Administración de suplementos de vitaminas antioxidantes y minerales para la prevención de la degeneración macular asociada a la edad
References
References to studies included in this review
References to studies excluded from this review
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Method of allocation: random. Sponsor provided coded capsules. Masking: participant: yes; provider: yes; outcome: yes Exclusions after randomisation: no Losses to follow‐up: 31%. Random sample for maculopathy study: 9%. | |
| Participants | Country: Finland Number of participants randomised: 29,133. Random sample of 1035 selected for maculopathy study. Age: 50 to 69 years in 1984. Maculopathy study 1992‐3 in people aged 65 plus. Sex: male Inclusion criteria: 5 or more cigarettes daily Exclusion criteria: history of cancer or serious disease limiting ability to participate; those taking supplements vitamin E, A, or beta‐carotene in excess of predefined doses; those treated with anticoagulants | |
| Interventions | Intervention:
Comparator:
Duration: 5 to 8 years (median 6.1) | |
| Outcomes | AMD: 4 grades: Quote "A person was considered to have ARM if he had a class I or higher change in either | |
| Notes | Compliance with treatment excellent; 4/5 active participants took more than 95% of scheduled capsules. Drop‐out rate and compliance similar between all 4 groups. Funding source: Quote "This study was supported by the Juho Vainio Foundation, Helsinki, Finland. The ATBC study was supported by Public Health Service Contract N01‐CN‐45165 from the Division of Cancer Prevention and Control, National Cancer Institute of the United States." Declarations of interest: NR Date study conducted: Quote "The ophthalmological examination took place during their final follow‐up trial visit between December 1992 and March 1993" Trial id: NCT00342992 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The participants were randomly assigned to one of four treatment groups: AT alone, AT and BC, BC alone, or placebo in a complete 2 x 2 factorial design" and "Randomization was performed in blocks of eight within each of the study areas." |
| Allocation concealment (selection bias) | Low risk | Quote "A coded reserve supply of capsule packs..." Not clearly stated that allocation concealed, but the study was described as being "double‐blind" |
| Blinding of participants and personnel (performance bias) | Low risk | Judgement comment: placebo‐controlled study. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote "The retinal specialist [...] examined six photographs (three per eye) of each participant without knowledge of the subject's treatment group" |
| Incomplete outcome data (attrition bias) | Low risk | Quote "A total of 941 persons participated (91%) and non‐participation rates were similar across the intervention groups." |
| Selective reporting (reporting bias) | Unclear risk | Visual acuity measured but not reported, but as the main results for AMD showed no difference between groups, it is not clear whether this was an example of selective reporting or whether, in fact, the investigators considered that visual acuity in this age group might be attributed to a variety of causes, and therefore, was not a relevant outcome. |
| Methods | Method of allocation: coded tablets Masking: participant: yes; provider: yes; outcome: yes 99% follow‐up 2 x 2 factorial design. | |
| Participants | Country: USA Number randomised: originally 22,071 men were randomised: Age: 40 to 84 years in 1982 Sex: male Inclusion criteria: physician aged 40 to 84 years in 1982 with no history of cancer, myocardial infarction, stroke, or transient cerebral ischaemia Exclusion criteria: personal history of cardiovascular disease or cancer; contraindications or current use of study medication; | |
| Interventions | Intervention:
Comparator:
There was also an aspirin arm (2 x 2 factorial arm), which was terminated early (January 1988) Mean duration 12 years range (range 11.6 to 14.2 years) | |
| Outcomes | Self report of AMD followed by medical record review and questionnaire to relevant ophthalmologist Primary endpoint: visually significant AMD, defined as a self‐report confirmed by medical record evidence of an initial diagnosis after randomisation, but before 31 December 1995, with a reduction in best‐corrected visual acuity to 20/30 or worse attributable to AMD Secondary endpoints: AMD with or without vision loss, composed of all incident cases; Advanced AMD, encompassed of cases of visually significant AMD with pathological signs of disciform scar, RPE detachment, geographic atrophy, or subretinal neovascular membrane Quote "Individuals, rather than eyes, were the unit of analysis because eyes were not examined independently, and participants were classified according to the status of the worse eye as defined by disease severity. When the worse eye was excluded because of visual acuity loss attributed to other ocular abnormalities the fellow eye was considered for classification." | |
| Notes | Funding source: Quote "Supported by research grants HL 26490, HL 34595, CA 34944, CA 40360, and EY 06633 from the National Institutes of Health" Declarations of interest: NR Date study conducted: August 1985 (from clinicaltrials.gov) to December 1995 Trial id: NCT00000500 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The PHS I was a randomised, double‐masked, placebo controlled trial..." "A total of 22,071 physicians were then randomised according to a two‐by‐two factorial design, with use of a computer‐generated list of random numbers..." |
| Allocation concealment (selection bias) | Low risk | Quote "The PHS I was a randomised, double‐masked, placebo controlled trial..." Judgement Comment: Although this aspect of the trial was not well described, the placebo control was described (placebo and supplement identical appearance and packaging) and the study was described as double‐blind |
| Blinding of participants and personnel (performance bias) | Low risk | Quote "The PHS I was a randomised, double‐masked, placebo controlled trial..." Judgement Comment: Although this aspect of the trial was not well described, the placebo control was described (placebo and supplement identical appearance and packaging) and the study was described as double‐blind |
| Blinding of outcome assessment (detection bias) | Low risk | Quote "The PHS I was a randomised, double‐masked, placebo controlled trial..." Judgement Comment: Although this aspect of the trial was not well described, the placebo control was described and the study was described as double‐blind. Diagnosis of AMD by self‐report based on health questionnaire (confirmed by ophthalmologist or optometrist). Patients and researchers unaware of intervention. |
| Incomplete outcome data (attrition bias) | Low risk | Quote "At the end of 11 years of follow‐up (the last year completed for all participants), 99.2% were still providing information on morbidity, and the follow‐up for mortality was 99.9% complete. Eighty percent of participants in the beta‐carotene group and in the placebo group were still taking the study pills, with a mean compliance among pill takers of more than 97%. Therefore, even after 11 years, 78% of the study pills assigned in the beta‐carotene group were reported as still being taken. In the placebo group, 6% of participants reported taking supplemental beta carotene or vitamin A." |
| Selective reporting (reporting bias) | Low risk | Judgement comment: reported AMD outcomes as expected |
| Methods | Method of allocation: coded tablets Masking: participant: yes; provider: yes; outcome: yes 95% follow‐up 2 x 2 x 2 x 2 factorial design. | |
| Participants | Country: USA Number randomised: 14,236 with no diagnosis of AMD at baseline according to vitamin C/E paper; 14,233 with no diagnosis of AMD at baseline according to multivitamin paper. Average age: 64 years Sex: male Inclusion criteria: US male physicians; 50 years and older; participants in PHS I and new physician participants; willing to forego use of supplements for new trial; for new participants, do not report personal history of cancer (except non‐melanoma skin cancer). CVD, current liver disease, current renal disease, peptic ulcer or gout. Compliance with pill‐taking regimen in run‐in period. Exclusion criteria:History of cirrhosis; active liver disease in past six months; participants reporting cataract or AMD at baseline. | |
| Interventions | Intervention:
Comparator:
Alternate day beta‐carotene (50 mg) component terminated in March 2003. Lutein (added to Centrum Silver during course of study (250 µg) and doses of other nutrients changed Follow‐up: the multivitamin component had a longer duration. "An average of 8 years of treatment and follow‐up" for vitamin E and vitamin C Median duration of treatment for multivitamin analyses 11.2 years, IQR 10.7 to 13.3 | |
| Outcomes | Age‐related macular degeneration: reported diagnosis followed up by contact with treating ophthalmologist/optometrist Quote "We considered individuals, rather than eyes, as the unit of analysis and we classified individuals according to the status of the worse eye as defined by disease severity. When the worse eye was excluded because of visual acuity loss attributed to other ocular abnormalities, the fellow eye was considered for classification." | |
| Notes | Funding source: Grants from National Eye Institute, National Institute on Ageing and the National Institutes of Health. BASF and DSM provided study agents and packaging. Declarations of interest: "The authors have no proprietary or commercial interest in any of the materials discussed in this article." Date study conducted: July 1997 to June 2011 (from clinicaltrials.gov) Trial id: NCT00270647 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "Randomisation to the other agents, using a computer generated list of random numbers, will be stratified according to age" |
| Allocation concealment (selection bias) | Low risk | Judgement comment: central randomisation |
| Blinding of participants and personnel (performance bias) | Low risk | Quote "The Physicians’ Health Study (PHS II) was a randomised, double‐blind, placebo‐controlled, factorial trial evaluating a daily multivitamin (Centrum Silver), alternate day vitamin E (400 IU synthetic α‐tocopherol), and daily vitamin C (500 mg synthetic ascorbic acid) in the prevention of cancer and CVD among 14,641 male physicians aged 50 years and older." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote "Random misclassification was reduced by the use of medical records to confirm the participant reports. Non‐random misclassification was unlikely since medical records were reviewed by an investigator (WGC) masked to treatment assignment, and study participants and treating ophthalmologists and optometrists were similarly unaware of treatment assignment." |
| Incomplete outcome data (attrition bias) | Low risk | Quote "Morbidity and mortality follow‐up were extremely high, at 95.3% and 97.9%, respectively." |
| Selective reporting (reporting bias) | Low risk | Judgement comment: reported AMD outcomes as expected |
| Methods | Method of allocation: coded bottles Masking: participant: yes; provider: yes; outcome: yes Losses to follow‐up: not known | |
| Participants | Country: Australia Number of participants randomised: 1204 Eyes: worse eye used as the study eye Age: 55 to 80 years, mean 66 Sex: 56% female Inclusion criteria: lens and retina of at least one eye available for documentation Exclusion criteria: previous cataract surgery or advanced cataract in both eyes; steroid or anticoagulation use; serious disease; regular use or sensitivity to vitamin E | |
| Interventions | Intervention:
Comparator:
Duration: 4 years | |
| Outcomes | 2 m logMAR visual acuity; clinical examination; colour stereoscopic fundus photographs graded using International Grading Scheme Quote "Participants were categorised by their worse eye." | |
| Notes | Funding source: Quote "The VECAT study was funded in part by grants from the National Health and Medical Research Council, the Jack Brockhoff Foundation, the Eirene Lucas Foundation, the Stoicesco Foundation, the Carleton Family Charitable Trust, JeHope Knell Trust Fund, Smith and Nephew, Australia, and Henkel Australia". Declarations of interest: none declared Date study conducted: January 1995 to April 1996 Trial id: NR | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "This random allocation was performed by using a “permuted blocks” allocation scheme" |
| Allocation concealment (selection bias) | Low risk | Quote "Study numbers were allocated sequentially by the study coordinator as participants were enrolled in the study. Participants were then randomly allocated to treatment group. The allocation list was stored at a remote site." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote "Participants randomly received either 500 IU natural vitamin E (335 mg dá tocopherol) in a soybean oil suspension encapsulated in gelatin or a matched placebo capsule containing only the soybean oil." [...] "Bulk medications were dispensed into labelled jars by a person not involved in the study. Vitamin E and placebo were dispensed on different days to avoid confusion. Identical containers were used. The jars were packed in numerical order and then dispensed by study personnel. Vitamin E and placebo capsules were of identical appearance and taste. Neither study staff nor examiners or participants were aware of the treatment allocation, although all knew that participants would be randomly assigned to receive either vitamin E or placebo." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote "Neither study staff nor examiners or participants were aware of the treatment allocation, although all knew that participants would be randomly assigned to receive either vitamin E or placebo." "At the end of the study we reassessed the initial and final photographs for any change with a “side by side” comparison in a masked and randomised fashion". |
| Incomplete outcome data (attrition bias) | Low risk | Quote "From the 1906 people who were screened by telephone, 1289 (69%) were examined and 1204 (93%) of these were enrolled and randomised. We excluded 11 participants after randomisation as they were outside the required age range at enrolment." "In the vitamin E group, eight people were excluded from final data analysis: six developed diabetic retinopathy, one had myopic degeneration, and one had missing data. Six people were excluded from the placebo group: two developed adult vitelliform macular degeneration and four had missing data." Figure 3: 1204 randomised, 11 excluded after randomisation, 14 excluded from analysis: 8/595 vitamin E group and 6/598 placebo group. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: AMD incidence and progression reported but no difference between groups; visual acuity not reported but "Analysis of best corrected visual acuity and visual function data showed no differences between the groups (data not shown)." Therefore, no evidence that outcomes with "better" results selectively reported. |
| Methods | Method of allocation: random allocation of coded bottles Masking: participant: yes; provider: yes; outcome: yes Losses to follow‐up: not known | |
| Participants | Country: USA Number of participants randomised: 39,876 women health professionals Age: 45+ Sex: female Inclusion/exclusion criteria: (a) Female; (b) aged 45 years or older; (c) postmenopausal or with no intention of becoming pregnant; (d) no reported personal history of cardiovascular disease, cancer (other than non‐melanoma skin cancer), gout, peptic ulcer, chronic renal or liver disease, or other serious illness precluding participation; (e) no reported history of serious side effects to the study treatments; (f) not currently taking aspirin, aspirin containing medication, or nonsteroidal anti‐inflammatory drugs (NSAIDs) more than 1 day per week or, if so doing, willing to forego use of these medications; (g) not currently taking individual supplements of vitamin E or beta‐carotene more than 1 day per week; (h) not currently taking anticoagulants or corticosteroids | |
| Interventions | Intervention:
Comparator:
| |
| Outcomes | Self‐report and review of medical records Quote "Individuals, rather than eyes, were the unit of analysis because eyes were not examined independently, and participants were classified according to the status of the worse eye as defined by disease severity. When the worse eye was excluded because of visual acuity loss attributed to other ocular abnormalities, the fellow eye was considered for classification." | |
| Notes | Funding source: Quote "Supported by research grants CA 47988, HL 43851, and EY 06633 from the National Institutes of Health, Bethesda, Md. Pills and packaging were provided by Bayer Healthcare and the Natural Source Vitamin E Association. Bayer Healthcare and the Natural Source Vitamin E Association had no role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript." Declarations of interest: Quote "The authors have no proprietary or commercial interest in any materials discussed in this article." Date study conducted: August 1993 to March 2004 Trial id: NCT00000161 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The WHS was a randomised, double‐blind, placebo‐controlled, 2 x 2 factorial trial..." "Randomization used blocks of size 16 within 5‐year age strata and took place from April 30, 1993, through January 24, 1996." |
| Allocation concealment (selection bias) | Low risk | Quote "The WHS was a randomised, double‐blind, placebo‐controlled, 2 x 2 factorial trial..." |
| Blinding of participants and personnel (performance bias) | Low risk | Quote "Study medications and end‐point ascertainment were continued in a blinded fashion through the scheduled end of the trial (March 31, 2004)." "Pill taking and end point ascertainment were continued in blinded fashion through the scheduled end of the trial on March 31, 2004. Morbidity and mortality follow‐up were 97.2% and 99.4% complete, respectively." |
| Blinding of outcome assessment (detection bias) | Low risk | Quote "Study medications and end‐point ascertainment were continued in a blinded fashion through the scheduled end of the trial (March 31, 2004)." "Pill taking and end point ascertainment were continued in blinded fashion through the scheduled end of the trial on March 31, 2004. Morbidity and mortality follow‐up were 97.2% and 99.4% complete, respectively." |
| Incomplete outcome data (attrition bias) | Low risk | Quote "Compliance (defined as taking at least two thirds of the study capsules) was 78.9% at 5 years and 71.6% at 10 years, and averaged 75.8% throughout the trial." "Pill taking and end point ascertainment were continued in blinded fashion through the scheduled end of the trial on March 31, 2004. Morbidity and mortality follow‐up were 97.2% and 99.4% complete, respectively." Follow‐up balanced across treatment groups. See figure 1. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. The outcome was limited to the study design ‐ medical record review. Primary and secondary outcomes were apparently defined a priori and were reported. |
AMD: age‐related macular degeneration
ETDRS: Early Treatment Diabetic Retinopathy Study
IU: international units
RPE: retinal pigment epithelium
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| No published data on AMD. No response from author. | |
| No published data on AMD. Unable to contact author. | |
| Age‐related maculopathy outcomes for people without age‐related maculopathy at baseline were not reported | |
| Participants had AMD | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No data on AMD collected | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| Biological availability study only | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| Study on people with AMD (non‐advanced AMD), therefore, not on prevention in healthy people | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. Unable to contact author. | |
| No data on AMD collected | |
| No published data on AMD. No response from author | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| Participants had early AMD | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No AMD outcomes | |
| No published data on AMD. Unable to contact author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No follow‐up data on AMD collected | |
| Reported on folic acid, pyridoxine and cyanocobalamin combination treatment | |
| Study of macular pigment only | |
| Study on people with AMD | |
| No AMD outcomes | |
| Study on people with AMD | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| Study of ocular blood flow and endothelial function only in model of oxidative stress in health volunteers | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| Study not a randomised controlled trial | |
| No published data on AMD. No response from author | |
| No AMD outcomes | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No data on AMD collected | |
| No data on AMD collected | |
| Participants had geographic atrophy | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. | |
| No published data on AMD. No response from author. |
AMD: age‐related macular degeneration
Characteristics of ongoing studies [ordered by study ID]
Jump to:
| Trial name or title | Lutein influence on macula of persons issued from AMD parents |
| Methods | Multicentre, double‐masked, randomised clinical trial of supplementation with 'Nutrof Total' (lutein and zeaxanthin) versus placebo |
| Participants | People at high genetic risk for AMD because their parents had AMD. Age 40 to 70 years. |
| Interventions | Nutrof Total or placebo |
| Outcomes | Primary outcome measure: Macular pigment density at 6 months after supplementation Secondary outcome measures: Best corrected visual acuity 12 months Cognitive ability 12 months Plasma fatty acids 12 months Macular pigment density during supplementation and after stopping supplementation |
| Starting date | ‐ |
| Contact information | Jean‐Francois Korobelnik |
| Notes | clinicaltrials.gov/show/NCT01269697 The principal Investigator was contacted in March 2016 and confirmed that the study should be published in the next year. |
| Trial name or title | Selenium and Vitamin E Cancer Prevention Trial (SELECT) for prostate cancer |
| Methods | This is a randomised, double‐masked, multi‐centre study. Participants are randomised to one of 4 prevention arms: Arm I: participants receive 2 different oral placebos once daily Arm II: participants receive oral selenium and oral placebo once daily Arm III: participants receive oral vitamin E and oral placebo once daily Arm IV: participants receive oral selenium and oral vitamin E once daily. Treatment continues for 7 to 12 years in the absence of unacceptable toxicity or diagnosis of prostate cancer Quality of life is assessed at baseline and then at 1, 3, 5, and 7 years Participants are followed annually |
| Participants | Healthy male volunteers. A total of 32,400 participants (8100 per prevention arm) will be accrued for this study within 5 years |
| Interventions | Dietary supplement: selenium |
| Outcomes | Primary outcome measures: Effect on the clinical incidence of cancer |
| Starting date | July 2001 |
| Contact information | ‐ |
| Notes | clinicaltrials.gov/show/NCT00006392 We contacted the principal investigator in March 2016; data collection and analysis of AMD outcomes is still ongoing. |
| Trial name or title | Women's Antioxidant Cardiovascular Study |
| Methods | ‐ |
| Participants | 8171 female health professionals aged 40+ with pre‐existing cardiovascular disease (CVD) or high risk for developing CVD |
| Interventions | 2 x 2 x 2 x 2 factorial design: Vitamin E (600 IU on alternate days) Vitamin C (500 mg daily) Beta‐carotene (5 mg on alternate days) Combination of folate (800 mg daily), vitamin B6 (25 mg daily) and vitamin B12 (1 mg daily) |
| Outcomes | Self‐report and review of medical records |
| Starting date | 1993 |
| Contact information | ‐ |
| Notes | clinicaltrials.gov/show/NCT00000541 We contacted the principal investigator in March 2016; no reply as yet. |
AMD: age‐related macular degeneration
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any AMD Show forest plot | 4 | 55614 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.06] |
| Analysis 1.1  Comparison 1 Vitamin E versus placebo, Outcome 1 Any AMD. | ||||
| 2 Late AMD (either neovascular AMD or geographic atrophy or both) Show forest plot | 4 | 55614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.89, 1.67] |
| Analysis 1.2 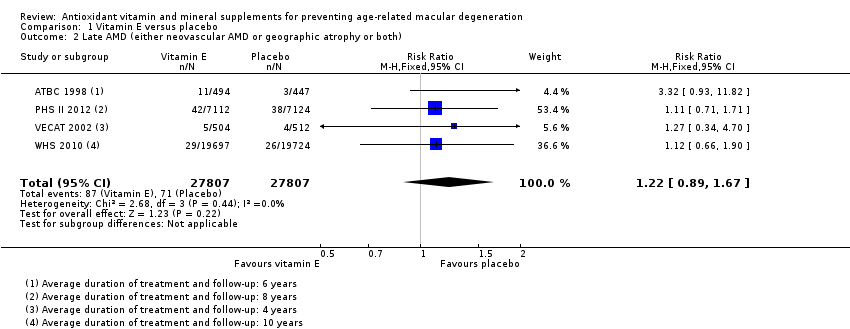 Comparison 1 Vitamin E versus placebo, Outcome 2 Late AMD (either neovascular AMD or geographic atrophy or both). | ||||
| 3 Neovascular AMD or geographic atrophy separately Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 1.3  Comparison 1 Vitamin E versus placebo, Outcome 3 Neovascular AMD or geographic atrophy separately. | ||||
| 3.1 Neovascular AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Geographic atrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any AMD Show forest plot | 2 | 22083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.14] |
| Analysis 2.1  Comparison 2 Beta‐carotene versus placebo, Outcome 1 Any AMD. | ||||
| 2 Late AMD (either neovascular AMD or geographic atrophy or both) Show forest plot | 2 | 22083 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.24] |
| Analysis 2.2 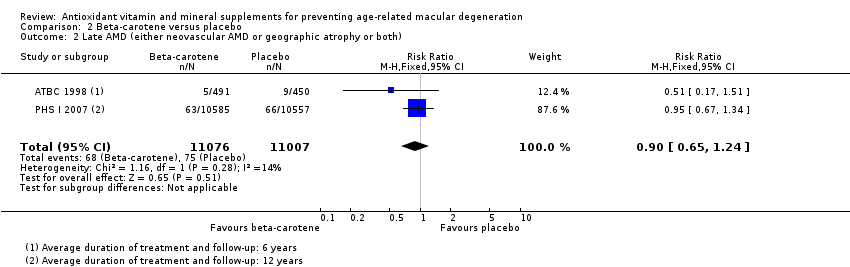 Comparison 2 Beta‐carotene versus placebo, Outcome 2 Late AMD (either neovascular AMD or geographic atrophy or both). | ||||
| 3 Neovascular AMD or geographic atrophy separately Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 2.3 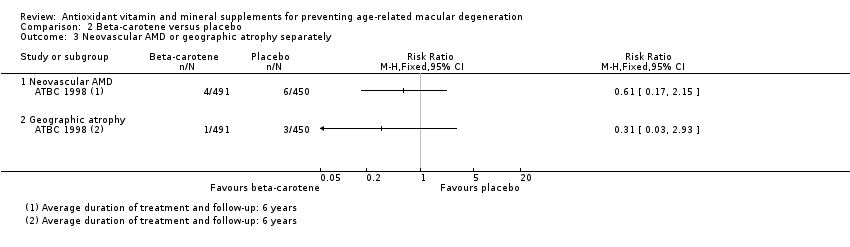 Comparison 2 Beta‐carotene versus placebo, Outcome 3 Neovascular AMD or geographic atrophy separately. | ||||
| 3.1 Neovascular AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Geographic atrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 3.1 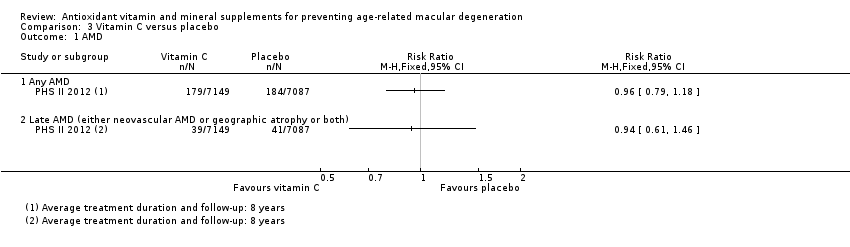 Comparison 3 Vitamin C versus placebo, Outcome 1 AMD. | ||||
| 1.1 Any AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Late AMD (either neovascular AMD or geographic atrophy or both) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| Analysis 4.1  Comparison 4 Multivitamin versus placebo, Outcome 1 AMD. | ||||
| 1.1 Any AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Late AMD (either neovascular AMD or geographic atrophy or both) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
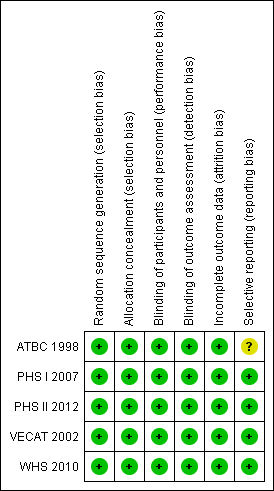
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Vitamin E versus placebo, Outcome 1 Any AMD.

Comparison 1 Vitamin E versus placebo, Outcome 2 Late AMD (either neovascular AMD or geographic atrophy or both).

Comparison 1 Vitamin E versus placebo, Outcome 3 Neovascular AMD or geographic atrophy separately.

Comparison 2 Beta‐carotene versus placebo, Outcome 1 Any AMD.

Comparison 2 Beta‐carotene versus placebo, Outcome 2 Late AMD (either neovascular AMD or geographic atrophy or both).

Comparison 2 Beta‐carotene versus placebo, Outcome 3 Neovascular AMD or geographic atrophy separately.

Comparison 3 Vitamin C versus placebo, Outcome 1 AMD.

Comparison 4 Multivitamin versus placebo, Outcome 1 AMD.
| Vitamin E versus placebo | ||||||
| Patient or population: general population | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo** | Risk with vitamin E | |||||
| Any AMD | 150 per 1000 | 146 per 1000 | RR 0.97 | 55,614 | ⊕⊕⊕⊕ | Average duration of treatment and follow‐up ranged from 4 years to 10 years |
| Late AMD (either neovascular AMD or geographic atrophy or both) | 5 per 1000 | 6 per 1000 | RR 1.22 | 55,614 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up ranged from 4 years to 10 years |
| Neovascular AMD | 3 per 1000 | 11 per 1000 | RR 3.62 | 941 | ⊕⊝⊝⊝ | Average duration of treatment and follow‐up was 6 years |
| Geographic atrophy | 2 per 1000 | 6 per 1000 | RR 2.71 | 941 | ⊕⊝⊝⊝ | Average duration of treatment and follow‐up was 6 years |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse effects (AE) | ‐ | ‐ | ‐ | ‐ | ⊕⊕⊝⊝ | Two trials reported similar numbers of AEs in vitamin E and placebo group. Another trial reported excess of haemorrhagic strokes in vitamin E group (39 vs 23 events, hazard ratio 1.74, 95% CI 1.04 to 2.91). |
| Resource use and costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| * Dose of vitamin E used in studies were: 50 mg/day, 400 IU/alternate days, 600 IU/alternate days, and 500 IU/day **The risk in the placebo group is the median risk in the placebo groups in the included studies. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision due to wide confidence intervals i.e. are below 0.8 or above 1.25. 2 Downgraded one level for indirectness (only one trial in male smokers) and downgraded two levels for imprecision as very few cases (10 neovascular AMD, 4 geographic atrophy) 3 Downgraded one level for imprecision due to wide confidence intervals and lower confidence near 1 and downgraded one level for inconsistency as effect only reported by one trial. | ||||||
| Beta‐carotene versus placebo | ||||||
| Patient or population: general population | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo** | Risk with beta‐carotene | |||||
| Any AMD | 150 per 1000 | 150 per 1000 | RR 1.00 | 22,083 | ⊕⊕⊕⊕ | Average duration of treatment and follow‐up was 6 years in one study and 12 years in the other study |
| Late AMD (either neovascular AMD or geographic atrophy or both) | 5 per 1000 | 5 per 1000 | RR 0.90 | 22,083 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up was 6 years in one study and 12 years in the other study |
| Neovascular AMD | 3 per 1000 | 2 per 1000 | RR 0.61 | 941 | ⊕⊝⊝⊝ | Average duration of treatment and follow‐up was 6 years |
| Geographic atrophy | 2 per 1000 | 1 per 1000 | RR 0.31 | 941 | ⊕⊝⊝⊝ | Average duration of treatment and follow‐up was 6 years |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse effects | ‐ | ‐ | ‐ | ⊕⊕⊕⊕ | Beta‐carotene associated with increased risk of lung cancer in people who smoke. | |
| Resource use and costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| * Dose of beta‐carotene used was 20 mg/day in one study and 50 mg/alternate days in the other study. **The risk in the placebo group is the median risk in the control groups of the four included studies in summary of findings Table for the main comparison. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision due to wide confidence intervals i.e. are below 0.8 or above 1.25. 2 Downgraded one level for indirectness (only one trial in male smokers) and downgraded two levels for imprecision as very few cases (10 neovascular AMD, 4 geographic atrophy) | ||||||
| Vitamin C versus placebo | ||||||
| Patient or population: general population | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo** | Risk with vitamin C | |||||
| Any AMD | 150 per 1000 | 144 per 1000 | RR 0.96 | 14,236 | ⊕⊕⊕⊕ | Average duration of treatment and follow‐up was 8 years |
| Late AMD (either neovascular AMD or geographic atrophy or both) | 5 per 1000 | 5 per 1000 | RR 0.94 | 14,236 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up was 8 years |
| Neovascular AMD | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Geographic atrophy | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse effects | ‐ | ‐ | ‐ | ‐ | None reported | |
| Resource use and costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| * Dose of vitamin C used was 500 mg/day. **The risk in the placebo group is the median risk in the control groups of the four included studies in summary of findings Table for the main comparison. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision due to wide confidence intervals i.e. are below 0.8 or above 1.25. | ||||||
| Multivitamin versus placebo for preventing AMD | ||||||
| Patient or population: general population | ||||||
| Outcomes | Anticipated absolute effects (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with placebo** | Risk with multivitamin | |||||
| Any AMD | 150 per 1000 | 182 per 1000 | RR 1.21 | 14,233 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up was 11 years |
| Late AMD | 5 per 1000 | 6 per 1000 | RR 1.22 | 14,233 | ⊕⊕⊕⊝ | Average duration of treatment and follow‐up was 11 years |
| Neovascular AMD | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Geographic atrophy | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Quality of life | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Adverse effects | ‐ | ‐ | ‐ | ‐ | ⊕⊕⊕⊝ | "Those taking the active versus placebo multivitamin were more likely to have skin rashes (2111 and 1973 men in corresponding active and placebo multivitamin groups; HR 1.08, 95% CI 1.01 to 1.15; P = 0.016)". PHS II |
| Resource use and costs | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| * Multivitamin used was Centrum Silver (zinc 15 mg, vitamin E 45 IU, vitamin C 60 mg, beta‐carotene 5000 IU vitamin A, 20% as beta carotene, folic acid 2.5 mg, vitamin B6 50 mg, vitamin B12 1 mg) **The risk in the placebo group is the median risk in the control groups of the four included studies in summary of findings Table for the main comparison. The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for imprecision | ||||||
| Definition of AMD used in this review | Study | ||
| No AMD | 0 = no ARM | Did not self‐report or no signs listed below in medical records | ‐ |
| Any AMD | I = dry maculopathy, with hard drusen, pigmentary changes, or both II = soft macular drusen III = disciform degeneration IV = geographic atrophy. | Drusen, RPE hypo or hyperpigmentation, geographic atrophy, RPE detachment, subretinal neovascular membrane, | Early AMD 1: Soft intermediate or soft distinct or soft indistinct or pigment changes (hyperpigmentation or hypopigmentation) Early AMD 2: Soft intermediate or soft distinct or soft indistinct and pigment changes (hyperpigmentation or hypopigmentation) Early AMD 3: Soft distinct or soft indistinct or pigment changes (hyperpigmentation or hypopigmentation) Early AMD 4: Soft distinct or soft indistinct and pigment changes (hyperpigmentation or hypopigmentation) Late AMD: Serous or haemorrhagic detachment of the RPE or sensory retina, characteristic haemorrhages, or subretinal fibrous scars, central areolar zone of retinal pigment epithelial atrophy with visible choroidal vessels, at least 175 µm in diameter |
| Late AMD | III = disciform degeneration IV = geographic atrophy. | Geographic atrophy, RPE detachment, subretinal neovascular membrane, or disciform scar | Serous or haemorrhagic detachment of the RPE or sensory retina, characteristic haemorrhages, or subretinal fibrous scars, central areolar zone of retinal pigment epithelial atrophy with visible choroidal |
| Neovascular AMD | III = disciform degeneration | RPE detachment, subretinal neovascular membrane, or disciform scar | Serous or haemorrhagic detachment of the RPE or sensory retina, characteristic haemorrhages, or subretinal fibrous scars |
| Geographic atrophy | IV = geographic atrophy. | Geographic atrophy | Central areolar zone of retinal pigment epithelial atrophy with visible choroidal |
| RPE: retinal pigment epithelial | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any AMD Show forest plot | 4 | 55614 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.90, 1.06] |
| 2 Late AMD (either neovascular AMD or geographic atrophy or both) Show forest plot | 4 | 55614 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.89, 1.67] |
| 3 Neovascular AMD or geographic atrophy separately Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Neovascular AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Geographic atrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Any AMD Show forest plot | 2 | 22083 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.88, 1.14] |
| 2 Late AMD (either neovascular AMD or geographic atrophy or both) Show forest plot | 2 | 22083 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.65, 1.24] |
| 3 Neovascular AMD or geographic atrophy separately Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Neovascular AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Geographic atrophy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Any AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Late AMD (either neovascular AMD or geographic atrophy or both) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 AMD Show forest plot | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 1.1 Any AMD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Late AMD (either neovascular AMD or geographic atrophy or both) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |


