Tipo de injertos para la cirugía de revascularización femoropoplítea
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Site: Femoral to AK popliteal Study design: Single‐centre RCT Method of randomisation: sealed envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: none | |
| Participants | Country: Holland No. of participants: 85 patients(93 limbs; 46 PTFE, 47 HUV) Age: 64 yrs Sex: 67 male, 18 female DM 16, critical 17 Exclusion criteria: those with previous femoro‐popliteal graft | |
| Interventions | 6 mm PTFE versus 6 mm HUV | |
| Outcomes | Primary patency, secondary patency, complications | |
| Notes | All had post‐op anticoagulants | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Random permuted blocks" |
| Allocation concealment (selection bias) | Unclear risk | Not specifically stated. Probably not done |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Some patients lost to follow‐up early on, but clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other obvious bias |
| Methods | Site: Femoral to AK popliteal Study design: Multicentre RCT Method of randomisation: central randomisation, but exact method unclear Blinding: unblinded, intention to treat Exclusions post randomisation: not discussed Losses to follow up: high rate of losses to follow‐up (37 within first 12 months of follow‐up) | |
| Participants | Country: USA Setting: multicentre No. of participants: 231 patients (240 limbs; 122 PTFE, 118 Dacron) Age: mean 67.1 yrs Sex: 145 male, 95 female Inclusion criteria: angiographically demonstrated superficial femoral artery occlusion with reconstitution of a popliteal segment above the knee Exclusion criteria: earlier infrainguinal vascular procedures Unclear whether patients had IC or critical ischaemia | |
| Interventions | PTFE versus Dacron (diameter at discretion of operating surgeon) | |
| Outcomes | Primary patency, secondary patency, peri‐operative complications | |
| Notes | 13 patients randomised but not described. Unclear how many patients had post‐op aspirin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised centrally after eligibility was determined by the operating surgeon and informed consent obtained." |
| Allocation concealment (selection bias) | Unclear risk | Not specifically stated. Probably not done |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | High risk | 37 patients randomised lost by 12 months |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Anticoagulation protocol not stated |
| Methods | Site: Femoral to AK popliteal Study design: RCT Method of randomisation: concealed randomisation using computer generated randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: none | |
| Participants | Country: Italy Setting: hospital No. of participants: 51 (102 limbs; 51 PTFE, 51 reversed vein) Age (mean): 62 yrs Sex: 33 males, 18 females Inclusion criteria: severe claudication, SFA occlusion with one to three runoff vessels Exclusion criteria: untreated inflow disease of ipsilateral pelvic arteries (more than 50% stenosis or occlusion); previous bypass procedure or stent in target SFA; multiple lesions exceeding 10 cm; acute critical limb ischaemia; an untreated ipsilateral iliac artery stenosis; known intolerance to study medications or contrast agents | |
| Interventions | 8 mm PTFE and reversed vein graft Oral warfarin from one day pre‐op and continued for 6 months; 325 mg aspirin afterwards | |
| Outcomes | Primary assisted patency as remedial surgery for late bypass stenosis was not considered a primary failure 5‐year data | |
| Notes | Compliance with medication not checked | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Concealed randomisation using computer generated randomisation envelopes." |
| Allocation concealment (selection bias) | Low risk | Envelopes sealed as above |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | No patients lost to long term follow up (mean 59 months) |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No other obvious bias |
| Methods | Site: Femoral to AK popliteal Study design: RCT Method of randomisation: not described Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: not specified | |
| Participants | Country: Serbia Setting: hospital No. of participants: 85 (43 ePTFE, 42 Dacron) Age (mean): 65.5 yrs Sex: 71 males, 14 females Inclusion criteria: severe claudication or critical ischaemia, "considered suitable for surgical revascularization using above‐knee prosthetic bypass graft" Exclusion criteria: previous procedures on aorto‐iliac or ipsilateral femoro‐politeal arterial segments | |
| Interventions | 8 mm FlowNit Biosel (Dacron) or 8mm FlowLine BioPore (ePTFE) bypass graft from femoral to above‐knee popliteal artery. All patients given 4 days' antibiotic prophylaxis with a second generation cephalosporine and started on acetylsalicylic acid immediately after surgery | |
| Outcomes | Primary: primary patency, early complications (mortality, bleeding and infection), early limb salvage Secondary: secondary patency, mid‐term complications (mortality, false anastomotic aneurysms and infection), mid‐term limb salvage | |
| Notes | Clear antibiotic and antiplatelet protocols | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not discussed |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | High risk | Numbers at risk not presented with survival curves, secondary patency presented as worse than primary patency, which is impossible |
| Selective reporting (reporting bias) | Low risk | All outcomes presented, but numbers at risk at different time points not given so impossible to discern significance of different rates |
| Other bias | Low risk | Clear antiplatelet and antibiotic protocols |
| Methods | Site: Femoral to AK and BK popliteal Study design: RCT Method of randomisation: concealed randomisation using computer generated randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: none | |
| Participants | Country: UK Setting: hospital No. of participants: 209 (AK: 88 PTFE, 91 HBD; BK: 15 PTFE, 15 HBD) Age (mean): 63 yrs Sex: 142 males, 67 females Inclusion criteria: severe claudication, SFA occlusion with one to three runoff vessels Exclusion criteria: emergency surgery for trauma, acute thrombosis, embolism, or popliteal artery thrombosis | |
| Interventions | HBD or PTFE (diameter at discretion of operating surgeon) Anticoagulation not stated | |
| Outcomes | Primary patency | |
| Notes | Anticoagulation not stated | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization, stratified for AK or BK and by surgeon, was performed for eligible patients, using a dedicated computer program." |
| Allocation concealment (selection bias) | Low risk | Quote: "Sealed randomization envelopes (1 for AK, 1 for BK) were delivered to the vascular surgeon before surgery." |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | No losses, but numbers at risk not given for below knee outcomes so attrition not clear for this outcome |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Anticoagulation protocol not stated |
| Methods | Site: Femoral to BK popliteal Study design: multicentre RCT Method of randomisation: sealed envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: none | |
| Participants | Country: Scandinavia Setting: hospital No. of participants: 105 (55 PTFE, 50 HUV) Age: 68 yrs Sex: 60 male, 45 female Inclusion criteria: DM 12, critical ischaemia 80. BK fem‐pop for short distance IC or critical ischaemia, if no vein or CABG intended Exclusion criteria: short life expectancy, previous graft, Buerger's, coagulopathy | |
| Interventions | PTFE versus HUV (diameter at discretion of operating surgeon) | |
| Outcomes | Secondary patency | |
| Notes | Post‐op anti‐thrombotic/coagulant therapy unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear as to how the randomisation sequence was generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Anticoagulation protocol not stated |
| Methods | Site: Ilio or femoral to AK popliteal Study design: single‐centre RCT Method of randomisation: not explicitly stated Blinding: stated to be single‐blind Exclusions post randomisation: not stated Losses to follow up: none Protocol violations: none stated | |
| Participants | Country: France Setting: hospital No. of participants: 18 (20 limbs; 10 PUR graft, 10 Dacron) Age (mean): PUR group: 70.7 years; Dacron: 70.5 years Sex: Overall 13 men, 7 women; PUR group: 6 men, 4 women; Dacron group: 7 men, 3 women Inclusion criteria: peripheral arterial occlusion of lower limb graded Fontaine stage IIb‐IV requiring AK synthetic ilio‐ or femoro‐popliteal bypass Exclusion criteria: obesity, emergency surgery, critical threat to limb | |
| Interventions | Iliac or Femoral to AK popliteal bypass graft with either 6 mm PUR or 6 mm Dacron | |
| Outcomes | Primary and secondary patency, complications in first 30 days, reintervention rate | |
| Notes | Clear anticoagulation/antiplatelet protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Unclear risk | Timing of randomisation not declared |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial, though participants were blinded |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors not obviously blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | No PRISMA flow chart, no mention of patients excluded prior to randomisation or after randomisation |
| Selective reporting (reporting bias) | Unclear risk | Primary and secondary patency as well as reinterventions reported, but no complications in first 30 days which did not lead to reintervention mentioned |
| Other bias | Low risk | Clear anticoagulation and antiplatelet protocol |
| Methods | Site: Femoral to AK or BK popliteal Study design: single‐centre RCT Method of randomisation: selecting a random card from an unsorted deck of cards marked with the choice of graft material Blinding: unblinded, no documented crossover so as treated/intention to treat analysis not discussed Exclusions post randomisation: none Losses to follow up: none Protocol violations: none | |
| Participants | Country: USA Setting: hospital No. of participants: 122 (59 AK of whom 29 ringed, 63 BK of whom 29 ringed) Age (mean): 71 yrs Sex: split not specified Inclusion criteria: patients without an available ipsilateral ASV long enough to serve as femoro‐popliteal bypass on the basis of a history of prior removal, duplex ultrasonography, saphenous venography or operative findings requiring an AK or BK femoro‐popliteal bypass. Patients whose life expectancy was judged to be less than 3 years were also included whether or not an ipsilateral ASV was available Exclusion criteria: patients with extensive necrosis requiring sequential grafts to distal arteries, patients requiring bypass for reasons other than arteriosclerotic occlusive disease | |
| Interventions | 6 mm ringed or unringed PTFE | |
| Outcomes | Primary patency, secondary patency, limb salvage (secondary patency and limb salvage not presented separately for above and below‐knee grafts so not included) | |
| Notes | Clear anticoagulation and antiplatelet protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by selection of "a random card from an unsorted deck of cards marked with the choice of graft material" |
| Allocation concealment (selection bias) | Unclear risk | Timing of randomisation not declared |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Clear anticoagulation and antiplatelet protocol |
| Methods | Site: Femoral to AK popliteal (POPUP study) Study design: RCT Method of randomisation: randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: 13 (8 Dacron, 5 PTFE) Losses to follow up: 51 (12%) | |
| Participants | Country: Scandinavia Setting: hospital (13 departments) No. of participants: 426 (413 for analysis due to exclusions; 205 PTFE, 208 Dacron) Age (mean): 66 yrs Sex: 152 males, 261 females Inclusion criteria: "chronic lower limb ischaemia" Exclusion criteria: less than 18, pregnant, could not obtain informed consent | |
| Interventions | 6 mm PTFE and 6 mm Dacron graft Anticoagulation as per individual centre protocol | |
| Outcomes | Primary patency, secondary patency and limb survival | |
| Notes | No common anticoagulation pathway. Multiple, different surgeons | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Grafts were contained in envelopes, however the randomisation procedure is unclear. Probably done as other papers from this unit clearly use random sequences (Eiberg 2006; Vogt 2007) |
| Allocation concealment (selection bias) | Low risk | Quote: "Immediately before surgery, the graft material was selected by a pre‐processed sealed envelope. Randomisation was stratified for each centre." |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | Anticoagulation as per individual centre protocol and therefore inconsistent |
| Methods | Site: Femoral to AK popliteal Study design: RCT Method of randomisation: concealed randomisation using computer generated randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: 11 (7%) | |
| Participants | Country: the Netherlands Setting: hospital No. of participants: 136 (151 limbs; 75 Saphenous vein, 76 PTFE) Age (median): 69 yrs Gender: 88 males, 48 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: patients with earlier bypass or previously removed long saphenous vein | |
| Interventions | 6 mm PTFE and reversed vein graft Oral warfarin from one day pre‐op continued for 6 months. 38 mg aspirin afterwards | |
| Outcomes | Primary and secondary patency 5‐year follow up | |
| Notes | No compliance checks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear. No specific description |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization took place with closed envelope allocation." |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | 13 patients lost to long term follow up, clearly described |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Oral warfarin from one day pre‐op continued for 6 months. 38mg aspirin afterwards |
| Methods | Site: Femoral to AK or BK popliteal Study design: multicentre RCT Method of randomisation: not stated Blinding: unblinded, as treated analysis Exclusions post randomisation: 3 (1.4%) Losses to follow up: 4 (1.9%) Protocol violations: 1 (treatment with a non test graft) | |
| Participants | Country: 18 centres in the USA and 7 in Europe Setting: hospital No. of participants: 209 (105 FUSION BIOLINE, 101 standard ePTFE, 2 no graft implanted, 1 non test graft implanted so latter 3 excluded) Age (median): 62 yrs in standard ePTFE group, 67 in FUSION BIOLINE group Sex: 145 males, 58 females; 2 excluded Inclusion criteria: patients requiring an AK or BK femoro‐popliteal bypass with the proximal anastomosis at the level of the distal external iliac, common femoral, profunda femoral, or proximal superficial femoral artery. The study protocol specified that a prosthetic femoro‐popliteal bypass must be medically necessary, but did not, per se, exclude those without an adequate autogenous conduit. Patients with Rutherford category 1 to 5 ischaemia were eligible, with symptoms of claudication, rest pain, or with superficial ulceration in the target lower extremity Exclusion criteria: acute arterial occlusion requiring urgent intervention; prior open surgical bypass in the target extremity; angioplasty or stenting at the site of a planned anastomosis within the previous 30 days; serum creatinine > 2.5 mg/dL; recent (< 6 weeks) MI or stroke; coagulation or bleeding disorders; receiving warfarin therapy where oral anticoagulation could not be withheld | |
| Interventions | FUSION BIOLINE heparin coated vascular graft or standard ePTFE graft (diameter at discretion of operating surgeon) | |
| Outcomes | Primary endpoints: efficacy: primary graft patency at 6 months as assessed by duplex ultrasound imaging and ABI. Safety: the composite of MALE and POD. MALE included major amputation, major graft reintervention with placement of a new graft or an interposition graft, open or percutaneous graft thrombectomy, pharmacologic thrombolysis, or graft excision. POD was defined as those that occurred within 30 days of the index procedure or any remedial procedure performed at the same anatomic site. Secondary endpoints: efficacy: primary assisted patency, secondary patency, and bleeding at the suture hole as judged subjectively by the operating surgeon and objectively by recording the time between restoration of flow into the graft and the absence of detectable bleeding from the suture holes | |
| Notes | No consistent anticoagulation protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation sequence generation technique |
| Allocation concealment (selection bias) | Unclear risk | Timing and method of randomisation allocation not stated |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | Only 4 patients had missing data at 6‐month follow‐up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
| Methods | Site: Femoral to AK and BK popliteal Study design: RCT Method of randomisation: concealed randomisation using computer generated randomisation envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: 3 (1%) Losses to follow up: 6 (2%) | |
| Participants | Country: Germany Setting: hospital No. of participants: 203 (194 limbs analysed. AK: 65 PTFE, 76 Dacron, BK: 26 PTFE, 27 Dacron) Age (median): 66 yrs Sex: 155 males, 48 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: infection, emergency surgery for acute ischaemia, distal anastomosis below anterior tibial origin, concomitant disease not expected to live past 3 years, contraindication to anticoagulants | |
| Interventions | PTFE and Dacron (diameter at discretion of operating surgeon) Post‐op warfarin, heparin or antiplatelet agents | |
| Outcomes | Primary patency 3‐year follow up | |
| Notes | No consistent anticoagulation protocol. No compliance checks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order of Secondary end‐points assignment had been generated by random digits from a statistical software package (SAS)." |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomised to either treatment arm intraoperatively by sealed envelopes." |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
| Methods | Site: BK popliteal and distal (the latter not included in this review) Study design: multicentre RCT Method of randomisation: concealed randomisation using sealed envelopes in blocks of 16 per centre Blinding: unblinded, intention to treat Exclusions post randomisation: 3 (1%) Losses to follow up: 0 (0%) Protocol violations: 3 (1 ‐ suitable vein available, 1 ‐ distal reconstruction below popliteal artery, 1 ‐ crossover from non‐collar to collar group) | |
| Participants | Country: 29 centres in Sweden and 3 in Denmark Setting: hospital No. of participants: 202 (87 PTFE, 115 PTFE with vein collar) Age (median): 79 yrs in PTFE group, 76 yrs in PTFE with collar group Gender: 77 males, 122 females; 3 excluded Inclusion criteria: rest pain, tissue loss Exclusion criteria: no suitable distal anastomotic target, distal anastomosis AK or below anterior tibial origin for BK popliteal group, or below‐ankle for distal group | |
| Interventions | Gore or Impra PTFE graft with or without distal vein cuff, diameter not specified (diameter at discretion of operating surgeon) | |
| Outcomes | Primary patency; secondary patency; amputation; death | |
| Notes | No consistent anticoagulation protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation sequence generation technique |
| Allocation concealment (selection bias) | Low risk | Envelope selected at random after confirmation of suitable target vessel |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | Only 3 patients had missing follow‐up data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
| Methods | Site: AK Study design: RCT Method of randomisation: controlled by the BOA‐trial agency using a dedicated computer program Blinding: unblinded, intention to treat Exclusions post randomisation: 8 (6%) Losses to follow up: 13 (9%) | |
| Participants | Country: the Netherlands Setting: hospital No. of participants: 137 (137 limbs with 8 excluded; 59 HBD, 70 HUV) Age (median): 65 yrs Sex: 87 males, 50 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: patients younger than 30 or older than 90 yrs of age; patients with an ABI higher than 0.8 at rest, emergency surgery for trauma, acute thrombosis or embolism of the popliteal artery, the diagnosis or treatment for malignancy within 12 months, hospital in‐patient treatment for cardiac failure in the previous 6 months, the absence of the possibility for adequate follow up or contraindications for anticoagulant drug therapy | |
| Interventions | Heparin bonded Dacron and HUV (diameter at discretion of operating surgeon) Aspirin 80 mg daily or coumarin derivates (Sintrom) | |
| Outcomes | Primary patency. 5‐year follow‐up | |
| Notes | No consistent anticoagulation protocol. No compliance checks | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was controlled by the BOA‐trial agency using a dedicated computer program." |
| Allocation concealment (selection bias) | Low risk | Not specifically stated but assumed done as BOA‐trial agency involved |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
| Methods | Site: AK popliteal Study design: single‐centre RCT Method of randomisation: concealed randomisation using sealed envelopes following intraoperative assessment of artery and vein Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: 9 (7%) Protocol violations: none | |
| Participants | Country: 1 centre in Bosnia Setting: hospital No. of participants: 109 patients, 121 limbs (12 patients had a second bypass in the contralateral limb during the study period). There were 60 reversed LSV bypasses and 61 prosthetic bypasses (PTFE or Dacron, material not further specified) Age (median): 70 yrs in reversed LSV group, 68 in prosthetic group Sex: 70 males, 51 females Inclusion criteria: rest pain, tissue loss, 'disabling claudication' Exclusion criteria: previous revascularisation in treated leg, LSV not available or suitable, CFA or AK popliteal not suitable site for anastomosis | |
| Interventions | Reversed LSV or 6 mm prosthetic bypass from CFA to above‐knee popliteal artery | |
| Outcomes | Primary patency, secondary patency | |
| Notes | All patients received prophylactic clexane at a dose of 0.5 ml/kg while in hospital and then 150 mg/day aspirin after discharge. Compliance with this protocol was not reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation sequence generation technique |
| Allocation concealment (selection bias) | Low risk | Envelope selected at random after confirmation of suitable target vessel and suitable vein |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | Only 7% of patients lost to follow‐up over 5 years |
| Selective reporting (reporting bias) | Low risk | Stated outcomes reported |
| Other bias | Unclear risk | Consistent anticoagulation protocol but no compliance checks reported |
| Methods | Site: Femoral to AK or BK popliteal Study design: multicentre RCT Method of randomisation: central randomisation centre assessment of artery and vein Blinding: unblinded, intention to treat Exclusions post randomisation: not specified Losses to follow up: not stated Protocol violations: none declared | |
| Participants | Country: UK Setting: multicentre No. of participants: 246 Inclusion criteria: femoro‐popliteal graft to AK (76 cuff, 74 no cuff) or BK (48 cuff, 47 no cuff) popliteal Exclusion criteria: trauma | |
| Interventions | 6 mm PTFE with and without a vein cuff | |
| Outcomes | Primary patency, secondary patency, limb salvage | |
| Notes | No consistent anticoagulation protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | No clear description |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Unclear risk | Attrition rates not clearly presented |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Unclear risk | No consistent anticoagulation protocol |
| Methods | Site: AK Study design: RCT Method of randomisation: unclear Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: 6 (6%) | |
| Participants | Country: France Setting: hospital No. of participants: 85 (103 limbs; 51 reversed vein, 52 polyester) Age (median): 69 yrs Sex: 49 males, 36 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: patients with earlier bypass or un‐useable LSV | |
| Interventions | 6 mm collagen‐impregnated woven polyester prosthesis and reversed vein graft Oral warfarin from one day pre‐op continued for 6 months. 38 mg aspirin afterwards | |
| Outcomes | Primary and secondary patency 5‐year follow‐up | |
| Notes | No medication compliance checks. Unclear randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation technique |
| Allocation concealment (selection bias) | Unclear risk | No clear description |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses, clear data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | No obvious other source of bias |
| Methods | Site: AK Study design: RCT Method of randomisation: sealed envelopes Blinding: unblinded, intention to treat Exclusions post randomisation: none Losses to follow up: 4 (%) | |
| Participants | Country: France Setting: hospital No. of participants: 228 (228 limbs; 114 Dacron, 114 PTFE) Age (median): 66 yrs Sex: 147 males, 81 females Inclusion criteria: severe claudication, rest pain, tissue loss Exclusion criteria: patients with earlier bypass contraindication to long term anticoagulant therapy, life expectancy less than 1 year | |
| Interventions | 6 mm PTFE or 6 mm Dacron. Warfarin post‐op (all patients) | |
| Outcomes | Primary, primary assisted and secondary patency 10‐year follow‐up | |
| Notes | Good anticoagulation protocol. Clear numbers of patients throughout (flow chart) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer program used for sequence generation |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes used |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | No losses, clear life table data |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Good anticoagulation protocol. Clear numbers of patients throughout (flow chart) |
| Methods | Site: Femoral to AK popliteal Study design: multicentre RCT Method of randomisation: concealed randomisation using sealed envelopes in blocks of 4 per centre Blinding: unblinded, as treated analysis Exclusions post randomisation: 1 (0.4%) Losses to follow up: 4 (1.5%) Protocol violations: 1 (1 ‐ crossover from allocated group) | |
| Participants | Country: 6 centres in the Netherlands Setting: hospital No. of participants: 266 (136 externally supported polyester, 129 non‐externally supported polyester, 1 not treated according to protocol so excluded) Age (median): 65 yrs in externally supported group, 67 in non externally supported group Sex: 199 males, 66 females; 1 excluded Inclusion criteria: all patients requiring AK femoro‐popliteal bypass for disabling claudication, rest pain, tissue loss in the absence of a suitable venous conduit Exclusion criteria: no suitable distal anastomotic target, distal anastomosis not above knee, previous ipsilateral femoro‐popliteal procedures, contra‐indication for the use of acetyl salicylic acid or anticoagulants, patients receiving chemo‐ or radiotherapy, malignancy diagnosed or treated within 12 months, known allergy to iodine or contrast medium, and impaired renal function | |
| Interventions | Fluoropassiv 6 mm knitted polyester, either externally supported thin‐wall fluoropolymer coated or 6 mm externally unsupported thin wall | |
| Outcomes | Primary endpoints: primary patency at 1 and 2 years post‐op. Secondary endpoints: mortality, primary assisted and secondary patency | |
| Notes | Clear anticoagulation protocol. Clear numbers of patients throughout (flow chart) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation sequence generation technique |
| Allocation concealment (selection bias) | Low risk | Envelope selected at random after confirmation of suitable target vessel |
| Blinding of participants and personnel (performance bias) | High risk | Operative blinding impossible in this type of trial |
| Blinding of outcome assessment (detection bias) | Unclear risk | Outcome assessors and patients not obviously blinded |
| Incomplete outcome data (attrition bias) | Low risk | Only 4 patients (1.5%) were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All stated outcomes reported |
| Other bias | Low risk | Good anticoagulation protocol. Clear numbers of patients throughout (flow chart) |
ABI: ankle brachial index
AK: above knee
ASV: autologous saphenous vein
BK: below knee
CABG: coronary bypass graft
CFA: common femoral artery
DM: diabetes mellitus
HBD: heparin bonded Dacron
HUV: human umbilical vein
IC: intermittent claudication
LSV: long saphenous vein
MALE: major adverse limb events
MI: myocardial infarction
POD: peri‐procedural death
post‐op: post‐operative/operatively
pt: patient
PTFE: polytetrafluoroethylene
PUR: polyurethane
RCT: randomised controlled trial
SFA: superficial femoral artery
yrs: years
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Results presented include non‐randomised patients. Randomisation technique unclear. Distal grafts included, not intention to treat | |
| Retrospective, non‐randomised study (not an RCT or CCT): retrospective study where data were collected from patient records | |
| The trial was performed in patients having femoro‐popliteal bypass both above and below the knee. Outcomes for the above‐ and below‐knee subgroups were not reported so it was not possible to include the trial | |
| Randomisation technique unclear, above‐knee, below‐knee and distal bypasses inseparable (English title states above‐knee but methods talk about below‐knee bypass) | |
| Case series, not randomised trial data | |
| Inadequate randomisation process. Quote: "the choice between a PTFE and HUV bypass graft was randomized in the operating room, initially to favour saphenous vein." The data were presented as vein versus HUV versus PTFE and was inseparable for analysis | |
| Bypass to any below‐knee artery, not just popliteal. Randomisation technique unclear | |
| Unclear randomisation process. Results never fully published in paper form, only as two abstracts. Data presented as vein versus PTFE versus Dacron and were inseparable for analysis | |
| The trial was performed in patients having femoro‐popliteal bypass both above and below the knee. Outcomes for the above‐ and below‐knee subgroups were not reported so it was not possible to include the trial | |
| The trial was performed in patients having femoro‐popliteal and more distal bypass. Outcomes for the subgroups of patients with distal anastomosis the above‐knee popliteal or below‐knee popliteal artery were not reported so the study could not be included | |
| The trial was performed in both patients having femoro‐popliteal bypass below the knee and patients having femoro‐distal bypass. Outcomes for the subgroup having femoro‐popliteal bypass alone were not reported | |
| Unable to separate above‐ and below‐knee data | |
| Trial failed to recruit 30% of planned patients, and lost 26% of these to follow up. Results only presented at 5 years follow‐up using an unusual system to impute missing data | |
| Unable to separate above‐ and below‐knee data | |
| Above‐knee, below‐knee and distal bypasses inseparable; unclear randomisation | |
| Trial terminated by sponsor due to slow recruitment. No results available | |
| Trial withdrawn prior to enrolment of any patients | |
| Unable to separate above‐ and below‐knee data. A proportion of both above‐ and below‐knee anastomoses included endarterectomies and or vein cuffs which the study authors concede produced a significant difference in patency without giving detailed subgroup analysis. Unclear randomisation | |
| Unable to separate above‐ and below‐knee data. Below‐knee anastomotic site described as 'distal' in some cases without detailed anatomical description. A proportion of both above‐ and below‐knee anastomoses included endarterectomies and or vein cuffs which the study authors concede produced a significant difference in patency without giving detailed subgroup analysis. Unclear randomisation | |
| Patients received both above‐ and below‐knee bypass grafts but results presented together. Poor randomisation (month of birth) | |
| Unable to separate above‐ and below‐knee data. Unclear randomisation technique | |
| Unable to separate above‐ and below‐knee data. Inadequate randomisation (hospital number, card pulling, random number generator) | |
| The trial was performed in patients having femoro‐popliteal bypass both above and below the knee. Outcomes for the above‐ and below‐knee subgroups were not reported so it was not possible to include the trial | |
| Unable to separate above‐ and below‐knee data, not intention to treat. Inadequate randomisation (random number generator, concealment not stated) |
CCT: clinically controlled trial
HUV: human umbilical vein
PTFE: polytetrafluoroethylene
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Multicentric, Prospective, Randomized, Comparing Trial Between Bypass of the Femoropoplitea by PTFE and Heparin Bounded PTFE |
| Methods | Randomised controlled trial |
| Participants | 18 years and older, peripheral vascular disease requiring above‐ or below‐knee femoro‐popliteal bypass |
| Interventions | PTFE versus PTFE with bonded heparin |
| Outcomes | Primary outcome measures: primary patency after 2 years Secondary outcome measures: secondary patency; limb salvage; mortality; re‐intervention |
| Starting date | April 2004 |
| Contact information | Frank Vermassen, MD, PhD, University Hospital, Ghent |
| Notes | A preliminary survival curve was presented at the Charing Cross Symposium in 2009. No useable data could be gleaned from this and no official abstract was published. The lead author was contacted for results but did not reply. The study is reported as completed on ClinicalTrials.gov but has not been published. ClinicalTrials.gov identifier: NCT00147979 |
| Trial name or title | GORE‐TEX PROPATEN Vascular Graft Study |
| Methods | Single‐blind randomised controlled trial |
| Participants | 21 years and older, peripheral vascular disease requiring above‐knee femoro‐popliteal bypass |
| Interventions | GORE‐TEX PROPATEN vascular grafts versus thin walled GORE‐TEX Stretch vascular grafts |
| Outcomes | Primary outcome measures: primary patency at 12 months; major device complication rates at 12 months |
| Starting date | February 2003. Trial completed recruitment in 2007 but still has not published results |
| Contact information | Enrico Ascher, MD Maimonides Hospital, Brooklyn NY |
| Notes | Sponsored by WL Gore & Associates ClinicalTrials.gov identifier: NCT00205790 |
PTFE: polytetrafluoroethylene
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 3 months Show forest plot | 4 | 466 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.58, 2.48] |
| Analysis 1.1  Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 1 Primary patency at 3 months. | ||||
| 1.1 Autologous vein v PTFE | 2 | 249 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.41, 3.97] |
| 1.2 Autologous vein v other graft types | 2 | 217 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.45, 2.96] |
| 2 Primary patency at 6 months Show forest plot | 4 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.56, 1.83] |
| Analysis 1.2  Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 2 Primary patency at 6 months. | ||||
| 2.1 Autologous vein v PTFE | 2 | 245 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.45, 2.78] |
| 2.2 Autologous vein v other graft types | 2 | 207 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.43, 2.05] |
| 3 Primary patency at 12 months Show forest plot | 4 | 440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.44, 1.22] |
| Analysis 1.3 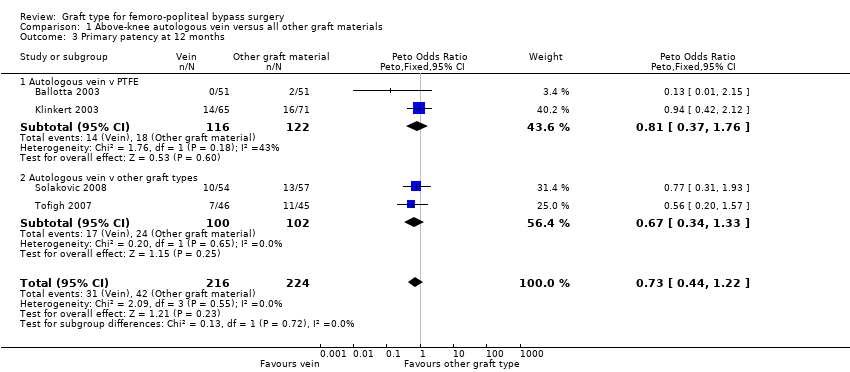 Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 3 Primary patency at 12 months. | ||||
| 3.1 Autologous vein v PTFE | 2 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.37, 1.76] |
| 3.2 Autologous vein v other graft types | 2 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.34, 1.33] |
| 4 Primary patency at 24 months Show forest plot | 4 | 422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.37, 0.94] |
| Analysis 1.4  Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 4 Primary patency at 24 months. | ||||
| 4.1 Autologous vein vs PTFE | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.34, 1.33] |
| 4.2 Autologous vein vs other graft types | 2 | 190 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.28, 0.99] |
| 5 Primary patency at 60 months Show forest plot | 3 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.28, 0.80] |
| Analysis 1.5  Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 5 Primary patency at 60 months. | ||||
| 5.1 Autologous vein v PTFE | 2 | 191 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.25, 0.95] |
| 5.2 Autologous vein vs other graft type | 1 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.18, 1.07] |
| 6 Secondary patency at 3 months Show forest plot | 3 | 364 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.47, 2.32] |
| Analysis 1.6  Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 6 Secondary patency at 3 months. | ||||
| 6.1 Autologous vein v PTFE | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.30, 3.87] |
| 6.2 Autologous vein v other graft types | 2 | 217 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.37, 2.83] |
| 7 Secondary patency at 6 months Show forest plot | 3 | 351 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.49, 1.82] |
| Analysis 1.7  Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 7 Secondary patency at 6 months. | ||||
| 7.1 Autologous vein v PTFE | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.36, 2.69] |
| 7.2 Autologous vein v other graft types | 2 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.39, 2.19] |
| 8 Secondary patency at 12 months Show forest plot | 3 | 338 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.45, 1.45] |
| Analysis 1.8 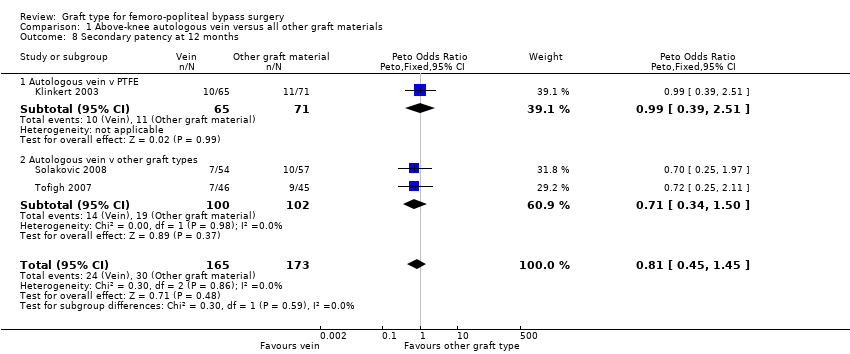 Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 8 Secondary patency at 12 months. | ||||
| 8.1 Autologous vein v PTFE | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.39, 2.51] |
| 8.2 Autologous vein v other graft types | 2 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.34, 1.50] |
| 9 Secondary patency at 24 months Show forest plot | 3 | 320 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.41, 1.19] |
| Analysis 1.9 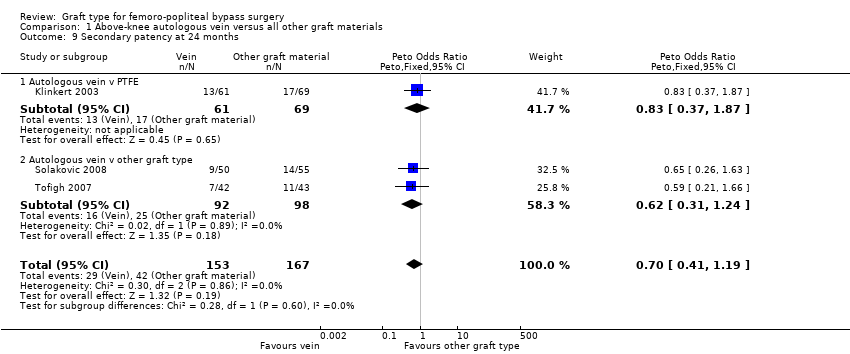 Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 9 Secondary patency at 24 months. | ||||
| 9.1 Autologous vein v PTFE | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.37, 1.87] |
| 9.2 Autologous vein v other graft type | 2 | 190 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.31, 1.24] |
| 10 Secondary patency at 60 months Show forest plot | 2 | 176 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.22, 0.74] |
| Analysis 1.10 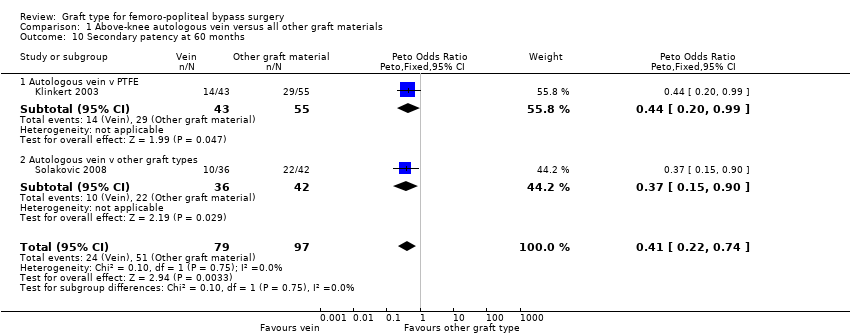 Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 10 Secondary patency at 60 months. | ||||
| 10.1 Autologous vein v PTFE | 1 | 98 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.20, 0.99] |
| 10.2 Autologous vein v other graft types | 1 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.15, 0.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 3 months Show forest plot | 2 | 312 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.36 [0.81, 6.87] |
| Analysis 2.1  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 1 Primary patency at 3 months. | ||||
| 1.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [0.26, 9.33] |
| 1.2 PTFE v Dacron | 1 | 219 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.97 [0.78, 11.25] |
| 2 Primary patency at 6 months Show forest plot | 5 | 824 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [1.37, 3.25] |
| Analysis 2.2  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 2 Primary patency at 6 months. | ||||
| 2.1 PTFE v HUV | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.56 [0.69, 9.47] |
| 2.2 PTFE v Dacron | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.79, 3.11] |
| 2.3 PTFE v PTFE with vein cuff | 1 | 139 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.80 [0.57, 5.60] |
| 2.4 PTFE v FUSION BIOLINE | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.99 [1.43, 6.26] |
| 3 Primary patency at 12 months Show forest plot | 6 | 1088 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.93, 1.64] |
| Analysis 2.3  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 3 Primary patency at 12 months. | ||||
| 3.1 PTFE v HUV | 1 | 83 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.17 [1.04, 9.64] |
| 3.2 PTFE v Dacron | 4 | 875 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.91, 1.70] |
| 3.3 PTFE v PTFE with vein cuff | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.26, 1.56] |
| 4 Primary patency at 24 months Show forest plot | 6 | 945 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [1.00, 1.71] |
| Analysis 2.4 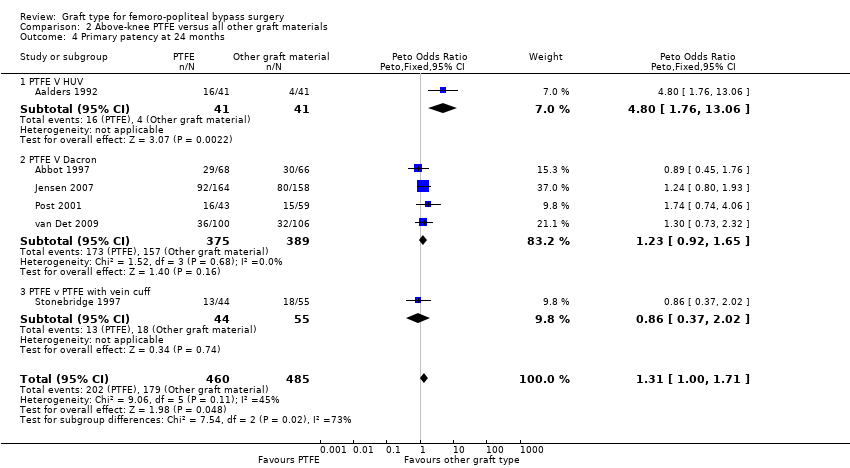 Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 4 Primary patency at 24 months. | ||||
| 4.1 PTFE V HUV | 1 | 82 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.80 [1.76, 13.06] |
| 4.2 PTFE V Dacron | 4 | 764 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.92, 1.65] |
| 4.3 PTFE v PTFE with vein cuff | 1 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.37, 2.02] |
| 5 Primary patency at 60 months Show forest plot | 3 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.06 [1.28, 3.31] |
| Analysis 2.5  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 5 Primary patency at 60 months. | ||||
| 5.1 PTFE v HUV | 1 | 69 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.75 [1.46, 9.62] |
| 5.2 PTFE v Dacron | 2 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [0.96, 2.90] |
| 6 Secondary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.6  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 6 Secondary patency at 3 months. | ||||
| 6.1 PTFE v HUV | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Secondary patency at 6 months Show forest plot | 2 | 318 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.48, 3.62] |
| Analysis 2.7  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 7 Secondary patency at 6 months. | ||||
| 7.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [0.42, 7.44] |
| 7.2 PTFE v Dacron | 1 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.25, 4.13] |
| 8 Secondary patency at 12 months Show forest plot | 4 | 806 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.80, 1.74] |
| Analysis 2.8  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 8 Secondary patency at 12 months. | ||||
| 8.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.43, 5.89] |
| 8.2 PTFE v Dacron | 2 | 581 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.76, 1.86] |
| 8.3 PTFE v PTFE with vein cuff | 1 | 132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.39, 2.52] |
| 9 Secondary patency at 24 months Show forest plot | 4 | 700 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [1.18, 2.33] |
| Analysis 2.9  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 9 Secondary patency at 24 months. | ||||
| 9.1 PTFE V HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.01 [1.44, 11.17] |
| 9.2 PTFE v Dacron | 2 | 528 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [1.04, 2.28] |
| 9.3 PTFE v PTFE with vein cuff | 1 | 79 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.48, 3.06] |
| 10 Secondary patency at 60 months Show forest plot | 2 | 260 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.86 [1.73, 4.72] |
| Analysis 2.10  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 10 Secondary patency at 60 months. | ||||
| 10.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.87 [1.65, 9.05] |
| 10.2 PTFE v Dacron | 1 | 167 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.43 [1.31, 4.53] |
| 11 Limb salvage at 1 month Show forest plot | 2 | 560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.12, 3.98] |
| Analysis 2.11  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 11 Limb salvage at 1 month. | ||||
| 11.1 PTFE v Dacron | 1 | 410 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.20] |
| 11.2 PTFE v PTFE with vein cuff | 1 | 150 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [0.21, 19.72] |
| 12 Limb salvage at 24 months Show forest plot | 2 | 389 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.33, 1.62] |
| Analysis 2.12  Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 12 Limb salvage at 24 months. | ||||
| 12.1 PTFE v Dacron | 1 | 322 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.27, 2.48] |
| 12.2 PTFE v PTFE with vein cuff | 1 | 67 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.20, 2.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 12 months Show forest plot | 2 | 294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| Analysis 3.1  Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 1 Primary patency at 12 months. | ||||
| 1.1 HBD v HUV | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.20, 1.12] |
| 1.2 HBD v PTFE | 1 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.34, 1.25] |
| 2 Primary patency at 24 months Show forest plot | 2 | 282 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| Analysis 3.2  Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 2 Primary patency at 24 months. | ||||
| 2.1 HBD v HUV | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.26, 1.33] |
| 2.2 HBD v PTFE | 1 | 165 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.34, 1.19] |
| 3 Primary patency at 60 months Show forest plot | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.33, 0.93] |
| Analysis 3.3 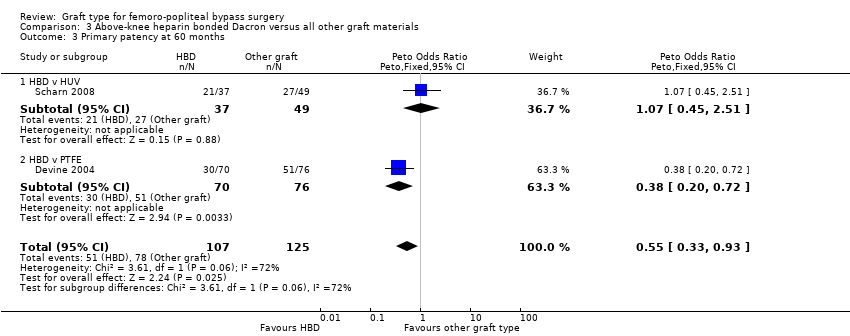 Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 3 Primary patency at 60 months. | ||||
| 3.1 HBD v HUV | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.45, 2.51] |
| 3.2 HBD v PTFE | 1 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.20, 0.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 6 months Show forest plot | 2 | 299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.71, 2.31] |
| Analysis 4.1  Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 1 Primary patency at 6 months. | ||||
| 1.1 Externally supported dacron versus unsupported dacron | 1 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.69, 2.39] |
| 1.2 Externally supported PTFE versus unsupported PTFE | 1 | 46 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.16, 9.25] |
| 2 Primary patency at 12 months Show forest plot | 2 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [1.06, 2.98] |
| Analysis 4.2  Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 2 Primary patency at 12 months. | ||||
| 2.1 Externally supported dacron versus unsupported dacron | 1 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.99, 2.93] |
| 2.2 Externally supported PTFE versus unsupported PTFE | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.73 [0.49, 15.28] |
| 3 Primary patency at 24 months Show forest plot | 2 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.08 [1.29, 3.35] |
| Analysis 4.3  Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 3 Primary patency at 24 months. | ||||
| 3.1 Externally supported dacron versus unsupported dacron | 1 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [1.26, 3.46] |
| 3.2 Externally supported PTFE versus unsupported PTFE | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.01 [0.46, 8.76] |
| 4 Secondary patency at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.4  Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 4 Secondary patency at 6 months. | ||||
| 5 Secondary patency at 12 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.5  Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 5 Secondary patency at 12 months. | ||||
| 6 Secondary patency at 24 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.6  Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 6 Secondary patency at 24 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.1  Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 1 Primary patency at 3 months. | ||||
| 2 Primary patency at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.2  Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 2 Primary patency at 6 months. | ||||
| 3 Primary patency at 12 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.3  Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 3 Primary patency at 12 months. | ||||
| 4 Secondary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.4  Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 4 Secondary patency at 3 months. | ||||
| 5 Secondary patency at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.5  Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 5 Secondary patency at 6 months. | ||||
| 6 Secondary patency at 12 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 5.6  Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 6 Secondary patency at 12 months. | ||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 6 months Show forest plot | 4 | 319 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.67, 1.87] |
| Analysis 6.1  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 1 Primary patency at 6 months. | ||||
| 1.1 PTFE v ringed PTFE | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.32, 6.71] |
| 1.2 PTFE v PTFE with vein cuff | 2 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.56, 1.78] |
| 1.3 PTFE v FUSION BIOLINE | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.39, 9.83] |
| 2 Primary patency at 12 months Show forest plot | 4 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.60, 1.55] |
| Analysis 6.2  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 2 Primary patency at 12 months. | ||||
| 2.1 PTFE v Dacron | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.12, 1.79] |
| 2.2 PTFE v PTFE with vein cuff | 2 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.59, 1.76] |
| 2.3 PTFE v ringed PTFE | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.35, 6.24] |
| 3 Primary patency at 24 months Show forest plot | 4 | 250 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.56, 1.57] |
| Analysis 6.3  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 3 Primary patency at 24 months. | ||||
| 3.1 PTFE v Dacron | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.12, 1.42] |
| 3.2 PTFE v PTFE with vein cuff | 2 | 182 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.58, 2.01] |
| 3.3 PTFE v ringed PTFE | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.31, 5.67] |
| 4 Primary patency at 36 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.4 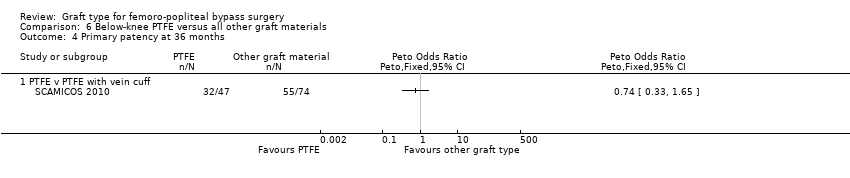 Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 4 Primary patency at 36 months. | ||||
| 4.1 PTFE v PTFE with vein cuff | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Secondary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.5  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 5 Secondary patency at 3 months. | ||||
| 5.1 PTFE v HUV | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Secondary patency at 6 months Show forest plot | 2 | 242 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.69, 2.13] |
| Analysis 6.6  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 6 Secondary patency at 6 months. | ||||
| 6.1 PTFE v HUV | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.01 [1.12, 8.07] |
| 6.2 PTFE v PTFE with vein cuff | 1 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.40, 1.56] |
| 7 Secondary patency at 12 months Show forest plot | 3 | 325 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.94, 2.34] |
| Analysis 6.7  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 7 Secondary patency at 12 months. | ||||
| 7.1 PTFE v HUV | 1 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.46 [1.10, 5.49] |
| 7.2 PTFE v PTFE with vein cuff | 2 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.66, 2.03] |
| 8 Secondary patency at 24 months Show forest plot | 3 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [1.05, 2.80] |
| Analysis 6.8  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 8 Secondary patency at 24 months. | ||||
| 8.1 PTFE v HUV | 1 | 88 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.40 [1.45, 7.97] |
| 8.2 PTFE v PTFE with vein cuff | 2 | 181 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.67, 2.23] |
| 9 Secondary patency at 36 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 6.9  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 9 Secondary patency at 36 months. | ||||
| 9.1 PTFE v PTFE with vein cuff | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Limb salvage at 12 months Show forest plot | 2 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.72, 2.55] |
| Analysis 6.10  Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 10 Limb salvage at 12 months. | ||||
| 10.1 PTFE v PTFE with vein cuff | 2 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.72, 2.55] |
| 11 Limb salvage at 24 months Show forest plot | 2 | 196 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.72, 2.49] |
| Analysis 6.11 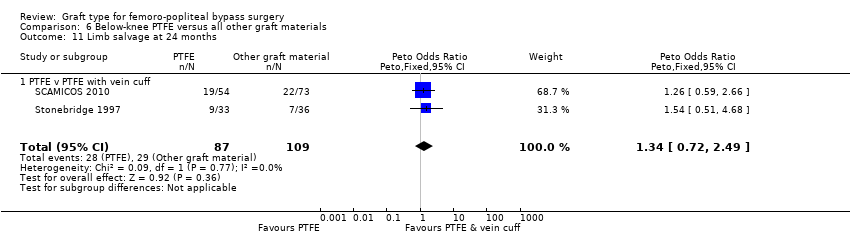 Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 11 Limb salvage at 24 months. | ||||
| 11.1 PTFE v PTFE with vein cuff | 2 | 196 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.72, 2.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.1  Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 1 Primary patency at 3 months. | ||||
| 1.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Primary patency at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.2  Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 2 Primary patency at 6 months. | ||||
| 2.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Primary patency at 12 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.3  Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 3 Primary patency at 12 months. | ||||
| 3.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Primary patency at 24 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.4  Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 4 Primary patency at 24 months. | ||||
| 4.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Primary patency at 60 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Analysis 7.5  Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 5 Primary patency at 60 months. | ||||
| 5.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 1 Primary patency at 3 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 2 Primary patency at 6 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 3 Primary patency at 12 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 4 Primary patency at 24 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 5 Primary patency at 60 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 6 Secondary patency at 3 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 7 Secondary patency at 6 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 8 Secondary patency at 12 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 9 Secondary patency at 24 months.

Comparison 1 Above‐knee autologous vein versus all other graft materials, Outcome 10 Secondary patency at 60 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 1 Primary patency at 3 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 2 Primary patency at 6 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 3 Primary patency at 12 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 4 Primary patency at 24 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 5 Primary patency at 60 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 6 Secondary patency at 3 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 7 Secondary patency at 6 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 8 Secondary patency at 12 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 9 Secondary patency at 24 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 10 Secondary patency at 60 months.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 11 Limb salvage at 1 month.

Comparison 2 Above‐knee PTFE versus all other graft materials, Outcome 12 Limb salvage at 24 months.

Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 1 Primary patency at 12 months.

Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 2 Primary patency at 24 months.

Comparison 3 Above‐knee heparin bonded Dacron versus all other graft materials, Outcome 3 Primary patency at 60 months.

Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 1 Primary patency at 6 months.

Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 2 Primary patency at 12 months.

Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 3 Primary patency at 24 months.

Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 4 Secondary patency at 6 months.

Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 5 Secondary patency at 12 months.

Comparison 4 Above‐knee externally supported graft versus unsupported graft materials, Outcome 6 Secondary patency at 24 months.

Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 1 Primary patency at 3 months.

Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 2 Primary patency at 6 months.

Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 3 Primary patency at 12 months.

Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 4 Secondary patency at 3 months.

Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 5 Secondary patency at 6 months.

Comparison 5 Above‐knee polyurethane (PUR) versus all other graft materials, Outcome 6 Secondary patency at 12 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 1 Primary patency at 6 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 2 Primary patency at 12 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 3 Primary patency at 24 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 4 Primary patency at 36 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 5 Secondary patency at 3 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 6 Secondary patency at 6 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 7 Secondary patency at 12 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 8 Secondary patency at 24 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 9 Secondary patency at 36 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 10 Limb salvage at 12 months.

Comparison 6 Below‐knee PTFE versus all other graft materials, Outcome 11 Limb salvage at 24 months.

Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 1 Primary patency at 3 months.

Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 2 Primary patency at 6 months.

Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 3 Primary patency at 12 months.

Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 4 Primary patency at 24 months.

Comparison 7 Below‐knee heparin bonded Dacron versus all other graft materials, Outcome 5 Primary patency at 60 months.
| Autologous vein compared to other graft types for above‐knee femoro‐popliteal bypass surgery | ||||||
| Patient or population: people with peripheral vascular disease requiring above‐knee femoro‐popliteal bypass surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of limbs | Quality of the evidence | Comments | |
| Risk with other graft types | Risk with autologous vein | |||||
| Primary patency (24 months) | Study population | OR 0.59 | 422 | ⊕⊕⊝⊝ | 92 fewer autologous vein grafts per 1000 (10 to 152 grafts per 1000) lose primary patency by 24 months compared to other grafts studied | |
| 275 per 1000 | 183 per 1000 | |||||
| Primary patency (60 months) | Study population | OR 0.47 | 269 | ⊕⊕⊕⊝ | 172 fewer autologous vein grafts per 1000 (54 to 264 grafts per 1000) lose primary patency by 60 months compared to other grafts studied | |
| 451 per 1000 | 279 per 1000 | |||||
| Secondary patency (60 months) | Study population | OR 0.41 | 176 | ⊕⊕⊝⊝ | 213 fewer autologous vein grafts per 1000 (75 to 330 grafts per 1000) lose secondary patency by 60 months compared to other grafts studied | |
| 526 per 1000 | 313 per 1000 | |||||
| Limb salvage | ‐ | ‐ | ‐ | ‐ | ‐ | No studies of these graft types reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to serious risk of bias resulting from lack of blinding and poor randomisation techniques | ||||||
| PTFE compared to Dacron for above‐knee femoro‐popliteal bypass surgery | ||||||
| Patient or population: people with peripheral vascular disease requiring above‐knee femoro‐popliteal bypass surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of limbs | Quality of the evidence | Comments | |
| Risk with Dacron | Risk with PTFE | |||||
| Primary patency (24 months) | Study population | OR 1.23 | 764 | ⊕⊕⊝⊝ | Our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 404 per 1000 | 454 per 1000 | |||||
| Primary patency (60 months) | Study population | OR 1.67 | 247 | ⊕⊕⊝⊝ | Our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 606 per 1000 | 720 per 1000 | |||||
| Secondary patency (24 months) | Study population | OR 1.54 | 528 | ⊕⊕⊝⊝ | 81 more PTFE grafts per 1000 (7 to 168 per 1000) suffer from failed secondary patency by 24 months compared to Dacron | |
| 212 per 1000 | 293 per 1000 | |||||
| Limb salvage (24 months) | Study population | OR 0.82 | 322 | ⊕⊕⊝⊝ | Our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 44 per 1000 | 37 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded because of serious risk of bias due to lack of blinding and poor randomisation techniques | ||||||
| Externally supported graft compared to unsupported graft for above‐knee femoro‐popliteal bypass surgery | ||||||
| Patient or population: people with peripheral vascular disease requiring above‐knee femoro‐popliteal bypass surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of limbs | Quality of the evidence | Comments | |
| Risk with unsupported graft | Risk with externally supported graft | |||||
| Primary patency (24 months) | Study population | OR 2.08 | 270 | ⊕⊕⊝⊝ | 180 fewer unsupported prosthetic grafts per 1000 (61 to 293 grafts per 1000) lose primary patency by 24 months compared to externally supported prosthetic grafts | |
| 376 per 1000 | 556 per 1000 | |||||
| Primary patency (60 months) | ‐ | ‐ | ‐ | ‐ | ‐ | No studies comparing supported and unsupported Dacron reported on primary patency at 60 months |
| Secondary patency (24 months) | Study population | OR 2.25 | 236 | ⊕⊕⊝⊝ | 143 fewer unsupported Dacron grafts per 1000 (32 to 281 grafts per 1,000) lose secondary patency by 24 months compared to externally supported Dacron grafts | |
| 165 per 1000 | 308 per 1000 | |||||
| Limb salvage | ‐ | ‐ | ‐ | ‐ | ‐ | No studies of these graft types reported on this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded because of serious risk of bias due to lack of blinding and poor randomisation techniques | ||||||
| PTFE compared to PTFE with vein cuff for below‐knee femoro‐popliteal bypass surgery | ||||||
| Patient or population: people with peripheral vascular disease requiring below‐knee femoro‐popliteal bypass surgery | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of limbs | Quality of the evidence | Comments | |
| Risk with PTFE with vein cuff | Risk with PTFE | |||||
| Primary patency (24 months) | Study population | OR 1.08 | 182 | ⊕⊝⊝⊝ | Findings from two small trials were inconsistent so our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 626 per 1000 | 644 per 1000 | |||||
| Primary patency (60 months) | ‐ | ‐ | ‐ | ‐ | ‐ | No studies comparing PTFE with and without a vein cuff for below‐knee bypass reported on primary patency at 60 months |
| Secondary patency (24 months) | Study population | OR 1.22 | 181 | ⊕⊝⊝⊝ | Findings from two small trials were inconsistent so our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 557 per 1000 | 605 per 1000 | |||||
| Limb salvage (24 months) | Study population | OR 1.34 | 196 | ⊕⊕⊝⊝ | Our confidence in the effect is limited and this may differ substantially from the estimate of the effect | |
| 266 per 1000 | 327 per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded due to serious risk of bias resulting from lack of blinding and poor randomisation techniques | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 3 months Show forest plot | 4 | 466 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.58, 2.48] |
| 1.1 Autologous vein v PTFE | 2 | 249 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.27 [0.41, 3.97] |
| 1.2 Autologous vein v other graft types | 2 | 217 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.45, 2.96] |
| 2 Primary patency at 6 months Show forest plot | 4 | 452 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.56, 1.83] |
| 2.1 Autologous vein v PTFE | 2 | 245 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.45, 2.78] |
| 2.2 Autologous vein v other graft types | 2 | 207 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.43, 2.05] |
| 3 Primary patency at 12 months Show forest plot | 4 | 440 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.44, 1.22] |
| 3.1 Autologous vein v PTFE | 2 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.37, 1.76] |
| 3.2 Autologous vein v other graft types | 2 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.34, 1.33] |
| 4 Primary patency at 24 months Show forest plot | 4 | 422 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.37, 0.94] |
| 4.1 Autologous vein vs PTFE | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.67 [0.34, 1.33] |
| 4.2 Autologous vein vs other graft types | 2 | 190 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.28, 0.99] |
| 5 Primary patency at 60 months Show forest plot | 3 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.28, 0.80] |
| 5.1 Autologous vein v PTFE | 2 | 191 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.25, 0.95] |
| 5.2 Autologous vein vs other graft type | 1 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.18, 1.07] |
| 6 Secondary patency at 3 months Show forest plot | 3 | 364 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.47, 2.32] |
| 6.1 Autologous vein v PTFE | 1 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.30, 3.87] |
| 6.2 Autologous vein v other graft types | 2 | 217 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.03 [0.37, 2.83] |
| 7 Secondary patency at 6 months Show forest plot | 3 | 351 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.49, 1.82] |
| 7.1 Autologous vein v PTFE | 1 | 143 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.36, 2.69] |
| 7.2 Autologous vein v other graft types | 2 | 208 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.39, 2.19] |
| 8 Secondary patency at 12 months Show forest plot | 3 | 338 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.81 [0.45, 1.45] |
| 8.1 Autologous vein v PTFE | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.39, 2.51] |
| 8.2 Autologous vein v other graft types | 2 | 202 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.34, 1.50] |
| 9 Secondary patency at 24 months Show forest plot | 3 | 320 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.41, 1.19] |
| 9.1 Autologous vein v PTFE | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.37, 1.87] |
| 9.2 Autologous vein v other graft type | 2 | 190 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.31, 1.24] |
| 10 Secondary patency at 60 months Show forest plot | 2 | 176 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.22, 0.74] |
| 10.1 Autologous vein v PTFE | 1 | 98 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.20, 0.99] |
| 10.2 Autologous vein v other graft types | 1 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.37 [0.15, 0.90] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 3 months Show forest plot | 2 | 312 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.36 [0.81, 6.87] |
| 1.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.55 [0.26, 9.33] |
| 1.2 PTFE v Dacron | 1 | 219 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.97 [0.78, 11.25] |
| 2 Primary patency at 6 months Show forest plot | 5 | 824 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.11 [1.37, 3.25] |
| 2.1 PTFE v HUV | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.56 [0.69, 9.47] |
| 2.2 PTFE v Dacron | 2 | 421 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.79, 3.11] |
| 2.3 PTFE v PTFE with vein cuff | 1 | 139 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.80 [0.57, 5.60] |
| 2.4 PTFE v FUSION BIOLINE | 1 | 174 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.99 [1.43, 6.26] |
| 3 Primary patency at 12 months Show forest plot | 6 | 1088 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.93, 1.64] |
| 3.1 PTFE v HUV | 1 | 83 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.17 [1.04, 9.64] |
| 3.2 PTFE v Dacron | 4 | 875 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.91, 1.70] |
| 3.3 PTFE v PTFE with vein cuff | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.26, 1.56] |
| 4 Primary patency at 24 months Show forest plot | 6 | 945 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.31 [1.00, 1.71] |
| 4.1 PTFE V HUV | 1 | 82 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.80 [1.76, 13.06] |
| 4.2 PTFE V Dacron | 4 | 764 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [0.92, 1.65] |
| 4.3 PTFE v PTFE with vein cuff | 1 | 99 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.37, 2.02] |
| 5 Primary patency at 60 months Show forest plot | 3 | 316 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.06 [1.28, 3.31] |
| 5.1 PTFE v HUV | 1 | 69 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.75 [1.46, 9.62] |
| 5.2 PTFE v Dacron | 2 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.67 [0.96, 2.90] |
| 6 Secondary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6.1 PTFE v HUV | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Secondary patency at 6 months Show forest plot | 2 | 318 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.48, 3.62] |
| 7.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [0.42, 7.44] |
| 7.2 PTFE v Dacron | 1 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.25, 4.13] |
| 8 Secondary patency at 12 months Show forest plot | 4 | 806 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.18 [0.80, 1.74] |
| 8.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.60 [0.43, 5.89] |
| 8.2 PTFE v Dacron | 2 | 581 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.76, 1.86] |
| 8.3 PTFE v PTFE with vein cuff | 1 | 132 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.39, 2.52] |
| 9 Secondary patency at 24 months Show forest plot | 4 | 700 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.66 [1.18, 2.33] |
| 9.1 PTFE V HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.01 [1.44, 11.17] |
| 9.2 PTFE v Dacron | 2 | 528 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.54 [1.04, 2.28] |
| 9.3 PTFE v PTFE with vein cuff | 1 | 79 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.48, 3.06] |
| 10 Secondary patency at 60 months Show forest plot | 2 | 260 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.86 [1.73, 4.72] |
| 10.1 PTFE v HUV | 1 | 93 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.87 [1.65, 9.05] |
| 10.2 PTFE v Dacron | 1 | 167 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.43 [1.31, 4.53] |
| 11 Limb salvage at 1 month Show forest plot | 2 | 560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.68 [0.12, 3.98] |
| 11.1 PTFE v Dacron | 1 | 410 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.20] |
| 11.2 PTFE v PTFE with vein cuff | 1 | 150 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.02 [0.21, 19.72] |
| 12 Limb salvage at 24 months Show forest plot | 2 | 389 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.33, 1.62] |
| 12.1 PTFE v Dacron | 1 | 322 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.27, 2.48] |
| 12.2 PTFE v PTFE with vein cuff | 1 | 67 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.20, 2.04] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 12 months Show forest plot | 2 | 294 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.58 [0.34, 0.98] |
| 1.1 HBD v HUV | 1 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.20, 1.12] |
| 1.2 HBD v PTFE | 1 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.34, 1.25] |
| 2 Primary patency at 24 months Show forest plot | 2 | 282 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.38, 1.02] |
| 2.1 HBD v HUV | 1 | 117 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.26, 1.33] |
| 2.2 HBD v PTFE | 1 | 165 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.64 [0.34, 1.19] |
| 3 Primary patency at 60 months Show forest plot | 2 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.33, 0.93] |
| 3.1 HBD v HUV | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.07 [0.45, 2.51] |
| 3.2 HBD v PTFE | 1 | 146 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.20, 0.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 6 months Show forest plot | 2 | 299 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.28 [0.71, 2.31] |
| 1.1 Externally supported dacron versus unsupported dacron | 1 | 253 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.29 [0.69, 2.39] |
| 1.2 Externally supported PTFE versus unsupported PTFE | 1 | 46 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.16, 9.25] |
| 2 Primary patency at 12 months Show forest plot | 2 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.78 [1.06, 2.98] |
| 2.1 Externally supported dacron versus unsupported dacron | 1 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.71 [0.99, 2.93] |
| 2.2 Externally supported PTFE versus unsupported PTFE | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.73 [0.49, 15.28] |
| 3 Primary patency at 24 months Show forest plot | 2 | 270 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.08 [1.29, 3.35] |
| 3.1 Externally supported dacron versus unsupported dacron | 1 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [1.26, 3.46] |
| 3.2 Externally supported PTFE versus unsupported PTFE | 1 | 30 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.01 [0.46, 8.76] |
| 4 Secondary patency at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Secondary patency at 12 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Secondary patency at 24 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2 Primary patency at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3 Primary patency at 12 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4 Secondary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5 Secondary patency at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 6 Secondary patency at 12 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 6 months Show forest plot | 4 | 319 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.67, 1.87] |
| 1.1 PTFE v ringed PTFE | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.32, 6.71] |
| 1.2 PTFE v PTFE with vein cuff | 2 | 247 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.56, 1.78] |
| 1.3 PTFE v FUSION BIOLINE | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.96 [0.39, 9.83] |
| 2 Primary patency at 12 months Show forest plot | 4 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.60, 1.55] |
| 2.1 PTFE v Dacron | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.47 [0.12, 1.79] |
| 2.2 PTFE v PTFE with vein cuff | 2 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.59, 1.76] |
| 2.3 PTFE v ringed PTFE | 1 | 36 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.35, 6.24] |
| 3 Primary patency at 24 months Show forest plot | 4 | 250 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.94 [0.56, 1.57] |
| 3.1 PTFE v Dacron | 1 | 40 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.41 [0.12, 1.42] |
| 3.2 PTFE v PTFE with vein cuff | 2 | 182 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.08 [0.58, 2.01] |
| 3.3 PTFE v ringed PTFE | 1 | 28 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.31, 5.67] |
| 4 Primary patency at 36 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4.1 PTFE v PTFE with vein cuff | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Secondary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5.1 PTFE v HUV | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Secondary patency at 6 months Show forest plot | 2 | 242 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.21 [0.69, 2.13] |
| 6.1 PTFE v HUV | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.01 [1.12, 8.07] |
| 6.2 PTFE v PTFE with vein cuff | 1 | 171 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.79 [0.40, 1.56] |
| 7 Secondary patency at 12 months Show forest plot | 3 | 325 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.48 [0.94, 2.34] |
| 7.1 PTFE v HUV | 1 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.46 [1.10, 5.49] |
| 7.2 PTFE v PTFE with vein cuff | 2 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.66, 2.03] |
| 8 Secondary patency at 24 months Show forest plot | 3 | 269 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.72 [1.05, 2.80] |
| 8.1 PTFE v HUV | 1 | 88 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.40 [1.45, 7.97] |
| 8.2 PTFE v PTFE with vein cuff | 2 | 181 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [0.67, 2.23] |
| 9 Secondary patency at 36 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 9.1 PTFE v PTFE with vein cuff | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Limb salvage at 12 months Show forest plot | 2 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.72, 2.55] |
| 10.1 PTFE v PTFE with vein cuff | 2 | 225 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.72, 2.55] |
| 11 Limb salvage at 24 months Show forest plot | 2 | 196 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.72, 2.49] |
| 11.1 PTFE v PTFE with vein cuff | 2 | 196 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.34 [0.72, 2.49] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Primary patency at 3 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 1.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Primary patency at 6 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 2.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Primary patency at 12 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 3.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Primary patency at 24 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 4.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Primary patency at 60 months Show forest plot | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 5.1 HBD v PTFE | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


