman randomised ‐ minimal stimulation Show forest plot | 7 | 1456 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.75, 1.18] |
| 8 Ongoing pregnancy rate per women randomised ‐ grouped by trigger Show forest plot | 37 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 hCG trigger | 29 | 5170 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.84, 1.08] |
| 8.2 Mixed trigger (hCG/GnRH agonist) | 1 | 66 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.23, 1.61] |
| 8.3 Unknown trigger | 7 | 3075 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.74, 1.03] |
| 9 Clinical pregnancy rate per woman randomised ‐ all women Show forest plot | 54 | 9959 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.83, 1.00] |
| 10 Clinical pregnancy rate per woman randomised ‐ minimal stimulation Show forest plot | 6 | 1102 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.50 [1.15, 1.96] |
| 11 Miscarriage rate per woman randomised Show forest plot | 34 | 7082 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
| 12 Miscarriage rate per clinical pregnancy Show forest plot | 34 | 2308 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.84, 1.37] |
| 13 Cycle cancellation rate per woman randomised Show forest plot | 34 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Cancellation due to high risk of OHSS | 19 | 4256 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.32, 0.69] |
| 13.2 Cancellation due to poor ovarian response | 25 | 5230 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.06, 1.65] |