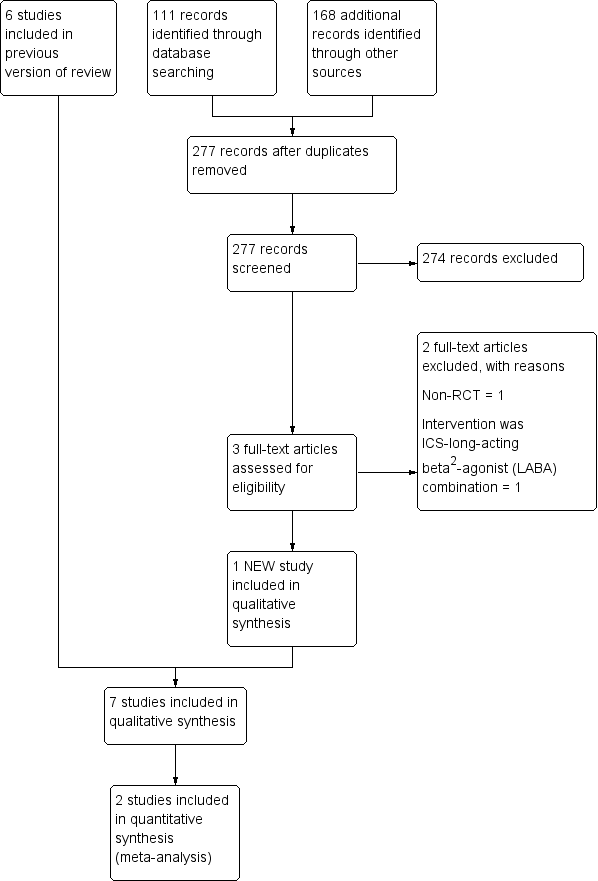
نقش کورتیکواستروئیدهای استنشاقی در درمان برونشکتازی
References
منابع مطالعات واردشده در این مرور
منابع مطالعات خارجشده از این مرور
منابع اضافی
منابع دیگر نسخههای منتشرشده این مرور
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | A prospective, double‐blind, placebo controlled, randomised cross‐over design with study duration of 6 weeks. There were five participants who dropped out; we are unsure when these occurred. Two participants declined to take part in second limb of study. No washout period mentioned. | |
| Participants | Twenty participants (12 females, mean age 50 years, range 30 to 65) were studied with bronchiectasis diagnosed by bronchogram in 18 and computed tomography scan in two. No participant received a course of antibiotics for at least 8 weeks prior to the study. Exclusion: participants with hypogammaglobulinaemia, cystic fibrosis, ABPA or primary ciliary dyskinesia, as well as those taking OCS or ICS. | |
| Interventions | Inhaled beclomethasone dipropionate 750 µg twice daily by MDI or placebo for 6 weeks. | |
| Outcomes |
| |
| Notes | Cross‐over design with no washout period. Separate results of first arm not available. Data not included in analysis. No funding source mentioned, though acknowledgement for ICS was given to Allen and Hanburys. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment was reported in the article. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "double‐blinded" and "matched placebo". |
| Incomplete outcome data (attrition bias) | High risk | There were 5 patients who dropped out; we are unsure when these occurred. Two patients declined to take part in second arm of study. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | Cross‐over design with no washout period. Separate results of first arm not available. |
| Methods | A prospective, double‐blind, parallel group placebo controlled trial. | |
| Participants | 77 participants included over a 3‐year period. Age range 40 to 85 years, mean age 68.06 years. Seven did not complete the study: three died from respiratory failure and four pulled out voluntarily. 37 analysed in the budesonide group and 33 in the placebo group. Mean age of the budesonide group was 68 years and that of the placebo group was 66 years. 19 (27.1%) participants in the budesonide group and 18 (25.7%) in the placebo group had Pseudomonas aeruginosa (P aeruginosa) cultured from their sputum. The mean (SD) baseline FEV1% predicted in the budesonide and the placebo group was 64.6% (25.1) and 64.7% (27), respectively. | |
| Interventions | 400 µg inhaled budesonide twice a day versus placebo for 6 months. | |
| Outcomes | Total symptom score which included I) cough and sputum production; and ii) dyspnoea.
| |
| Notes | Additional information provided by the authors. The study was supported by the Catalan Foundation of Pneumology grant. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hospital pharmacy (third party) performed randomisation and dispensed inhalers. |
| Allocation concealment (selection bias) | Unclear risk | No mention how allocation was done. |
| Blinding (performance bias and detection bias) | Low risk | Quote "parallel, placebo‐masked clinical trial". Probably done. |
| Incomplete outcome data (attrition bias) | High risk | 7 dropouts, 6 of whom were from the placebo group. Data of dropouts not included. Since 3 of these died of respiratory failure, all belonging to the placebo group, potential for bias on outcome if data not included. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | Low risk | No other risk of bias identified |
| Methods | Randomised double‐blind, placebo controlled, cross‐over study with study duration of 4 weeks. Two‐week washout period between cross‐over. Details of dropouts not clear. | |
| Participants | 20 participants (9 females) age range 15 to 60 years were prospectively enrolled. All participants treated with oral salbutamol 2 mg four times daily and oral theophylline 200 mg four times daily throughout the trial period. 14 participants had unilateral disease and six had bilateral disease. Inclusion: bronchiectasis confirmed by HRCT, chest in stable state (no exacerbation in previous 1 month) demonstrating significant post‐bronchodilator response (> 12% change) on spirometry Exclusion: atopy, bronchial asthma or smoking | |
| Interventions | Inhaled beclomethasone 800 µg/day in two divided doses by MDI or placebo for 4 weeks. | |
| Outcomes |
| |
| Notes | SD calculated from P value. Only first arm of the study before cross‐over used in analysis. Additional information provided by the authors. Spirometeric data not included in the analysis since baseline values not reported separately for the two groups and a common mean given for the group of 20. No mention of funding source. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No mention how allocation was done. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "patients were randomised in a double blind manner". Comment: Probably done. |
| Incomplete outcome data (attrition bias) | Unclear risk | No mention of dropouts. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | Inclusion of participants who had a significant post‐bronchodilator response biased the study in favour of response to ICS since those with positive bronchodilator response are more likely to improve with ICS due to the asthma‐like reversibility in their airway. |
| Methods | Randomised (stratified for prior smoking habit in pack year), double‐blind (only for dose of steroid), non‐placebo controlled prospective trial with study duration 6 months. | |
| Participants | Of the 132 participants initially included in the study, 39 were excluded prior to randomisation (23 had high probability of asthma, 4 did not give consent, 1 had Down's syndrome and 3 each had psychiatric disorder, systemic ICS and had disease of significant severity). 93 patients enrolled in the study. Seven dropouts during the study, three from the no steroid group and two each from the 500 µg and 1000 µg group. Study completed in 86 participants.
Inclusion: all participants with HRCT diagnosed bronchiectasis diagnosed between 1993 and June 2003 in Requena General Hospital. The participants were required to be free from acute exacerbation for at least 4 weeks. Exclusion: participants with asthma, cystic fibrosis and on whom ICS could not be stopped. | |
| Interventions | Inhaled fluticasone 500 µg twice daily by MDI versus 250 µg fluticasone twice daily versus no treatment for 6 months | |
| Outcomes | Baseline data collection started 6 months prior to randomisation. During this period data prospectively collected on number of acute exacerbations, antibiotic use and hospital admissions.
During randomisation visit, information collected on dyspnoea score, daily sputum production (average of sputum produced over three days); cough and need for short‐acting bronchodilator in the 1‐month prior to randomisation, and HRQoL using the validated Spanish version of the SGRQ. After randomisation, TLC, RV and diffusion capacity analysed again after 6 months. HRQoL assessment at 3 and 6 months and all other tests at 1, 3 and 6 months. > 4‐point change in SGRQ considered significant. > 1‐point change is dyspnoea score considered significant. | |
| Notes | Wherever available, data on the 250 µg twice daily group was combined with 500 µg twice daily group for comparison with no steroid group. Additional information provided by the authors. Study was supported by Grant Red Respira. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation was done. |
| Blinding (performance bias and detection bias) | High risk | Quote: "The study was conducted on a double blind basis regarding the effective inhalatory steroid dose administered (500 vs. 1000 µg/day), but not as relates to the administration or not of steroid treatment (i.e. 0 vs. 500 or 1000 µg/day)." Comment: No blinding for the two groups assessed by us (0 versus 1000 µg/day). |
| Incomplete outcome data (attrition bias) | High risk | The patient characteristics and outcome data of those excluded or dropped out not described and not compared with those included for analysis. |
| Selective reporting (reporting bias) | High risk | Information on TLC, RV and DLCO gathered but not reported. Some outcomes that were reported in the 1000 µg group were not reported in the 500 µg group (such as sputum volume, TLC, RV and cough and wheeze clinical variables). |
| Other bias | High risk | Intention‐to‐treat analysis not done. Of the 39 participants excluded before randomisation, no details available for the reason for exclusion for 2 participants. |
| Methods | Randomised double‐blind, placebo controlled prospective trial with study duration of 4 weeks. There were no dropouts. | |
| Participants | 24 participants (mean age 51 years, 12 females) with HRCT proven bronchiectasis. Fluticasone group: n = 12, 6 females, age: mean 43 (SD 11). Placebo group: n = 12, 8 females, age: mean 56.8 (SD 11). Inclusion: daily sputum > 10 mL, absence of asthma or other unstable systemic disease; and "steady state" bronchiectasis (< 10% alteration of 24‐hour sputum volume, FEV1 and FVC). Exclusion: unreliable clinic attendance, known adverse reaction to fluticasone, regular use of ICS and asthma. | |
| Interventions | Inhaled fluticasone 500 µg twice daily by accuhaler or placebo for 4 weeks. | |
| Outcomes |
Sputum leukocyte density, bacterial densities and concentrations of interleukin (IL)1B, IL 8, TNF alpha and leukotriene B4 were all measured at the time of randomisation and at 4 weeks. | |
| Notes | Geometric mean was used instead of arithmetic mean. Hence, none of the lung function data were included in the final analysis. No mention of funding source. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No mention of how allocation was done. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "performed a double blind, placebo controlled study". Comment: Probably done. |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | The baseline values for lung functions, sputum amount and sputum inflammatory markers were significantly different, thus were subject to bias. |
| Methods | Randomised double‐blind, prospective placebo controlled trial with study duration of 52 weeks. There were no dropouts. | |
| Participants | 60 participants (mean age 56.4 years, 38 females) with HRCT proven bronchiectasis. Fluticasone group: n = 30, age: mean 56.1 (SD 14). Placebo group: n = 30, age: mean 56.7 (SD 11.3). 16 were P aeruginosa colonised and 44 were not. Inclusion: absence of asthma or other unstable systemic disease; and "steady state" bronchiectasis (< 20% alteration of 24‐hour sputum volume, FEV1 and FVC) and absence of deterioration in respiratory symptoms at baseline visit. Exclusion: unreliable clinic attendance, known adverse reaction to fluticasone and asthma. | |
| Interventions | Inhaled fluticasone 500 µg twice daily by accuhaler or placebo for 52 weeks. | |
| Outcomes | The participants were followed up at ‐2, ‐1, 0, 4, 12, 24, 36, 48 and 52 weeks after commencement of therapy for measurement of FeNO. | |
| Notes | Data not included in the final analysis since medians and inter‐quartile range reported in this study. The study was supported by a CRCG Grant of the University of Hong Kong. The Accuhalers were donated by GlaxoWellcome, China. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information was provided within the published article about generation of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | No mention on allocation concealment. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "performed a double blind, placebo controlled study". |
| Incomplete outcome data (attrition bias) | Low risk | There were no dropouts. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | Baseline value of the 2 groups were significantly different. |
| Methods | Randomised double‐blind, prospective placebo controlled trial with study duration of 52 weeks. 5 dropouts in the placebo arm (1 at 4 weeks, 3 at 24 weeks and 1 at 52 weeks) and 8 dropouts in the fluticasone arm (2 at 4 weeks and 1 each at 6, 22, 32, 36, 50 and 52 weeks). | |
| Participants | 89 patients recruited. Three participants withdrew. 86 participants (57 females, mean age 58.5 years) with HRCT proven bronchiectasis randomised between fluticasone and placebo. Fluticasone group: n = 43, 23 females, age: mean 57.7 (SD 14.4). Placebo group: n = 43, 34 females, age: mean 59.2 (SD 14.2). 23 were P aeruginosa colonised. Inclusion: absence of asthma or other unstable systemic disease; and "steady state" bronchiectasis (< 20% alteration of 24‐hour sputum volume, FEV1 and FVC) and absence of deterioration in respiratory symptoms at baseline visit. Exclusion: unreliable clinic attendance, known adverse reaction to fluticasone or quinolones and regular usage of ICS. | |
| Interventions | Inhaled fluticasone 500 µg twice daily by accuhaler device or matched placebo for 52 weeks. | |
| Outcomes | The participants were followed up at ‐2, ‐1, 0, 4, 12, 24, 36, 48 and 52 weeks after commencement of therapy. Primary outcomes
Secondary outcomes
Improvement or deterioration was defined as > 20% change from baseline. | |
| Notes | SD calculated from P value for sputum volume and exacerbation frequency and from confidence interval for the rest. The study was partially funded by GlaxoWelcome (Hong Kong). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomisation (block of 4)", however, no information about who generated the randomisation codes. |
| Allocation concealment (selection bias) | Unclear risk | No information about concealment was reported in the published article. |
| Blinding (performance bias and detection bias) | Low risk | Quote: "double blind, placebo controlled study". |
| Incomplete outcome data (attrition bias) | High risk | Data of dropouts not compared to those included in analysis. |
| Selective reporting (reporting bias) | Low risk | No suggestion that selective reporting may have been done. |
| Other bias | High risk | Significant differences at the baseline on clinical features of "cough" and "dyspnoea" between the two groups to allow for post‐treatment comparison. |
ABPA: allergic bronchopulmonary aspergillosis
DLCO: diffusing capacity of the lungs for carbon monoxide
FeNO: fractional exhaled nitric oxide
FEV1: forced expiratory volume (in 1 second)
FVC: forced vital capacity
HRCT: high resolution computed tomography
HRQoL: health‐related quality of life
ICS: inhaled corticosteroids
MDI: metered dose inhaler
OCS: oral corticosteroids
PEFR: peak expiratory flow rate
RV: residual volume
SD: standard deviation
SGRQ: St George's Respiratory Questionnaire
TLC: total lung capacity
TNF: tumour necrosis factor
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Study compared effects of inhaled budesonide with inhaled ipratropium and not placebo | |
| Not a RCT, withdrawal study with before‐and‐after outcomes | |
| Study compared budesonide with budesonide‐formoterol combination and not a placebo | |
| Study included patients with pneumonia and not bronchiectasis. Used systemic steroids, not inhaled | |
| Study not a RCT. Studies subjective benefits of inhaler therapy including both ICS and bronchodilators |
ICS: inhaled corticosteroids
RCT: randomised controlled trial
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung function (spirometry indices) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 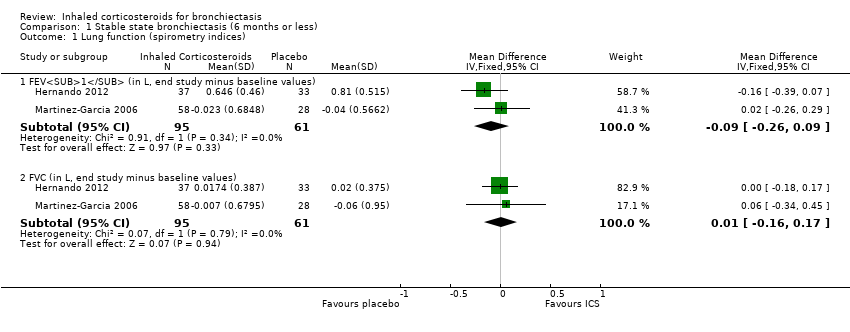 Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 1 Lung function (spirometry indices). | ||||
| 1.1 FEV1 (in L, end study minus baseline values) | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.26, 0.09] |
| 1.2 FVC (in L, end study minus baseline values) | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.16, 0.17] |
| 2 Lung function (other indices) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 2 Lung function (other indices). | ||||
| 2.1 Diffusion capacity % predicted (end of study) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐2.49, 7.89] |
| 2.2 RV % predicted (end of study values) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐9.41, 13.41] |
| 2.3 TLC % predicted (end of study values) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐1.99, 8.39] |
| 3 Clinical severity indices Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 3 Clinical severity indices. | ||||
| 3.1 Number of participants with regular wheeze (combined) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Number of participants without sputum reduction of > 50% (combined) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Number of participants with no improvement in dyspnoea score > 1 (min important difference) (1000F) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Number of participants with no clinically significant improvement in HRQoL | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Exacerbations Show forest plot | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.56, 0.22] |
| Analysis 1.4 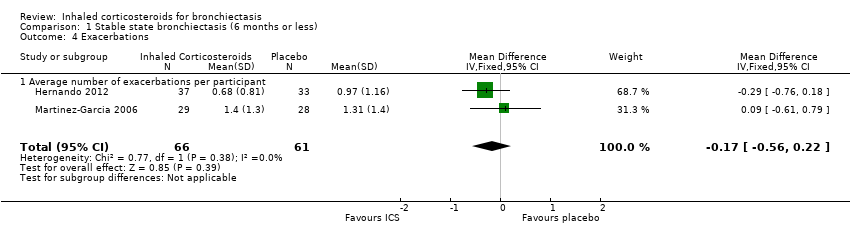 Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 4 Exacerbations. | ||||
| 4.1 Average number of exacerbations per participant | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.56, 0.22] |
| 5 Sputum and biomarkers characteristics Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.5 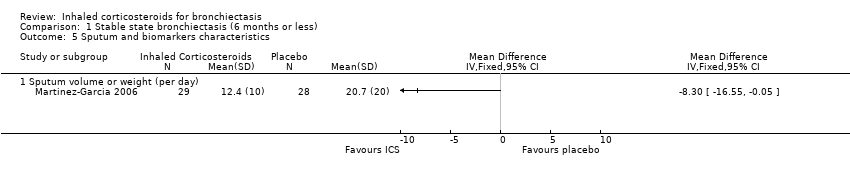 Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 5 Sputum and biomarkers characteristics. | ||||
| 5.1 Sputum volume or weight (per day) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pseudomonas aeruginosa colonisation Show forest plot | 2 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.45, 1.96] |
| Analysis 1.6  Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 6 Pseudomonas aeruginosa colonisation. | ||||
| 7 St George HRQoL (end of study minus baseline) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 7 St George HRQoL (end of study minus baseline). | ||||
| 7.1 Total score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐3.54 [‐8.00, 0.92] |
| 7.2 Symptom score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐4.75 [‐10.42, 0.92] |
| 7.3 Activity score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐6.21 [‐12.40, ‐0.01] |
| 7.4 Impact score | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.63 [‐9.35, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung function indices Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.1  Comparison 2 Stable state (> 6 months), Outcome 1 Lung function indices. | ||||
| 1.1 FEV1% predicted (end study minus baseline values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 FVC % predicted (end study minus baseline values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Exacerbations Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.2  Comparison 2 Stable state (> 6 months), Outcome 2 Exacerbations. | ||||
| 2.1 Number of participants improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sputum and biomarker characteristics Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 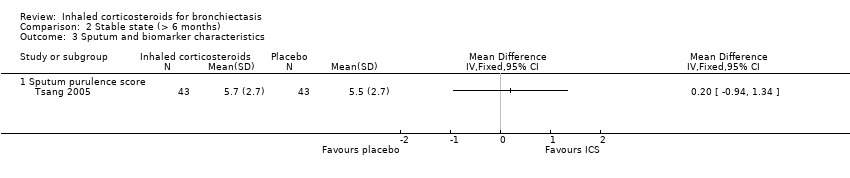 Comparison 2 Stable state (> 6 months), Outcome 3 Sputum and biomarker characteristics. | ||||
| 3.1 Sputum purulence score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 1 Lung function (spirometry indices).

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 2 Lung function (other indices).

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 3 Clinical severity indices.

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 4 Exacerbations.

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 5 Sputum and biomarkers characteristics.

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 6 Pseudomonas aeruginosa colonisation.

Comparison 1 Stable state bronchiectasis (6 months or less), Outcome 7 St George HRQoL (end of study minus baseline).

Comparison 2 Stable state (> 6 months), Outcome 1 Lung function indices.

Comparison 2 Stable state (> 6 months), Outcome 2 Exacerbations.

Comparison 2 Stable state (> 6 months), Outcome 3 Sputum and biomarker characteristics.
| Inhaled corticosteroids compared to placebo for bronchiectasis (< 6 months) | ||||||
| Patient or population: people with bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inhaled corticosteroids | |||||
| Lung function (spirometry indices) ‐ FEV1 (in L, end study minus baseline values) | Mean change from baseline ranged from ‐0.038 to 0.805 | MD 0.09 lower | ‐ | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (spirometry indices) ‐ FVC (in L, end study minus baseline values) | Mean change from baseline ranged from ‐0.062 to 0.0218 | MD 0.01 higher | ‐ | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ diffusion capacity % predicted (end of study) | Mean end of study value 84.2 | MD 2.70 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ RV % predicted (end of study values) | Mean end of study value 106 | MD 2.00 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Lung function (other indices) ‐ TLC % predicted (end of study values) | Mean end of study value 86.4 | MD 3.20 higher | ‐ | 57 | ⊕⊕⊝⊝ Low1,2,3 | |
| Average number of exacerbations per participant | Average number of exacerbations per patient ranged from 0.97 to 1.31 | MD 0.17 lower | ‐ | 127 | ⊕⊕⊝⊝ Low1,2,3 | |
| Pseudomonas aeruginosa (P aeruginosa) colonisation | 410 per 1000 | 395 per 1000 | OR 0.94 | 156 | ⊕⊕⊝⊝ Low1,2,3 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1The largest study was not a placebo controlled trial (Martinez‐Garcia 2006). | ||||||
| Inhaled corticosteroids compared to placebo for bronchiectasis (medium‐ to long‐term outcomes) | ||||||
| Patient or population: people with bronchiectasis | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with Inhaled corticosteroids | |||||
| Lung function indices ‐ FEV1% predicted (end study minus baseline values) | Mean change from baseline 0 | MD 0.30 higher | ‐ | 86 | ⊕⊕⊝⊝ Low1,2 | |
| Lung function indices ‐ FVC % predicted (end study minus baseline values) | Mean change from baseline 0.9 | MD 0.90 lower | ‐ | 86 | ⊕⊕⊝⊝ Low1,2 | |
| Number of participants improved | 628 per 1000 | 490 per 1000 | OR 0.57 | 86 | ⊕⊕⊝⊝ Low1,2 | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Only a single study with small participant numbers; we downgraded outcome one point for imprecision. | ||||||
| Study ID | Intervention | Control | Duration of intervention |
| Beclomethasone dipropionate 750 µg twice daily by MDI | Placebo | 6 weeks | |
| Budesonide 400 µg twice daily | Placebo | 6 months | |
| Beclomethasone 800 µg per day over 2 doses | Placebo | 4 weeks | |
| Fluticasone 500 µg twice daily by MDI or 250 µg fluticasone twice daily | No treatment | 6 months | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 4 weeks | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 52 weeks | |
| Fluticasone 500 µg twice daily by accuhaler | Placebo | 52 weeks | |
| MDI: metered dose inhaler | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung function (spirometry indices) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 FEV1 (in L, end study minus baseline values) | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | ‐0.09 [‐0.26, 0.09] |
| 1.2 FVC (in L, end study minus baseline values) | 2 | 156 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.16, 0.17] |
| 2 Lung function (other indices) Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Diffusion capacity % predicted (end of study) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐2.49, 7.89] |
| 2.2 RV % predicted (end of study values) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐9.41, 13.41] |
| 2.3 TLC % predicted (end of study values) | 1 | 57 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [‐1.99, 8.39] |
| 3 Clinical severity indices Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Number of participants with regular wheeze (combined) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Number of participants without sputum reduction of > 50% (combined) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Number of participants with no improvement in dyspnoea score > 1 (min important difference) (1000F) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.4 Number of participants with no clinically significant improvement in HRQoL | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Exacerbations Show forest plot | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.56, 0.22] |
| 4.1 Average number of exacerbations per participant | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐0.56, 0.22] |
| 5 Sputum and biomarkers characteristics Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.1 Sputum volume or weight (per day) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Pseudomonas aeruginosa colonisation Show forest plot | 2 | 156 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.45, 1.96] |
| 7 St George HRQoL (end of study minus baseline) Show forest plot | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Total score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐3.54 [‐8.00, 0.92] |
| 7.2 Symptom score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐4.75 [‐10.42, 0.92] |
| 7.3 Activity score | 2 | 127 | Mean Difference (IV, Fixed, 95% CI) | ‐6.21 [‐12.40, ‐0.01] |
| 7.4 Impact score | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐3.63 [‐9.35, 2.09] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Lung function indices Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 FEV1% predicted (end study minus baseline values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 FVC % predicted (end study minus baseline values) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Exacerbations Show forest plot | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 Number of participants improved | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sputum and biomarker characteristics Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Sputum purulence score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |