Program kawalan merokok bagi keluarga dan penjaga untuk mengurangkan pendedahan kanak‐kanak kepada asap tembakau persekitaran
Abstract
Background
Children's exposure to other people's tobacco smoke (environmental tobacco smoke, or ETS) is associated with a range of adverse health outcomes for children. Parental smoking is a common source of children's exposure to ETS. Older children in child care or educational settings are also at risk of exposure to ETS. Preventing exposure to ETS during infancy and childhood has significant potential to improve children's health worldwide.
Objectives
To determine the effectiveness of interventions designed to reduce exposure of children to environmental tobacco smoke, or ETS.
Search methods
We searched the Cochrane Tobacco Addiction Group Specialised Register and conducted additional searches of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, PsycINFO, Embase, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Education Resource Information Center (ERIC), and the Social Science Citation Index & Science Citation Index (Web of Knowledge). We conducted the most recent search in February 2017.
Selection criteria
We included controlled trials, with or without random allocation, that enrolled participants (parents and other family members, child care workers, and teachers) involved in the care and education of infants and young children (from birth to 12 years of age). All mechanisms for reducing children's ETS exposure were eligible, including smoking prevention, cessation, and control programmes. These include health promotion, social‐behavioural therapies, technology, education, and clinical interventions.
Data collection and analysis
Two review authors independently assessed studies and extracted data. Due to heterogeneity of methods and outcome measures, we did not pool results but instead synthesised study findings narratively.
Main results
Seventy‐eight studies met the inclusion criteria, and we assessed all evidence to be of low or very low quality based on GRADE assessment. We judged nine studies to be at low risk of bias, 35 to have unclear overall risk of bias, and 34 to have high risk of bias. Twenty‐one interventions targeted populations or community settings, 27 studies were conducted in the well‐child healthcare setting and 26 in the ill‐child healthcare setting. Two further studies conducted in paediatric clinics did not make clear whether visits were made to well‐ or ill‐children, and another included visits to both well‐ and ill‐children. Forty‐five studies were reported from North America, 22 from other high‐income countries, and 11 from low‐ or middle‐income countries. Only 26 of the 78 studies reported a beneficial intervention effect for reduction of child ETS exposure, 24 of which were statistically significant. Of these 24 studies, 13 used objective measures of children's ETS exposure. We were unable to pinpoint what made these programmes effective. Studies showing a significant effect used a range of interventions: nine used in‐person counselling or motivational interviewing; another study used telephone counselling, and one used a combination of in‐person and telephone counselling; three used multi‐component counselling‐based interventions; two used multi‐component education‐based interventions; one used a school‐based strategy; four used educational interventions, including one that used picture books; one used a smoking cessation intervention; one used a brief intervention; and another did not describe the intervention. Of the 52 studies that did not show a significant reduction in child ETS exposure, 19 used more intensive counselling approaches, including motivational interviewing, education, coaching, and smoking cessation brief advice. Other interventions consisted of brief advice or counselling (10 studies), feedback of a biological measure of children's ETS exposure (six studies), nicotine replacement therapy (two studies), feedback of maternal cotinine (one study), computerised risk assessment (one study), telephone smoking cessation support (two studies), educational home visits (eight studies), group sessions (one study), educational materials (three studies), and school‐based policy and health promotion (one study). Some studies employed more than one intervention. 35 of the 78 studies reported a reduction in ETS exposure for children, irrespective of assignment to intervention and comparison groups. One study did not aim to reduce children's tobacco smoke exposure but rather sought to reduce symptoms of asthma, and found a significant reduction in symptoms among the group exposed to motivational interviewing. We found little evidence of difference in effectiveness of interventions between the well infant, child respiratory illness, and other child illness settings as contexts for parental smoking cessation interventions.
Authors' conclusions
A minority of interventions have been shown to reduce children's exposure to environmental tobacco smoke and improve children's health, but the features that differentiate the effective interventions from those without clear evidence of effectiveness remain unclear. The evidence was judged to be of low or very low quality, as many of the trials are at a high risk of bias, are small and inadequately powered, with heterogeneous interventions and populations.
PICOs
Ringkasan bahasa mudah
Bolehkah intervensi untuk ibu bapa dan orang yang menjaga kanak‐kanak mengurangkan pendedahan kanak‐kanak kepada asap tembakau?
Latar belakang
Kanak‐kanak yang terdedah kepada asap rokok (asap tembakau persekitaran) berisiko lebih besar mengalami masalah paru‐paru, jangkitan, dan komplikasi serius termasuklah sindrom kematian bayi mengejut. Mencegah pendedahan kepada asap rokok dalam kalangan bayi dan kanak‐kanak mungkin memperbaiki kesihatan kanak‐kanak di seluruh dunia secara signifikan. Ibu bapa yang merokok adalah sumber yang lazim pendedahan rokok bagi kanak‐kanak. Kanak‐kanak yang lebih berusia juga berisiko terhadap pendedahan kepada asap rokok di persekitaran penjagaan kanak‐kanak atau persekitaran pendidikan.
Ciri‐ciri kajian
Kami mencari enam pangkalan data untuk penyelidikan yang relevan. Ini adalah kemas kini ulasan terbitan terdahulu dan tarikh carian yang terbaru adalah Februari 2017. Kami menemui 78 kajian tentang kesan intervensi yang menyasarkan keluarga dan penjaga dengan matlamat mengurangkan pendedahan kanak‐kanak kepada asap tembakau. Kajian‐kajian ini memasukkan ibu bapa dan ahli keluarga lain, pekerja penjagaan kanak‐kanak, dan guru yang terlibat dalam penjagaan dan pendidikan bayi dan kanak‐kanak (dari lahir hingga 12 tahun), dan menggunakan pelbagai intervensi, termasuk kaunseling, nasihat ringkas, dan bahan‐bahan pendidikan.
Keputusan utama
Hanya 26 kajian melaporkan intervensi berjaya mengurangkan pendedahan kanak‐kanak kepada asap tembakau. Kajian‐kajian ini menggunakan pelbagai intervensi. Sembilan kajian menggunakan kaedah kaunseling yang lebih intensif atau wawancara bermotivasi, tetapi dalam kajian yang lain, intervensi‐intervensi jenis ini tidak berkesan. Daripada 52 kajian yang tidak menunjukkan pengurangan yang signifikan dalam pendedahan asap tembakau kepada kanak‐kanak, 19 kajian menggunakan kaedah kaunseling intensif atau wawancara bermotivasi. Satu kajian berjaya mengurangkan simptom asma kanak‐kanak dengan menggunakan wawancara bermotivasi. Ulasan ini tidak menunjukkan sama ada sebarang intervensi tertentu mengurangkan ibu bapa merokok dan pendedahan asap kepada kanak‐kanak lebih berkesan daripada yang lain.
Kualiti bukti
Kualiti bukti adalah antara rendah ke sangat rendah. Kajian masa depan harus bertujuan menyediakan bukti berkualiti lebih baik dengan menangani masalah reka bentuk kajian, lebih ramai peserta, dan menerangkan intervensi dengan lebih terperinci.
Authors' conclusions
Summary of findings
| Community‐based interventions for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
| Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: community Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 | Quality of the evidence | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 3 to 12 months | Of 7 studies in this group, 3 found that the intervention group was significantly more likely than the control group to implement full home smoking bans. One study found that the geometric mean hair nicotine level in the intervention group significantly decreased from 0.30 ng/mg to 0.23 ng/mg (P = 0.024), but not in the control group. Four studies found no significant differences in the change in cotinine levels between intervention and control groups. | 2880 | +‐‐‐ VERY LOW3 | |
| Multi‐comoponent, education‐based interventions assessed with biochemical validation of ETS exposure length of follow‐up: 6 months | One study, with similar children’s urinary cotinine levels at baseline, found that cotinine levels were significantly lower (Z = ‐3.136; P = 0.002) in the intervention group (1.29 ng/mL) than in the control group (1.78 ng/mL) at 6 month follow‐up. The other study found no significant differences between intervention and control groups in child urine cotinine levels. | 307 | +‐‐‐ VERY LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 1 to 12 months | Of the 6 studies in this group, 3 found significantly greater reductions in cotinine levels in the intervention compared with the control group. Two studies found that the intervention group was significantly more likely to implement home smoking bans. Two studies found no significant intervention impacts. | 1001 | +‐‐‐ VERY LOW5 | |
| Telephone counselling assessed with biochemical validation of ETS exposure length of follow‐up: 9 months | One study found no significant difference in the proportion of children with low urinary cotinine levels (< 10 ng/mL) amongst parents receiving telephone counselling or a note regarding their child’s cotinine result. | 347 | ++‐‐ LOW6 | |
| ETS: environmental tobacco smoke | ||||
| 1 Not all studies reported length of follow‐up; length given based on those that reported. 2 Not all studies reported numbers of participants; number provided based on those that reported. 3 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 4 Downgraded one level due to risk of bias: one of two studies at high risk of bias. Downgraded two levels due to inconsistency: one study detected an effect and one did not; studies were clinically heterogeneous. 5 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 6 Downgraded one level due to risk of bias: one study at unclear risk of bias. Downgraded one level due to imprecision: only 186 participants with measured outcomes at nine‐month follow‐up. | ||||
| Interventions in the ill‐child setting for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
| Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: healthcare ‐ ill‐child setting Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 | Quality of the evidence | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 5 to 12 months | Three studies found no significant differences between intervention and control groups. | 746 (3 studies) | +‐‐‐ VERY LOW3 | |
| Multi‐component, education‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 6 to 13 months | One study reported significantly lower child's ETS exposure at home by any smoker at 12 months' follow‐up (52% vs 58%; P = 0.03). Six studies found no significant differences between intervention and control groups. | 2936 (7 studies) | +‐‐‐ VERY LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 3 to 18 months | Eight studies appeared to show intervention benefits based on self‐reported ETS exposures but no significant differences between intervention and control groups in objective measures of exposure (e.g. cotinine). | 1835 (8 studies) | +‐‐‐ VERY LOW5 | |
| Telephone counselling | No studies examined telephone counselling delivered in the ill‐child setting and measured ETS exposure. | |||
| Brief interventions Assessed with presence of home and car smoking ban length of follow‐up: 24 weeks | One study showed no significant differences between intervention and control groups in changed smoking policy: OR 2.0 (95% CI 0.166 to 24.069). | 100 (1 study) | +‐‐‐ VERY LOW6 | |
| GRADE Working Group grades of evidence | ||||
| 1 Not all studies reported length of follow‐up; length given based on those that reported. 2 Not all studies reported numbers of participants; number provided based on those that reported. 3 Downgraded one level due to risk of bias: two studies at unclear risk of bias. Downgraded one level due to imprecision. Downgraded one level due to indirectness: all studies were set in the USA and cannot be generalised to low income countries where smoking is more prevalent. 4 Downgraded two levels due to risk of bias: five of seven studies at high or unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 5 Downgraded two levels due to risk of bias: all eight studies at high or unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 6 Downgraded two levels due to risk of bias: only study was at high risk of bias. Downgraded one level due to imprecision: small study with a small number of events and wide confidence interval. | ||||
| Interventions in the well‐child setting for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
| Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: health care ‐ well‐child setting Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 | Quality of the evidence | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 2 to 12 months | One study found significant reduction in ETS exposure at home in the intervention group at age 6 years, but only on per‐protocol analysis (OR 0.71, 95% CI 0.59 to 0.87). One study found an increase in smoking bans in the home (19.3%) and in the car (7%) after 8 weeks' follow‐up in the intervention group, but not in the comparison group (2.5% increase in home ban and 0% change in car ban). One study found no significant difference between intervention and control groups in children’s urinary cotinine levels. | 8005 (3 studies) | +‐‐‐ VERY LOW3 | |
| Multi‐component, education‐based interventions assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 2 to 12 months | One study found that maternal self‐reported smoking at home around the infant was significantly less in the intervention group (8.6%) than in the control group (23.8%) (P < 0.05). Three studies found no evidence of effect of the intervention. | 1401 (4 studies) | ++‐‐ LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 3 to 90 months | One study found significantly greater reductions in geometric mean urinary cotinine in the intervention group (decrease from 48.72 ng/mg to 28.68 ng/mg) compared to the control group (decrease from 40.43 to 36.32 ng/mg). In addition, the intervention group had a significantly greater increase in the proportion of households with smoking bans at home (15% to 33.3%) compared to the control group (11.5% to 19.5%). One study found a significantly beneficial reduction in kitchen and TV room air nicotine levels in the intervention group than in the control group (P < 0.05). One study found no difference in serum cotinine concentrations between the intervention and control groups. | 1483 (3 studies) | ++‐‐ LOW5 | |
| Telephone counselling assessed with self‐report length of follow‐up: 6 months | One study found a greater proportion with partial home smoking bans in the intervention group (62.7%) than in the control group (56.4%), as well as a higher biochemically validated quit rate for the intervention group (10.6%) than for the control group (4.5%) at 6 months. | 952 (1 study) | ++‐‐ LOW6 | |
| Brief interventions assessed with self‐report length of follow‐up: not specified | One study found no significant difference in home (OR 1.04, 95 CI 0.47 to 2.28) or car smoking bans (OR 1.47, 95 CI 0.69 to 3.11) between intervention and control groups. | 218 (1 study) | +‐‐‐ VERY LOW7 | |
| CI: confidence interval; OR: odds ratio | ||||
| 1 Not all studies reported length of follow‐up; length given based on those that reported. 2 Not all studies reported numbers of participants; number provided based on those that reported. 3 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 4 Downgraded one level due to risk of bias: one study was at high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 5 Downgraded one level due to risk of bias: two of three studies at unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 6 Downgraded one level due to risk of bias: included study at unclear risk of bias. Downgraded one level due to indirectness: ETS exposure was measured indirectly as reported smoking restrictions in home. 7 Downgraded one level due to risk of bias: included study at unclear risk of bias. Downgraded one level due to indirectness: ETS exposure was measured indirectly as reported smoking restrictions in home and car. Downgraded one level due to imprecision: one study with a small number of participants and events. | ||||
Background
Active smoking has been recognised as harmful to the smoker for over six decades, since the landmark Doll and Hill publication (Doll 1950), but it was not until 1974 that the medical literature first discussed parental smoking, exposure to environmental tobacco smoke (ETS), and the effects of ETS on children (Harlap 1974). Overwhelming evidence indicates that parental smoking is associated with a range of adverse health effects for children (NHMRC 1997). Perhaps its most obvious association is with increased risk, increased severity, and greater likelihood of admission to hospital of children with lower and upper respiratory tract disease (Strachan 1997; Strachan 1998, respectively). An increasing body of evidence describes an association between parental smoking and increased risk of serious bacterial infections such as meningitis among children (Iles 2001). In addition, Lam 2001 reported that ETS exposure increases health service use and costs, and Chiswell 2017 described associated poorer surgical outcomes.
Furthermore, parental smoking confers a significantly increased risk of sudden infant death syndrome (SIDS) (Golding 1997). This effect is present regardless of which parent is the smoker (Blair 1999), and it is the strongest modifiable risk factor for SIDS. In addition, research across several continents over the last two decades has found that children of smokers have an increased risk of uptake in adolescence, perhaps as a result of role modelling and/or increased access to cigarettes (Mays 2014). There is also an increased risk of respiratory symptoms persisting into adulthood among children exposed to ETS from their parents or carers, but who do not themselves take up smoking later in life (Pugmire 2014).
Parental smoking is a common but preventable source of infant and childhood morbidity. The World Health Organization (WHO) has identified the need to reduce parental smoking as a key element of action to encourage health and development in early childhood, particularly among those living in difficult social and economic circumstances (WHO 1999; WHO 2013). In some countries, strong relationships between socioeconomic status and environmental quality are evident (Moore 2012), and strategies to reduce smoking and improve child health outcomes must be underpinned by recognition of finite resources and the limited control that some individuals and families have over environmental and social situations.
Infants' and toddlers' exposure to smoking occurs primarily within the home environment, as this is where they spend most of their time. Older children may also be exposed to smoking in a variety of child care and educational settings in which they spend their time. As children increase their time spent in commercial and informal child care settings, the importance of child care workers' behaviours increases. Similarly, environments in which young children are exposed extend beyond the home and include shopping centres, meeting places, and other social environments.
Tobacco cessation strategies and interventions to reduce ETS have had mixed success, often providing small benefits on an individual level (Rosen 2014). Systematic reviews have previously demonstrated that individual counselling increases cessation rates (Lancaster 2017), and that simple advice from a physician may have a positive effect in triggering quit attempts (Stead 2013). In relation to children's exposure in utero and during the early years, smoking cessation interventions for pregnant women can be effective in reducing smoking (Coleman 2015; Chamberlain 2017). Although smoke‐free legislation in England has contributed to the 79% reduction in children’s ETS exposure since 1998 (Jarvis 2015), variability is ongoing, and children in families from lower socioeconomic status remain at greater risk of ETS exposure (Moore 2012). Globally, 80% of the world's smokers live in low‐ and middle‐income countries (WHO 2014), which have demonstrated less political will to enforce smoke‐free legislation (Pugmire 2017).
Objectives
To determine the effectiveness of interventions designed to reduce exposure of children to environmental tobacco smoke, or ETS.
Methods
Criteria for considering studies for this review
Types of studies
Controlled trials with or without random allocation.
Types of participants
People (parents and other family members, child care workers, and teachers) involved in the care and education of infants and young children (from birth to 12 years of age).
Types of interventions
We included all mechanisms for the reduction of children's ETS exposure, including smoking prevention, smoking cessation, and any other tobacco control programmes targeting the participants described above. These included health promotion, social‐behavioural therapy, technology, and educational and clinical interventions.
We included studies in which the primary aim was to reduce children's exposure to ETS (thereby preventing adverse health outcomes), but where secondary outcomes included reduction or cessation of familial/parental/carer smoking, or changes in infant and child health measures. We also included studies where the primary outcome was reduction or cessation of familial/parental/carer smoking, resulting in reduced exposure for children.
We excluded studies on uptake of smoking by minors.
We did not restrict inclusion based on who delivered the programmes. These could include researchers, general practitioners, midwives, paediatricians, community and hospital nurses, health promotion agencies, tobacco control and anti‐cancer organisations, or health departments.
Types of outcome measures
The primary outcome measures were children's exposure to tobacco smoke, child illness and health service utilisation, and the smoking behaviours of children's parents and carers. We included studies where the only outcome was parental or carer smoking status.
We used biological verification of exposure to or absorption of ETS as the 'gold standard', but we did not require this as an inclusion criterion. Where biological verification of exposure/absorption conflicted with the parental report of exposure, we regarded the biologically verified result as correct.
Outcomes for children
-
Exposure to ETS: biochemical measures of children's exposure to ETS based on air monitoring for levels of nicotine or other measures of ETS (including parent‐reported behaviour change, as described in the next section)
-
Absorption of ETS: biochemical measures of children's absorption of ETS through cotinine in urine, blood, saliva, or hair
-
Frequency of childhood illness events, respiratory problems (changes in lung function or symptom scores)
-
Use of health services: admission to hospital; frequency of use of general practitioners (GPs); frequency of medication use
Outcomes for parents and carers
-
Behaviour change in relation to children's exposure to ETS: We noted any reported bans or restrictions on smoking at home or in other environments or in designated smoking areas outside the home
-
Smoking behaviour, including cessation, reduction, or uptake, using biochemically validated measures of smoking behaviour (e.g. thiocyanates; cotinine levels in blood, urine, or saliva), or self‐report
-
Maternal postpartum smoking status
-
Costs and cost‐effectiveness associated with interventions and outcomes
We reported biochemical confirmation of parental self‐reported quit status or changes in behaviour such as moves to smoke outside, but we did not exclude studies without this measurement. Most studies did not use biochemical validation. However, there is conflicting evidence regarding the validity of self‐report of smoking status. Some trial authors suggest that self‐report is reasonably accurate in community settings (Dwyer 1986; Velicer 1992; Patrick 1994), whereas others suggest that parental self‐reports of smoke consumption and ETS are frequently underestimated (Jarvis 1987; Ford 1997; Matthews 1999). For example, in clinical situations where a clinician is the interviewer, social bias may influence the report towards the socially desired response.
Researchers and clinicians often prefer to use levels of nicotine or its breakdown products, by contrast, as a measure of real reductions in smoking or ETS. Cotinine is a metabolic breakdown product of nicotine with a half‐life of about one day (Haley 1983). Its half‐life is longer in non‐smokers such as infants and young children (Idle 1990). Smoke exposure can be detected by hair cotinine (Zahlsen 1994; Nafstad 1997; Al‐Delaimy 2002a; Al‐Delaimy 2002b), and absorption by urinary cotinine (Jarvis 1984; Bakoula 1995). Long‐term exposure is best estimated by hair cotinine, whereas urinary cotinine is more informative of short‐term exposure. Saliva cotinine approximates to blood cotinine concentrations, and collection is simple and non‐invasive.
Search methods for identification of studies
This is the fourth update of this review. Search methods for the previous searches are described in previously published versions of this review (Roseby 2002; Priest 2008; Baxi 2014).
Nia Wyn Roberts, Outreach Librarian, Bodleian Health Care Libraries, updated the search. We searched the Cochrane Central Register of Controlled Trials (Issue 2011) in the Cochrane Library, MEDLINE (OvidSP) (1948 to the present), Embase (OvidSP) (1974 to the present), the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EbscoHOST) (1980 to the present), PsycINFO (OvidSP) (1967 to the present), and the Education Resource Information Center (ERIC) (ProQuest) (1966 to the present). In June 2011, we conducted a search for articles from 2007 to 2011. The Trial Search Co‐ordinator searched the CochraneTobacco Addiction Group Specialised Register. We conducted the most recent search in February 2017.
We obtained and reviewed reports of all references identified as possibly describing randomised controlled trials (RCTs) or controlled trials (CTs), and we checked the reference lists of all identified RCTs and CTs to identify potentially relevant citations. We made enquiries regarding other known published and unpublished studies so that we could include these results in our review.
We have presented search strategies for the key databases in Appendix 1 (MEDLINE); Appendix 2 (Embase); Appendix 3 (CINAHL); Appendix 4 (PsycINFO); Appendix 5 (ERIC); and Appendix 6 (the Cochrane Library).
Data collection and analysis
Two review authors (BB and MS) independently screened studies for inclusion using Covidence. Three review authors independently undertook assessment of quality and extraction of included study details and results. For this update, BB reviewed all studies; and MS, RB, and RR each reviewed one‐third of the studies and compared results. We created a data extraction spreadsheet in Microsoft Excel.
We extracted information on methods, participants, intervention and control conditions, and outcomes. We were particularly interested in aspects of intervention development that may have contributed to a stronger, more appropriate or sustained intervention. We extracted information on the theory underlying the intervention development and content, process indicators and descriptions of community consultation and/or participation in the planning and implementation of the intervention, incentives (if present), and concerns regarding intervention programmes. We also recorded any information about costs, either in terms of evaluations of cost‐effectiveness, or simply where costs were mentioned. Where possible, we examined outcomes by gender, age, and socioeconomic status.
We resolved differences between reviewers' screening and extraction results by discussion or by consultation with a third review author. Given the heterogeneity of study design and characteristics, we considered a quantitative estimate of effect to be inappropriate and therefore provided a narrative synthesis.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for all included studies, including those included in previous versions of this review. We categorised risk of bias as high, low, or unclear for randomisation, allocation concealment, incomplete data, blinding of outcome assessment, and other bias, in accordance with methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved differences by discussion.
Sequence generation (checking for possible selection bias)
We have described the methods used to generate the allocation sequence and have assessed these methods as having:
-
low risk of bias (any truly random process, e.g. random number table, computer random number generator);
-
high risk of bias (any non‐random process, e.g. odd or even date of birth, hospital or clinic record number); or
-
unclear risk of bias (insufficient information provided with which to judge).
Allocation concealment (checking for possible selection bias)
We have described the methods used to conceal the allocation sequence in sufficient detail to determine whether intervention allocation could have been foreseen in advance of, or during, recruitment, or changed after assignment. We have assessed these methods as having:
-
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
-
high risk of bias (open allocation; unsealed or non‐opaque envelopes; alternation; date of birth); or
-
unclear risk of bias (insufficient information provided with which to judge).
Blinding (checking for possible detection bias)
We have described the methods reported, if any, to blind study participants and personnel from knowledge of which intervention a participant received. With educational interventions (such as those assessed in this review) it is often not possible to blind participants to group allocation, and hence we did not evaluate blinding based on performance bias but rather based solely on the potential to introduce detection bias. It is possible for outcome assessors to be blinded to group allocation and we have noted where there was partial blinding. We have assessed study methods as having high risk of bias, low risk of bias, or unclear risk.
When investigators objectively measured findings (e.g. biochemical validation, household air nicotine monitors), we assessed blinding as adequate to prevent detection bias.
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, or protocol deviations)
Within each included study, we have described for each outcome or class of outcomes the completeness of data, including attrition and exclusions from analysis. We have noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups.
Other bias (e.g. selective reporting bias)
We have noted any other potential sources of bias that were not related to the four sources discussed above.
Overall risk of bias
We made explicit judgements about whether studies were at high, moderate, or low risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to the specific types of bias discussed above, we assessed the likely magnitude and direction of bias, and whether we considered it likely to impact study findings.
Results
Description of studies
We included 78 studies in this review, 21 of which were identified in the most recent update; see the search study flow diagram in Figure 1 (Abdullah 2015; Blaakman 2015; Borrelli 2016; Chen 2016; Collins 2015; Cooper 2014; Daly 2016; Eakin 2014; Hafkamp‐de 2014; Harutyunyan 2013; Joseph 2014; Kegler 2015; Nicholson 2015; Ortega 2015; Pollak 2015; Schuck 2014; Streja 2014; Ulbricht 2014; Walker 2015; Wang 2015; Yucel 2014). We have summarised the characteristics of included studies below, and have provided further detail in the Characteristics of included studies table.

Study flow diagram.
We identified five additional studies for which outcome data are not yet available; we identified three of these in the previous update (Johnston 2010; Rosen 2011; Wagener 2012; Hutchinson 2013; Risica 2016). We have provided information about these ongoing studies in the Characteristics of ongoing studies table.
We have listed 35 studies as excluded. The most common reasons for exclusion were study design; participants not meeting inclusion criteria; outcomes not related to environmental tobacco smoke exposure; and lack of outcome data. Further information is available in the Characteristics of excluded studies table.
Intervention setting
One study evaluated outcomes for smoking mothers who called a telephone smoking cessation assistance counselling service (Davis 1992), and another recruited participants from callers to a 2‐1‐1 service (Kegler 2015). Seven studies introduced interventions in a school setting (Zhang 1993; Elder 1996; Ekerbicer 2007; Halterman 2011; Schuck 2014; Wang 2015; Chen 2016). Five further studies introduced interventions in other community settings (Conway 2004; Herbert 2011; Prokhorov 2013; Eakin 2014; Ulbricht 2014; see Characteristics of included studies for futher details).
Eight studies recruited from general healthcare settings (Harutyunyan 2013; Streja 2014; Yucel 2014; Abdullah 2015; Blaakman 2015; Collins 2015; Pollak 2015; Walker 2015; see Characteristics of included studies for futher details). Twenty‐five studies took place in well‐child healthcare settings, and recruited participants postnatally, at well‐child health visits or at infant immunisation clinics. Fourteen of these studies were peripartum, recruiting participants via maternity hospitals, from their records, or through midwives and general practitioners (Woodward 1987; Greenberg 1994; Severson 1997; Armstrong 2000; Van't Hof 2000; Emmons 2001; Ratner 2001; Pulley 2002; Schonberger 2005; Wiggins 2005; Culp 2007; French 2007; Hannover 2009; Cooper 2014). Chilmonczyk 1992, Vineis 1993, Eriksen 1996, Fossum 2004, Zakarian 2004, Abdullah 2005, Kallio 2006, Winickoff 2010, Baheiraei 2011, Hafkamp‐de 2014, Joseph 2014, and Daly 2016 used well‐child health check visits to a doctor or maternal child health nurse. Chellini 2013 recruited from hospital and public health facility waiting rooms, as well as from supermarkets.
Twenty‐six studies reported interventions conducted in an ill‐child healthcare setting. Fourteen of these identified families through their children's respiratory problems (Hughes 1991; McIntosh 1994; Wahlgren 1997; Irvine 1999; Wilson 2001; Hovell 2002; Krieger 2005; Ralston 2008; Borrelli 2010; Butz 2011; Halterman 2011 (recruited from school rather than healthcare setting); Wilson 2011; Stotts 2012; Borrelli 2016). Investigators conducted 10 studies in non‐respiratory ill‐child healthcare settings (Groner 2000; Hovell 2000; Wakefield 2002; Kimata 2004; Chan 2005; Chan 2006a; Hovell 2009; Phillips 2012; Tyc 2013; Nicholson 2015). Patel 2012 and Ralston 2013 targeted children presenting to the emergency department, approximately 40% of whom had a respiratory presenting complaint. Hovell 2000 and Hovell 2009 recruited mothers from a Special Supplemental Nutrition Program for Women, Infants, and Children, and looked at the effectiveness of counselling on smoking rates and children's ETS exposure among women of low income, high risk, and ethnically diverse backgrounds.
Two additional studies conducted in paediatric clinics did not specify whether they were conducted in the context of well‐child or ill‐child health visits (Curry 2003; Nuesslein 2006), and Yilmaz 2006 recruited children visiting paediatric clinics for treatment of primary conditions or for a well‐child visit.
Main target of intervention
Children's ETS exposure can be reduced by encouraging avoidance of children's exposure to cigarettes smoked, for example, by moving the child or the smoker to a different location, reducing the number of cigarettes smoked by the parent or carer, or having the smoker cease smoking altogether. The aims of studies identified by this review were heterogeneous. Here, we consider only smoking and ETS targets; we do not describe other intervention components, such as healthy eating (e.g. Elder 1996), asthma management (e.g. Hughes 1991), or household safety (e.g. Culp 2007).
Of the 78 included studies, 18 aimed solely for parental or carer smoking cessation or reduction (Vineis 1993; Zhang 1993; Severson 1997; Groner 2000; Emmons 2001; Wakefield 2002; Curry 2003; Kimata 2004; Chan 2005; Wiggins 2005; Kallio 2006; Nuesslein 2006; Ralston 2008; Borrelli 2010; Ralston 2013; Cooper 2014; Pollak 2015; Borrelli 2016). Twenty‐five studies aimed solely for reducing children's exposure to cigarettes smoked (Chilmonczyk 1992; Davis 1992; Elder 1996; Wahlgren 1997; Hovell 2000; Wilson 2001; Pulley 2002; Baheiraei 2011; Butz 2011; Herbert 2011; Wilson 2011; Stotts 2012; Chellini 2013; Prokhorov 2013; Tyc 2013; Harutyunyan 2013; Hafkamp‐de 2014; Schuck 2014; Streja 2014; Ulbricht 2014; Collins 2015; Kegler 2015; Nicholson 2015; Ortega 2015; Chen 2016), while 30 studies aimed for a combination of parental or carer cessation, reduction, or avoidance (Woodward 1987; Hughes 1991; Greenberg 1994; McIntosh 1994; Eriksen 1996; Irvine 1999; Armstrong 2000; Hovell 2000; Conway 2004; Fossum 2004; Zakarian 2004; Abdullah 2005; Krieger 2005; Schonberger 2005; Chan 2006a; Yilmaz 2006; Culp 2007; Ekerbicer 2007; Hovell 2009; Winickoff 2010; Halterman 2011; Patel 2012; Eakin 2014; Joseph 2014; Yucel 2014; Abdullah 2015; Blaakman 2015; Walker 2015; Wang 2015; Daly 2016). Five studies aimed to prevent reuptake of smoking postpartum (Van't Hof 2000; Ratner 2001; French 2007; Hannover 2009; Phillips 2012).
All studies aimed to achieve changes in behaviour in some way to reduce child ETS exposure. Eleven studies did not expressly include an educational or knowledge‐building component in their interventions but instead targeted change in attitudes and behaviours (Chilmonczyk 1992; Zhang 1993; Wahlgren 1997; Hovell 2000; Curry 2003; Zakarian 2004; Chan 2005; Nuesslein 2006; Cooper 2014; Abdullah 2015; Ortega 2015).
Location of studies
Most studies were reported from high‐income countries. Forty‐five studies were from North America, with 42 from the USA and three from Canada. Four studies were from Australia, and one was conducted in both Australia and New Zealand (Walker 2015). Three studies were from each of the UK, Germany, and the Netherlands. Two studies were from Italy (Vineis 1993; Chellini 2013). One study was reported from each of Finland (Kallio 2006), Japan (Kimata 2004), Sweden (Fossum 2004), Norway (Eriksen 1996), Taiwan (Chen 2016), and Spain (Ortega 2015). Fifteen of the studies conducted in high‐income countries specifically targeted disadvantaged, low‐income, and/or culturally diverse populations. Eleven studies were reported from low‐ or middle‐income countries, with six from China (Zhang 1993; Abdullah 2005; Chan 2005; Chan 2006a; Abdullah 2015; Wang 2015), three from Turkey (Yilmaz 2006; Ekerbicer 2007; Yucel 2014), and one from each of Iran (Baheiraei 2011) and Armenia (Harutyunyan 2013).
Participants
Twenty‐four studies targeted mothers only. Hovell 2009, Yucel 2014, and Pollak 2015 targeted mothers but invited partners or other family members to participate in counselling. One study targeted fathers by educating their non‐smoking wives (Chan 2006a). Thirty‐six studies targeted both parents. Zhang 1993 targeted fathers only; Borrelli 2010, Wilson 2011, Patel 2012, and Ralston 2013 targeted carers; Elder 1996 targeted teachers only; Wahlgren 1997, Butz 2011, and Stotts 2012 targeted families; and Krieger 2005, Halterman 2011, Harutyunyan 2013, Prokhorov 2013, and Kegler 2015 targeted households.
Age group
We stratified studies according to age groups of children: infants (younger than one year); preschoolers (up to age six); and school age (six to twelve years). Twenty‐three studies examined measures to reduce ETS exclusively for infants. Nineteen studies examined measures to reduce ETS for children up to and including preschool age, and 18 studies considered measures for children up to and including school age. One study followed pregnant women between 13 and 29 weeks' gestation for 12 months (Pollak 2015). Eight studies examined interventions to reduce ETS that included older age groups: Wahlgren 1997 included parents of children aged 6 to 17 years; Hovell 2002 and Borrelli 2016 included parents of children aged 3 to 17 years; Chan 2006a included parents of children from birth to 15 years; Yilmaz 2006 included mothers of children younger than 16 years of age; Streja 2014 included parents or guardians of children from 2 to 14 years of age; and Borrelli 2010, Chellini 2013, Prokhorov 2013, Tyc 2013,Kegler 2015, and Nicholson 2015 included children younger than 18 years of age. Five studies did not provide children's ages (Curry 2003; Chan 2005; Nuesslein 2006; Ralston 2008; Ralston 2013).
Theoretical framework
Forty‐five of the 78 studies expressly employed a theoretical framework in the design and/or development of the intervention. Fifteen studies used motivational interviewing (Emmons 2001; Curry 2003; Chan 2005; French 2007; Hannover 2009; Borrelli 2010; Baheiraei 2011; Halterman 2011; Phillips 2012; Stotts 2012; Ralston 2013; Eakin 2014; Blaakman 2015; Kegler 2015; Borrelli 2016). Seven used a social learning model (Greenberg 1994; Elder 1996; Conway 2004; Fossum 2004; Harutyunyan 2013; Ulbricht 2014; Blaakman 2015), and six used the stages of change component of Prochaska's transtheoretical model (Abdullah 2005; Krieger 2005; Ralston 2008; Winickoff 2010; Patel 2012; Ralston 2013). Chen 2016 combined transtheoretical and I‐change models, and Winickoff 2010 combined the transtheoretical stages of change model with social learning theory, the health beliefs model, cognitive‐behavioural theory, Wagner's chronic care model, and behavioural and systems theory. Several studies combined motivational interviewing with other frameworks, including stages of change (Ralston 2013; Wang 2015), Maori and Aboriginal holistic models of health (Walker 2015), the teachable moment (Borrelli 2016), cognitive‐behavioural therapy (Joseph 2014), cognitive‐behavioural skill building (Schuck 2014), and social cognitive theory. Kegler 2015 combined motivational interviewing with both the transtheoretical stages of change model and social cognitive theory, while Pollak 2015 combined motivational interviewing with both the teachable moment model and cognitive‐behavioural couples therapy.
McIntosh 1994 developed activities for the parent manual based on behaviour modification theory. Wahlgren 1997 tailored the programme to individual families and incorporated several behavioural modification techniques, including stimulus control, shaping, personal feedback, and contingency contracting. Groner 2000 employed the health belief model, and Wakefield 2002 used a harm minimisation approach that was based on previous research indicating that restrictions produced significantly lower urinary cotinine levels. Ratner 2001 utilised Marlatt's relapse model. Chan 2006a used Fishbein's theory of reasoned action and Ajzen's theory of planned behaviour in developing its educational intervention. Hovell 2009 used the behavioural ecological model in developing the counselling intervention. Herbert 2011 used a family‐centred assessment and intervention model to empower families to reduce cigarettes smoked in the home. Tyc 2013 and Nicholson 2015 used behavioural contracting, problem solving, and social reinforcement. Ortega 2015 used the 5 As (Ask, Advise, Assess, Assist, and Arrange) approach, and Streja 2014 employed the Health Behaviour Framework (previously the Adherence Model).
Acceptability of intervention to participants
Six studies appear to have involved consultation with potential participants as part of the development of the intervention (Hughes 1991; Davis 1992; Hovell 2000; Borrelli 2010; Streja 2014; Chen 2016). Davis 1992 employed focus groups with smokers and non‐smokers to understand their beliefs and attitudes towards smoking and cessation in order to develop improved self‐help materials. Borrelli 2010 conducted focus groups to better understand Latino culture and to modify the motivational interviewing technique accordingly.
Process indicators
Process indicators provide important information regarding the integrity of the way in which interventions were implemented. However, only 32 of the 78 studies described process indicators well (Hughes 1991; Chilmonczyk 1992; Davis 1992; Greenberg 1994; McIntosh 1994; Eriksen 1996; Severson 1997; Hovell 2000; Emmons 2001; Hovell 2002; Wakefield 2002; Fossum 2004; Zakarian 2004; Abdullah 2005; Wiggins 2005; Culp 2007; Hannover 2009; Hovell 2009; Borrelli 2010; Winickoff 2010; Stotts 2012; Tyc 2013; Cooper 2014; Eakin 2014; Hafkamp‐de 2014; Joseph 2014; Schuck 2014; Abdullah 2015; Blaakman 2015; Kegler 2015; Borrelli 2016; Daly 2016). More specifically, 11 studies reported that they maintained regular monitoring and support with those responsible for providing the intervention (Hughes 1991; Greenberg 1994; Emmons 2001; Culp 2007; Hannover 2009; Hovell 2009; Borrelli 2010; Eakin 2014; Hafkamp‐de 2014; Abdullah 2015; Daly 2016), and 19 reported that they evaluated the extent to which participants received, read, undertook, or adhered to the intervention as intended (Davis 1992; McIntosh 1994; Severson 1997; Hovell 2002; Wakefield 2002; Zakarian 2004; Abdullah 2005; Wiggins 2005; Culp 2007; Hovell 2009; Winickoff 2010; Stotts 2012; Cooper 2014; Joseph 2014; Schuck 2014; Abdullah 2015; Blaakman 2015; Kegler 2015; Borrelli 2016). Among those that commented on the monitoring of study implementation, one study recommended prompting providers over the course of the study to ensure appropriate implementation (Severson 1997). Another study reported the collection of qualitative data showing the opinions of nurses delivering the intervention (Fossum 2004).
Biological verification of children's exposure and absorption
Thirty studies used biological evidence of children's ETS absorption by measuring cotinine in urine or saliva, and 14 studies used environmental monitors of children's exposure to ETS. Eight of the 14 used passive sampling nicotine monitors as a primary study outcome. One study also measured particulate matter in the child's bedroom and living room (Butz 2011). The remaining studies used air nicotine monitors to promote or verify the accuracy of parent reporting of smoking behaviours. Wahlgren 1997 reported using air nicotine monitors in a room where greatest exposure to ETS was reported for two weeks before clinic visits to verify parent reports of cigarette consumption. Hovell 2000, Hovell 2002, Zakarian 2004, and Hovell 2009 used inactive air nicotine monitors placed in three rooms where children’s greatest ETS exposure was reported, to promote accurate self‐reporting of smoking behaviours by mothers. These studies also placed active air monitors for a selected proportion of the total sample: Hovell 2000 in a randomly selected half of the sample; both Hovell 2002 and Zakarian 2004 in 20% of the sample; and Hovell 2009 in a randomly selected 24% of the sample at six months. Zakarian 2004 reported randomly selecting these homes and placing monitors in the homes one week before data collection, while Hovell 2002 did not report how the 20% of homes were selected but reported that they were used only for baseline and post‐test measures. Cost was given as a reason for not using active air nicotine monitors across the whole sample. Eakin 2014 placed two monitors for seven days in the room where the child slept and in another room identified as a major activity room by the carer. Streja 2014 placed two monitors, each for one of two consecutive seven‐day periods in a major activity room. Kegler 2015 used passive air monitors after the three‐month visit for all participants reporting full or no bans, and for half of the participants reporting partial bans. However, investigators did not specify the location of the monitors. Borrelli 2016 placed two monitors for seven days at baseline and after call 5, they placed one in the room where the child spent the most time, and the child wore one.
Eleven interventions used feedback to parents of biological evidence of children's ETS absorption as a stimulus for parental behaviour change (Chilmonczyk 1992; McIntosh 1994; Wilson 2001; Wakefield 2002; Ekerbicer 2007; Wilson 2011; Harutyunyan 2013; Ulbricht 2014; Yucel 2014; Wang 2015; Daly 2016). Twenty‐three studies used biological validation of parental smoking cessation by measuring cotinine in urine, saliva, or serum (Woodward 1987; Irvine 1999; Hovell 2000; Hovell 2002; Fossum 2004; Zakarian 2004; Abdullah 2005; Kallio 2006; Nuesslein 2006; French 2007; Hovell 2009; Winickoff 2010; Phillips 2012; Tyc 2013; Cooper 2014), and/or expired carbon monoxide (Emmons 2001; Ratner 2001; Curry 2003; Abdullah 2005; Schonberger 2005; Borrelli 2010; Stotts 2012; Cooper 2014).
Length of follow‐up
For this review we determined length of follow‐up as extending from completion of the intervention to time of data collection. Length of follow‐up is important to determine, as it affects the extent to which sustainability and long‐term outcomes can be assessed. While short‐term reductions in children's ETS exposure have provided some benefit for children's health outcomes, the ultimate goal is long‐term and sustained change in order to maximise the positive impact on children's health and well‐being as they grow and develop. Twenty‐eight studies included in this review reported follow‐up of at least 12 months from the end of the intervention. Another 24 studies reported shorter follow‐up periods of between 6 and 12 months. Wahlgren 1997 debriefed participants at the six‐month follow‐up and reported ongoing follow‐up 8 and 18 months after that. Long‐term effectiveness was particularly difficult to assess in the remaining studies, specifically those with follow‐up periods of six months or less. McIntosh 1994 reported follow‐up periods that ranged between four and six months. Stotts 2012 reported a follow‐up period of six months from baseline, but it was unclear what the follow‐up was post intervention. The remaining studies (24) used a follow‐up time of less than six months.
Sample size
Thirty‐nine of the 78 studies mention conducting a power calculation in the design of their studies (Woodward 1987; Greenberg 1994; McIntosh 1994; Severson 1997; Wahlgren 1997; Irvine 1999; Armstrong 2000; Groner 2000; Hovell 2000; Emmons 2001; Wakefield 2002; Conway 2004; Krieger 2005; Schonberger 2005; Wiggins 2005; French 2007; Ralston 2008; Hannover 2009; Hovell 2009; Borrelli 2010; Baheiraei 2011; Butz 2011; Halterman 2011; Wilson 2011; Phillips 2012; Chellini 2013; Harutyunyan 2013; Prokhorov 2013; Ralston 2013; Cooper 2014; Ulbricht 2014; Abdullah 2015; Ortega 2015; Pollak 2015; Walker 2015; Wang 2015; Borrelli 2016; Chen 2016; Daly 2016). Of these, McIntosh 1994, Wahlgren 1997, Borrelli 2010, Harutyunyan 2013, Cooper 2014, Pollak 2015, and Daly 2016 explicitly mention that the statistical power of their study was limited by the small sample size. Although Streja 2014 did not present a power calculation, the authors did include a lack of statistical power as one of their limitations.
Risk of bias in included studies
To meet inclusion criteria for this review, studies had to be controlled trials. For this update, we assessed risk of bias for all of the included studies. We have summarised this assessment in Figure 2 and Figure 3.
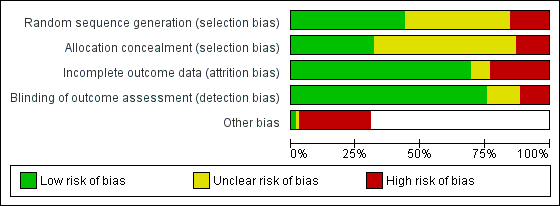
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
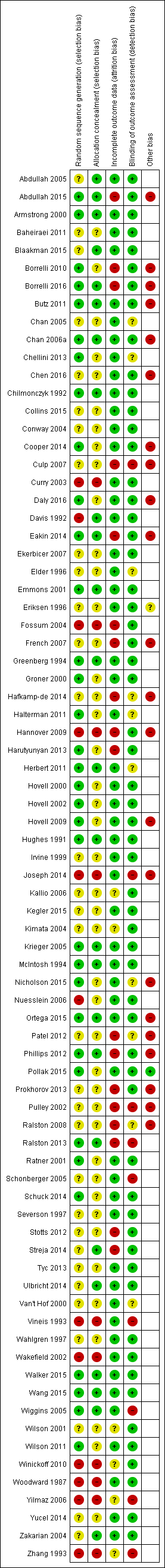
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators rarely described the method of randomisation in sufficient detail to permit assessment of whether allocation was concealed at the time of trial entry. For example, it was common for studies to merely state that participants were randomised. Quasi‐randomisation was not uncommon even in large trials. Twelve and 32 studies, respectively, were at high and unclear risk of bias from poor randomisation and lack of randomisation. Ten and 43 studies, respectively, were at high and unclear risk of bias from allocation concealment, with many studies not describing allocation concealment.
Blinding (detection bias)
Very few trials had any blinding of participants or providers, largely due to pragmatic issues associated with administering an educational intervention. We have noted in the Characteristics of included studies tables where there was blinding of outcome assessors. We classified those trials without adequate blinding of outcome assessors or that used a subjective measure of outcome assessment as having high risk of bias. Nine and 10 studies, respectively, were at high and unclear risk of bias from blinding of outcome assessment.
Incomplete outcome data
Attrition from withdrawals and exclusions from trials were common, and often studies did not clearly specify the reasons for this. Attrition presents a potentially serious risk of bias in these studies. We have provided in the Characteristics of included studies table levels of attrition for each study, and information about any intention‐to‐treat analyses performed. Eighteen and six studies, respectively, were at high and unclear risk of bias due to incomplete outcome data.
Other potential sources of bias
We judged 22 studies to be at high risk of "other potential sources of bias". In 12 of these studies, this related to systematic differences in the characteristics of treatment groups (Pulley 2002; Culp 2007; French 2007; Ralston 2008; Hovell 2009; Butz 2011; Phillips 2012; Prokhorov 2013; Hafkamp‐de 2014; Abdullah 2015; Ortega 2015; Borrelli 2016). In four studies, this was due to potential exposure misclassification (Eakin 2014; Hafkamp‐de 2014; Joseph 2014; Daly 2016); in four this was due to a lack of intention‐to‐treat analysis (Pulley 2002; Hannover 2009; Patel 2012; Prokhorov 2013); in three this was due to the possibility of contamination between groups (Chan 2006a; Hafkamp‐de 2014; Abdullah 2015); in one it was due to a Hawthorne effect (Ortega 2015); and in another to the possibility of social desirability bias resulting from the interview format (Abdullah 2015).
Effects of interventions
See: Summary of findings for the main comparison Summary of findings: community‐based interventions for reducing children's exposure to environmental tobacco smoke; Summary of findings 2 Summary of findings: interventions in the ill‐child setting for reducing children's exposure to environmental tobacco smoke; Summary of findings 3 Summary of findings: interventions in the well‐child setting for reducing children's exposure to environmental tobacco smoke
We provide study results by outcome and by setting and child age below. We have discussed specific intervention types within individual outcomes, and more generally in the Discussion section. For further information, including effect sizes of interventions, see Analysis 1.1.
Tobacco smoke exposure outcomes
Of the 78 studies, 26 reported success in achieving reduced children's ETS exposure between intervention and control groups, 24 of which presented statistically significant findings (N = 33,811). Thirteen (N = 3640) used biochemical or environmental measures of children's ETS exposure (biological verification of cotinine in urine or saliva of the child, or use of environmental monitors) (Wahlgren 1997; Emmons 2001; Kimata 2004; Borrelli 2010; Baheiraei 2011; Harutyunyan 2013; Prokhorov 2013; Collins 2015; Kegler 2015; Ortega 2015; Wang 2015; Borrelli 2016; Chen 2016) and 11 (N = 30,171) did not use such measures (Zhang 1993; Armstrong 2000; Curry 2003; Abdullah 2005; Schonberger 2005; Yilmaz 2006; French 2007; Phillips 2012; Hafkamp‐de 2014; Abdullah 2015; Blaakman 2015). Of these, we judged 11 to be at high risk of bias, three at low risk of bias, and 10 at unclear risk of bias. We provide a brief summary of outcomes below, along with further details of available outcome measures in the section Analysis 1.1.
Of the 13 studies using biochemical or environmental measures of children's ETS exposure, five (N = 645) reported children's urinary cotinine measures (Kimata 2004; Baheiraei 2011; Collins 2015; Wang 2015; Chen 2016), two (N = 1351) reported children's hair nicotine measures (Harutyunyan 2013; Ortega 2015), and six (N = 1644) recorded household air nicotine assessed with monitors (Wahlgren 1997; Emmons 2001; Borrelli 2010; Prokhorov 2013; Kegler 2015; Borrelli 2016). Seven (N = 1580) of these 13 studies used in‐person counselling (Wahlgren 1997; Emmons 2001; Borrelli 2010; Baheiraei 2011; Collins 2015; Borrelli 2016; Chen 2016), two (N = 748) used complex interventions consisting of counselling plus additional components (Harutyunyan 2013; Kegler 2015), one (N = 65) used a complex intervention consisting of education plus additional components (Wang 2015), one (N = 1101) used a brief intervention (Ortega 2015), and one (N = 71) used "fotonovelas" and a comic book (Prokhorov 2013). In one study (N = 75) intervention methods are unclear as investigators do not describe how they encouraged participants to stop smoking, but do state that those in the intervention group "agreed to stop smoking" (Kimata 2004).
Eight studies reported success based on parents' reports of smoking cessation, with or without salivary cotinine verification, or reduction in smoking in the presence of children but without verification of children's ETS exposure. These studies employed a range of interventions including school‐based interventions (children writing letters to their fathers urging them to quit), intensive counselling, a home visiting programme, education and advice, and an intervention based on the Behavioural Action Model (BAM). Zhang 1993 (N = 19,533) used a school‐based intervention and reported the proportion of fathers who quit smoking for at least 180 days as 800/9953 (11.7%) for the intervention group, and as 14/6274 (0.2%) for the control group. At follow‐up, Armstrong 2000 (N = 181) reported smoking in the house around an infant (maternal self‐report) for the intervention group as 8.6% and for the control group as 23.8% when the intervention group received a home visiting programme. Curry 2003 (N = 303) reported smoking abstinence at 12 months as 13.5% in the intervention group, following a brief motivational message and telephone counselling, and as 6.9% in the control group. Abdullah 2005 (N = 952) used telephone counselling and reported a biochemically validated quit rate of 47/444 (10.6%) for the intervention group and 21/459 (4.5%) for the control group at six months. Schonberger 2005 (N = 476) reported that 52% (14/27) of postnatal mothers quit smoking in the intervention group, compared with 28% (8/30) in the control group, at six months' follow‐up when the intervention group received home visits. Yilmaz 2006 (N = 363) included two intervention groups that had discussions about effects of smoking on child or maternal health. Quit rates at follow‐up were as follows: child intervention group 24.3%; mother intervention group 13%; and control group 0.8%. French 2007 (N = 61) used motivational interviewing; and at six months' follow‐up, 26 (22%) participants in the intervention group and 9 (10%) in the control group were saliva cotinine‐verified non‐smokers. Phillips 2012 (N = 44) used motivational interviewing for both groups, and provided information about infant bonding to the intervention group. The study reported that at eight weeks postpartum, there were significantly more smoke‐free mothers in the intervention (81%) group compared with the control (46%) group.
Fifty‐two studies (N = 19,758) failed to detect an intervention effect on ETS outcomes (Woodward 1987; Hughes 1991; Chilmonczyk 1992; Davis 1992; Vineis 1993; Greenberg 1994; McIntosh 1994; Elder 1996; Eriksen 1996; Severson 1997; Irvine 1999; Groner 2000; Hovell 2000; Van't Hof 2000; Ratner 2001; Wilson 2001; Hovell 2002; Pulley 2002; Wakefield 2002; Conway 2004; Fossum 2004; Zakarian 2004; Chan 2005; Krieger 2005; Wiggins 2005; Chan 2006a; Kallio 2006; Nuesslein 2006; Culp 2007; Ekerbicer 2007; Ralston 2008; Hannover 2009; Hovell 2009; Winickoff 2010; Butz 2011; Halterman 2011; Herbert 2011; Wilson 2011; Stotts 2012; Chellini 2013; Patel 2012; Ralston 2013; Tyc 2013; Cooper 2014; Eakin 2014; Joseph 2014; Schuck 2014; Streja 2014; Yucel 2014; Pollak 2015; Walker 2015; Daly 2016). Three (N = 824) of these studies reported significant reduction in self‐reported parental smoking based on intensive counselling without a corresponding reduction in children’s urinary cotinine measurements (Hovell 2000; Hovell 2009; Schuck 2014). In Culp 2007 (N = 263), the intervention group received home visits, and whilst there was no significant reduction in smoking, the other outcome of relevance to our review was mothers' knowledge of the effects of smoking on child development. At 12 months, the intervention group answered two out of six questions better than the control group.
In all, 21 of these 52 studies (N = 6485) used biochemical measures of children's ETS exposure (child urinary, hair, or salivary cotinine levels) (Woodward 1987; Chilmonczyk 1992; Greenberg 1994; McIntosh 1994; Irvine 1999; Hovell 2000; Wilson 2001; Hovell 2002; Wakefield 2002; Conway 2004; Zakarian 2004; Kallio 2006; Ekerbicer 2007; Hovell 2009; Halterman 2011; Wilson 2011; Tyc 2013; Eakin 2014; Streja 2014;Yucel 2014; Walker 2015), while the rest used self‐reports of smoking behaviour, with or without salivary cotinine verification. Interventions used in these studies were varied; 29 studies (N = 8930) used complex interventions predominantly including counselling and/or education (Hughes 1991; Chilmonczyk 1992; Davis 1992; Vineis 1993; Greenberg 1994; McIntosh 1994; Eriksen 1996; Irvine 1999; Groner 2000; Wilson 2001; Hovell 2002; Pulley 2002; Wakefield 2002; Zakarian 2004; Krieger 2005; Chan 2006a; Ralston 2008; Winickoff 2010; Butz 2011; Wilson 2011; Ralston 2013; Tyc 2013; Eakin 2014; Joseph 2014; Schuck 2014; Streja 2014; Yucel 2014; Walker 2015; Daly 2016).
Thirty‐four of the 78 studies reported reduced children's ETS exposure among study participants regardless of assignment to intervention or control groups (Woodward 1987; Hughes 1991; Davis 1992; Vineis 1993; Elder 1996; Eriksen 1996; Severson 1997; Wahlgren 1997; Irvine 1999; Groner 2000; Ratner 2001; Wilson 2001; Hovell 2002; Wakefield 2002; Curry 2003; Fossum 2004; Abdullah 2005; Chan 2005; Krieger 2005; Chan 2006a; Kallio 2006; Nuesslein 2006; Ekerbicer 2007; Hovell 2009; Winickoff 2010; Halterman 2011; Herbert 2011; Wilson 2011; Chellini 2013; Prokhorov 2013; Ralston 2013; Tyc 2013; Eakin 2014; Nicholson 2015).
Household air quality
Eleven studies (N = 2636) reported household air nicotine measures (Wahlgren 1997; Emmons 2001; Hovell 2009; Borrelli 2010; Butz 2011; Stotts 2012; Prokhorov 2013; Eakin 2014; Streja 2014; Kegler 2015; Borrelli 2016). Of these studies, two did not use air nicotine measures to evaluate the impact of interventions; Hovell 2009 used air nicotine measures to validate reported exposures, while Kegler 2015 used air nicotine measures to validate home smoking bans. Of the remaining nine studies, five (N = 1385) found a statistically significant benefit of the intervention in reducing air nicotine levels (Emmons 2001; Borrelli 2010; Prokhorov 2013; Eakin 2014; Borrelli 2016).
Borrelli 2010 reported a significant decrease in nicotine concentrations as measured by home monitors in the Behaviour Action Model (BAM) group (intervention to increase self‐efficacy; baseline Mean = 1.07, standard error (SE) 0.19; three‐month Mean = 0.28, SE 0.11; P = 0.01) but not in the Precaution Adoption Model (PAM) (motivational interviewing) group at three‐month follow‐up. Borrelli 2016 used the PAM for two aims: first, to determine whether second‐hand smoke exposure (SHSe) feedback motivates cessation among parents of children with asthma versus parents of healthy children (HC) ‐ the study reported significant differences in levels of SHS exposure detected by home monitors (PAM 92.1% vs HC 97.2%; P = 0.04), but not by child monitors (PAM 91.4% vs HC 95.6%); second, to evaluate whether greater intervention intensity (enhanced‐precaution adoption model (PAM)) produces greater cessation than a previously tested intervention (PAM). However, data show no significant between‐group differences.
Emmons 2001 used motivational interviewing and telephone counselling and reported reduced household air nicotine measurements over time in the intervention groups (kitchen and TV room air nicotine at six months (log‐transformed units): intervention 3.7 and 3.1, falling to 2.6 and 2.3; Control 3.0 and 3.5, changing to 6.9 and 3.5; P < 0.05). As there was no change in the number of cigarettes smoked per day, nor in the cessation rate, the implication of the difference was that parents and carers had changed smoking location and had moved outside to smoke.
Eakin 2014 found that motivational interviewing and education resulted in significantly lower air nicotine levels compared to education alone (0.29 vs 0.40 mg) amongst carers of preschool children in a Head Start programme in the USA.
Prokhorov 2013 reported a significant decrease in nicotine concentrations for the intervention group, which received a comic book and "fotonovelas" for the "high‐exposure" room (1.14 μg/m³ to 0.20 μg/m³; P < 0.01) but not for the "low‐exposure" room, whilst the decrease noted in the control group was not significant.
Of the four studies (N = 603) that did not show a significant benefit, three used counselling, motivational interviewing, or a combination of air cleaners and health coaching in ill‐child settings (Wahlgren 1997; Butz 2011; Stotts 2012); while one used a combination of a video and a booklet with educational and risk reduction strategies, together with visual reminders, in a community setting (Streja 2014).
Child health outcomes
Sixteen studies (N = 12,520) assessed child health outcomes (Hughes 1991; Greenberg 1994; Armstrong 2000; Wilson 2001; Pulley 2002; Kimata 2004; Krieger 2005; Schonberger 2005; Wiggins 2005; Culp 2007; Borrelli 2010; Butz 2011; Halterman 2011; Wilson 2011; Hafkamp‐de 2014; Walker 2015), and five studies measured child health outcomes, although they were not regarded as a primary outcome variable (N = 2184; see Analysis 1.1) (Wahlgren 1997; Cooper 2014; Abdullah 2015; Blaakman 2015; Borrelli 2016). Of these, the child health outcome of interest in 10 studies was asthma related (symptom scores, quality of life, functional morbidity, symptom‐free days, and asthma‐related health services utilisation). In three studies, the health outcome of interest was respiratory illness, and another two reported health service utilisation alone ‐ community services in one, and hospital admissions and emergency visits in another. One study measured changes in neurotrophin levels but did not specify which neurotrophins were measured.
Nine studies found improvement in child health outcomes. Hughes 1991 (N = 95) embedded an intervention to reduce children’s ETS exposure in a study of a comprehensive asthma education intervention. Although asthma control was improved there was no change in exposure to ETS. Greenberg 1994 (N = 933) targeted ETS exposure in infants younger than six months of age and aimed to reduce the incidence of lower respiratory tract illness and the prevalence of respiratory symptoms. For infants of smoking mothers, the study demonstrated a lower prevalence of persistent symptoms in the intervention group (17.8%) compared with the control group (30.9%; risk difference 13.1%; 95% confidence interval (CI) 1.0% to 27.0%). There was no difference in the incidence of illness. Wilson 2001 (N = 87) examined the effects of an intervention targeting smoking behaviour change and asthma education on healthcare utilisation and asthma hospitalisations, and explored other measures of asthma control. The study demonstrated a reduction in the prevalence of children making more than one acute care asthma visit in the year following the intervention. Given that there was no apparent benefit of the smoking‐related counselling on smoking‐related outcomes, it is likely that asthma education, rather than the smoking behaviour programme, achieved improvement in asthma morbidity. Kimata 2004 (N = 75) found that cessation of smoking had no effect on skin wheal responses nor on plasma neurotrophins among normal children, but achieved a significant reduction in skin wheal response, responses to house dust mite, and cat dander, along with lower neutrophil levels for those with atopic eczema/dermatitis syndrome. Neurotrophins are a subset of growth factors with a range of functions throughout the body and include nerve growth factor and brain‐derived neurotrophic factor, as reported in Lackie 1999, which was the only study identified by this review to consider neurotrophin levels, and it does not specify which particular neurotrophins were measured. Krieger 2005 (N = 274) delivered a community home intervention to address conditions affecting childhood asthma and reported that the high‐intensity intervention group showed clinically significant improvement in paediatric carer asthma quality of life scores and a decline in urgent health service utilisation, but no significant difference in symptom‐free days, compared to the low‐intensity intervention group. However, they did not achieve a statistically significant intervention effect for carer reports of smoking in the home nor for reports of no smoking allowed in the home, so the child health intervention effect is probably due to other aspects of the intervention. Culp 2007 (N = 263) conducted home visits with the goal of promoting the health and development of first‐time mothers and infants and found no significant differences between groups in terms of numbers of hospital admissions or emergency room visits. At 12 months, intervention mothers were more likely to make use of health department clinics for well‐child care as compared to the control group (P = 0.04). Borrelli 2010 (N = 133) reported that the child’s level of functional morbidity due to asthma decreased significantly (P < .001) in both the BAM (intervention to increase self‐efficacy) and PAM (motivational interviewing) groups over time. Butz 2011 (N = 126) reported that after the two groups that used air cleaners were combined, children assigned to those groups showed a significant increase in symptom‐free days during the previous two weeks: 1.36 compared with 0.24 symptom‐free days for control group children from baseline to follow‐up. Halterman 2011 (N = 530) used motivational interviewing to counsel the primary carer and an additional smoker who spent the most time with the child and observed inhaler administration at school by a nurse. This study only measured child health outcomes and found a significant improvement in many asthma‐related outcome measures in the intervention compared to the control group. We have provided further details in the Analysis 1.1 table.
Seven studies (N = 9619) did not detect a significant intervention effect on child health outcomes (Wahlgren 1997; Armstrong 2000; Pulley 2002; Wiggins 2005; Wilson 2011; Hafkamp‐de 2014; Walker 2015). See Analysis 1.1 for further details. Of these seven studies, three used complex interventions consisting of counselling and additional components (Wilson 2011; Hafkamp‐de 2014; Walker 2015), two used complex interventions consisting of education and additional components (Armstrong 2000; Pulley 2002), one used in‐person counselling (Wahlgren 1997), and one used community support groups for mothers (Wiggins 2005).
Schonberger 2005 (N = 476) reported associations of exposure to passive smoking with parentally reported asthma symptoms without group allocation. Therefore it is not possible to determine an intervention effect on child health outcomes.
Results according to child age
A smaller proportion of studies of infants detected beneficial intervention effects compared with studies of older age groups. Four (N = 1187) of the 23 studies that examined measures to reduce ETS exclusively among infants detected a beneficial intervention effect (Abdullah 2005; French 2007; Baheiraei 2011; Phillips 2012). Eight (N = 10,576) of the nine studies examining measures to reduce ETS among children up to and including preschool age demonstrated a beneficial intervention effect (Emmons 2001; Schonberger 2005; Harutyunyan 2013; Hafkamp‐de 2014; Abdullah 2015; Collins 2015; Ortega 2015; Wang 2015). Ten (N = 22,078) of the 18 studies examining measures to reduce ETS among children up to and including school age and older demonstrated an intervention effect (Zhang 1993; Greenberg 1994; Wahlgren 1997; Kimata 2004; Krieger 2005; Yilmaz 2006; Borrelli 2010; Halterman 2011; Prokhorov 2013; Chen 2016).
Results according to setting
In the ill‐child respiratory setting, four (N = 1028) of 13 studies demonstrated a beneficial intervention effect (Wahlgren 1997; Krieger 2005; Borrelli 2010; Halterman 2011). Krieger 2005 and Halterman 2011 showed a significant effect on child health outcomes but not on tobacco smoke exposure outcomes. Three of these four studies used intensive counselling or motivational interviewing, whilst one used a community home intervention with elements of education and behaviour change. Of the nine studies that did not demonstrate an intervention effect, three used intensive counselling, one used motivational interviewing, one used a motivational health coach in addition to air cleaners, two used brief counselling methods, and two used home visits.
In the ill‐child non‐respiratory setting, two (N = 119) of nine studies showed a beneficial intervention effect (Kimata 2004; Phillips 2012). Kimata 2004 did not describe the intervention, and Phillips 2012 used motivational interviewing for both groups, with the intervention group also receiving information about infant bonding. Of the seven studies that did not demonstrate an intervention effect, three used brief counselling methods and four used more intensive counselling, including one study that used motivational interviewing, one that used a booklet, and one that used cotinine feedback.
In the clinical setting (not designated well‐child or ill‐child), one study (N = 303) out of two demonstrated a beneficial intervention effect (Curry 2003). This study used a brief motivational message and a motivational interview, along with follow‐up telephone counselling. Nuesslein 2006 (N = 40) did not find an intervention effect and used parental cotinine feedback.
In the clinical setting (both well‐child and ill‐child), Yilmaz 2006 (N = 3636) and Ortega 2015 (N = 1101) demonstrated a beneficial intervention effect. We included no other studies in this group.
In the well‐child clinical setting, seven (N = 9866) of the 27 studies demonstrated a beneficial intervention effect (Armstrong 2000; Emmons 2001; Abdullah 2005; Schonberger 2005; French 2007; Baheiraei 2011; Hafkamp‐de 2014). Three of these seven studies used motivational interviewing, two used home visiting interventions, one used telephone smoking cessation counselling, and one used a combination of counselling and education. Of the 20 studies that did not demonstrate an intervention effect, five used brief counselling methods; five used intensive counselling methods; four used home visits; one used cotinine feedback; one used telephone counselling; one used nicotine replacement therapy; one used an information kit and letter; one used a combination of counselling, education, and feedback on exposure level; and another used a combination of feedback on a computer risk assessment and nurse brief advice.
In the community setting, eight (N = 20,975) of 21 studies showed a beneficial intervention effect (Zhang 1993; Harutyunyan 2013; Prokhorov 2013; Abdullah 2015; Blaakman 2015; Kegler 2015; Wang 2015; Chen 2016). Four of these eight studies used counselling, one of which used motivational interviewing; two used a combination of counselling, education, and feedback on exposure level; one was a school‐based intervention; and one used a combination of telephone motivational interviewing and mailings. Of the 13 studies that did not demonstrate an intervention effect, two used telephone and two used in‐person counselling; four provided a combination of counselling and education, smoking cessation brief advice, or feedback on cotinine exposure level; two provided a combination of education with a video and visual reminders or culturally tailored couples‐based intervention with nicotine replacement therapy; one adopted a tobacco‐free school policy; and one used a support health visitor intervention consisting of monthly supportive listening home visits.
Biological validation of parents' self‐report
Of the 30 studies providing biological evidence of child ETS absorption, 16 (N = 4057) allowed an assessment of validation of parent‐reported change in exposure versus child ETS absorption (Greenberg 1994; McIntosh 1994; Hovell 2000; Wilson 2001; Hovell 2002; Wakefield 2002; Kimata 2004; Zakarian 2004; Kallio 2006; Hovell 2009; Baheiraei 2011; Tyc 2013; Streja 2014; Walker 2015; Wang 2015; Daly 2016). Of these studies, seven (N = 2116) did not show a discrepancy between reported exposure and an objective measure of absorption (Wilson 2001; Wakefield 2002; Kimata 2004; Kallio 2006; Streja 2014; Walker 2015; Wang 2015). Kallio 2006 (N = 1062) reported that parent serum cotinine values showed that parents reported smoking habits accurately but did not provide data. Of the studies using environmental monitors of child exposure to ETS, Wahlgren 1997 (N = 91) and Hovell 2009 (N = 150) allowed an assessment of validation of parent‐reported change in exposure versus objective measure. Wahlgren 1997 did not demonstrate a correlation between parental report and environmental monitoring, whilst Hovell 2009 reported a significant moderate correlation. For Hovell 2009, however, the results showed a significant reduction in child second‐hand smoke exposure associated with the intervention according to reports, but not according to child urinary cotinine. Tyc 2013 (N = 135) also noted a significant decrease in reported child second‐hand smoke exposure but not in child urinary cotinine in the intervention group. Borrelli 2010 (N = 133) noted that, according to monitors in the home, but not those on the child, there was a significantly greater reduction in exposure to children in the BAM (intervention to increase self‐efficacy) group, although quit rates in the PAM (motivational interviewing) group were higher. This was thought to have occurred as the result of a greater change in the number of cigarettes smoked in front of the child in the BAM group, rather than following use of monitors as a validation measure.
Cost data and cost‐effectiveness
Thirteen of the included studies made some reference to costs. However, this was generally limited to some statement of implementation costs. McIntosh 1994 (N = 92) mentioned the cost of the manual, and Severson 1997 (N = 1875) mentioned staff and intervention costs of the intervention per person. Conway 2004 (N = 143) and Wiggins 2005 (N = 731) also mentioned the costs of implementing the intervention but indicated that investigators did not conduct further analysis of cost‐effectiveness because of a lack of an intervention effect. Krieger 2005 (N = 274) reported reduced urgent healthcare costs during the two months before the exit interview among those receiving the intervention relative to those in the comparison group, but investigators did not provide an extensive cost‐benefit analysis. Cooper 2014 (N = 1050) reported total mean costs that were approximately £91 higher in the nicotine replacement therapy group and indicated that the incremental cost‐effectiveness ratio (ICER) associated with nicotine replacement therapy use was £4926 per additional quitter (95% CI ‐£114128 to £126747).
Discussion
Of the 78 included studies, a minority (26 studies) detected an effect in favour of the intervention, 24 of which reported statistically significant findings. Although the proportion of studies targeting the population or community level has increased since review authors conducted the previous update (Baxi 2014), most studies that detected an effect (15) were performed in clinical settings (eight well‐child; five ill‐child; two well‐ and ill‐child), with eight successful interventions delivered in community settings and one in an unspecified setting. The intervention most frequently used in 16 of the 24 successful studies was counselling, two instances of which were provided in combination with education and feedback on measures of exposure. Seven of the eight studies in community settings used counselling successfully ‐ five of the eight studies in well‐child clinical settings, and three of the five studies in ill‐child clinical settings.
However, counselling was also used in 29 of the 52 studies showing no effect of the intervention; most of which delivered the intervention in clinical settings (11 well‐child; 9 ill‐child), with nine delivering the intervention in community settings.
Our findings suggest that strategies that are effective in the adult healthcare setting may not be generalisable to the paediatric setting. Brief advice for adult smokers when they attend clinical services for their health has a positive effect in triggering quit attempts (Stead 2013). Trials of interventions for parents attending clinical paediatric or child health services did not detect this effect. However, this finding might suggest that either a different sort of brief intervention should be employed, or that this context should not be used for brief advice. Also, studies may have been underpowered to detect a small effect. Examination of the dynamics of the doctor‐child‐parent relationship may assist the development of brief strategies with a greater likelihood of success in this clinical setting. Given the unknowns about the doctor‐child‐parent interaction, interventions provided in this setting may potentially cause harm. One study reported a trend for mothers in the intervention group to smoke more than mothers in the control group after receiving the intervention (Irvine 1999). Several studies used only one‐tailed t‐tests to look for statistical significance. When an intervention may cause harm, even if the hypothesis is unidirectional, investigators should always employ two‐tailed tests of significance. Hovell 2009 undertook a regression analysis to examine factors associated with the longest participant smoking quit attempts following counselling. The odds favouring the longest quit attempt were significantly increased when participants had made a 24‐hour quit attempt in the year prior to baseline, had tried a greater number of methods to quit in the past, and had reduced permissiveness of home smoking. Researchers did not find significant associations between longer quit attempts and level of education, heaviness of smoking or the smoking status of a partner.
There are relatively high rates of smoking cessation in pregnancy, both spontaneously and with clinical interventions (Chamberlain 2017; Coleman 2015). With high postnatal relapse rates reported among women who have quit during pregnancy (Lelong 2001), prevention of relapse for this group is an obvious means of preventing environmental tobacco smoke (ETS) exposure for their children. Ratner 2001 and Van't Hof 2000 identified risk factors for relapse. Risk factors identified by Ratner 2001 included having a partner who smoked and smoking a greater number of sticks per day before quitting; data show that prolonged breast feeding and higher scores on a scale measuring mental health were protective. Van't Hof 2000 found that a lower level of confidence in maintaining cessation, a lower level of encouragement by family and friends to maintain cessation, and greater numbers of family and friends who smoked were all associated with significantly higher odds of postpartum relapse. Further work in this area will make an important contribution.
Many of the studies identified for this review demonstrated reduced child exposure to ETS among participants, regardless of assignment to intervention or control groups, which suggests that studies may be describing the natural history of smoking among parents. Parents may reduce their own smoking or their children's exposure over time, possibly as a result of social pressures. Indeed the prevalent social trend in many developed countries over the past decade has been increased community concern about exposing non‐smokers to ETS (although arguably more so among non‐smokers than among active smokers). This is especially true for adults in the workplace and in public spaces such as bars and restaurants, particularly in North America, Australia, and some countries within the EU, where total smoking bans for these settings are increasingly legislated. Campaigns and community concerns about children's exposure to ETS at home and in cars have also increased. It is possible that these studies have recorded parents responding to this social trend by limiting their children's exposure in the home. This being the case, studies need to aim not just for a reduction in children's ETS exposure, but for a greater than background reduction in ETS exposure. For a study to produce a significant effect, the impact of interventions must be greater than the rate of decline in comparison groups. It may be true that as most studies used comparison groups rather than control groups (i.e. no cessation or avoidance advice and no information), the comparison interventions may have been more effective than anticipated. As studies have generally involved comparison groups receiving a limited intervention rather than strict control groups, this is certainly possible. Moreover, measurement of tobacco smoke exposure outcomes alone may produce an intervention effect and thus may be an important component of any intervention.
We judged the inconclusive evidence presented in this review to be of low or very low quality, despite the fact that this review includes 78 studies (summary of findings Table for the main comparison; summary of findings Table 2; summary of findings Table 3). Limitations include risk of bias, heterogeneity among study interventions and populations, and small sample sizes with low statistical power. Continuing to perform studies without adequate sample size, quality, or comparable interventions and populations will not allow for any conclusions to be reached regarding the clinical effects or cost‐effectiveness of interventions. Moreover, additional low‐quality studies may be an unethical use of resources and participants’ time.
Limitations of methods employed
The heterogeneity of study designs and characteristics rendered quantitative analysis inappropriate for this review. However, there is currently no best approach in narrative, rather than quantitative, syntheses of published studies. As we have included 78 studies, it would not be feasible to list results of each in the main text. Therefore, we have highlighted key results in our narrative summary and have recorded further results data in Analysis 1.1. However, we are aware that in some places, this means that studies with statistically significant results have been described in greater detail in the text than those that did not detect an effect. We have attempted to mitigate any impact of this by explicitly describing studies that tested similar interventions but did not detect an effect.
An additional limitation is that, of the 20 studies that used objective measures of children's ETS exposure or absorption, only four showed no discrepancy between parental reports of children's exposure and the biological measures. As most studies did not use objective measures, this calls into question the validity of self‐reported data provided in this review.
As noted above, many of the included studies had small sample sizes, and fewer than half (N = 28) reported a power calculation. For studies that did not detect an effect, this makes it difficult to establish whether the intervention was genuinely not effective, or if a result was not detected because the sample size was too small.
Included studies reported varying lengths of follow‐up. We used the longest reported follow‐up for the results. However, some studies reported short lengths of follow‐up, with 20 studies reporting follow‐up of less than six months. It is difficult to determine the sustainability and long‐term effectiveness of interventions when study follow‐up is short. Indeed, of the studies reporting longer follow‐up, some did show an initial difference between intervention and control groups that was not sustained at the final follow‐up period.
Finally, given that the burden of ETS is shifting more and more towards low‐ and middle‐income countries, and that in high‐income countries the burden is disproportionately falling on disadvantaged households, findings of the studies included in this review may not be generalisable, as these trials were conducted mainly in high‐income countries.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
| Study | |
| Abdullah 2005 | Counselling strategies based on the stages of change component of Prochaska's transtheoretical model. Results as N (%), intervention N = 444, control N = 459. Biochemically validated quit rate: Intervention 47 (10.6) Control 21 (4.5) No measure of children's exposure or absorption via cotinine. |
| Abdullah 2015 | ETS exposure: 6 month follow‐up: 1) higher proportion of the intervention (62%) than the comparison (45%) group households adopted complete smoking restrictions at home (P = 0.022); 2) higher proportion of the intervention (38%) than the comparison (17%) group households did not smoke at home at all (P = 0.002); 3) total exposure from household members inside home in the past 7 days (measured by mean number of cigarettes smoked per week in front of the child by household members) was lower in the intervention (3.29) than the comparison (7.41) group (P = 0.021); 4) total exposure from all smokers indoors and outdoors in the past 7 days (measured by mean number of cigarettes smoked per week in front of the child) was significantly lower among children in the intervention (15.2) than the comparison (25.7) group (P = 0.005); 5) Comparison group: mean cotinine levels increased from baseline to 2 months and maximum at 6 months, with no statistically significant difference in time effects. Intervention group: mean cotinine levels increased at 2 months from baseline level but decreased again at 6 months, with statistically significant difference in time effects only from 2 to 6 months (P < 0.05); 6) No significant difference in allowing others to smoke around the child (P = 0.908). Air quality: At 6 month follow‐up: 1) mean number of cigarettes smoked daily was significantly lower in the intervention (11.02) than the comparison (13.6) group (P = 0.021); 2) significantly more participants in the intervention (48%) than the comparison (28%) group reduced the number of cigarettes smoked at home daily (P = 0.006) Child health: Perceived overall respiratory health of the child improved significantly in the intervention (35%) than the comparison (20%) group (P = 0.024). There were no significant differences in the reports respiratory symptoms of the child (P = 0.258). |
| Armstrong 2000 | Targeted disadvantaged mothers. Smoking in house around infant (maternal self report verified by researcher observation during home visit) included education about smoking near infants as a Sudden Infant Death Syndrome (SIDS) prevention strategy in a post‐natal nurse home visiting programme aimed to improve the quality of maternal‐child attachment, maternal health and child health parameters. At four months the intervention group had significantly more completed immunizations than the controls, although both groups had high immunization rates. At 12 months there was no statistically significant difference between the groups for immunization status. There was also no significant difference at four or 12 months for rates of utilisation of community services. |
| Baheiraei 2011 | Motivational Interviewing used. In 3 months geometric mean urinary cotinine: intervention decreased from 48.72 ng/mg to 28.68 ng/mg, control decreased from 40.43 to 36.32 ng/mg, differences between two groups statistically significant using one tailed t‐test. Greater decrease in total daily cigarette consumption in the presence of child in the intervention group than the control group (statistically significant with one tailed t‐test). Intervention median cigarettes at 3 month 0 (IQR 1 to 2.71), control 1 (IQR 0 to 3.21). Home smoking bans: intervention 15% to 33.3% (statistically significant increase), control 11.5% to 19.5% (not statistically significant increase), differences between two groups statistically significant using a one tailed t‐test. Car smoking bans in the intervention group increased from 4% to 8%, and didn't change in the control group. This was not a statistically significant difference. |
| Blaakman 2015 | ETS exposure: 5 months after discharge from NICY, caregivers in treatment group were sig more likely to report a home smoking ban than the comparison group (96% vs 84%; P = 0.03), and less likely to report routine infant contact with a smoker (40% vs 58%, P = 0.03). Differences in reported home bans (92% vs 83%, P = 0.14) and routine infant contact with smokers (44% vs 53%, P = 0.33) were no longer significantly different at study end (8 months after NICU discharge). No difference in car smoking bans or total smoking bans at any time. 8 months after NICU discharge, infants in intervention group had lower salivary cotinine and a greater decrease in salivary cotinine since baseline than infants in the comparison group. Air quality: Overall, very few caregivers quit smoking, which didn't differ between groups after intervention or at study end. Of the 29 total caregivers who reported smoking 5 months after NICU discharge, caregivers in the intervention group reported significantly higher confidence to quit than smoking caregivers in the comparison group at the 5‐month survey, but not at study end. No significant difference between groups in caregiver motivation to quit. Child health: No significant differences between groups in respiratory symptoms or use of health care services. |
| Borrelli 2010 | Latino families targeted. Used two interventions with different theoretical frameworks: one intervention used motivational interviewing, whilst the other intervention used the social cognitive theory. At 3 months 61.7% home monitors were returned and 98.8% were in good condition, whilst 60.9% child monitors returned and 100% in good condition. Household air nicotine significantly decreased from pretreatment to the 3 month follow‐up in the BAM condition, (baseline M = 1.07, SE 0.19, and 3‐month M = 0.28, SE 0.11, P = 0.01), whereas the decrease observed in the PAM condition was not statistically significant. Changes in secondhand smoke concentrations as assessed by the child monitors were not statistically significant. Continuous abstinence at 3 months 12.3% BAM group and 19.1% PAM group (OR 1.68, 95% CI 0.64 to 4.37). The child's level of functional morbidity due to asthma decreased significantly (P < 0.001) in both groups over time. Secondhand smoke exposure as measured by monitors directly on the child did not show a significant decrease in either group. |
| Borrelli 2016 | ETS exposure: SELF‐REPORTED: 1) PAM had significant reductions over time on one SHS exp variable, while HC had reductions on 4 of the 5 SHS exp variables, with a significant group x time interaction. 2) Enhanced PAM showed sig within‐group decreases in SHS exp over time on all 5 variables and HC showed sig within group decreases in SHS exp over time on 4 of the 5. Sig group x time interaction, such that enhanced PAM showed greater decreases in SHS exp over time versus HC for 3 of the 5 SHS exp variables; 3) Comparing PAM with enhanced PAM, no significant group x time interaction. OBJECTIVE: 1) No significant differences in levels of SHS exp at baseline; 2) At follow‐up, there were significant differences in detectable levels of SHS exp in the HOME monitors (PAM 92.1% vs HC 97.2%, P = 0.04), but NOT the CHILD monitors (PAM 91.4% vs HC 95.6%); 3) At follow up, no significant between‐group differences in detectable levels of SHS exp in either the home or child monitors, when comparing PAM with enhanced PAM. Air quality: 1) PAM more than 2x as likely to achieve 7‐day and 30‐day point‐prevalence abstinence than HC (statistically significant); 2) Enhanced PAM more than 2x as likely to achieve 7‐day PPA, 3x as likely to achieve 30‐day PPA than HCs, and 5x as likely to be continuously abstinent than HCs (statistically significant); 3) At 4‐months, enhanced PAm were more than 2x as likely to achieve 30‐day PPA versus PAM (significant). Child health: 1) At 6‐months, enhanced PAM had significantly lower child asthma hospitalisations than PAM; 2) At 2, 4 and 6 month follow‐up, enhanced PAM had sig lower missed school days due to asthma than PAM; 3) Odds of at least 1 day with asthma symptoms was sig lower in enhanced PAM than PAM at 6‐months; 3) No sig diff between groups in changes in asthma functional morbidity. |
| Butz 2011 | Low income households targeted. No statistically significant differences in urinary cotinine between baseline and follow up by group After combining the air cleaner groups, children assigned to those groups had a significant increase in symptom‐free days (SFDs) during the past 2 weeks (1.36 SFDs) compared with 0.24 SFDs for control group children from baseline to follow‐up No statistically significant differences In air nicotine at baseline and follow‐up by group Comparison of the combined air cleaner groups and the control group indicated that the combined air cleaner groups had significant mean differences in PM2.5 and PM2.5‐10 levels from baseline to follow‐up (mean differences for PM2.5: control, 3.5 [SD, 20.0]; combined air cleaner groups, ‐18.0 [SD, 33.2; P 0.001]; and for PM2.5‐10: control, 2.4 [SD, 20.8]; combined air cleaner groups, ‐9.6 [SD, 16.0; P = 0.009]) |
| Chan 2005 | Motivational Interviewing used. No statistically significant evidence of effect. |
| Chan 2006a | Fishbein's theory of reasoned action and Ajzen's theory of planned behaviour used in the development of the educational intervention. Three most frequently reported actions taken by the mother to protect the child from passive smoking at home: opening the windows (N = 641, 43.9%), asking the father not to smoke near the child (N = 608, 41.6%), and moving the child away from the smoke (N = 482, 33%). |
| Chellini 2013 | Post‐intervention smoke free homes were not significantly different between groups (increased in both): percentage increase in intervention group 12.7% and control group 11.1% (OR 1.04, 95 CI 0.47 to 2.28) . For cars: intervention group 18.2%, and control group 12.0% (OR 1.47 95 CI 0.69 to 3.11. Of the N = 131 smokers there was no significant difference in change of smoking habits. between intervention and control group (7% total stopped smoking, 5% stopped smoking indoors and n = 9 stopped smoking in the car). |
| Chen 2016 | ETS exposure: After intervention, the percentage of children with a urine cotinine concentration higher than 6ng/ml (indicating exposure) in the intervention group was significantly lower than that in the control group at both 8 weeks (P < 0.0001) and 6 months (P = 0.007). Air quality: Significantly less smoking in presence of children in intervention group at both 8 weeks and 6 months. Child health: N/A |
| Chilmonczyk 1992 | No evidence of effect. |
| Collins 2015 | ETS exposure: Associated with lower child urine cotinine compared with the control group. Air quality: Twenty (18.3%) of intervention group mothers and three (1.9%) of the control group mothers had bioverified quit status) P < 0.01). Child health: N/A |
| Conway 2004 | Participants (Latino families) for this study were recruited through advertising at community organisations and venues. Social learning model used. No significant effect. |
| Cooper 2014 | ETS exposure: N/A Air quality: After delivery, there were no statistically significant differences in cessation; self‐reported abstinence at 2 years was 2.9% in the NRT group and 1.7% in the placebo group. However, few participants reported using a full 8‐week course of NRT; 7.2% in NRT group and 2.8% in placebo group used their trial medications for over 1 month. Child health: At birth, significantly more Caesarian births occurred in the NRT group (20.7% vs 15.3%); at 2 years, significantly more infants in the NRT group (72.6% vs 65.5%) survived with 'no impairment'; 3) However, no sig difference between groups in infants' reported respiratory problems. |
| Culp 2007 | At 12 months the intervention group smokers smoked mean 2.1 fewer than control, which was not statistically significant: intervention 7.28 (s.d. 6.79), control 9.41 (s.d. 7.09) (t(147) = 1.82, P = 0.071). There were no significant differences between groups on number of hospital admissions or emergency room visits. At 12 months, intervention mothers were more likely to make use of health department clinics for well child care as compared to control group (chi square P =0.04) Knowledge of secondhand smoke exposure on child development: at 12 months significantly more intervention (N = 90, 58.1%) than control (N = 51, 47.7%) knew about SHS and impaired brain development, and significantly more intervention (N = 126, 80.6%) than control (N = 77, 72.0%) knew it takes longer to get well. No other significant differences with questions. |
| Curry 2003 | Ethnically diverse low income women targeted. Motivational Interviewing used. Abstinence rates: 3 mth Intervention 7.7% vs Control 3.4%; 12mth Intervention 13.5% vs Control 6.9% ‐ 12 mth difference statistically significant. Validation of smoking cessation by carbon monoxide expiration was completed by only a small subsample (13/156 in the intervention group and 5/147 in the control group). |
| Daly 2016 | ETS exposure: At 12 month follow‐up, 13% of all infants were reported to be exposed to SHS; however with urine cotinine validation, 17% overall were exposed. No significant time by group difference detected from baseline to follow‐up for either of the 2 treatment arms when compared with the control group. Air quality: At follow‐up, 47% of all parent/carers reported they were smokers. No significant time by group differences detected comparing either treatment arm with the control group. Child health: N/A |
| Davis 1992 | This study recruited participants through an advertising campaign that invited them to call a telephone smoking cessation assistance counselling service run by the National Cancer Institute in the USA. No evidence of difference between self‐help guides. |
| Eakin 2014 | ETS exposure: Differences in salivary cotinine were not significant. However, among all families who reported a home smoking ban, salivary cotinine and air nicotine levels declined in both groups (P < 0.05). Air quality: Participants in the MI and education group had significantly lower air nicotine levels (0.29 vs 0.40 mg), 17% increase in prevalence of caregiver‐reported home smoking bans, and a 13% decrease in caregiver smokers compared with education‐alone group (all P values < 0.05). Child health: N/A |
| Ekerbicer 2007 | This study from Turkey recruited ETS exposed children from a primary school. Parents of identified children received telephone counselling or a note regarding their child's urinary cotinine result. At 9 months follow‐up: Group one 74/93 students had urinary cotinine levels < 10 ng/ml; group two 69/93 had urinary cotinine < 10 ng/ml. "The proportion of children with urinary cotinine values < 10ng/ml were statistically similar (P > 0.05) in both groups". |
| Elder 1996 | Social learning model used. No evidence of effect on tobacco‐free school policy after 3 years: |
| Emmons 2001 | Motivational Interviewing used. Quit rates: Intervention 7.5%, Control 10.1%, P > 0.05 |
| Eriksen 1996 | No evidence of effect. |
| Fossum 2004 | Social learning model used. Self‐reported smoking (number of cigarettes) 1 month before childbirth: Intervention 13.1 vs Control 10.8 NS; 3 months after childbirth Intervention 12.8 vs Control 8.2 (significant); Past 24 hrs Intervention 11.8 vs Control 7.8 (significant). |
| French 2007 | Six month follow‐up data Saliva cotinine verified non smoker: intervention (N = 26, 22%), control (N = 9, 10%) ‐ P < 0.025 Self‐reported non‐smoker: intervention (N = 40, 33%), control (N = 21, 22%) ‐ P < 0.10 |
| Greenberg 1994 | Social learning model used. Targeted ETS exposure in infants less than six months of age, and aimed to reduce the incidence of lower respiratory tract illness and the prevalence of respiratory symptoms. For infants of smoking mothers it demonstrated a lower prevalence of persistent symptoms in the intervention group (17.8%) compared with control group (30.9%; risk difference 13.1%; 95% CI: 1.0 to 27.0%). There was no difference in the incidence of illness. |
| Groner 2000 | No evidence of effect. |
| Hafkamp‐de 2014 | ETS exposure: No significant difference in ETS exposure at home between intervention and control groups at age 6 years in the intention to treat analyses (OR 0.82, 95% CI: 0.66, 1.03); though this reached statistical significance in per‐protocol analysis with intervention group having less ETS exposure at age 6 years than the control group (OR 0.71, 95% CI: 0.59, 0.87). No effect modification by sociodemographic characteristics (data not shown). Air quality: N/A Child health: No significant differences between groups in asthma, wheezing frequency, airway inflammation (exhaled NO), or airway resistance (Rint). |
| Halterman 2011 | Motivational Interviewing used. Symptom‐free days/2 wk (difference) 0.96 (95% CI 0.39 to 1.52) |
| Hannover 2009 | Motivational Interviewing used. At 24 months follow‐up Sustained abstinence: intervention (N = 36, 12%, 95% CI 8.8 to16.2), control (N = 39, 11%, 95% CI 8.4 to15.1), no statistically significant difference in proportions (0.7, 95% CI ‐4.2 to 5.8) Four week point prevalence: intervention (N = 72, 24% 95% CI 19.6 to29.2), control (N = 67, 19%, 95% CI 15.6 to23.9), no statically significant difference in proportions (4.7, 95 CI ‐1.7 to 11.1). |
| Harutyunyan 2013 | ETS exposure: Adjusting for baseline hair nicotine concentration, child's age and gender, the follow‐up geometric mean hair nicotine concentration in the intervention group was 17% lower than the control group (P = 0.239). The GM of hair nicotine in the intervention group significantly decreased from 0.30 ng/mg to 0.23 ng/mg (P = 0.024), but not in the control group. Adjusted odds of children's less than daily exposure to SHS at follow‐up was 1.87 times higher in the intervention group than in the control group (P = 0.077). Air quality: According to mothers, 4.5% intervention households and 5.4% control households completely banned indoor smoking at follow‐up. Also 4.5% smokers in the intervention group and 0.9% in the control group have reportedly stopped smoking at follow‐up. Child health: N/A |
| Herbert 2011 | Recruited families to participate in the study through five public health nursing offices, eight daycare centres, and kindergartens on Prince Edward Island. Used a family‐centred assessment and intervention model to empower families to reduce cigarettes smoked in the home. Those identified as having children exposed to ETS were then invited to participate in group counselling sessions. Intervention: decrease from median of 17 to 4.5 cigarettes/day and Control: decrease from 18.5 to 3.5 cigarettes/day. Both decreases statistically significant so did not detect a beneficial effect of the intervention. At 6 months follow‐up intervention participants smoked 0.65 (95% CI ‐5.68 to 6.98) more cigarettes per day compared to control participants |
| Hovell 2000 | Reduction in parent‐reported child exposure to cigarettes in the home and in total. At home reported exposure Intervention baseline 3.9 CPD, follow up 0.52 CPD vs Control 3.51 CPD baseline, 1.20 CPD follow up. The trend for parent‐reported total CPD exposure was similar. Achieved a reduction in the number of parent‐reported cigarettes smoked in the presence of children per day at 12 months, following a three‐month intensive counselling intervention. There was, however, no change in cigarette smoke absorption as measured by children's urinary cotinine (ng/ml) for the intervention group over the 12 months (with measures collected at 3, 6 and 12 months). Cigarette smoke absorption for the control group increased from 9.4 ng/ml to 17.5 ng/ml over this time period, whereas there was almost no change in the intervention group (10.9 at baseline and 10.5 at 12 months). This increase in absorption observed for children in the control group appears to account for the apparent benefit of the intervention group. However the argument that this is solely due to reduced exposure in the home is uncertain, as the mothers in both the intervention and control groups reported falls in mothers' cigarettes smoked in the presence of the child from 3.9 to 0.5 (intervention) and 3.5 to 1.2 (control) cigarettes per day. In addition, they reported falls in total exposure to any source of cigarettes per day from 7.3 to 1.2 (intervention) and 7.2 to 2.8 (control). As the cotinine indicates a minimal fall for the intervention group and almost a doubling in urinary cotinine for the control group, either the cotinine measurement is unreliable or, more probably, that the parental report of cigarette exposure is not reliable. |
| Hovell 2002 | Latino families targeted. No significant effect. |
| Hovell 2009 | Low income households targeted. Behavioural ecological model used for development of the counselling intervention. Children's total SHSe showed a significant group by linear time interaction (P = 0.012) and a linear time effect (P < 0.001) from baseline to 6 months. Children's urinary cotinine showed no significant difference. Exposure from mothers in home (reported cigarettes/week) intervention 1.93 (95% CI 0.92 to3.48) control 6.16 (95% CI 3.61 to10.12); total reported exposure (cigarettes/week) intervention 5.15 (95% CI 2.71 to9.17) control 22.97 (95% CI 15.14 to34.58); mothers smoking reported cigarettes/week intervention 77.91 (95% CI 64.22 to91.60) control 92.88 (95% CI 80.59 to105.16); reported smoking by mothers indoors at home (cigarettes/week) intervention 3.94 (95% CI 2.06 to6.97) control 10.37 (95 CI 6.16 to17.06); reported smoking by all indoors at home (cigarettes/week) intervention 6.46 (95% CI 3.16 to12.40) control 19.18 (95% CI 11.15 to32.52). Children's urinary cotinine concentration and mother's reported smoking showed a significant group main effect, but did not show a significant difference in rates between intervention and control groups at 18 months. |
| Hughes 1991 | Intervention to reduce children's ETS exposure in a study of a comprehensive asthma education intervention. The outcome was improved asthma control but no change in exposure to ETS. |
| Irvine 1999 | No evidence of effect. |
| Joseph 2014 | ETS exposure: Little change in household or car rules about smoking 8 weeks after index visit, but parents reported a high rate of total restriction at baseline. Air quality: 8 weeks after index visit, 11 of 38 (29%) parents in the intervention group reported 7‐day point‐prevalent abstinence. In contrast, only one parent in the comparison group reported abstinence from smoking (P = 0.001). There were fewer quit attempts and less readiness to quit in the comparison group. Child health: Not reported |
| Kallio 2006 | At child 8 years of age 10.1% (29/287) of mothers and 19.7% (43/218) fathers in the intervention group smoked regularly. The corresponding %s for the control group were 15.1% (45/298) mothers and 25.1% (60/239) fathers. Additionally 5.9% (17/287) of intervention group mothers and 8.3% (18/218) of intervention group fathers smoked occasionally compared with 5.7% (17/298) of control group mothers and 6.7% (16/239) of control group fathers (NS). |
| Kegler 2015 | ETS exposure: Significantly more intervention participants reported a full ban on smoking in the home than control participants at both 3 months (30.4% vs 14.9%, P < 0.001) and 6 months (40.0% vs 25.4%, P = 0.002) post‐baseline. The longitudinal intent‐to‐treat analysis showed that the difference in change was significant over time. When defining success more stringently by including only those reporting a full ban and no enforcement challenges, we found again that more intervention than control participants were successful in having and enforcing their smoke‐free home rule at 3 months (11.0% vs 5.6%; P = 0.03) and at 6 months post baseline (18.3% vs 8.7%; P = ).002). Air quality: Larger reduction in self‐reported exposure to SHS in the home among intervention participants at both follow‐up points, with a significantly larger decrease in the intervention group. In addition, significantly higher percentage of intervention participants (26.2% vs 18.0%) reported a full smoking ban in cars at 3 months (P = 0.02), although this difference was not observed 6 months post baseline. Child health: Not reported |
| Kimata 2004 | After 1 month urinary cotinine levels reduced 285±43 ngmL‐1 to 2.2±0.85 ngmL‐1 in AEDS cessation group, 257±31 ngmL‐1 to 1.8±52 ngmL‐1 in normal child cessation group and 274±42 ngmL‐1 vs 298±52 ngmL‐1 in control group of children with AEDS. AEDS children showed significant reduction in SCORAD index skin wheal (mm) from 9.9 baseline to 7.5; Control group 9.6 baseline to 9.3. Also significant changes in response to house dust mite & cat dander & lower neutrophil levels. |
| Krieger 2005 | Intervention guided by the transtheoretical stages of change model, as well as by social cognitive theory. Report that 20% of the sample quit smoking and that among smokers who did not go outside to smoke prior to intervention, a quarter did so after education, but data are not provided and it is unclear whether intervention outcomes were different between groups. |
| McIntosh 1994 | Number of smokers who moved outside: Intervention 7/30, Control 4/30. Not statistically significant. |
| Nicholson 2015 | ETS exposure: At the end of the follow‐up phase, 45.4% of families reported a home ban (intervention: 47.2%; control: 43.6%) and 20.4% employed a full ban (intervention: 24.5%; control: 16.4%). Group assignment (intervention or control) was not a significant predictor of adopting a home ban. There was a marginal difference between intervention and control groups for the adoption of full bans (OR = 1.81, P = .060). Air quality: Not reported Child health: Not reported |
| Nuesslein 2006 | Calculated nicotine consumption Intervention: 12 micrograms to 4.65 micrograms vs Control: 12 micrograms to 7.5 micrograms NS |
| Ortega 2015 | ETS exposure: TSP‐avoidance strategies improved more in the intervention group than in the control: 35.4% and 26.9% (P = 0.006) at home, and 62.2% and 53.1% in cars (P = 0.008). Logistic regression showed adjusted ORs for appropriate measures in the intervention group vs control group of 1.59 (95% CI 1.21 to 2.09) at home and 1.30 (95% CI 0.97 to 1.75) in cars. Air quality: Not reported Child health: Not reported |
| Patel 2012 | No significant differences between intervention compared to control groups in: |
| Phillips 2012 | Where both saliva cotinine and self‐report were available, saliva cotinine was used. At eight weeks post‐partum, there was a significantly more smoke free mothers in the intervention (81%) compared with the control group (46%) ‐ P < 0.001. |
| Pollak 2015 | ETS exposure: Not reported Air quality: Found high rates of cessation but no arm differences in smoking rates at the end of pregnancy (0.31 vs. 0.30, materials only vs. counselling, respectively) and 12 months after randomisation (postpartum: 0.39 vs. 0.38). Found high quit rates among non daily smokers but no arm differences (0.43 vs. 0.46 in pregnancy and 0.52 vs. 0.48 postpartum). Among daily smokers, found lower quit rates with no arm differences but effects favouring the intervention arm (0.13 vs. 0.16 in pregnancy and 0.17 vs. 0.24 postpartum). Child health: Not reported |
| Prokhorov 2013 | Smoking status of smoker; 90% on baseline smokers in each group still using tobacco (N = 36 intervention, N = 35 control) Results for the environmental monitors: two monitors ‐ one in a "higher exposure" room than the other. In the high exposure room there was a significant main effect for time (P < 0.001) and time by condition effect (P < 0.05); for the intervention group the mean ambient nicotine level decreased from baseline at 12 months (1.14 μg/m3 to 0.20μg/m3, P < 0.01). There was a decrease in mean of control group but not significant (0.55 μg/m3 to 0.17μg/m3, P = .99), and a significant difference between average rate of change for intervention and control groups. In the low exposure there was a significant main effect for time but not time‐by‐condition and similar reductions in the intervention and control groups. Percentage of households banning smoking at 12 months: 73% of the intervention group and 56% of the control group. |
| Pulley 2002 | Follow‐up three weeks post‐intervention Cigarettes/day: intervention 16.17 (sd 9.10), control 11.33 (sd 4.69) ‐ P = 0.132 Mothers in the intervention group smoked more at enrolment compared with control group, an effect not present at the 2 week visit (baseline) but present again three weeks post intervention Respiratory illness: intervention N = 5 (42%), control N = 6 (66%) ‐ P = 0.666 |
| Ralston 2008 | Counselling strategies based on the stages of change component of Prochaska's transtheoretical model. N = 42, 33% (N = 14) lost to follow‐up. The quit rate: 14% intervention, 5% control group which did not reach statistical significance |
| Ralston 2013 | Differences between intervention and control groups were not significant (fisher's test): Self‐reported quit ‐ control 6/30 (20%, 95% CI 9 to 38%) and intervention 5/30 (17%, 95% CI 7 to 34%); any quit attempt during follow‐up ‐ control 11/30 (37%, 95 CI 22 to 55%) and intervention 16/30 (53%, 95% CI 36 to 70%); cut down ‐ control 11/30 (27%, 95% CI 22 to 55%) and intervention 15/30% (15%, 95 CI 33 to 67%); used quitline ‐ control 2/30 (7%, 95% CI 8 to 22%) and intervention 0/30 (0%, 95% CI 0 to 13%). |
| Ratner 2001 | 6 month Follow up: 36% abstinent, 26% occasional, 38% daily smoking. 76% homes smoke‐free. |
| Schonberger 2005 | At 6 month Follow up |
| Schuck 2014 | ETS exposure: Not reported Air quality: Parents who received quitline counselling were more likely to report 7‐day point‐prevalence abstinence at 12‐month assessment (34.0 versus 18.0%, odds ratio (OR) = 2.35, confidence interval (CI) = 1.56–3.54) than those who received a standard self‐help brochure. Parents who received quitline counselling were more likely to use nicotine replacement therapy (P < 0.001) than those who received a standard self‐help brochure. Among parents who did not achieve abstinence, those who received quitline counselling smoked fewer cigarettes at 3‐month (P < 0.001) and 12‐month assessment (P < 0.001), were more likely to make a quit attempt (P < 0.001), to achieve 24 hours’ abstinence (P < 0.001) and to implement a complete home smoking ban (P < 0.01). Child health: Not reported |
| Severson 1997 | Cessation at 6 & 12 months: Intervention 25/1073 (2.3%), Control 10/802 (1.2%), P < 0.05*, 1‐tailed test Only 35 of the 97 12‐month quitters had quit by six months, with more early quitters in the intervention group (25/59) compared with the control group (10/38). |
| Stotts 2012 | Lower rates of total smoking bans in the usual care‐reduced measurement group (P < 0.012 for total ban, P < 0.01 for car) but not significantly different for home alone. 63.6% receiving motivational interviewing had a ban by 1 month post‐discharged compared to 20% of the usual care group. No significant differences in environmental nicotine monitors measurements |
| Streja 2014 | ETS exposure: No significant difference between intervention and control groups in child urine cotinine levels. Air quality: No significant difference between intervention and control groups in any of the measures. Child health: Not reported |
| Tyc 2013 | Group difference for average cigarettes smoked and child SHSe was not significantly different as the 12‐month follow‐up (P > 0.05). Child SHSe was significantly lower at 12 months from baseline for each group (P < 0.05). Children's urinary cotinine showed no significant difference, and did not change significantly over time in either group. |
| Ulbricht 2014 | ETS exposure: The child urine cotinine level difference between follow‐up and baseline was smaller in the control than in the intervention group, but the effect was not significant. Air quality: Not reported Child health: Not reported |
| Van't Hof 2000 | There was no statistically significant difference in the smoking relapse rate between women in the intervention (41%) and control (37%) groups. |
| Vineis 1993 | Smoking cessation for mothers: Intervention 12/74 vs Control 10/84, OR 1.4, 95% CI 0.6 to 3.5 showed a trend towards smoking cessation for mothers classified as white collar workers in the intervention arm (5/33) versus the control arm (2/36) (Odds Ratio [OR] 3.0; 95% confidence intervals [CI] 0.6 to 16.0). No difference was detected for the other participants, comprising 80 blue collar mothers and a total of 411 men defined as white or blue collar workers. |
| Wahlgren 1997 | Intensive intervention was able to demonstrate a statistically significant but very small reduction in cigarette exposure from parents' cigarettes reported by parents without biological verification. Mean number of parent cigarettes smoked in presence of child fell in Intervention group: 5.8CPD baseline, 3.4CPD at clinic pre‐intervention to 1.2 CPD at 6 months following completion of intervention. In control group, parent reported exposure fell from 8.0 baseline, 5.7 pre‐intervention to 4.6 CPD at 6 month follow up. P for trend < 0.01. The effect size was small, however, and curiously, the largest fall in this measure occurred in the period after recruitment but before the intervention. After the intervention, parents reported a reduction of 1.1 cigarettes per day smoked in the presence of the children for the control group, and 2.2 cigarettes per day for the intervention group. There was no validation by measurement of children's exposure or absorption via cotinine, or validation of the parental reports, and the clinical significance of such a fall is unclear |
| Wakefield 2002 | Home smoking ban: |
| Walker 2015 | ETS exposure: No significant difference between group in urine cotinine level change over time, self‐reported SHS exposure, smoking ban, smoking cessation. Air quality: No significant change in smoking prevalence and intensity was seen by group. Child health: No significant difference in infant cough, acute respiratory illness or rate of hospitalisations between treatment groups. |
| Wang 2015 | ETS exposure: Children's urinary cotinine was significantly lower (Z = ‐3.136; P = 0.002) in the intervention group (1.29 ng/mL) than the control group (1.78 ng/mL). After 6months, reported mean ETS exposure from caregivers decreased 40.6% from baseline among the intervention group and 3.4% among controls. Air quality: Caregiver's 7‐day quit rate was significantly higher (34.4% versus 0%) (p < 0.001; adjusted OR = 1.13; 95% CI: 1.02‐1.26) in the intervention group. Child health: Not reported |
| Wiggins 2005 | Mothers living in disadvantaged inner city areas targeted. No significant effect of either intervention. |
| Wilson 2001 | Of 51 children with complete urinary cotinine: creatinine ratio (CCR) data. Log CCR (ng/mg) Intervention baseline 1.82, Follow up 1.27 vs Control baseline 2.34, Follow up 1.93, adjusted Diff ‐0.38, adjusted P = 0.26. examined the effect of an intervention targeting smoking behaviour change and asthma education on health care utilisation and asthma hospitalisations, and explored other measures of asthma control. It demonstrated a reduction in the prevalence of children making more than one acute care asthma visit in the year following the intervention. Given that there was no apparent benefit of the smoking‐related counselling on smoking‐related outcomes, it is likely that it was the asthma education that achieved the improvement in asthma morbidity, rather than the smoking behaviour programme. |
| Wilson 2011 | Mean urinary cotinine creatinine ratio (CCR) decreased in both groups (not shown data for 6 and 12 month follow‐up). The natural log of the urinary CCR decreased more in the intervention arm but it did not reach statistical significance (B coefficient ‐0.307 95% CI ‐0.633 to 0.018, P = 0.64) Decrease in asthma symptoms at follow‐up visits in both groups. The decrease in the intervention group did not reach statistical significance (B coefficient 0.035, 95% CI ‐0.208 to 0.277, P = 0.78) At 12 months 84.0% of the intervention group (N = 142) and 77.1% of the control group (N = 131) had home smoking bans (P = 0.11). |
| Winickoff 2010 | Prevalence of self‐reported 7 day abstinence 38% at baseline and 30% at follow up in the control group vs 31% at baseline and 30% at follow up in the intervention group (Effect size = 13% P = NS) Cotinine‐confirmed 7 day abstinence for baseline current smokers NS. |
| Woodward 1987 | No evidence of effect. |
| Yilmaz 2006 | Quit smoking: Child intervention group 24.3%; Mother intervention group 13%; Control 0.8%. (χ2 = 29.5, P < 0.0001) |
| Yucel 2014 | ETS exposure: No significant difference between intensive and minimal intervention groups in change in child urine cotinine levels. Air quality: No significant difference in any outcome. Child health: Not reported |
| Zakarian 2004 | Low income ethnically diverse population. Both groups showed significant decline in reported exposure to mother's cigarette's/week (intervention group 18.89 at baseline to 5.41 at 12 months, control group 13.25 at baseline to 5.23 at 12 months) (P < 0.001). Total exposure to cigarettes/week (intervention group 53.2 at baseline to 21.99 at 12 months, control 54.48 at baseline to 18.22 at 12 months) (P < 0.001) however, no significant difference between groups. |
| Zhang 1993 | This was a study designed to increase public knowledge of the health consequences of cigarette smoking and to promote healthier attitudes among elementary school students in China, and encouraged these students to help their fathers to quit smoking. Schools in one district used a tobacco control curriculum, and the control group were students in another district. The other school‐based study was a cardiovascular health promotion programme that included an intervention designed to limit children's ETS exposure and negative role modelling from staff and visitors smoking at school (Elder 1996). Conducted in the USA, this study used a cluster‐randomized design with schools as the unit of allocation.Number (proportion) of smoking fathers: Intervention baseline 6843/9953 (68.8%) & follow up 60.7% vs Control baseline 6274/9580 (65.5%), follow up "approximately the same" [numbers are not stated] |
Comparison 1 Results, Outcome 1 Main outcomes.
| Community‐based interventions for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
| Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: community Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 | Quality of the evidence | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 3 to 12 months | Of 7 studies in this group, 3 found that the intervention group was significantly more likely than the control group to implement full home smoking bans. One study found that the geometric mean hair nicotine level in the intervention group significantly decreased from 0.30 ng/mg to 0.23 ng/mg (P = 0.024), but not in the control group. Four studies found no significant differences in the change in cotinine levels between intervention and control groups. | 2880 | +‐‐‐ VERY LOW3 | |
| Multi‐comoponent, education‐based interventions assessed with biochemical validation of ETS exposure length of follow‐up: 6 months | One study, with similar children’s urinary cotinine levels at baseline, found that cotinine levels were significantly lower (Z = ‐3.136; P = 0.002) in the intervention group (1.29 ng/mL) than in the control group (1.78 ng/mL) at 6 month follow‐up. The other study found no significant differences between intervention and control groups in child urine cotinine levels. | 307 | +‐‐‐ VERY LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 1 to 12 months | Of the 6 studies in this group, 3 found significantly greater reductions in cotinine levels in the intervention compared with the control group. Two studies found that the intervention group was significantly more likely to implement home smoking bans. Two studies found no significant intervention impacts. | 1001 | +‐‐‐ VERY LOW5 | |
| Telephone counselling assessed with biochemical validation of ETS exposure length of follow‐up: 9 months | One study found no significant difference in the proportion of children with low urinary cotinine levels (< 10 ng/mL) amongst parents receiving telephone counselling or a note regarding their child’s cotinine result. | 347 | ++‐‐ LOW6 | |
| ETS: environmental tobacco smoke | ||||
| 1 Not all studies reported length of follow‐up; length given based on those that reported. 2 Not all studies reported numbers of participants; number provided based on those that reported. 3 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 4 Downgraded one level due to risk of bias: one of two studies at high risk of bias. Downgraded two levels due to inconsistency: one study detected an effect and one did not; studies were clinically heterogeneous. 5 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 6 Downgraded one level due to risk of bias: one study at unclear risk of bias. Downgraded one level due to imprecision: only 186 participants with measured outcomes at nine‐month follow‐up. | ||||
| Interventions in the ill‐child setting for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
| Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: healthcare ‐ ill‐child setting Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 | Quality of the evidence | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 5 to 12 months | Three studies found no significant differences between intervention and control groups. | 746 (3 studies) | +‐‐‐ VERY LOW3 | |
| Multi‐component, education‐based interventions assessed with biochemical validation of ETS exposure and self‐report length of follow‐up: 6 to 13 months | One study reported significantly lower child's ETS exposure at home by any smoker at 12 months' follow‐up (52% vs 58%; P = 0.03). Six studies found no significant differences between intervention and control groups. | 2936 (7 studies) | +‐‐‐ VERY LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 3 to 18 months | Eight studies appeared to show intervention benefits based on self‐reported ETS exposures but no significant differences between intervention and control groups in objective measures of exposure (e.g. cotinine). | 1835 (8 studies) | +‐‐‐ VERY LOW5 | |
| Telephone counselling | No studies examined telephone counselling delivered in the ill‐child setting and measured ETS exposure. | |||
| Brief interventions Assessed with presence of home and car smoking ban length of follow‐up: 24 weeks | One study showed no significant differences between intervention and control groups in changed smoking policy: OR 2.0 (95% CI 0.166 to 24.069). | 100 (1 study) | +‐‐‐ VERY LOW6 | |
| GRADE Working Group grades of evidence | ||||
| 1 Not all studies reported length of follow‐up; length given based on those that reported. 2 Not all studies reported numbers of participants; number provided based on those that reported. 3 Downgraded one level due to risk of bias: two studies at unclear risk of bias. Downgraded one level due to imprecision. Downgraded one level due to indirectness: all studies were set in the USA and cannot be generalised to low income countries where smoking is more prevalent. 4 Downgraded two levels due to risk of bias: five of seven studies at high or unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 5 Downgraded two levels due to risk of bias: all eight studies at high or unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 6 Downgraded two levels due to risk of bias: only study was at high risk of bias. Downgraded one level due to imprecision: small study with a small number of events and wide confidence interval. | ||||
| Interventions in the well‐child setting for reducing children's exposure to environmental tobacco smoke (ETS) | ||||
| Patient or population: people who smoke and are involved in the care of young children (birth to 12 years of age) Settings: health care ‐ well‐child setting Intervention: behavioural interventions Comparison: usual care or minimal intervention | ||||
| Intervention type and outcomes1 | Impact | No. of participants2 | Quality of the evidence | Comments |
| Multi‐component, counselling‐based interventions assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 2 to 12 months | One study found significant reduction in ETS exposure at home in the intervention group at age 6 years, but only on per‐protocol analysis (OR 0.71, 95% CI 0.59 to 0.87). One study found an increase in smoking bans in the home (19.3%) and in the car (7%) after 8 weeks' follow‐up in the intervention group, but not in the comparison group (2.5% increase in home ban and 0% change in car ban). One study found no significant difference between intervention and control groups in children’s urinary cotinine levels. | 8005 (3 studies) | +‐‐‐ VERY LOW3 | |
| Multi‐component, education‐based interventions assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 2 to 12 months | One study found that maternal self‐reported smoking at home around the infant was significantly less in the intervention group (8.6%) than in the control group (23.8%) (P < 0.05). Three studies found no evidence of effect of the intervention. | 1401 (4 studies) | ++‐‐ LOW4 | |
| In‐person counselling (no additional components) assessed with biochemical validation of ETS exposure, self‐report length of follow‐up: 3 to 90 months | One study found significantly greater reductions in geometric mean urinary cotinine in the intervention group (decrease from 48.72 ng/mg to 28.68 ng/mg) compared to the control group (decrease from 40.43 to 36.32 ng/mg). In addition, the intervention group had a significantly greater increase in the proportion of households with smoking bans at home (15% to 33.3%) compared to the control group (11.5% to 19.5%). One study found a significantly beneficial reduction in kitchen and TV room air nicotine levels in the intervention group than in the control group (P < 0.05). One study found no difference in serum cotinine concentrations between the intervention and control groups. | 1483 (3 studies) | ++‐‐ LOW5 | |
| Telephone counselling assessed with self‐report length of follow‐up: 6 months | One study found a greater proportion with partial home smoking bans in the intervention group (62.7%) than in the control group (56.4%), as well as a higher biochemically validated quit rate for the intervention group (10.6%) than for the control group (4.5%) at 6 months. | 952 (1 study) | ++‐‐ LOW6 | |
| Brief interventions assessed with self‐report length of follow‐up: not specified | One study found no significant difference in home (OR 1.04, 95 CI 0.47 to 2.28) or car smoking bans (OR 1.47, 95 CI 0.69 to 3.11) between intervention and control groups. | 218 (1 study) | +‐‐‐ VERY LOW7 | |
| CI: confidence interval; OR: odds ratio | ||||
| 1 Not all studies reported length of follow‐up; length given based on those that reported. 2 Not all studies reported numbers of participants; number provided based on those that reported. 3 Downgraded two levels due to risk of bias: all studies at unclear or high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 4 Downgraded one level due to risk of bias: one study was at high risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 5 Downgraded one level due to risk of bias: two of three studies at unclear risk of bias. Downgraded one level due to inconsistency: interventions and populations were clinically heterogeneous. 6 Downgraded one level due to risk of bias: included study at unclear risk of bias. Downgraded one level due to indirectness: ETS exposure was measured indirectly as reported smoking restrictions in home. 7 Downgraded one level due to risk of bias: included study at unclear risk of bias. Downgraded one level due to indirectness: ETS exposure was measured indirectly as reported smoking restrictions in home and car. Downgraded one level due to imprecision: one study with a small number of participants and events. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Main outcomes Show forest plot | Other data | No numeric data | ||

