Antibióticos para la faringitis
Appendices
Appendix 1. Details of previous searches
For the 2011 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2011, Issue 2, part of The Cochrane Library, www.thecochranelibrary.com (accessed 18 May 2011), which contains the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (November 2008 to May week 1, 2011) and EMBASE (November 2008 to May 2011).
In the previous update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) and the Database of Abstracts of Reviews of Effects (DARE) (The Cochrane Library 2008, Issue 4) which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (January 1966 to November 2008) and EMBASE (January 1990 to November 2008).
MEDLINE and CENTRAL were searched using the search strategy shown below. We combined the MEDLINE search string with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity and precision‐maximising version (2008 revision) (Lefebvre 2011). We adapted the search string for EMBASE.
MEDLINE (Ovid)
# 1 explode Pharyngitis/
# 2 pharyngit$.mp.
# 3 explode Nasopharyngitis/
# 4 nasopharyngit$.mp.
# 5 explode Tonsillitis/
# 6 tonsillit$.mp.
# 7 sore throat*.mp.
# 8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7
# 9 explode Anti‐Bacterial Agents/
# 10 antibiot$.mp.
# 11 #9 OR #10
# 12 #8 AND #11
(Embase.com used in 2011 update)
#1. 'pharyngitis'/exp AND [embase]/lim
#2. pharyngit*:ti,ab AND [2004‐2008]/py
#3. 'rhinopharyngitis'/exp AND [embase]/lim
#4. rhinopharyngit*:ti,ab OR nasopharyngit*:ti,ab [embase]/lim
#5. 'tonsillitis'/exp AND [embase]/lim
#6. tonsillit*:ti,ab AND [embase]/lim
#7. 'sore throat'/exp AND [embase]/lim
#8. 'sore throat':ti,ab OR 'sore throats':ti,ab embase]/lim
#9. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8
#10. 'antibiotic agent'/exp AND [embase]/lim
#11. antibiotic*:ti,ab AND [embase]/lim
#12. #10 OR #11 619,306
#13. random*:ti,ab OR factorial*:ti,ab OR crossover*:ti,ab OR 'cross over':ti,ab OR placebo*:ti,ab OR assign*:ti,ab OR allocat*:ti,ab OR volunteer*:ti,ab AND [embase]/lim
#14. 'double blind':ti,ab OR 'double blinded':ti,ab OR 'single blind':ti,ab OR 'single blinded':ti,ab AND [embase]/lim
#15. 'crossover procedure'/exp AND [embase]/lim
#16. 'double blind procedure'/exp AND [embase]/lim
#17. 'single blind procedure'/exp AND [embase]/lim
#18. 'randomized controlled trial'/exp AND [embase]/lim
#19. #13 OR #14 OR #15 OR #16 OR #17 OR #18
#20. #9 AND #12 AND #19
(EMBASE search used in earlier versions of the review)
EMBASE (WebSPIRS)
#1 explode 'pharyngitis‐' / all subheadings in DEM,DER,DRM,DRR
#2 (pharyngit* in ti) or (pharyngit* in ab)
#3 explode 'rhinopharyngitis‐' / all subheadings in DEM,DER,DRM,DRR
#4 (nasopharyngit* in ti) or (nasopharyngit* in ab)
#5 explode 'tonsillitis‐' / all subheadings in DEM,DER,DRM,DRR
#6 (tonsillit* in ti) or (tonsillit* in ab)
#7 explode 'sore‐throat' / all subheadings in DEM,DER,DRM,DRR
#8 (sore throat in ti) or (sore throat in ab)
#9 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8
#10 'antibiotic‐agent' / all subheadings in DEM,DER,DRM,DRR
#11 (antibiotic* in ti) or (antibiotic* in ab)
#12 #10 or #11
#13 #9 and #12
#14 explode 'randomized‐controlled‐trial' / all subheadings
#15 explode 'controlled‐study' / all subheadings
#16 explode 'single‐blind‐procedure' / all subheadings
#17 explode 'double‐blind‐procedure' / all subheadings
#18 explode 'crossover‐procedure' / all subheadings
#19 explode 'phase‐3‐clinical‐trial' / all subheadings
#20 (randomi?ed controlled trial in ti) or (randomi?ed controlled trial in ab)
#21 ((random* or placebo* or double‐blind*)in ti) or ((random* or placebo* or double‐blind*)in ab)
#22 (controlled clinical trial* in ti) or (controlled clinical trial* in ab)
#23 (explode 'randomized‐controlled‐trial' / all subheadings) or (explode 'controlled‐study' / all subheadings) or (explode 'single‐blind‐procedure' / all subheadings) or (explode 'double‐blind‐procedure' / all subheadings) or (explode 'crossover‐procedure' / all subheadings) or (explode 'phase‐3‐clinical‐trial' / all subheadings) or ((randomi?ed controlled trial in ti) or (randomi?ed controlled trial in ab)) or (((random* or placebo* or double‐blind*)in ti) or ((random* or placebo* or double‐blind*)in ab)) or ((controlled clinical trial* in ti) or (controlled clinical trial* in ab))
#24 (nonhuman in der) not ((human in der)and (nonhuman in der))
#25 ((explode 'randomized‐controlled‐trial' / all subheadings) or (explode 'controlled‐study' / all subheadings) or (explode 'single‐blind‐procedure' / all subheadings) or (explode 'double‐blind‐procedure' / all subheadings) or (explode 'crossover‐procedure' / all subheadings) or (explode 'phase‐3‐clinical‐trial' / all subheadings) or ((randomi?ed controlled trial in ti) or (randomi?ed controlled trial in ab)) or (((random* or placebo* or double‐blind*)in ti) or ((random* or placebo* or double‐blind*)in ab)) or ((controlled clinical trial* in ti) or (controlled clinical trial* in ab))) not ((nonhuman in der) not ((human in der)and (nonhuman in der)))
#26 #13 and #25
Appendix 2. EMBASE (Elsevier) search strategy
#16 #11 AND #15
#15 #12 OR #13 OR #14
#14 azithromycin*:ab,ti OR clarithromycin*:ab,ti OR erythromycin*:ab,ti OR roxithromycin*:ab,ti OR macrolide*:ab,ti OR cefamandole*:ab,ti OR cefoperazone*:ab,ti OR cefazolin*:ab,ti OR cefonicid*:ab,ti OR
cefsulodin*:ab,ti OR cephacetrile*:ab,ti OR cefotaxime*:ab,ti OR cephalothin*:ab,ti OR cephapirin*:ab,ti OR cephalexin*:ab,ti OR cephaclor*:ab,ti OR cephadroxil*:ab,ti OR cephaloglycin*:ab,ti OR
cephradine*:ab,ti OR cephaloridine*:ab,ti OR ceftazidime*:ab,ti OR cephamycin*:ab,ti OR cefmetazole*:ab,ti OR cefotetan*:ab,ti OR cefoxitin*:ab,ti OR cephalosporin*:ab,ti OR cefpodoxime*:ab,ti OR
cefuroxime*:ab,ti OR cefixime*:ab,ti OR amoxicillin*:ab,ti OR amoxycillin*:ab,ti OR ampicillin*:ab,ti OR sulbactum*:ab,ti OR tetracyclin*:ab,ti OR clindamycin*:ab,ti OR lincomycin*:ab,ti OR doxycyclin*:ab,ti OR fluoroquinolone*:ab,ti OR ciprofloxacin*:ab,ti OR fleroxacin*:ab,ti OR enoxacin*:ab,ti OR norfloxacin*:ab,ti OR ofloxacin*:ab,ti OR pefloxacin*:ab,ti OR moxifloxacin*:ab,ti OR esparfloxacin*:ab,ti OR clindamicin*:ab,ti OR penicillin*:ab,ti OR ticarcillin*:ab,ti OR 'beta‐lactam':ab,ti OR 'beta‐lactams':ab,ti OR levofloxacin*:ab,ti OR trimethoprim*:ab,ti OR 'co‐trimoxazole':ab,ti
#13 antibiot*:ab,ti
#12 'antibiotic agent'/exp
#11 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10
#10 (sore* NEAR/2 throat*):ab,ti
#9 ((throat* OR pharyn*) NEAR/3 (infect* OR inflam* OR strep*)):ab,ti
#8 'sore throat'/de
#7 (tonsil* NEAR/2 (infect* OR inflam*)):ab,ti
#6 tonsillit*:ab,ti
#5 'tonsillitis'/exp
#4 rhinopharyngit*:ab,ti OR nasopharyngit*:ab,ti
#3 'rhinopharyngitis'/de
#2 pharyngit*:ab,ti
#1 'pharyngitis'/exp
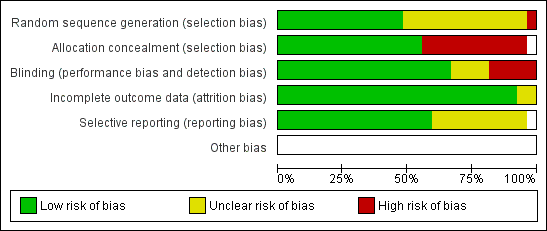
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 1 Symptom of sore throat on day 3.

Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 2 Symptom of sore throat on day 3: blind versus unblinded studies.

Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 3 Symptom of sore throat on day 3: antipyretics versus no antipyretics.

Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 4 Symptom of sore throat on day 3: GABHS‐positive throat swab, negative swab, untested/inseparable.
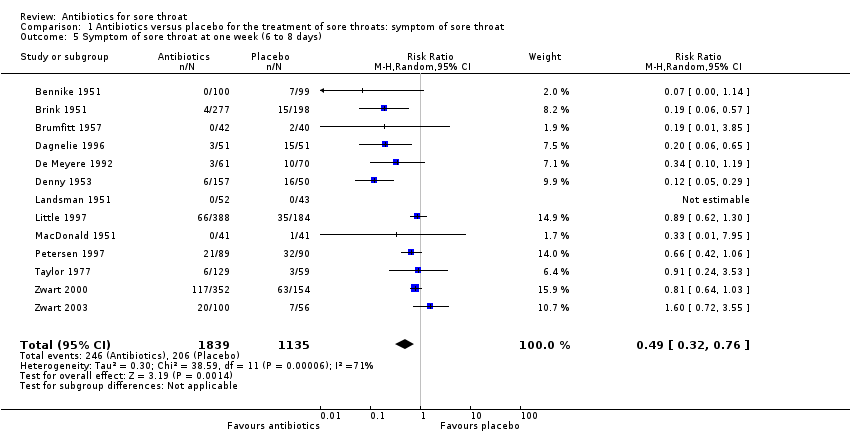
Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 5 Symptom of sore throat at one week (6 to 8 days).

Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 6 Symptom of sore throat at one week (6 to 8 days): blind versus unblinded studies.
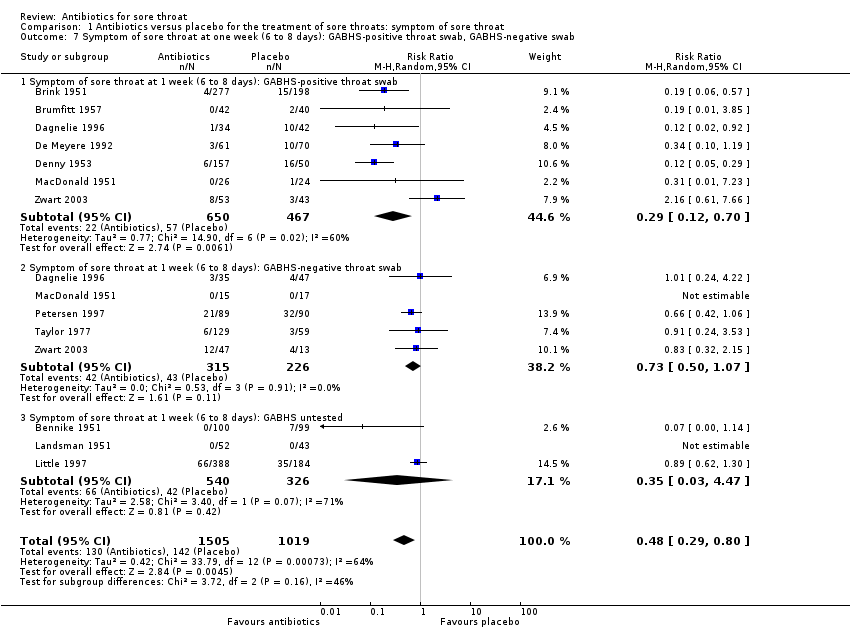
Comparison 1 Antibiotics versus placebo for the treatment of sore throats: symptom of sore throat, Outcome 7 Symptom of sore throat at one week (6 to 8 days): GABHS‐positive throat swab, GABHS‐negative swab.

Comparison 2 Antibiotics versus control for the treatment of sore throat: symptom of fever, Outcome 1 Symptom of fever on day 3.
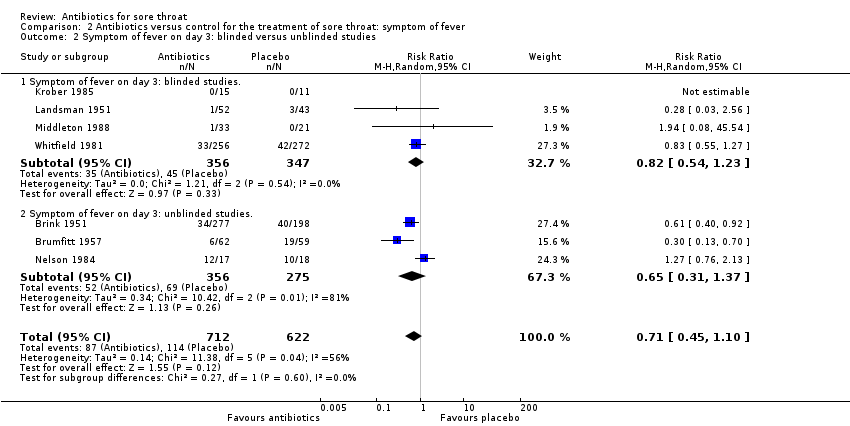
Comparison 2 Antibiotics versus control for the treatment of sore throat: symptom of fever, Outcome 2 Symptom of fever on day 3: blinded versus unblinded studies.

Comparison 2 Antibiotics versus control for the treatment of sore throat: symptom of fever, Outcome 3 Symptom of fever on day 3: children compared with adults.
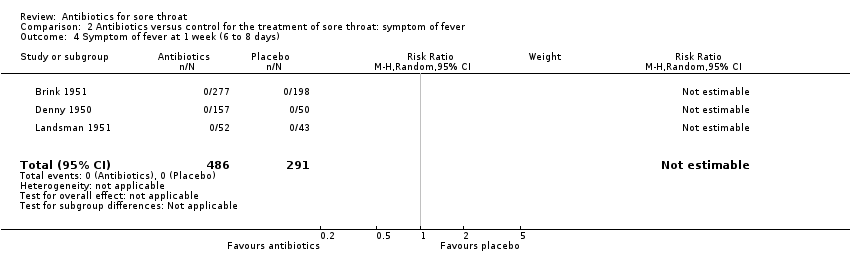
Comparison 2 Antibiotics versus control for the treatment of sore throat: symptom of fever, Outcome 4 Symptom of fever at 1 week (6 to 8 days).

Comparison 3 Antibiotics versus control for the treatment of sore throat: symptom of headache, Outcome 1 Symptom of headache on day 3.
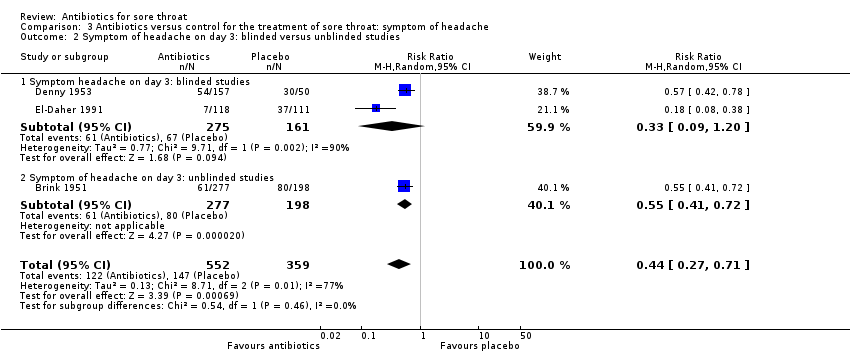
Comparison 3 Antibiotics versus control for the treatment of sore throat: symptom of headache, Outcome 2 Symptom of headache on day 3: blinded versus unblinded studies.

Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 1 Incidence of acute rheumatic fever within 2 months. Rheumatic fever defined by clinical diagnosis.

Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 2 Incidence of acute rheumatic fever within 2 months. Penicillin versus placebo.

Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 3 Incidence of acute rheumatic fever within 2 months: early (pre‐1975) versus late studies (post‐1975).

Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 4 Incidence of otitis media within 14 days. Otitis media defined by clinical diagnosis.
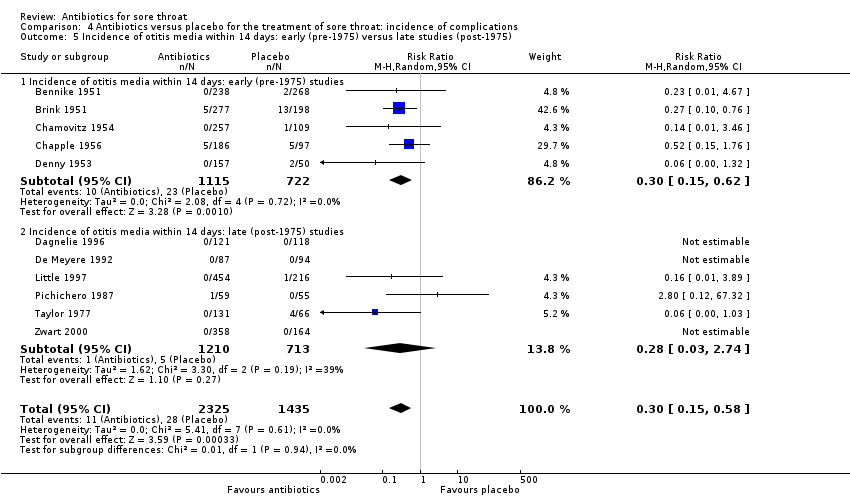
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 5 Incidence of otitis media within 14 days: early (pre‐1975) versus late studies (post‐1975).
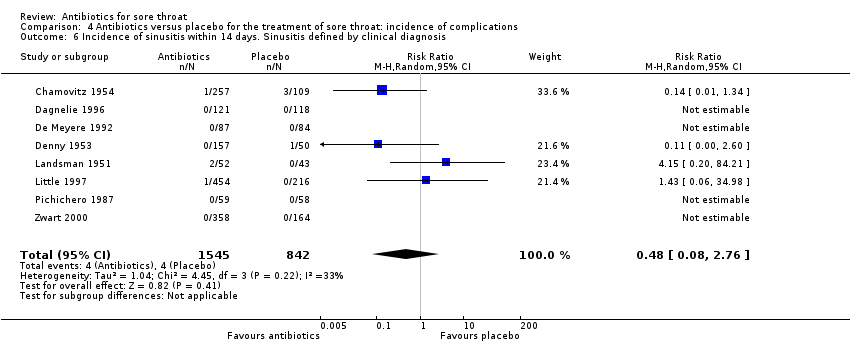
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 6 Incidence of sinusitis within 14 days. Sinusitis defined by clinical diagnosis.
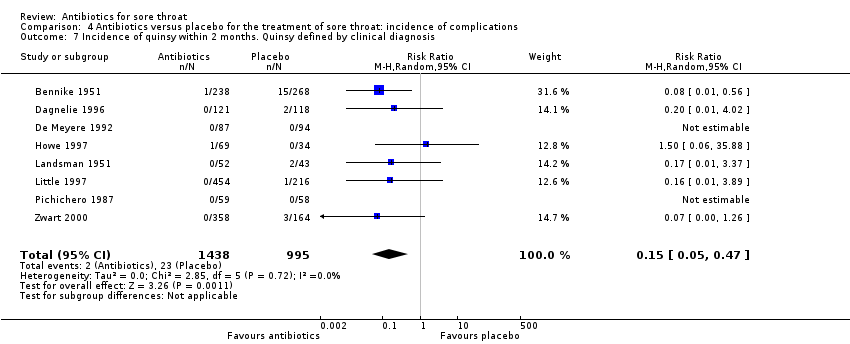
Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 7 Incidence of quinsy within 2 months. Quinsy defined by clinical diagnosis.

Comparison 4 Antibiotics versus placebo for the treatment of sore throat: incidence of complications, Outcome 8 Incidence of acute glomerulonephritis within 1 month. Acute glomerulonephritis defined by clinical diagnosis.
| Antibiotics compared with placebo for sore throat | ||||||
| Patient or population: patients presenting with sore throat Settings: community Intervention: antibiotics Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Corresponding risk | Assumed risk | |||||
| Antibiotics | Placebo | |||||
| Sore throat: day 3 | 0.66 | 0.72 | 0.68 to 0.76 | 3621 (15) | High | |
| Sore throat: day 7 | 0.18 | 0.65 | 0.55 to 0.76 | 2974 (13) | High | |
| Rheumatic fever | 0.017 | 0.29 | 0.18 to 0.44 | 10,101 (16) | High | Based largely on risk in pre‐1960 trials |
| Glomerulonephritis | 0.001 | 0.22 | 0.07 to 1.32 | 5147 (10) | Low | Sparse data: 2 cases only |
| Quinsy | 0.023 | 0.14 | 0.05 to 0.39 | 2433 (8) | High | |
| Otitis media | 0.02 | 0.28 | 0.15 to 0.52 | 3760 (11) | High | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptom of sore throat on day 3 Show forest plot | 15 | 3621 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.59, 0.79] |
| 2 Symptom of sore throat on day 3: blind versus unblinded studies Show forest plot | 15 | 3621 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.59, 0.79] |
| 2.1 Symptom of sore throat on day 3: blinded studies | 12 | 2662 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.54, 0.78] |
| 2.2 Symptom of sore throat on day 3: unblinded studies | 3 | 959 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.60, 1.05] |
| 3 Symptom of sore throat on day 3: antipyretics versus no antipyretics Show forest plot | 5 | 1137 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.70] |
| 3.1 Symptom of sore throat on day 3: antipyretics administered | 3 | 455 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.33, 0.81] |
| 3.2 Symptom of sore throat on day 3: no antipyretics administered | 2 | 682 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.55, 0.70] |
| 4 Symptom of sore throat on day 3: GABHS‐positive throat swab, negative swab, untested/inseparable Show forest plot | 15 | 3600 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.59, 0.78] |
| 4.1 Symptom of sore throat on day 3: GABHS‐positive throat swab | 11 | 1839 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.48, 0.71] |
| 4.2 Symptom of sore throat on day 3: GABHS‐negative throat swab | 6 | 736 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.63, 0.97] |
| 4.3 Symptom of sore throat on day 3: untested for GABHS culture or combined inseparable data | 3 | 1025 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 1.00] |
| 5 Symptom of sore throat at one week (6 to 8 days) Show forest plot | 13 | 2974 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.32, 0.76] |
| 6 Symptom of sore throat at one week (6 to 8 days): blind versus unblinded studies Show forest plot | 13 | 2944 | Risk Ratio (M‐H, Random, 95% CI) | 0.57 [0.38, 0.86] |
| 6.1 Symptom of sore throat at 1 week (6 to 8 days): blinded studies | 9 | 1616 | Risk Ratio (M‐H, Random, 95% CI) | 0.62 [0.38, 1.03] |
| 6.2 Symptom of sore throat at 1 week (6 to 8 days): unblinded studies | 4 | 1328 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.08, 1.15] |
| 7 Symptom of sore throat at one week (6 to 8 days): GABHS‐positive throat swab, GABHS‐negative swab Show forest plot | 12 | 2524 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.29, 0.80] |
| 7.1 Symptom of sore throat at 1 week (6 to 8 days): GABHS‐positive throat swab | 7 | 1117 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.12, 0.70] |
| 7.2 Symptom of sore throat at 1 week (6 to 8 days): GABHS‐negative throat swab | 5 | 541 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.50, 1.07] |
| 7.3 Symptom of sore throat at 1 week (6 to 8 days): GABHS untested | 3 | 866 | Risk Ratio (M‐H, Random, 95% CI) | 0.35 [0.03, 4.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptom of fever on day 3 Show forest plot | 7 | 1334 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.10] |
| 2 Symptom of fever on day 3: blinded versus unblinded studies Show forest plot | 7 | 1334 | Risk Ratio (M‐H, Random, 95% CI) | 0.71 [0.45, 1.10] |
| 2.1 Symptom of fever on day 3: blinded studies. | 4 | 703 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.54, 1.23] |
| 2.2 Symptom of fever on day 3: unblinded studies. | 3 | 631 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.31, 1.37] |
| 3 Symptom of fever on day 3: children compared with adults Show forest plot | 4 | 657 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.46] |
| 3.1 Symptom of fever on day 3: children | 2 | 61 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.76, 2.13] |
| 3.2 Symptom of fever on day 3: adults | 2 | 596 | Risk Ratio (M‐H, Random, 95% CI) | 0.29 [0.06, 1.51] |
| 4 Symptom of fever at 1 week (6 to 8 days) Show forest plot | 3 | 777 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Symptom of headache on day 3 Show forest plot | 3 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.71] |
| 2 Symptom of headache on day 3: blinded versus unblinded studies Show forest plot | 3 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.44 [0.27, 0.71] |
| 2.1 Symptom headache on day 3: blinded studies | 2 | 436 | Risk Ratio (M‐H, Random, 95% CI) | 0.33 [0.09, 1.20] |
| 2.2 Symptom of headache on day 3: unblinded studies | 1 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.41, 0.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Incidence of acute rheumatic fever within 2 months. Rheumatic fever defined by clinical diagnosis Show forest plot | 16 | 10101 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.60] |
| 2 Incidence of acute rheumatic fever within 2 months. Penicillin versus placebo Show forest plot | 14 | 8175 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.14, 0.50] |
| 3 Incidence of acute rheumatic fever within 2 months: early (pre‐1975) versus late studies (post‐1975) Show forest plot | 16 | 10101 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.60] |
| 3.1 Incidence of acute rheumatic fever within 2 months: early (pre‐1975) studies | 10 | 7617 | Risk Ratio (M‐H, Random, 95% CI) | 0.27 [0.12, 0.60] |
| 3.2 Incidence of acute rheumatic fever within 2 months: late (post‐1975) studies | 6 | 2484 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Incidence of otitis media within 14 days. Otitis media defined by clinical diagnosis Show forest plot | 11 | 3760 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.58] |
| 5 Incidence of otitis media within 14 days: early (pre‐1975) versus late studies (post‐1975) Show forest plot | 11 | 3760 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.58] |
| 5.1 Incidence of otitis media within 14 days: early (pre‐1975) studies | 5 | 1837 | Risk Ratio (M‐H, Random, 95% CI) | 0.30 [0.15, 0.62] |
| 5.2 Incidence of otitis media within 14 days: late (post‐1975) studies | 6 | 1923 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.03, 2.74] |
| 6 Incidence of sinusitis within 14 days. Sinusitis defined by clinical diagnosis Show forest plot | 8 | 2387 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.08, 2.76] |
| 7 Incidence of quinsy within 2 months. Quinsy defined by clinical diagnosis Show forest plot | 8 | 2433 | Risk Ratio (M‐H, Random, 95% CI) | 0.15 [0.05, 0.47] |
| 8 Incidence of acute glomerulonephritis within 1 month. Acute glomerulonephritis defined by clinical diagnosis Show forest plot | 10 | 5147 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.02, 2.08] |


