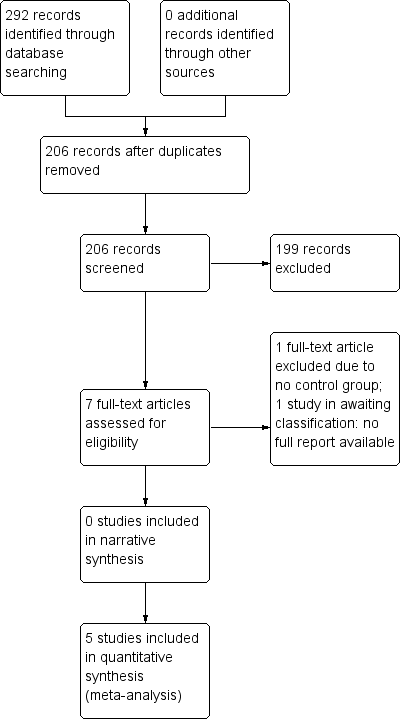
Tratamiento complementario con zonisamida para la epilepsia parcial resistente a fármacos
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | Multicentre study, 49 centres in Europe and 5 in South Africa | |
| Interventions | Placebo, 100 mg, 300 mg or 500 mg placebo, randomised in 2:1:1:2 ratio | |
| Outcomes | Reduction in seizure frequency, proportion with a 50% or greater reduction in seizure frequency | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised sequentially in blocks of six" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details were provided |
| Blinding of participants and personnel (performance bias) | Low risk | "Treatments were blinded using a double dummy technique throughout the study" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient details about blinding of outcome assessors was provided |
| Incomplete outcome data (attrition bias) | Low risk | A modified ITT analysis was conducted as "all patients who received at least one dose of study drug were included in the safety analysis". 4 participants not included in the analysis were spread fairly evenly among groups (1 participant lost from 2 groups, 2 participants lost from 1 group) |
| Selective reporting (reporting bias) | Low risk | This study was deemed to be at a low risk of selective reporting |
| Other bias | Low risk | Allocation to groups led to different durations of stable‐dose phase |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study. 2 treatment arms: 1 placebo and 1 zonisamide | |
| Participants | Multicentre (20) US study | |
| Interventions | Zonisamide 400 mg/day or placebo (weeks 8 to 12) | |
| Outcomes | Primary: median percentage reduction from baseline of all partial seizures | |
| Notes | Of the randomised participants 8 failed to complete week 5 in the placebo group, 15 in the zonisamide group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization codes were generated centrally, with separate randomization sequences for each site." |
| Allocation concealment (selection bias) | Low risk | "Each investigator had a sealed copy of the code to be opened in an emergency. Otherwise, assignments were not revealed until all patients at all sites had completed the study." |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient detail was provided about blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient detail was provided about blinding of outcome assessors |
| Incomplete outcome data (attrition bias) | Unclear risk | The analysis for "the primary populations was a modified ITT" as it included participants who had received at least one dose of study medication |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of any other source of bias |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel trial. 12‐week baseline phase, 4‐week titration phase, 12‐week stable treatment phase. Placebo or zonisamide treatment interventions | |
| Participants | Single centre in China. 104 participants randomised, 53 received zonisamide (29 M:24 F) and 51 received placebo (32 M: 19 F). Mean age of zonisamide group = 36.83 years +/‐ 10.77 and mean age in placebo group = 29.81 years +/‐ 8.24. All participants had simple partial seizures, complex partial seizures or secondary generalised seizures | |
| Interventions | Placebo or zonisamide (titrated to 300 mg/day or 400 mg/day) | |
| Outcomes | The following outcomes were measured: 1. Responder rate (50% or greater reduction in seizures frequency during treatment phase compared to baseline) 2. Seizure freedom 3. Adverse effects | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information given other than "zonisamide and placebo were assigned to our centre in a ratio of 1:1" (page 223) |
| Allocation concealment (selection bias) | Low risk | "Random allocation of patients to their treatment group was concealed via the use of numbered containers" Comment: it is not explicitly stated whether the containers were opaque or not |
| Blinding of participants and personnel (performance bias) | Low risk | "Investigators were blind to treatment each patient received until the end of the study" and "Zonisamide and placebo tablets had the same size, colour and shape. The tablets were randomly numbered by the study sponsors" |
| Blinding of outcome assessment (detection bias) | Unclear risk | It is unclear whether the investigators who were blinded were also the outcome assessors or not |
| Incomplete outcome data (attrition bias) | Low risk | One patient was lost from each group, therefore missing data were balanced between groups |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | Both providers of zonisamide were manufacturers of the drug |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel‐group study | |
| Participants | Conducted at 4 US centres between August 1983 and July 1986 | |
| Interventions | Zonisamide median dosage 400 mg/day (100 mg capsules) | |
| Outcomes | Primary: median percentage reduction in seizure frequency of all partial seizures from baseline | |
| Notes | Because of the variable baseline periods, baseline seizure frequency was recalculated for the 8 weeks immediately before entry into the treatment period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation codes were generated by the study sponsor". "Each patient who qualified to receive double‐blind treatment was assigned a randomisation number and given zonisamide or placebo" |
| Allocation concealment (selection bias) | Low risk | "Random allocation of patients to their treatment groups was concealed via the use of numbered containers" |
| Blinding of participants and personnel (performance bias) | Low risk | This study was deemed to be at low risk of performance bias |
| Blinding of outcome assessment (detection bias) | Low risk | This study was deemed to be at low risk of detection bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient or unclear details were provided with regard to attrition bias |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | Baseline period was extended if the frequency of seizures did not meet a prespecified threshold |
| Methods | Randomised, double‐blind, placebo‐controlled, parallel group study | |
| Participants | Participants from 10 European centres recruited between June 1984 and October 1986 | |
| Interventions | Zonisamide median dosage 400 mg/day (100 mg capsules) | |
| Outcomes | Primary: median percentage reduction in seizure frequency of all partial seizures from baseline | |
| Notes | Because of the variable baseline periods, baseline seizure frequency was recalculated for the 8 weeks immediately before entry into the treatment period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised sequentially in blocks of four" |
| Allocation concealment (selection bias) | Unclear risk | Insufficient details provided |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient details were provided about blinding of personnel |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of outcome assessors was not detailed |
| Incomplete outcome data (attrition bias) | Unclear risk | A modified ITT analysis was conducted including patients who had received "at least 7 days of treatment" |
| Selective reporting (reporting bias) | Low risk | This study was deemed to be at low risk of selective reporting |
| Other bias | Low risk | Baseline period was extended if the frequency of seizures did not meet a prespecified threshold |
AED: antiepileptic drug
F: female
ITT: intention‐to‐treat
M: male
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| No control group was used |
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | No details |
| Participants | No details |
| Interventions | Zonisamide add‐on versus sodium valproate add‐on |
| Outcomes | No details |
| Notes | No details |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% responder rate ‐ whole treatment period Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 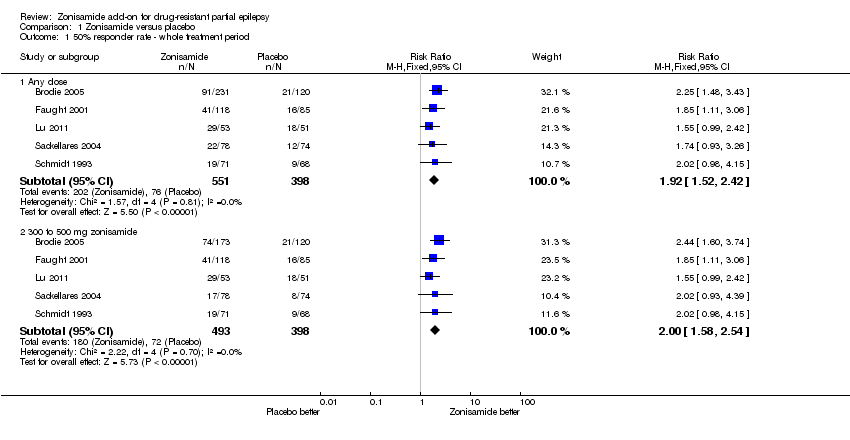 Comparison 1 Zonisamide versus placebo, Outcome 1 50% responder rate ‐ whole treatment period. | ||||
| 1.1 Any dose | 5 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.52, 2.42] |
| 1.2 300 to 500 mg zonisamide | 5 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.58, 2.54] |
| 2 50% responder rate ‐ best‐case Show forest plot | 5 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.78, 2.79] |
| Analysis 1.2  Comparison 1 Zonisamide versus placebo, Outcome 2 50% responder rate ‐ best‐case. | ||||
| 3 50% responder rate ‐ worst‐case Show forest plot | 5 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.16, 1.75] |
| Analysis 1.3  Comparison 1 Zonisamide versus placebo, Outcome 3 50% responder rate ‐ worst‐case. | ||||
| 4 50% responder rate ‐ dose effect Brodie 2005 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.4 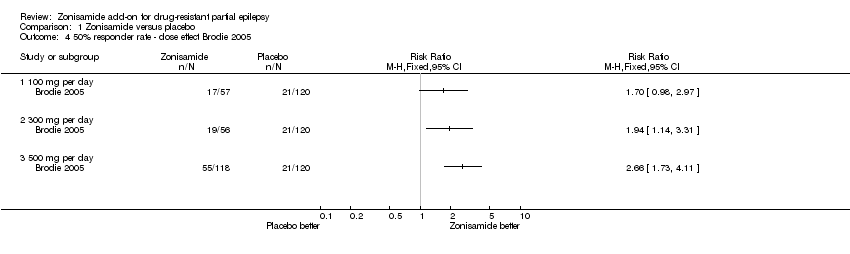 Comparison 1 Zonisamide versus placebo, Outcome 4 50% responder rate ‐ dose effect Brodie 2005. | ||||
| 4.1 100 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 300 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 500 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 50% responder rate ‐ dose effect Faught 2001 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 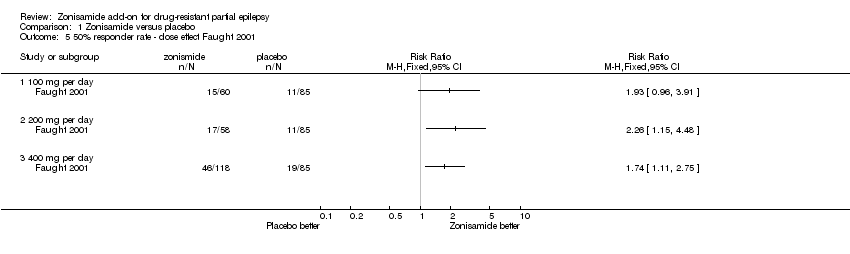 Comparison 1 Zonisamide versus placebo, Outcome 5 50% responder rate ‐ dose effect Faught 2001. | ||||
| 5.1 100 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 200 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 400 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Withdrawal rates Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.6 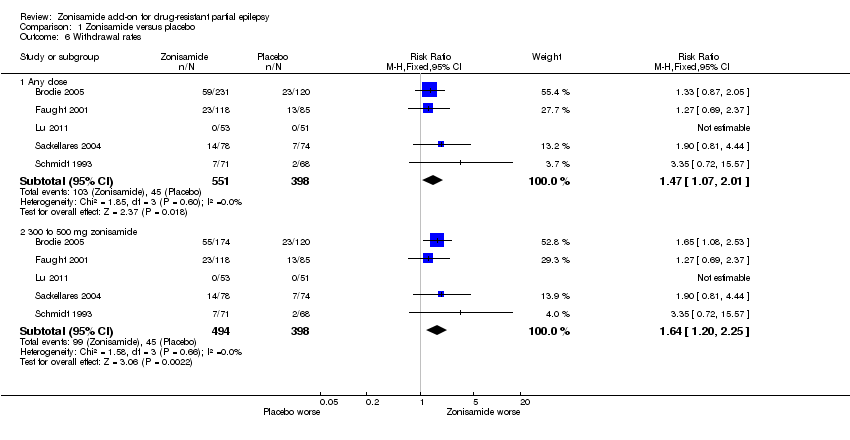 Comparison 1 Zonisamide versus placebo, Outcome 6 Withdrawal rates. | ||||
| 6.1 Any dose | 5 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.07, 2.01] |
| 6.2 300 to 500 mg zonisamide | 5 | 892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.20, 2.25] |
| 7 Adverse effects Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Zonisamide versus placebo, Outcome 7 Adverse effects. | ||||
| 7.1 Ataxia | 3 | 494 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.77 [1.28, 11.11] |
| 7.2 Dizziness | 5 | 949 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.46 [0.88, 2.44] |
| 7.3 Nausea | 4 | 598 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.21 [0.61, 2.40] |
| 7.4 Fatigue | 4 | 598 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.41 [0.76, 2.62] |
| 7.5 Somnolence | 5 | 949 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.83 [1.08, 3.11] |
| 7.6 Agitation/irritability | 4 | 598 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.35 [1.05, 5.27] |
| 7.7 Anorexia | 3 | 494 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.71 [1.29, 5.69] |

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Zonisamide versus placebo, Outcome 1 50% responder rate ‐ whole treatment period.

Comparison 1 Zonisamide versus placebo, Outcome 2 50% responder rate ‐ best‐case.

Comparison 1 Zonisamide versus placebo, Outcome 3 50% responder rate ‐ worst‐case.

Comparison 1 Zonisamide versus placebo, Outcome 4 50% responder rate ‐ dose effect Brodie 2005.

Comparison 1 Zonisamide versus placebo, Outcome 5 50% responder rate ‐ dose effect Faught 2001.

Comparison 1 Zonisamide versus placebo, Outcome 6 Withdrawal rates.

Comparison 1 Zonisamide versus placebo, Outcome 7 Adverse effects.
| Zonisamide versus placebo for drug‐resistant partial epilepsy | ||||||
| Patient or population: patients with drug‐resistant partial epilepsy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Zonisamide versus placebo | |||||
| 50% responder rate ‐ whole treatment period ‐ any dose | Study population | RR 1.92 | 949 | ⊕⊕⊕⊕ | 3 out of 5 studies found a non‐significant effect of zonisamide on responder rate but the combined overall effect was statistically significant | |
| 191 per 1000 | 367 per 1000 | |||||
| Moderate | ||||||
| 175 per 1000 | 336 per 1000 | |||||
| Withdrawal rates ‐ any dose | Study population | RR 1.47 | 949 | ⊕⊕⊕⊕ | No study produced a statistically significant effect of zonisamide on withdrawal rates independently but a statistically significant effect was found overall | |
| 113 per 1000 | 166 per 1000 | |||||
| Moderate | ||||||
| 95 per 1000 | 140 per 1000 | |||||
| Adverse effects ‐ ataxia | Study population | 3.77 | 494 | ⊕⊕⊕⊕ | No study found a statistically significant effect of zonisamide on ataxia independently, but there was a significant effect when study data were combined | |
| 26 per 1000 | 100 per 1000 | |||||
| Moderate | ||||||
| 24 per 1000 | 90 per 1000 | |||||
| Adverse effects ‐ dizziness | Study population | 1.46 | 949 | ⊕⊕⊕⊕ | No study found a statistically significant effect of zonisamide on dizziness independently | |
| 88 per 1000 | 128 per 1000 | |||||
| Moderate | ||||||
| 118 per 1000 | 172 per 1000 | |||||
| Adverse effects ‐ nausea | Study population | 1.21 | 598 | ⊕⊕⊕⊕ | No study found a statistically significant effect of zonisamide on nausea independently | |
| 76 per 1000 | 91 per 1000 | |||||
| Moderate | ||||||
| 69 per 1000 | 83 per 1000 | |||||
| Adverse effects ‐ fatigue | Study population | 1.41 | 598 | ⊕⊕⊕⊕ | No study found a statistically significant effect of zonisamide on fatigue independently | |
| 86 per 1000 | 122 per 1000 | |||||
| Moderate | ||||||
| 79 per 1000 | 111 per 1000 | |||||
| Adverse effects ‐ somnolence | Study population | 1.83 | 949 | ⊕⊕⊕⊕ | 4 out of 5 studies found no statistically significant difference in the occurrence of somnolence between the zonisamide group and the control group | |
| 75 per 1000 | 138 per 1000 | |||||
| Moderate | ||||||
| 118 per 1000 | 216 per 1000 | |||||
| Adverse effects ‐ agitation/irritability | Study population | 2.35 | 598 | ⊕⊕⊕⊕ | 1 out of 4 studies found a significant effect of zonisamide on agitation/irritability and there was a statistically significant effect overall | |
| 43 per 1000 | 101 per 1000 | |||||
| Moderate | ||||||
| 44 per 1000 | 103 per 1000 | |||||
| Adverse effects ‐ anorexia | Study population | 2.71 | 494 | ⊕⊕⊕⊕ | None of 3 studies found significant effects of zonisamide on anorexia, but overall there was a significant effect when data were combined | |
| 62 per 1000 | 167 per 1000 | |||||
| Moderate | ||||||
| 81 per 1000 | 220 per 1000 | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 50% responder rate ‐ whole treatment period Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Any dose | 5 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.52, 2.42] |
| 1.2 300 to 500 mg zonisamide | 5 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.58, 2.54] |
| 2 50% responder rate ‐ best‐case Show forest plot | 5 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [1.78, 2.79] |
| 3 50% responder rate ‐ worst‐case Show forest plot | 5 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.16, 1.75] |
| 4 50% responder rate ‐ dose effect Brodie 2005 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.1 100 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 300 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 500 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 50% responder rate ‐ dose effect Faught 2001 Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 100 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 200 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 400 mg per day | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Withdrawal rates Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Any dose | 5 | 949 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.07, 2.01] |
| 6.2 300 to 500 mg zonisamide | 5 | 892 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.20, 2.25] |
| 7 Adverse effects Show forest plot | 5 | Risk Ratio (M‐H, Fixed, 99% CI) | Subtotals only | |
| 7.1 Ataxia | 3 | 494 | Risk Ratio (M‐H, Fixed, 99% CI) | 3.77 [1.28, 11.11] |
| 7.2 Dizziness | 5 | 949 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.46 [0.88, 2.44] |
| 7.3 Nausea | 4 | 598 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.21 [0.61, 2.40] |
| 7.4 Fatigue | 4 | 598 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.41 [0.76, 2.62] |
| 7.5 Somnolence | 5 | 949 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.83 [1.08, 3.11] |
| 7.6 Agitation/irritability | 4 | 598 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.35 [1.05, 5.27] |
| 7.7 Anorexia | 3 | 494 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.71 [1.29, 5.69] |