Fármacos anticolinérgicos para la discinesia tardía inducida por antipsicóticos
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jump to:
| Methods | Allocation: random, further details not reported. Blindness: not reported. Duration: 40 weeks. Design: parallel. Setting: outpatients, USA. | |
| Participants | Diagnosis: chronic schizophrenia treated with phenothiazine for several years and demonstrating obvious dyskinetic manifestations. Duration of tardive dyskinesia (TD): ≥2 years. N = 20. Sex: 16 female, 4 male. Age: range 45 to 62 years. | |
| Interventions | 1. Procyclidine (anticholinergic), 5 mg B.I.D. + chlorpromazine, 100 mg T.I.D. N = 10. Continuous phenothiazine‐antiparkisonian treatment for at least 2 years. Other concomitant medication: not reported. | |
| Outcomes | TD symptoms: improvement (clinical evaluation, scale not reported). Leaving the study early. Adverse events. Unable to use ‐
| |
| Notes | Sponsorship source: not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “The patients were divided at random into groups of 10 each”, no further details reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | High risk | Blinding of participants and personnel not reported. |
| Blinding of outcome assessment (detection bias) | High risk | Blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) | Low risk | “One male patient on chlorpromazine and isocarboxazid was discontinued after 6 weeks as he became increasingly tense, apprehensive and sleepless.” |
| Selective reporting (reporting bias) | High risk | Unclear if all outcomes have been reported. A protocol is not available for verification. Adverse effects reported only as those related to treatment. Mental state data not reported for group 2. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
| Methods | Allocation: "randomly assigned" no further details. Blind: "double‐blind" no further details. Design: parallel group. Setting: not reported if inpatients or outpatients or both; Germany. Duration: 7 weeks. | |
| Participants | Diagnosis: chronic schizophrenics (ICD‐9) with tardive dyskinesia based on the presence of a 'typical' bucco‐linguo‐masticatory syndrome and the absence of other adequate explanations for the movement disorder. Duration of tardive dyskinesia: ≥1 year, severity of the symptoms stable for at least one month before admission to the study. N = 10. Sex: 7 female, 3 male. Age: mean 56.6 (SD 9.2) years; range 35 to 65 years. | |
| Interventions | 1. Biperiden (same dose as before the trial) stopped after 4 weeks followed by placebo for 3 weeks. N = 4. 2. Biperiden (same dose as before the trial) stopped after 1 week followed by placebo for 6 weeks. N = 6. All stable on antipsychotics and anticholinergics for at least 5 months before entry and during the trial. Other concomitant medication: not reported. | |
| Outcomes | Leaving the study early. Unable to use (results not reported per randomised group) ‐
Study author was contacted for additional data but no reply was received. | |
| Notes | Sponsorship source: not reported. Knoll AG supplied placebo. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly assigned", further details not reported. |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported. |
| Blinding of participants and personnel (performance bias) | Low risk | "double‐blind" "investigators were not informed about the study design" |
| Blinding of outcome assessment (detection bias) | Unclear risk | Blinding of raters was not mentioned. |
| Incomplete outcome data (attrition bias) | Low risk | "Nine patients completed the trial. One patient dropped out one week after biperiden withdrawal because of severe parkinsonism; in this patient, only one rating could be carried out while on the placebo." |
| Selective reporting (reporting bias) | High risk | TD symptoms data were not reported per randomised group, but before biperiden removal versus after biperiden removal. |
| Other bias | Unclear risk | Insufficient information to make a judgement. |
Characteristics of excluded studies [ordered by study ID]
Jump to:
| Study | Reason for exclusion |
| Allocation: not randomised, controlled clinical study. | |
| Allocation: randomised. | |
| Allocation: randomised. | |
| Allocation: randomised. Participants: people with chronic schizophrenia, no TD. | |
| Allocation: randomised. | |
| Allocation: randomised. Participants: antipsychotic‐induced akathisia, parkinsonism and hyperkinetic movements, 11/15 participants had tardive dyskinesia. Dr Gerlach (author) contacted and replied promptly. Data were destroyed and no more information is available. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: not randomised ‐ randomisation was only in one arm of the study “haloperidol + biperiden for 4 weeks (phase 2 and phase 3 in randomized sequence)”; all other arms were not randomised (thioridazine for 3 months, haloperidol for 4 weeks, thioridazine for 4 weeks, clozapine for 4 weeks). | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: randomised. Intervention: benztropine withdrawal vs maintenance. Dr Klett (author) contacted and replied promptly. Data were destroyed and no more information is available. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: randomised. Participants: schizophrenia, schizoaffective disorder, major affective disorder attention deficit disorder and TD (criteria of Schooler & Kane). Intervention: physostigmine vs bromocriptine vs benztropine vs haloperidol. Outcomes: no outcome data were been provided for the first period before crossover. Study author was contacted for data; no additional information was received and as this study is over 25 years old, we excluded this trial. | |
| Allocation: randomised. Intervention: L‐dopa 500 mg + carbidopa 50 mg/d + low dose antipsychotics (N = 35) vs placebo + anticholinergic medication + low dose antipsychotic (N = 25). Outcomes: no outcome data could be used. The study is over 25 years old and we were unable to identify contact details for the author. | |
| Allocation: randomised crossover. Intervention: chlorprothixene vs haloperidol vs perphenazine or haloperidol + biperiden with placebo periods in between phases. Outcomes: no outcome data has been provided for the first period before crossover. Author was contacted but did not reply. Study is over 30 years old and we excluded it. | |
| Allocation: randomised. Participants: people with schizophrenia (DSM‐III‐R), with and without TD. Interventions: biperiden vs amantadine. Outcomes: no outcome data has been provided for the first period before crossover. We were unable to find up‐to‐date contact details for the authors and, as this study is over 20 years old, we excluded this trial. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: not randomised, ABA design. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: not randomised, controlled clinical trial. | |
| Allocation: not randomised, controlled clinical trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
Jump to:
| Methods | Allocation: randomised. |
| Participants | Diagnosis: schizophrenia with drug‐induced tremor. N = 68. |
| Interventions | 1. Dexetimide. N = 36. |
| Outcomes | Movement disorder: clinical response. Adverse events: TESS. |
| Notes | Language: Chinese ‒ assessed by Sai Zhao. Study authors have been contacted to find out if participants were diagnosed with tardive dyskinesia. |
TESS ‒ Treatment Emergent Symptom Scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TD symptoms: no clinically significant improvement Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1 Anticholinergic medications versus other compounds, Outcome 1 TD symptoms: no clinically significant improvement. | ||||
| 1.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 4.2 [1.40, 12.58] |
| 2 TD symptoms: not any improvement Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.2  Comparison 1 Anticholinergic medications versus other compounds, Outcome 2 TD symptoms: not any improvement. | ||||
| 2.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 7.0 [1.57, 31.15] |
| 3 Adverse effects Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.3 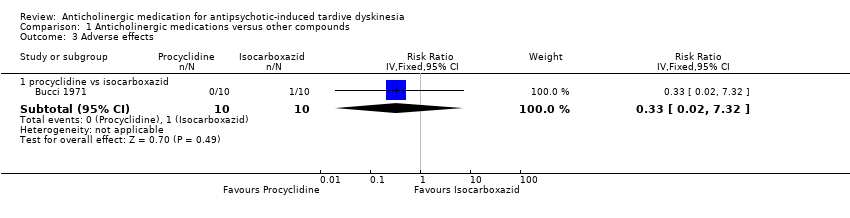 Comparison 1 Anticholinergic medications versus other compounds, Outcome 3 Adverse effects. | ||||
| 3.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 4 Leaving the study early Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 1.4 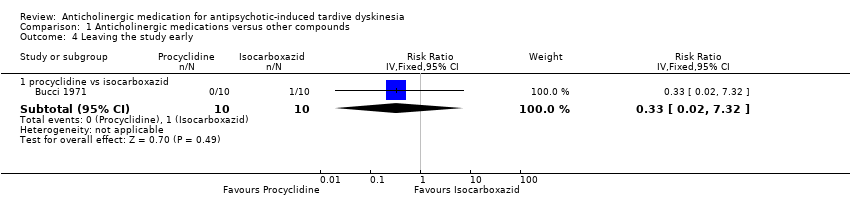 Comparison 1 Anticholinergic medications versus other compounds, Outcome 4 Leaving the study early. | ||||
| 4.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| Analysis 2.1 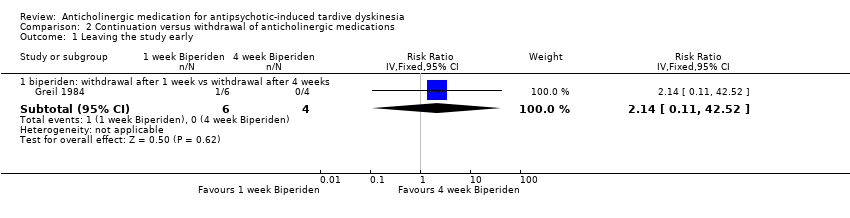 Comparison 2 Continuation versus withdrawal of anticholinergic medications, Outcome 1 Leaving the study early. | ||||
| 1.1 biperiden: withdrawal after 1 week vs withdrawal after 4 weeks | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 2.14 [0.11, 42.52] |

Message from one of the participants of the Public and patient involvement consultation of service user perspectives on tardive dyskinesia research.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Study flow diagram for 2015 and 2017 searches.

Comparison 1 Anticholinergic medications versus other compounds, Outcome 1 TD symptoms: no clinically significant improvement.

Comparison 1 Anticholinergic medications versus other compounds, Outcome 2 TD symptoms: not any improvement.

Comparison 1 Anticholinergic medications versus other compounds, Outcome 3 Adverse effects.

Comparison 1 Anticholinergic medications versus other compounds, Outcome 4 Leaving the study early.

Comparison 2 Continuation versus withdrawal of anticholinergic medications, Outcome 1 Leaving the study early.
| Methods | Allocation: randomised, with sequence generation and concealment of allocation clearly described. |
| Participants | People with antipsychotic‐induced tardive dyskinesia.* |
| Interventions | 1. Anticholinergic withdrawal (N = 150) versus anticholinergic continuation (N = 150). OR 2. Specific anticholinergic (N = 150) versus placebo (N = 150). |
| Outcomes | Tardive dyskinesia: any clinically important improvement in TD, any improvement, deterioration.*** |
| Notes | * This could be diagnosed by clinical decision. If funds were permitting, all participants could be screened using operational criteria; otherwise a random sample should suffice. ** Size of study with sufficient power to highlight about a 10% difference between groups for primary outcome. |
| Anticholinergic medication compared with other treatments for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: patients with antipsychotic‐induced tardive dyskinesia Settings: anywhere. Intervention: any anticholinergic Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| risk with placebo | risk with anticholinergic drugs | |||||
| Tardive dyskinesia: not improved to a clinically important extent | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Tardive dyskinesia: deterioration of symptoms | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Mental state | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: any adverse effects | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Social confidence, social inclusion, social networks, or personalised quality of life | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: the included study did not adequately describe randomisation procedure or allocation concealment, and there was no mention of the study being blinded. 2 Downgraded two levels for imprecision: very small sample size (n = 20). 3 Downgraded two levels for imprecision: very wide CI that includes appreciable benefit for both groups; very small sample size (n = 20). 4 Downgraded one level for indirectness: leaving the study early can give an indication, but is not a direct measurement, of treatment acceptability. | ||||||
| Anticholinergic medication compared with other treatments for antipsychotic‐induced tardive dyskiesia | ||||||
| Patient or population: chronic schizophrenia patients with antipsychotic‐induced tardive dyskinesia Settings: outpatients in the USA. Intervention: procyclidine (anticholinergic), 5 mg twice/day Comparison: isocarboxazid (MAO‐inhibitor), 10 mg twice/day | ||||||
| Outcomes | Illustrative comparative risks* (CI) | Relative effect | No of Participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| risk with MAO‐inhibitor | risk with anticholinergic drugs | |||||
| Tardive dyskinesia: Not improved to a clinically important extent | 200 per 1000 | 840 per 1000 | RR 4.20 (1.40 to 12.58) | 20 | ⊕⊝⊝⊝ | |
| Tardive dyskinesia: deterioration of symptoms | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Mental state | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effect: any adverse effects | 100 per 1000 | 33 per 1000 | RR 0.33 (0.02 to 7.32) | 20 | ⊕⊝⊝⊝ | |
| Acceptability of the treatment: leaving the study early | 100 per 1000 | 33 per 1000 | RR 0.33 (0.02 to 7.32) | 20 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life | see comment | see comment | not estimable | (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: the included study did not adequately describe randomisation procedure or allocation concealment, and there was no mention of the study being blinded. 2 Downgraded two levels for imprecision: very small sample size (n = 20). 3 Downgraded two levels for imprecision: very wide CI that includes appreciable benefit for both groups; very small sample size (n = 20). 4 Downgraded one level for indirectness: leaving the study early can give an indication, but is not a direct measurement, of treatment acceptability. | ||||||
| Withdrawal of anticholinergic medication compared to continuing anticholigergic medication for antipsychotic‐induced tardive dyskinesia | ||||||
| Patient or population: chronic schizophrenia patients with antipsychotic‐induced tardive dyskinesia | ||||||
| Outcomes | Anticipated absolute effects* (CI) | Relative effect | № of participants | Quality of the evidence | Comments | |
| Risk with continuation of anticholinergic drugs | Risk with withdrawal of anticholinergic drugs | |||||
| Tardive dyskinesia: not improved to a clinically important extent | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Tardive dyskinesia: deterioration of symptoms | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Mental state | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Adverse effects | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| Acceptability of the treatment: leaving the study early | 0 per 1,000 | 0 per 1,000 | RR 2.14 | 10 | ⊕⊝⊝⊝ | |
| Social confidence, social inclusion, social networks, or personalised quality of life | see comment | see comment | not estimable | 0 (0 studies) | ‐ | None of the included studies reported on this outcome. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level for risk of bias: the included study did not adequately describe randomisation procedure or allocation concealment. 2 Downgraded one level for indirectness: leaving the study early can give an indication, but is not a direct measurement, of treatment acceptability. In addition, the continuation of anticholinergic medication group stopped biperiden after 4 weeks but the results were measured after 7 weeks. 3 Downgraded two levels for imprecision: very wide CI that includes appreciable benefit for both groups; very small sample size (n = 10). | ||||||
| Interventions | Reference |
| Anticholinergic medication | This review |
| Benzodiazepines | Bhoopathi 2006; update to be published |
| Calcium channel blockers | Essali 2011; update to be published |
| Cholinergic medication | Tammenmaa 2002; update to be published |
| Gamma‐aminobutyric acid agonists | Alabed 2011; update to be published |
| Miscellaneous treatments | Soares‐Weiser 2003; update to be published |
| Neuroleptic reduction and/or cessation and neuroleptics | Soares‐Weiser 2006; update to be published |
| Non‐neuroleptic catecholaminergic drugs | El‐Sayeh 2006; update to be published |
| Vitamin E | Soares‐Weiser 2011; update to be published |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 TD symptoms: no clinically significant improvement Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 4.2 [1.40, 12.58] |
| 2 TD symptoms: not any improvement Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 7.0 [1.57, 31.15] |
| 3 Adverse effects Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 4 Leaving the study early Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 procyclidine vs isocarboxazid | 1 | 20 | Risk Ratio (IV, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Leaving the study early Show forest plot | 1 | Risk Ratio (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 biperiden: withdrawal after 1 week vs withdrawal after 4 weeks | 1 | 10 | Risk Ratio (IV, Fixed, 95% CI) | 2.14 [0.11, 42.52] |

