Tratamiento antiplaquetario oral para el accidente cerebrovascular isquémico agudo
References
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
References to other published versions of this review
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | C = centrally produced, prepacked, sequentially numbered envelopes | |
| Participants | China | |
| Interventions | Rx: aspirin 160 mg once daily orally or via nasogastric tube | |
| Outcomes | Death | |
| Notes | Ex: not specified by protocol but by responsible physician, possibly including increased risk of adverse effects, or little likelihood of any worthwhile benefit in hospital | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | R = random number list | |
| Participants | Italy | |
| Interventions | Rx: ticlopidine 250 mg orally 12 hourly | |
| Outcomes | Death | |
| Notes | Ex: cerebral oedema | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | C = telephone randomisation | |
| Participants | International | |
| Interventions | Rx: subcutaneous heparin (5000 IU or 12 500 IU 12 hourly), aspirin 300 mg, both, or neither (factorial design) | |
| Outcomes | Death | |
| Notes | Ex: small likelihood of worthwhile benefit; high risk of adverse effect (e.g. hypersensitivity of aspirin, recent GI bleed or peptic ulcer disease, already on long‐term anticoagulation) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | C = telephone central office | |
| Participants | Europe | |
| Interventions | Factorial design of streptokinase and aspirin versus no treatment; only aspirin alone versus no aspirin included to prevent confounding influence of streptokinase | |
| Outcomes | Death plus cause of death | |
| Notes | Ex: coma, bleeding risk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | R = sealed envelope | |
| Participants | France | |
| Interventions | Rx: aspirin 330 mg 8 hourly (oral) plus dipyridamole 75 mg 8 hourly (oral) | |
| Outcomes | Death | |
| Notes | Ex: bleeding risk, aspirin allergy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
| Methods | R = randomisation tables, stratified for gender | |
| Participants | Sweden | |
| Interventions | Rx: aspirin 325 mg orally once daily | |
| Outcomes | Death | |
| Notes | Ex: specified by protocol ‐ includes severe concomitant medical conditions or pre‐existing neurological illness, bleeding risk, blood pressure above 240/140 mmHg | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | R = identical, sequentially numbered bottles from pharmacy | |
| Participants | Canada | |
| Interventions | Rx: ticlopidine 250 mg orally 12 hourly | |
| Outcomes | Death | |
| Notes | Ex: unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
| Methods | R = unknown | |
| Participants | Japan | |
| Interventions | Rx: ticlopidine 100 mg orally 8 to 12 hourly | |
| Outcomes | Death | |
| Notes | Ex: unknown | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
ADL: activities of daily living
C: concealment
CT: computerised tomography
DVT: deep venous thrombosis
Ex: exclusions
FU: follow‐up
GI: gastrointestinal
iv: intravenous
R: randomisation
Rx: treatment
SSSS: Scandinavian Stroke Supervision Scale
Systematic: outcome sought by systematically scanning all patients at a predefined time, irrespective of presence or absence of symptoms
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Intravenous antiplatelet therapy | |
| Intravenous antiplatelet therapy | |
| Intravenous antiplatelet therapy | |
| Intravenous antiplatelet therapy | |
| Intravenous antiplatelet therapy | |
| Not adequately randomised; participants allocated on alternating basis | |
| Not acute stroke therapy | |
| Not adequately randomised; confounded; people with acute and non‐acute stroke included | |
| Intravenous antiplatelet therapy | |
| Not randomised | |
| Not acute stroke therapy; people included up to 30 days post‐stroke | |
| Not adequately randomised | |
| Intravenous antiplatelet therapy | |
| Confounded trial | |
| No control group which did not receive antiplatelet therapy (picotamide versus aspirin versus aspirin and picotamide) | |
| Ligustrazine, a kind of Chinese herbal medicine, is not an antiplatelet agent | |
| No control group (clopidogrel plus aspirin versus aspirin alone in minor stroke and TIA within 24 hours of onset) | |
| No control group (aspirin versus ligustrazine versus betahistine) | |
| No control group (aspirin versus levamisol) | |
| Not randomised | |
| Not acute stroke therapy | |
| No control group (extended‐release dipyridamole 200 mg plus acetylsalicylic acid 50 mg in a capsule, 2 capsules twice daily versus acetylsalicylic acid 81 mg,1 tablet once daily) Not acute stroke therapy; participants included between 1 week and 6 months after stroke | |
| Not adequately randomised | |
| Not randomised | |
| No results published, described as 'discontinued' | |
| No control group which did not receive antiplatelet therapy (aspirin and dipyridamole combined versus aspirin alone) | |
| Not acute stroke therapy | |
| Non‐randomised, dose escalation study | |
| Intravenous antiplatelet therapy | |
| Method of allocation unclear, no relevant clinical outcomes reported | |
| Multiple antiplatelet agents | |
| No relevant clinical outcomes reported | |
| Intravenous antiplatelet therapy | |
| Intravenous antiplatelet therapy | |
| No relevant clinical outcomes reported | |
| No relevant clinical outcomes reported | |
| Not acute stroke therapy; participants included up to 6 months after stroke | |
| Multiple antiplatelet agents. Ongoing trial | |
| Ligustrazine, a kind of Chinese herbal medicine, is not an antiplatelet agent | |
| Not adequately randomised | |
| Method of allocation unclear, no relevant clinical outcomes reported |
TIA: transient ischaemic attack
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | |
| Participants | People with non‐cardioembolic ischaemic stroke within 72 hours of the onset and NIHSS score ≤ 7 |
| Interventions | Cilostazol (200 mg/d) plus intravenous antithrombotic agents (heparin, ozagrel sodium or argatroban) versus intravenous antithrombotic agents (heparin, ozagrel sodium or argatroban) |
| Outcomes | Neurological deterioration and stroke recurrence at 14 days, and mRS at 3 months |
| Notes |
| Methods | Randomised, placebo‐controlled trial |
| Participants | People with ischaemic stroke within 48 hours of the onset and demonstrated by CT |
| Interventions | Aspirin (160 mg/d for 4 weeks) versus placebo |
| Outcomes | |
| Notes |
CT: computerised tomography
mRS: modified Rankin Scale
NIHSS: National Institutes of Health Stroke Scale
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Cilostazol in Acute Ischemic Stroke Trial |
| Methods | Randomised, single‐blind ‐ participants are blinded |
| Participants | People with ischaemic stroke within 72 hours after the onset and NIHSS score ≥ 14 |
| Interventions | Cilostazol versus conventional therapy |
| Outcomes | Primary outcome: mRS 0 to 2 at 3 months Secondary outcome: recurrence of stroke, NIHSS, JSS, Barthel Indes, mRS, MMSE, neurological deterioration, pneumonia |
| Starting date | 2006 |
| Contact information | Norihiro Suzuki Keio University School of Medicine Department of Neurology 35 Shinanomachi Shinjuku‐ku Tokyo 165‐8582 Japan |
| Notes |
| Trial name or title | A randomised clinical trial of an antiplatelet agent in the treatment of acute stroke: cilostazol in the prevention of acute progressing stroke (CAPS) study |
| Methods | Randomised, open‐label |
| Participants | People with non‐cardioembolic cerebral infarction within 24 hours after the onset, aged 20 to 80 years |
| Interventions | Cilostazol (200 mg/d) plus standard therapy versus standard therapy |
| Outcomes | mRS at 3 months, progressing stroke, incidence of cardiovascular events |
| Starting date | 2009 |
| Contact information | Teiji Tominaga Tohoku University Graduate School of Medicine Department of Neurosurgery 1‐1, Seiryo‐machi, Aoba‐ku, SENDAI 980‐8574 |
| Notes |
JSS: Japan Stroke Scale
MMSE: Mini Mental State Exmanination
mRS: modified Rankin Scale
NIHSS: National Institutes of Health Stroke Scale
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependence at end of follow‐up Show forest plot | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.91, 0.99] |
| Analysis 1.1 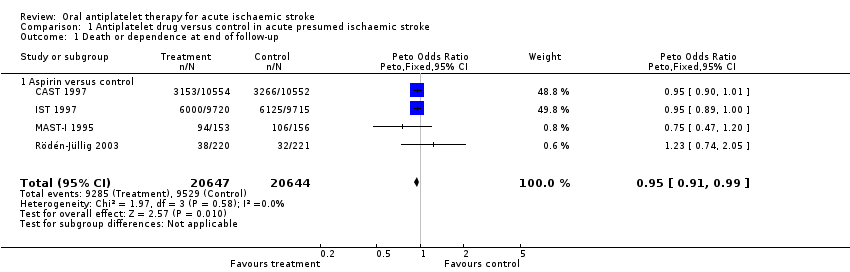 Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 1 Death or dependence at end of follow‐up. | ||||
| 1.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.91, 0.99] |
| 2 Deaths from all causes during treatment period Show forest plot | 8 | 41483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| Analysis 1.2  Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 2 Deaths from all causes during treatment period. | ||||
| 2.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 2.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.43, 4.99] |
| 2.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 1.20] |
| 3 Deaths from all causes during follow‐up Show forest plot | 8 | 41483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| Analysis 1.3  Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 3 Deaths from all causes during follow‐up. | ||||
| 3.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 3.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.43, 4.99] |
| 3.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.88] |
| 4 Deep venous thrombosis during treatment period Show forest plot | 2 | 133 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.36, 1.67] |
| Analysis 1.4  Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 4 Deep venous thrombosis during treatment period. | ||||
| 4.1 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.13, 0.95] |
| 4.2 Ticlopidine versus control | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.37 [0.72, 7.73] |
| 5 Pulmonary embolism during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.53, 0.96] |
| Analysis 1.5  Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 5 Pulmonary embolism during treatment period. | ||||
| 5.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.53, 0.97] |
| 5.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 5.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.06] |
| 6 Recurrent ischaemic/unknown stroke during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.69, 0.87] |
| Analysis 1.6  Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 6 Recurrent ischaemic/unknown stroke during treatment period. | ||||
| 6.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.69, 0.87] |
| 6.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Symptomatic intracranial haemorrhage during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [1.00, 1.50] |
| Analysis 1.7  Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 7 Symptomatic intracranial haemorrhage during treatment period. | ||||
| 7.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [1.00, 1.50] |
| 7.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.38] |
| 7.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [0.36, 20.26] |
| 8 Any recurrent stroke/intracranial haemorrhage during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.79, 0.97] |
| Analysis 1.8 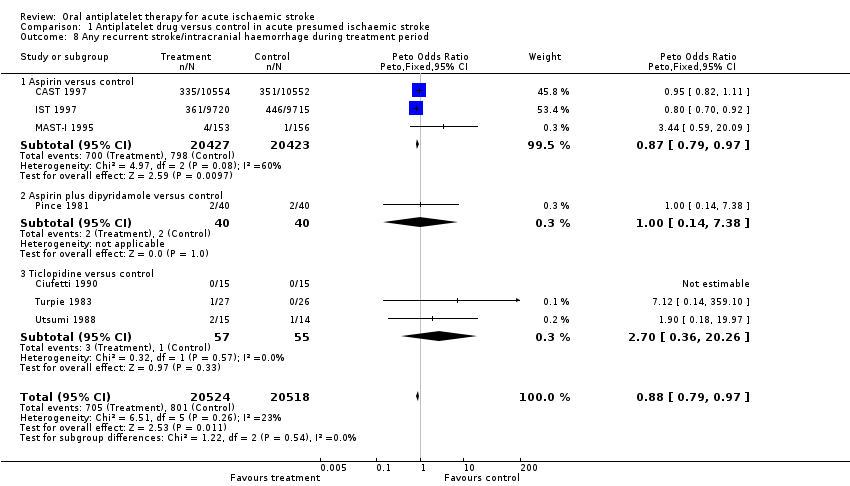 Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 8 Any recurrent stroke/intracranial haemorrhage during treatment period. | ||||
| 8.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.79, 0.97] |
| 8.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.38] |
| 8.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [0.36, 20.26] |
| 9 Major extracranial haemorrhage during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.35, 2.11] |
| Analysis 1.9 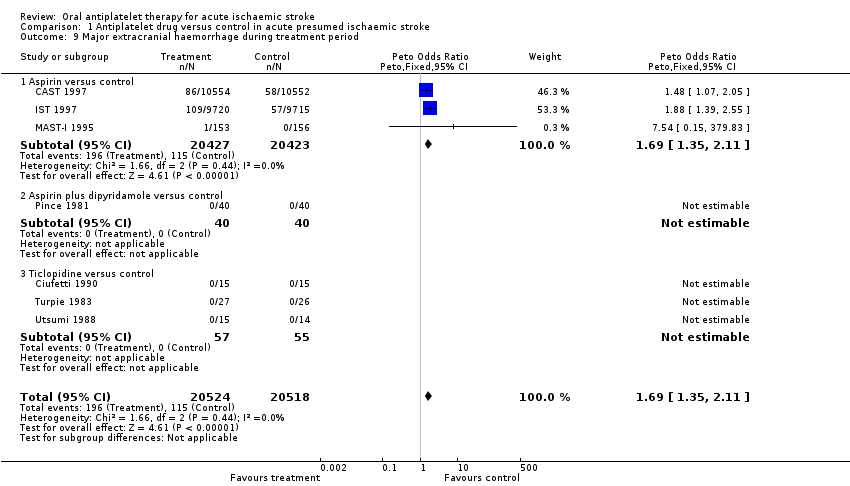 Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 9 Major extracranial haemorrhage during treatment period. | ||||
| 9.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.35, 2.11] |
| 9.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Complete recovery from stroke (post hoc) Show forest plot | 2 | 40541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [1.01, 1.11] |
| Analysis 1.10 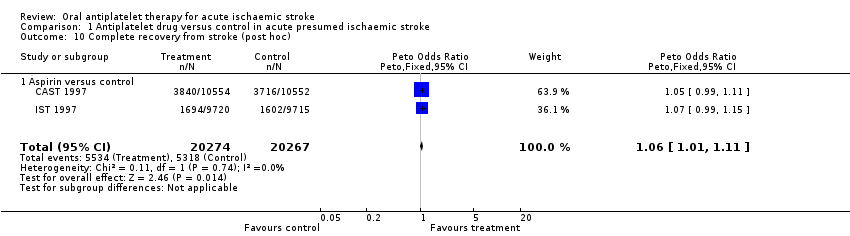 Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 10 Complete recovery from stroke (post hoc). | ||||
| 10.1 Aspirin versus control | 2 | 40541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [1.01, 1.11] |
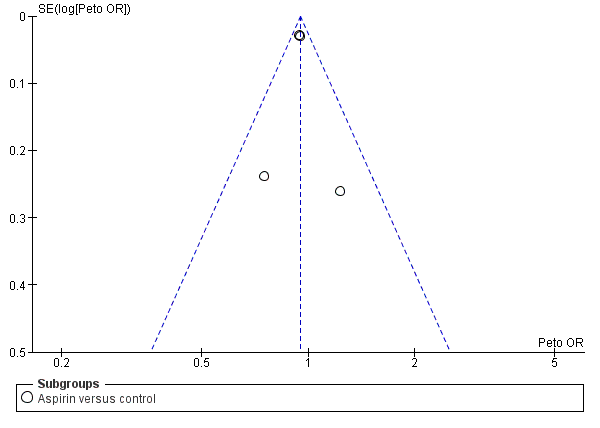
Funnel plot of comparison: 1 Antiplatelet agent versus control in acute presumed ischaemic stroke, outcome: 1.1 Death or dependence at end of follow‐up.
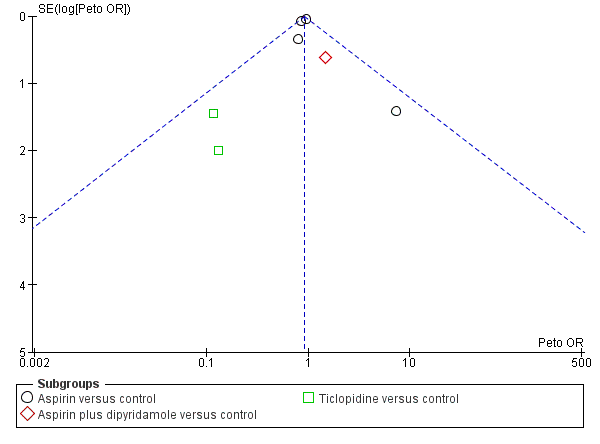
Funnel plot of comparison: 1 Antiplatelet agent versus control in acute presumed ischaemic stroke, outcome: 1.2 Deaths from all causes during treatment period.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 1 Death or dependence at end of follow‐up.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 2 Deaths from all causes during treatment period.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 3 Deaths from all causes during follow‐up.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 4 Deep venous thrombosis during treatment period.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 5 Pulmonary embolism during treatment period.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 6 Recurrent ischaemic/unknown stroke during treatment period.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 7 Symptomatic intracranial haemorrhage during treatment period.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 8 Any recurrent stroke/intracranial haemorrhage during treatment period.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 9 Major extracranial haemorrhage during treatment period.

Comparison 1 Antiplatelet drug versus control in acute presumed ischaemic stroke, Outcome 10 Complete recovery from stroke (post hoc).
| Outcome | Control event rate | No of events avoided | NNTB or NNTH |
| Per 1000 people treated (95% CI) | Data are number needed to treat to benefit (NNTB) (95% CI) unless otherwise indicated. NNTH = number needed to treat to harm | ||
| Estimated from the average of the control event rate in the 2 largest trials (CAST 1997 and IST 1997) | Estimated by applying the odds ratio for the outcome for studies of aspirin. Calculator is available at: http://www.dcn.ed.ac.uk/csrg/entity/entity_NNT2.asp | Estimated by applying the odds ratio for the outcome for studies of aspirin. Calculator is available at: http://www.dcn.ed.ac.uk/csrg/entity/entity_NNT2.asp | |
| Death or dependence at end of follow‐up | 0.47 | 13 (3 to 23) | 79 (43 to 400) |
| Deaths from all causes during follow‐up | 0.13 | 9 (2 to 15) | 108 (66 to 436) |
| Pulmonary embolism during treatment period | 0.01 | 1 (0 to 2) | 693 (427 to 6700) |
| Recurrent ischaemic/unknown stroke during treatment period | 0.03 | 7 (4 to 10) | 140 (104 to 248) |
| Symptomatic intracranial haemorrhage during treatment period | 0.01 | ‐2 (i.e. 2 extra) (‐4 to 0) | NNTH 574 (254 to 126 010) |
| Any recurrent stroke/intracranial haemorrhage during treatment | 0.04 | 5 (1 to 8) | 200 (123 to 868) |
| Major extracranial haemorrhage during treatment period | 0.01 | ‐4 (i.e. 4 extra) (‐7 to ‐2) | NNTH 245 (153 to 481) |
| Complete recovery from stroke (post hoc) | 0.26 | 11 (2 to 21) | 89 (49 to 523) |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Death or dependence at end of follow‐up Show forest plot | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.91, 0.99] |
| 1.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.95 [0.91, 0.99] |
| 2 Deaths from all causes during treatment period Show forest plot | 8 | 41483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 2.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.85, 1.00] |
| 2.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.43, 4.99] |
| 2.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.01, 1.20] |
| 3 Deaths from all causes during follow‐up Show forest plot | 8 | 41483 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 3.1 Aspirin versus control | 4 | 41291 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.87, 0.98] |
| 3.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.43, 4.99] |
| 3.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.02, 0.88] |
| 4 Deep venous thrombosis during treatment period Show forest plot | 2 | 133 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.36, 1.67] |
| 4.1 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.13, 0.95] |
| 4.2 Ticlopidine versus control | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.37 [0.72, 7.73] |
| 5 Pulmonary embolism during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.53, 0.96] |
| 5.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.53, 0.97] |
| 5.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 5.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.06] |
| 6 Recurrent ischaemic/unknown stroke during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.69, 0.87] |
| 6.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.77 [0.69, 0.87] |
| 6.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Symptomatic intracranial haemorrhage during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.23 [1.00, 1.50] |
| 7.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.22 [1.00, 1.50] |
| 7.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.38] |
| 7.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [0.36, 20.26] |
| 8 Any recurrent stroke/intracranial haemorrhage during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.79, 0.97] |
| 8.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.79, 0.97] |
| 8.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.0 [0.14, 7.38] |
| 8.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.70 [0.36, 20.26] |
| 9 Major extracranial haemorrhage during treatment period Show forest plot | 7 | 41042 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.35, 2.11] |
| 9.1 Aspirin versus control | 3 | 40850 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [1.35, 2.11] |
| 9.2 Aspirin plus dipyridamole versus control | 1 | 80 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Ticlopidine versus control | 3 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Complete recovery from stroke (post hoc) Show forest plot | 2 | 40541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [1.01, 1.11] |
| 10.1 Aspirin versus control | 2 | 40541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.06 [1.01, 1.11] |