母乳添加脂肪補充劑促進早產兒生長
References
References to studies included in this review
References to studies excluded from this review
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Parallel randomised controlled trial in two neonatal units. | |
| Participants | Inclusion criteria: birth weight < 1500 g, appropriate‐for‐gestational‐age, tolerance of complete enteral feeding (170 mL/kg/day), no obvious disease or major malformations, no oxygen therapy, and informed parental consent and acceptance of a blind trial | |
| Interventions | 1.0 g of human milk fat per 100 mL unpasteurised human milk (maternal or term banked donor) (n = 7) versus unsupplemented human milk (n = 7). | |
| Outcomes | The outcomes were not specified as primary or secondary but the following were assessed: short‐term growth parameters (weight, crown‐heel length, occipito‐frontal head circumference), intake of protein, fat, carbohydrates, energy, and electrolytes (sodium, potassium, calcium). | |
| Notes | Conflicts of interest: no details This study had four arms: unsupplemented versus supplemented with protein versus supplemented with fat versus supplemented with fat and protein. The analyses of the protein and combined fat and protein arms are discussed in other reviews on multi‐component and protein supplementation, respectively (Brown 2016; Kuschel 2000). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details |
| Allocation concealment (selection bias) | Unclear risk | The study used closed envelopes without specifying if they were opaque or not. |
| Blinding of participants and personnel (performance bias) | Unclear risk | The study was stated to be double‐blinded, but who was blinded was not specified |
| Blinding of outcome assessment (detection bias) | Unclear risk | It was not specified whether outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) | Unclear risk | The missing data (i.e. from 6 infants) was less than 20%. They were excluded for the following reasons: 2 had apnoea, 3 developed feeding intolerance, and 1 needed intravenous therapy. However, the authors did not report whether there were any differences between infants excluded and included in the study. |
| Selective reporting (reporting bias) | Unclear risk | No details were given as to which were primary and secondary outcomes, and no protocol was viewed to clarify whether the outcomes reported were the only ones collected. |
| Other bias | Unclear risk | The authors stated 'there was a difference in sex distribution between the groups and later analyses confirmed that this difference had no implications on the results'. However, no further details were provided as to how this conclusion was reached. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Irrelevant intervention | |
| A commentary of a trial with irrelevant intervention | |
| Fat supplementation of maternal diet of lactating mothers | |
| Unable to extract data for infants supplemented with fat alone |
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Growth ‐ weight Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1 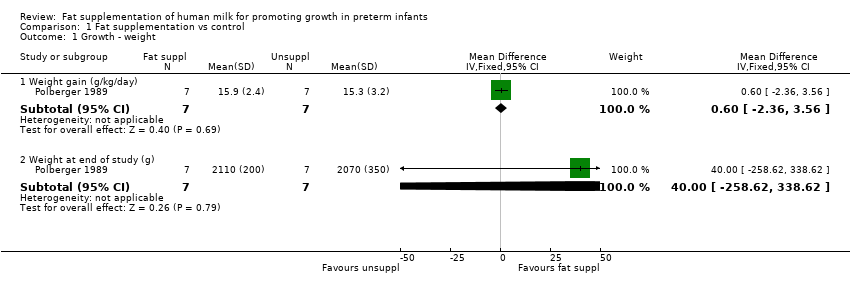 Comparison 1 Fat supplementation vs control, Outcome 1 Growth ‐ weight. | ||||
| 1.1 Weight gain (g/kg/day) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.36, 3.56] |
| 1.2 Weight at end of study (g) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 40.0 [‐258.62, 338.62] |
| 2 Growth ‐ length Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Fat supplementation vs control, Outcome 2 Growth ‐ length. | ||||
| 2.1 Length gain (cm/week) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.08, 0.28] |
| 3 Growth ‐ head circumference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3  Comparison 1 Fat supplementation vs control, Outcome 3 Growth ‐ head circumference. | ||||
| 3.1 Head growth (cm/week) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.07, 0.37] |
| 4 Feeding intolerance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4  Comparison 1 Fat supplementation vs control, Outcome 4 Feeding intolerance. | ||||

81, Study flow diagram: review update

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Fat supplementation vs control, Outcome 1 Growth ‐ weight.

Comparison 1 Fat supplementation vs control, Outcome 2 Growth ‐ length.

Comparison 1 Fat supplementation vs control, Outcome 3 Growth ‐ head circumference.

Comparison 1 Fat supplementation vs control, Outcome 4 Feeding intolerance.
| Fat supplementation compared to control for promoting growth in preterm infants | ||||||
| Patient or population: preterm infants | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with control | Risk with Fat supplementation | |||||
| Growth ‐ weight ‐ weight gain (g/kg/day) | The mean weight gain in the unsupplemented human milk group was 15.3 g/kg/day. | MD 0.6 g/kg/day higher | ‐ | 14 | ⊕⊝⊝⊝ | |
| Growth ‐ length ‐ length gain (cm/week) | The mean length gain in the unsupplemented human milk group was 0.8 cm/week. | MD 0.1 cm/week higher | ‐ | 14 | ⊕⊝⊝⊝ | |
| Growth ‐ head circumference ‐ head growth (cm/week) | The mean head growth in the unsupplemented human milk group was 0.9 cm/week. | MD 0.2 cm/week higher | ‐ | 14 | ⊕⊝⊝⊝ | |
| Neurodevelopmental outcomes | ‐ | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on neurodevelopmental outcomes. |
| Duration of hospital admission (days) | ‐ | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on duration of hospital admission. |
| Feeding intolerance | 0 per 1000 | 0 per 1000 | RR 3.00 | 16 | ⊕⊝⊝⊝ | . |
| Necrotising enterocolitis | ‐ | ‐ | ‐ | ‐ | ‐ | None of the included studies reported on necrotising enterocolitis. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Risk of bias: most of the trials lacked methodological details so that we were unable to judge risk of bias. This could have an impact on assessment of growth parameters and possibly the estimate of effect. Single trial. We downgraded one level. 2 Imprecision: few patients and wide confidence intervals, which included meaningful benefit and harm. Single trial. We downgraded two levels. 3 Imprecision: few patients, few events and wide confidence intervals, which include meaningful benefit and harm. Single trial. We downgraded two levels. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Growth ‐ weight Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Weight gain (g/kg/day) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [‐2.36, 3.56] |
| 1.2 Weight at end of study (g) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 40.0 [‐258.62, 338.62] |
| 2 Growth ‐ length Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Length gain (cm/week) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.08, 0.28] |
| 3 Growth ‐ head circumference Show forest plot | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Head growth (cm/week) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.07, 0.37] |
| 4 Feeding intolerance Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |

