Green and black tea for the primary prevention of cardiovascular disease
Abstract
Background
There is increasing evidence that both green and black tea are beneficial for cardiovascular disease (CVD) prevention.
Objectives
To determine the effects of green and black tea on the primary prevention of CVD.
Search methods
We searched the following databases on 12 October 2012 without language restrictions: CENTRAL in The Cochrane Library, MEDLINE (OVID), EMBASE (OVID) and Web of Science (Thomson Reuters). We also searched trial registers, screened reference lists and contacted authors for additional information where necessary.
Selection criteria
Randomised controlled trials (RCTs) lasting at least three months involving healthy adults or those at high risk of CVD. Trials investigated the intake of green tea, black tea or tea extracts. The comparison group was no intervention, placebo or minimal intervention. The outcomes of interest were CVD clinical events and major CVD risk factors. Any trials involving multifactorial lifestyle interventions or focusing on weight loss were excluded to avoid confounding.
Data collection and analysis
Two review authors independently selected trials for inclusion, abstracted data and assessed the risk of bias. Trials of green tea were analysed separately from trials of black tea.
Main results
We identified 11 RCTs with a total of 821 participants, two trials awaiting classification and one ongoing trial. Seven trials examined a green tea intervention and four examined a black tea intervention. Dosage and form of both green and black tea differed between trials. The ongoing trial is examining the effects of green tea powder capsules.
No studies reported cardiovascular events.
Black tea was found to produce statistically significant reductions in low‐density lipoprotein (LDL) cholesterol (mean difference (MD) ‐0.43 mmol/L, 95% confidence interval (CI) ‐0.56 to ‐0.31) and blood pressure (systolic blood pressure (SBP): MD ‐1.85 mmHg, 95% CI ‐3.21 to ‐0.48. Diastolic blood pressure (DBP): MD ‐1.27 mmHg, 95% CI ‐3.06 to 0.53) over six months, stable to sensitivity analysis, but only a small number of trials contributed to each analysis and studies were at risk of bias.
Green tea was also found to produce statistically significant reductions in total cholesterol (MD ‐0.62 mmol/L, 95% CI ‐0.77 to ‐0.46), LDL cholesterol (MD ‐0.64 mmol/L, 95% CI ‐0.77 to ‐0.52) and blood pressure (SBP: MD ‐3.18 mmHg, 95% CI ‐5.25 to ‐1.11; DBP: MD ‐3.42, 95% CI ‐4.54 to ‐2.30), but only a small number of studies contributed to each analysis, and results were not stable to sensitivity analysis. When both tea types were analysed together they showed favourable effects on LDL cholesterol (MD ‐0.48 mmol/L, 95% CI ‐0.61 to ‐0.35) and blood pressure (SBP: MD ‐2.25 mmHg, 95% CI ‐3.39 to ‐1.11; DBP: MD ‐2.81 mmHg, 95% CI ‐3.77 to ‐1.86). Adverse events were measured in five trials and included a diagnosis of prostate cancer, hospitalisation for influenza, appendicitis and retinal detachment but these are unlikely to be directly attributable to the intervention.
Authors' conclusions
There are very few long‐term studies to date examining green or black tea for the primary prevention of CVD. The limited evidence suggests that tea has favourable effects on CVD risk factors, but due to the small number of trials contributing to each analysis the results should be treated with some caution and further high quality trials with longer‐term follow‐up are needed to confirm this.
Plain language summary
Green and black tea to prevent cardiovascular disease
Cardiovascular disease (CVD) is a worldwide healthcare burden. However, it is thought that CVD risk can be lowered by changing a number of modifiable risk factors such as diet, and this includes the intake of tea. This review assessed the effectiveness of green tea, black tea or black/green tea extracts in healthy adults and those at high risk of CVD. We found 11 randomised controlled trials, four of which examined black tea interventions and seven examined green tea interventions. There were variations in the dosage and form (drink, tablets or capsules) of the black and green tea interventions, and the duration of the interventions ranged from three months to six months. Adverse events were reported in five of the included trials. These included a diagnosis of prostate cancer, hospitalisation for influenza, appendicitis and retinal detachment; these are unlikely to be associated with the intervention. The results showed black and green tea to have a beneficial effect on lipid levels and blood pressure, but these results were based on only a small number of trials that were at risk of bias. Analysis conducted over both tea types showed beneficial effects of tea on LDL‐cholesterol and blood pressure but again this was based on only a few trials that were at risk of bias. To date the small number of studies included suggest some benefits of green and black tea on blood pressure and lipid levels but more longer‐term trials at low risk of bias are needed to confirm this.
Authors' conclusions
Background
Description of the condition
Cardiovascular diseases (CVD) are the result of complications in the heart and blood vessels (WHO 2011), and include cerebrovascular disease, coronary heart disease (CHD), and peripheral arterial disease (PAD). Around 29.6% of total global deaths can be attributed to CVD (World Health Report 2003) and it is estimated that 17 million deaths per year are caused by CVD (Mackay 2004).
One of the main mechanisms thought to cause CVD is atherosclerosis, where the arteries become blocked by plaques or atheromas (NHS 2010). Atherosclerosis can cause CVD when the arteries are completely blocked by a blood clot or when blood flow is restricted by a narrowed artery limiting the amount of blood and oxygen that can be delivered to organs or tissue (British Heart Foundation 2012). While arteries may naturally become harder and narrower with age this process may be accelerated by factors such as smoking, high cholesterol, high blood pressure, obesity, a sedentary lifestyle and ethnicity (NHS 2010). Ruptures of unstable plaque can also lead to CVD. Unstable plaques are thought to activate an inflammatory response in the body. This inflammatory response causes the structure of atherosclerotic plaque to weaken and rupture leading to the formation of blood clots (Spagnoli 2007).
A number of dietary factors have been found to be associated with CVD risk such as a low consumption of fruit and vegetables (Begg 2007), a high intake of saturated fat (Siri‐Tarino 2010) and a high consumption of salt (He 2011). These factors are important since they can be modified in order to lower CVD risk making them a prime target for interventions aimed at primary prevention and management of CVD.
Description of the intervention
According to Deka (Deka 2011), records show tea has been used largely due to its medicinal purposes from as far back as the 10th century and it is now consumed worldwide. Tea leaves come from the plant Camillia sinesis and there are three main types of tea: green, black and oolong. The type of tea produced from the leaves depends on how the leaves are processed. For instance, partly fermented leaves produce oolong tea, fermented leaves produced black tea while non‐fermented leaves create green tea (Deka 2011).
All types of tea made from Camillia sinesis are rich in flavonoids. These are water‐soluble plant pigments that belong to the larger group of polyphenolic compounds (Corradini 2011; Scalbert 2005). The main class of flavonoids found in tea are flavanols. These include epigallocatechin (EGC), epigallocatechin gallate (EGCG), epicatechin gallate (ECG) and epicatechin (EC) (Kris‐Etherton 2002). Whilst the total flavonoid content in green and black tea is similar, their chemical structures differ. This is mainly due to the oxidation process used in the manufacture of black tea that converts flavonoids, such as catechin found in green tea, into more complex varieties, mainly thearubigins and theaflavins (Deka 2011; Stangl 2006). In green tea catechin constitutes around 80% to 90% of total flavonoids, whereas in black tea they account for 20% to 30% of total flavonoids (Stangl 2006).
Green tea is also thought to have a high content of vitamins and minerals with five cups a day providing between 5% to 10% of a person’s daily requirement of riboflavin, niacin, folic acid and pantothenic acid. Furthermore, this amount of green tea a day provides 45% of the daily requirement of manganese, 25% of potassium and 5% of magnesium (Shukla 2007).
How the intervention might work
Observational, epidemiological and experimental evidence have indicated that the consumption of green and black tea may have a beneficial effect on cardiovascular function (Deka 2011; Kuriyama 2008; Mineharu 2010; Nagao 2007; Sesso 1999). In particular, observational studies suggest that a high intake of both green and black tea is related to a reduction in CVD risk (Kuriyama 2008; Sesso 1999). For example, de Koning Gans et al (de Koning Gans 2010), in a prospective cohort study of 37,514 participants in the Netherlands, found that consuming three to six cups of tea (mainly black tea) a day was associated with a reduction in the risk of CHD mortality (hazard ratio (HR) = 0.55, 95% confidence interval (CI) 0.31 to 0.97) (cup size was not stated in the article). This is supported by Mineharu et al (Mineharu 2010) who reported a strong inverse relationship between the intake of more than six cups of green tea daily and CVD mortality in a cohort of 76,979 Japanese adults. These observational findings, however, must be cautiously interpreted because of the potential for confounding effects by factors commonly associated with tea drinking, such as healthier lifestyles, which might contribute to the observed inverse associations. Indeed, some studies have failed to show any relationship between the intake of tea and CVD risk (Brown 1993; Hertog 1997).
Meta‐analyses of observational studies corroborate the findings from individual studies showing an inverse relationship between tea and CVD risk (Arab 2009; Peters 2001). Peters and colleagues examined the association between tea and CVD by analysing 10 cohort studies and seven case‐control studies (Peters 2001). They found an 11% reduction in the risk of myocardial infarction when consuming three or more cups of tea a day. However, these authors suggest that their findings must be cautiously interpreted since there was evidence of publication bias of smaller positive studies.
Evidence from intervention studies also show the benefit of tea consumption in reducing the risk factors for CVD (Brown 2009; Nagao 2007). Fujita et al. (Fujita 2008), for instance, conducted a randomised double‐blind placebo‐controlled study to investigate the benefits of taking black tea extract in 47 Japanese patients with borderline hypercholesterolaemia. They found that black tea extract significantly lowered patients low‐density lipoprotein (LDL) cholesterol and blood total cholesterol levels. A systematic review that searched for RCTs until 2006 and included 12 trials of green tea, and 12 of black tea compared with control (all assessed as at moderate to high risk of bias) found little effect of either type of tea on systolic or diastolic blood pressure or high‐density lipoprotein (HDL) cholesterol (Hooper 2008). However, it was not stated in the primary studies of this review whether tea was caffeinated or decaffeinated. This is important since the impact of tea on CVD risk factors may be due, in part, to the acute effects of caffeine. Nonetheless, there was some evidence from moderate to poor quality trials that green tea reduced LDL cholesterol (black tea had no effect) and black tea improved flow mediated dilatation, an emerging risk factor for CVD (Hooper 2008). None of the included studies assessed mortality or cardiovascular events.
A more recent systematic review examined green tea consumption and its antioxidant effects in 31 controlled intervention studies published up to June 2010 (Ellinger 2011). The findings indicated that there was some evidence that regular green tea consumption of at least 0.6 ‐ 1.5 L/day reduced lipid peroxidation and increased antioxidant capacity. Ellinger et al (Ellinger 2011) therefore concluded that there was evidence, although limited, for the antioxidant effects of green tea which are suggested to protect against CVDs. However, many of the included studies were very short term and it is unclear whether benefits are sustained over longer periods.
The reduction of CVD risk by tea may be largely due to the high levels of polyphenols, in particular flavonoids, which both green and black tea contain. However, the exact mechanisms through which increased tea consumption reduces CVD risk are unknown. Some potential mechanisms include reducing weight, improving insulin sensitivity, improving dyslipidaemia, improving endothelial function by lowering oxidative stress, platelet inhibition and anti‐inflammatory effects (Deka 2011). Furthermore, tea and their flavonoids have antioxidant properties that help to reduce CVD risk (Deka 2011; Gardner 2007).
Why it is important to do this review
Tea is the second most consumed beverage worldwide after water (Kris‐Etherton 2002) and due to such high frequency of intake worldwide, even a small impact of tea on human health could have large implications for public health (Peters 2001). However, there is still inconclusive evidence from interventional and observational studies of tea consumption on clinical cardiovascular endpoints. An up‐to‐date systematic review is needed to clarify the association between tea consumption and CVD risk, which will then provide guidance for national and international governments, local authorities, practitioners and members of the public.
The current review updates and expands the most recent systematic reviews (Ellinger 2011; Hooper 2008). We have included only randomised controlled trials of either green or black tea and examined the effects over longer time periods (at least three months) as these are most relevant for public health interventions.
Objectives
The primary objective was to determine the effectiveness of green and black tea consumption for the primary prevention of CVD.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs). Cross‐over trials were eligible for inclusion in this review if identified, but we would have used data only from the first half as a parallel group design.
Types of participants
Adults aged 18 and over from the general population and adults at high risk of CVD. This review focused on the effects of green and black tea intake on participants in primary prevention trials. We therefore excluded studies where more than 25% of participants had CVD at baseline including those who have experienced a previous myocardial infarction (MI), stroke, revascularisation procedure (coronary artery bypass grafting (CABG) or percutaneous transluminal coronary angioplasty (PTCA)), those with angina, or angiographically‐defined CHD, cerebrovascular disease (stroke) and PAD. We also excluded studies where more than 25% of the participants had type 2 diabetes. While patients with type 2 diabetes are at increased risk of CVD, interventions for diabetes are covered specifically by the Cochrane Metabolic and Endocrine Disorders review group.
Types of interventions
The intervention was the intake of green or black tea as a beverage or the intake of tea extracts. No limit was placed on the amount of tea consumed. Studies examining green tea and green tea extracts were examined separately from those examining black tea and black tea extracts. We intended to examine the effect of "dose" and duration of tea intake if there were sufficient studies, and the effects of caffeine intake.
We focused on follow‐up periods of six months or more as these are most relevant for public health interventions, and considered trials with follow‐up periods of three months or more where longer term trials were lacking. Trials were only considered where the comparison group was no intervention, placebo or minimal intervention (e.g. leaflet to follow a dietary pattern with no person‐to‐person intervention or reinforcement). Trials using multifactorial lifestyle interventions and trials focusing on weight loss were excluded from the review to avoid confounding.
Types of outcome measures
Primary outcomes
-
Cardiovascular mortality
-
All‐cause mortality
-
Non‐fatal endpoints such as MI, CABG, PTCA, angina, or angiographically‐defined CHD, stroke, carotid endarterectomy, or PAD
Secondary outcomes
-
Changes in blood pressure (systolic and diastolic blood pressure) and blood lipids (total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides)
-
Occurrence of type 2 diabetes as a major CVD risk factor
-
Health‐related quality of life
-
Adverse effects
-
Costs
Search methods for identification of studies
Electronic searches
We searched the following electronic databases without language restrictions on 12 October 2012:
-
Cochrane Central Register of Controlled Trials (CENTRAL) (Issue 9 of 12, September 2012) in The Cochrane Library;
-
MEDLINE (OVID) (1946 to Week 1 October 2012);
-
EMBASE Classic + EMBASE (OVID) (1947 to 2012 Week 40);
-
Web of Science (Thomson Reuters) (1970 to 12 October 2012);
-
Database of Abstracts of Reviews of Effects (DARE), Health Technology Assessment Database (HTA) and Health Economics Evaluations Database (HEED) (Issue 3 of 4, July 2012) on The Cochrane Library.
Medical subject headings (MeSH) or equivalent and text word terms were used. The Cochrane sensitive‐maximising RCT filter (Lefebvre 2011) was used for MEDLINE and adaptations of it were used for EMBASE and Web of Science.
Searches were tailored to individual databases. The search strategies are shown in Appendix 1.
Searching other resources
We checked reference lists of reviews and retrieved articles for additional studies.
We searched the metaRegister of controlled trials (mRCT) (www.controlled‐trials.com/mrct), Clinical trials.gov (www.clinicaltrials.gov), the WHO International Clinical Trials Registry platform (ICTRP) (http://apps.who.int/trialsearch/) for ongoing trials and Google Scholar for additional studies. We also searched OpenGrey to identify any relevant grey literature.The search strategies are shown in Appendix 2.
We also performed citation searches on key articles. We contacted experts in the field for unpublished and ongoing trials and authors were contacted where necessary for any additional information.
Data collection and analysis
Selection of studies
Two review authors (Louise Hartley (LH), Nadine Flowers (NF)) reviewed the title and abstract of each paper and retrieved potentially relevant references. We then obtained the full text of potentially relevant studies and the same two authors (LH, NF) independently selected studies to be included in the review by using predetermined inclusion criteria. In all cases we resolved all disagreements about study inclusion by consensus and consulted a third review author (Karen Rees (KR)) if disagreements persisted.
Data extraction and management
Two review authors independently (LH, NF or Jennifer Holmes (JH)) extracted data using a proforma. We also contacted chief investigators to provide additional relevant information when necessary. Details of the study design, participant characteristics, study setting, intervention (including number of components, tea or extract, duration, flavonoid and caffeine dose), and outcome data (including details of outcome assessment, adverse effects) and methodological quality (randomisation, blinding and attrition) were extracted from each included study. We resolved any disagreements about extracted data by consensus and consulted a third author (KR) if disagreements persisted.
Assessment of risk of bias in included studies
We assessed risk of bias by examining the random sequence generation and allocation concealment, description of drop‐outs and withdrawals (including analysis by intention‐to‐treat), blinding (participants, personnel and outcome assessment) and selective outcome reporting (Higgins 2011) in each trial. Two authors (LH, NF) independently assessed the risk of bias of included studies and rated each domain as having a low risk of bias, a high risk of bias or an unclear risk of bias.
Measures of treatment effect
Data were processed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). For continuous outcomes net changes were compared (i.e. intervention group minus control group differences) and a mean difference (MD) and 95% confidence interval (CIs) calculated for each study.
Assessment of heterogeneity
For each outcome, tests of heterogeneity were conducted (using the Chi2 test of heterogeneity and I2 statistic). Where there was no heterogeneity a fixed‐effect meta‐analysis was performed. If substantial heterogeneity (I2 greater than 50%) was detected the review authors looked for possible explanations for this (for example, participants and intervention). If the heterogeneity could not be explained, the review authors considered the following options: provide a narrative overview and not aggregate the studies at all or use a random‐effects model with appropriate cautious interpretation.
Subgroup analysis and investigation of heterogeneity
Results were stratified by i) black tea, ii) green tea. It was our intention to stratify studies by “dose” of tea intake and duration of the intervention but there were insufficient trials that met the inclusion criteria to do this. Similarly, the lack of included studies meant that we were unable to examine the effects of caffeine intake.
Sensitivity analysis
We carried out sensitivity analysis excluding studies with inadequate or unclear allocation concealment. There were insufficient trials to examine the effects of publication bias using funnel plots and tests of asymmetry (Egger 1997).
Results
Description of studies
Results of the search
The searches generated 2319 hits and 1736 after de‐duplication. Screening the titles and abstracts identified 135 papers for formal inclusion or exclusion. Of these, 11 RCTs (12 papers) met the inclusion criteria. We identified one ongoing trial (one paper) and there are two trials (two papers) awaiting classification. Details of the flow of studies through the review are given in Figure 1.

Study flow diagram.
Included studies
Details of the methods, participants, intervention, comparison group and outcome measures for each of the studies included in the review are shown in the Characteristics of included studies table. Eleven trials with 11 trial arms were included with 821 participants randomised. None of the included studies reported on both black and green tea.
Four included studies examined black tea (Bahorun 2012; Fujita 2008; Hodgson 2012; Mukamal 2007). The health status of participants varied between studies; one of the studies recruited participants with borderline, or mild to moderate hypercholesterolaemia (Fujita 2008); one study recruited participants with either diabetes or two other cardiovascular disease risk factors (Mukamal 2007) and the remaining studies recruited healthy participants (Bahorun 2012; Hodgson 2012;). All four trials examining black tea recruited both male and female participants. One trial was conducted in the USA (Mukamal 2007) while the other studies were conducted in Japan (Fujita 2008), Mauritius (Bahorun 2012) and Australia (Hodgson 2012). The duration of the intervention and follow‐up periods varied between three months (Bahorun 2012; Fujita 2008) and six months (Hodgson 2012; Mukamal 2007).
All four studies used black tea extracts, in tablet form (Fujita 2008) or as a drink (Bahorun 2012; Hodgson 2012; Mukamal 2007). Again, the dosage and type of black tea extracts varied between studies; 1 g black tea extract per day (Fujita 2008); 1.29 g black tea polyphenols per day (Hodgson 2012); three servings of 200 mL of black tea a day (Bahorun 2012) and 318 mg black tea catechins per day (Mukamal 2007).
Seven of the included studies examined green tea (Bogdanski 2012; Janjua 2009; Maron 2003; Nantz 2009; Shen 2010; Smith 2010; Stendell‐Hollis 2010).
In these studies the health status of participants varied; one of the studies recruited participants with borderline, or mild to moderate hypercholesterolaemia (Maron 2003); one study recruited participants with hypertension (Bogdanski 2012); one study recruited breast cancer survivors (Stendell‐Hollis 2010) and one recruited postmenopausal women with osteopenia (Shen 2010). The remaining three studies recruited healthy participants (Janjua 2009; Nantz 2009; Smith 2010). Four trials examining green tea recruited female participants only (154 randomised) (Janjua 2009; Shen 2010; Smith 2010; Stendell‐Hollis 2010). Five trials were conducted in the USA ( Janjua 2009; Nantz 2009; Shen 2010; Smith 2010; Stendell‐Hollis 2010). The remaining studies were conducted in China (Maron 2003) and Poland (Bogdanski 2012). The duration of the intervention and follow‐up periods varied between three months (Bogdanski 2012; Maron 2003; Nantz 2009; Smith 2010), six months (Shen 2010; Stendell‐Hollis 2010) and two years (Janjua 2009).
Five of the studies used green tea extracts, in the form of tablets or capsules. Dosage and type of green tea extracts varied between studies; 500 mg per day of green tea polyphenols (Shen 2010); 375 mg green tea extract (Bogdanski 2012); 250 mg twice a day of green tea polyphenols (Janjua 2009); 200 mg theanine and 400 mg decaffeinated catechin green tea extract per day (Nantz 2009); and 75 mg theaflavins, 150 mg green tea catechins, and 150 mg other tea polyphenols per day (Maron 2003). One study provided participants with one beverage a day containing green tea extract (Smith 2010) while the remaining study provided participants with green tea bags containing 58.91 mg of catechins (Stendell‐Hollis 2010).
One ongoing trial (one paper) was identified. Details of this trial are provided in the Characteristics of ongoing studies table. Briefly, the trial (Mitsuhiro Yamada 2009) examines the effects of 10 green tea powder capsules three times a day for 12 weeks in adults prone to metabolic syndrome. The outcomes measured include blood pressure and lipid levels. No anticipated end date was provided for this trial.
Excluded studies
Details and reasons for exclusion for the studies that most closely missed the inclusion criteria are presented in the Characteristics of excluded studies table. Reasons for exclusion for the majority of studies was their short‐term duration (< three months). Other reasons for exclusion include the control not being a minimal intervention or no intervention/placebo, and no outcomes of interest.
Short‐term studies
As stated above, the reason for exclusion for the majority of studies was that they were short term (< three months follow‐up). We focused on three or more months follow‐up as we were interested in the sustained effects of tea intake which are most relevant for public health interventions. Other systematic reviews have included some of these short‐term studies (Ellinger 2011) and for interest we have listed them in Table 1.
| Study | Green/Black or extracts? | Dose | Duration |
| Black and Green tea | 6 g/d | 2 wks | |
| Green tea and Green tea extract | 4 cups/d or 2 capsules and 4 cups of water /d | 8 wks | |
| Green tea and Green tea extract | 4 cups/d or 2 capsules and 4 cups of water /d | 8 wks | |
| Green tea extract | 250 mg/d | 8 wks | |
| Green tea extract | 500 mg | 4 hrs | |
| Black tea | 6 mugs/d | 4 wks | |
| Green tea extract | 530 mg twice a day | 6 wks | |
| Black tea | 5 servings a day | 3 wks | |
| Black tea, Green tea and Green tea extract | 6 cups (150 mL)/day or 6 x 4 capsules/day with 6 x 150 mL of control beverage | 4 wks | |
| Green tea extract | In a beverage consumed once a day | 21 days | |
| Black tea | 5 servings (200 mL)/d | 6 wks | |
| Green tea extract | 6 capsules/d | 3 wks | |
| Green tea extract | 3 g a day | 4 wks | |
| Black tea | 0mg, 100 mg, 200 mg, 400 mg or 800 mg twice a day | 1 wk | |
| Black tea | 450 mL | 2hrs | |
| Black tea | 1250 mL/d | 4 wks | |
| Black or Green tea | 5 cups/d | 7 d | |
| Black tea | 5 cups/d | 4 wks | |
| Green tea extract | 500 mg | 4 wks | |
| Black tea | 5 cups/d (750 mL) | 4 wks | |
| Black tea | > 200 mL twice a day | 8 wks | |
| Green tea | 400ml | 2 hrs | |
| Green tea or Green tea extract | 4 cups a day or 2 capsules and 4 cups of water a day | 8 wks | |
| Black or Green tea | 6 cups/d of Black or Green tea or 3,6g tablet of Green tea polyphenols/day | 4 wks | |
| Black tea | 400 mL | 60 mins | |
| Black tea | 300 mL | 105 mins | |
| Black tea | 5 cups per day | 2 weeks | |
| Black tea | 1 litre | 7 hours | |
| Black tea extract | one capsule/d | 11 wks | |
| Black or Green tea | 6 gm | 3 hrs | |
| Black and Green tea | 1000 mL/d or 250 mL/d | 7 d or 4 wks | |
| Black tea | one cup | 4 hrs | |
| Green tea extract | 1.06 g | 90 mins | |
| Green tea | 2 cups | 42 days | |
| Green tea extract | 400 mg or 800 mg per day | 2 mths | |
| Green tea | 6 g | 120 mins | |
| Black tea | 1 cup | 6 hours | |
| Tea | 10, 224 or 674 mg of tea catechins | 6 hours | |
| Tea | 1069 mg/day of total catechins | 1 wk | |
| Black tea | 4 sachets a day | 6 wks |
d:day
Risk of bias in included studies
Details are provided for each of the included studies in the 'Risk of bias' tables in Characteristics of included studies and summarised in Figure 2; Figure 3.
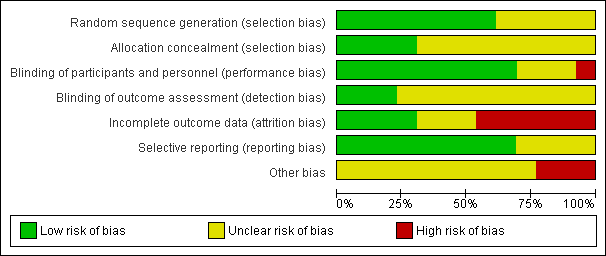
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
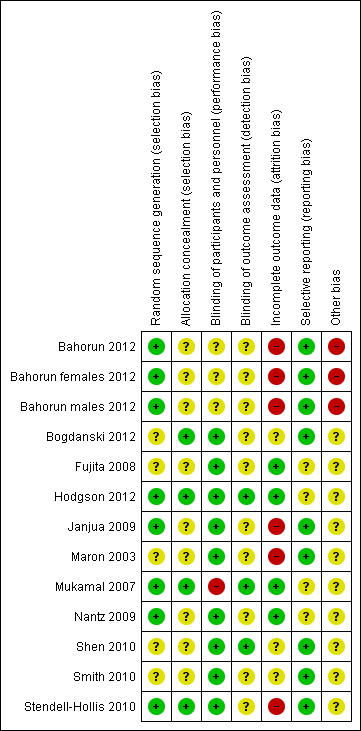
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Three of the trials that examined black tea clearly stated the methods of random sequence generation (Bahorun 2012; Hodgson 2012; Mukamal 2007) and were judged to be of low risk of bias. The methods of allocation concealment were not stated in two of the studies that examined black tea. In the two trials where this was clear, the methods were judges to be of low risk of bias (Hodgson 2012; Mukamal 2007).
The methods of random sequence generation were unclear in four of the green tea trials (Bogdanski 2012; Maron 2003; Shen 2010; Smith 2010). In the three trials where this was clear, the methods used were judged to be of low risk of bias (Janjua 2009; Nantz 2009; Stendell‐Hollis 2010). Allocation concealment methods were not stated in five of the seven green tea trials. In the remaining two trials, the methods of allocation concealment were clear and so judged to be of low risk of bias (Bogdanski 2012; Stendell‐Hollis 2010).
Blinding
Two trials examining black tea stated that they were double blind (participants and personnel were blind to treatment allocation, as were outcome assessors) and were regarded at low risk of bias (Fujita 2008; Hodgson 2012). One trial was stated as single blind (participants were not blinded to treatment allocation, but outcome assessors were blinded to the treatment allocation) (Mukamal 2007) while one trial did not state if it had used blinding (Bahorun 2012).
All of the trials examining green tea stated that they were double blind and placebo‐controlled and so were regarded as being at low risk of performance bias. However, in six of the trials no details were provided as to whether outcome assessors were blinded (Bogdanski 2012; Janjua 2009; Maron 2003; Nantz 2009; Smith 2010; Stendell‐Hollis 2010) and so were regarded as being at unclear risk of detection bias.
Incomplete outcome data
Loss to follow‐up was reported in all four of the black tea trials. Three of the four included studies were judged as low risk of bias as they clearly reported reasons for withdrawals, exclusions and losses to follow‐up (Fujita 2008; Hodgson 2012; Mukamal 2007). Two of these studies had also performed intention‐to‐treat (ITT) analyses (Hodgson 2012; Mukamal 2007). One of the trials examining black tea was judged at high risk of bias as losses to follow‐up had not been reported by group and no ITT analysis had been performed (Bahorun 2012).
Four of the seven trials looking at green tea reported loss to follow‐up (Nantz 2009; Janjua 2009; Shen 2010; Stendell‐Hollis 2010) and one trial was judged as low risk of bias as withdrawals and exclusions were clearly reported (Nantz 2009). Three studies were judged at high risk of bias as either losses to follow‐up were not reported and no ITT analysis had been performed (Maron 2003), or losses to follow‐up were unbalanced between groups and no ITT analysis was performed (Janjua 2009; Stendell‐Hollis 2010). The remaining three studies were judged as unclear as in two studies no information on loss to follow‐up was provided (Bogdanski 2012; Smith 2010) and in the third study no reasons were reported for loss to follow‐up but an ITT analysis was performed (Shen 2010).
Selective reporting
Three of the four studies looking at black tea were judged as unclear as there was insufficient information to judge the risk of selective reporting (Fujita 2008; Hodgson 2012; Mukamal 2007). The remaining study was judged as low risk as all expected outcomes were reported (Bahorun 2012).
One study examining green tea was judged as unclear due to there being insufficient information to judge the risk of selective reporting (Nantz 2009). The remaining six trials have been judged as low risk as all expected outcomes were reported (Bogdanski 2012; Janjua 2009; Maron 2003; Shen 2010; Smith 2010; Stendell‐Hollis 2010).
Other potential sources of bias
For studies of both black and green tea, there was insufficient information to judge the risk of bias from other potential sources.
Effects of interventions
Cardiovascular events
None of the included studies provided clinical event data.
Mortality
None of the included studies provided mortality data.
Cardiovascular risk factors
Black tea
Three of the four included trials examining the effects of black tea measured lipid levels (Bahorun 2012; Fujita 2008; Mukamal 2007) and contribute to the meta‐analysis. For one study (Bahorun 2012), the reported results for all lipid measurements were split by gender.
For LDL‐cholesterol (three studies, one study reporting males and females separately, 147 participants) moderate heterogeneity was observed between the studies (I2 = 33%) so a random‐effects meta‐analysis was performed. From the pooled analysis, black tea was found to lower LDL‐cholesterol (mean difference (MD) ‐0.43 mmol/L, 95% confidence interval (CI) ‐0.56 to ‐0.31) (Analysis 1.1). Results were similar for the fixed‐effect model, the random‐effects results were reported as the effect estimate is more conservative with wider confidence intervals. Sensitivity analysis, removing studies with unclear allocation concealment, retained one study and statistical significance (MD ‐0.39 mmol/L, 95% CI ‐0.54 to ‐0.24).
A random‐effects meta‐analysis was also conducted for HDL‐cholesterol (three studies, one reporting males and females separately, 146 participants) where heterogeneity was again observed between studies (I2 = 36%). We found no evidence of effect of black tea on HDL levels in the four trials reporting this (Analysis 1.2).
For triglyceride levels, significant heterogeneity existed between the trials (three studies, one reporting males and females separately, I2 = 64%) and a meta‐analysis was not performed (Analysis 1.3). In one trial, black tea was found to significantly reduce triglyceride levels (MD ‐0.17 mmol/L. 95% CI ‐0.30 to ‐0.04) (Fujita 2008) whilst for female participants in another trial, triglycerides were found to increase in those given black tea (MD 0.55 mmol/L, 95% CI ‐0.01 to 1.11) but this was not statistically significant (Bahorun 2012). For their male counterparts, black tea was found to have no effect on triglyceride levels (MD ‐0.32 mmol/L, 95% CI ‐1.06 to 0.42) (Bahorun 2012), a finding supported by the final study (MD 0.03 mmol/L, 95% CI ‐0.17 to 0.23) (Mukamal 2007) (and the only study with low risk of bias from allocation concealment).
Two trials (one reporting males and females separately) of black tea measured total cholesterol levels (Bahorun 2012; Fujita 2008) (Analysis 1.4). However, significant heterogeneity was found to exist between the trials (I2 = 84%) and therefore a meta‐analysis was not performed. One trial showed a statistically significant reduction in total cholesterol (MD ‐0.54 mmol/L, 95% CI ‐0.63 to ‐0.45) (Fujita 2008) whilst in the second trial black tea was found to have no effect on total cholesterol in females (MD 0.41 mmol/L, 95% CI ‐0.19 to 1.01) or males (MD 0.00 mmol/L, 95% CI ‐0.59 to 0.59) (Bahorun 2012).
Two included trials examined the effect of black tea on blood pressure (Hodgson 2012; Mukamal 2007). The meta‐analysis (123 participants) showed a statistically significant reduction in systolic blood pressure (SBP) (MD ‐1.85 mmHg, 95% CI ‐3.21 to ‐0.48) (Analysis 1.5). Diastolic blood pressure (DBP) (123 participants) was also reduced with the black tea intervention, however, this result did not reach statistical significance (MD ‐1.27 mmHg, 95% CI ‐3.06 to 0.53) (Analysis 1.6). Sensitivity analysis removing studies at unclear risk of bias from allocation concealment did not remove either study, so results were unchanged.
Green tea
Four of the seven included trials examined the effects of green tea on lipid levels (Bogdanski 2012; Maron 2003; Smith 2010; Stendell‐Hollis 2010) and so contributed to the meta‐analysis. From the pooled analysis, the green tea intervention showed a statistically significant reduction in total cholesterol (327 participants) (MD ‐0.62 mmol/L, 95% CI ‐0.77 to ‐0.46) (Analysis 2.1) and LDL‐cholesterol (327 participants) (MD ‐0.64 mmol/L, 95% CI ‐0.77 to ‐0.52) (Analysis 2.2) compared to placebo. For triglycerides (327 participants) the pooled analysis found green tea to lower triglyceride levels (MD ‐0.08 mmol/L, 95% CI ‐0.24 to 0.07) (Analysis 2.3), however, this result did not reach statistical significance.
For HDL cholesterol (four studies, 327 participants), moderate heterogeneity was found between studies (I2= 39%) so a random‐effects meta‐analysis was performed (Analysis 2.4). From the pooled analysis, there was no evidence of effect of green tea on HDL cholesterol levels (MD 0.01 mmol/L, 95% CI ‐0.08 to 0.11). Results were similar for the fixed‐effect model; the random‐effects results were reported as the effect estimate is more conservative with wider confidence intervals. Sensitivity analysis, removing studies with unclear allocation concealment, retained one study (MD ‐0.10 mmol/L, 95% CI ‐0.27 to 0.07).
Three trials examining green tea measured blood pressure (Bogdanski 2012; Nantz 2009; Smith 2010) but one did not provide any individual group data and could not be included in a meta‐analysis. The pooled analysis showed a statistically significant reduction in SBP (167 participants) (MD ‐3.18 mmHg, 95% CI ‐5.25 to ‐1.11) (Analysis 2.5) and DBP (167 participants) (MD ‐3.42, 95% CI ‐4.54 to ‐2.30) (Analysis 2.6) in the green tea group. The trial not included in the meta‐analysis showed no significant change in blood pressure in either the tea (SBP change 1.10%, DBP change ‐7.20%) or control group (SBP decrease 0%, DBP decrease 1.10%) (Smith 2010). For all three trials allocation concealment was unclear, and sensitivity analyses removed them from the pooled analysis.
All tea (Green and Black)
Six trials (four using green tea and two using black tea, one reporting males and females separately) measured total cholesterol levels (Bahorun 2012; Bogdanski 2012; Fujita 2008; Maron 2003; Smith 2010; Stendell‐Hollis 2010). Significant heterogeneity (I2 = 66%) was found between the trials and so a meta‐analysis was not performed. Two of the four trials showed tea to significantly reduce total cholesterol (Fujita 2008; Maron 2003) while in two trials, tea was found to reduce total cholesterol but this result did not reach statistical significance (Bogdanski 2012; Smith 2010). In the remaining two trials, tea was found to have no effect on total cholesterol levels (Bahorun 2012; Stendell‐Hollis 2010).
Seven trials (one examining males and females separately) looked at HDL‐cholesterol (473 participants), LDL‐cholesterol (474 participants) and triglyceride levels (476 participants) (three using black tea and four using green tea) and contributed to the meta‐analysis (Bahorun 2012; Bogdanski 2012; Fujita 2008; Maron 2003; Mukamal 2007; Smith 2010; Stendell‐Hollis 2010). For LDL cholesterol (I2= 49%), HDL‐cholesterol (I2 = 33%) and triglycerides (I2 = 37%), moderate heterogeneity was observed between the studies so random‐effects meta‐analyses were performed. The pooled analysis showed tea to significantly reduce LDL‐cholesterol (MD ‐0.48 mmol/L, 95% CI ‐0.61 to ‐0.35) (Analysis 3.2), have no effect on HDL‐cholesterol (MD 0.00 mmol/L, 95% CI ‐0.04 to 0.04) (Analysis 3.3) and decrease triglycerides (MD ‐0.06 mmol/L, 95% CI ‐0.19 to 0.06) (Analysis 3.4), although this did not reach statistical significance.
Five trials (three using green tea and two using black tea) measured blood pressure (Bogdanski 2012; Hodgson 2012; Mukamal 2007, Nantz 2009:Smith 2010). Only four of these contributed to the meta‐analysis as one trial did not report individual group data (Smith 2010). The meta‐analysis showed a statistically significant reduction in SBP (290 participants) (MD ‐2.25 mmHg, 95% CI ‐3.39 to ‐1.11) (Analysis 3.5) and DBP (290 participants) (MD ‐2.81 mmHg, 95% CI ‐3.77 to ‐1.86) (Analysis 3.6) with the tea intervention.
Adverse effects
Adverse effects were monitored in five trials. Generally, side effects were mild and not attributable to the tea interventions as there were no significant differences in adverse events between the treatment and placebo groups (Janjua 2009; Maron 2003; Nantz 2009; Shen 2010). However, in one study (Mukamal 2007), adverse events included a new diagnosis of prostate cancer and a single hospitalisation for influenza among participants assigned to tea and in another study adverse events in the tea group included appendicitis and retinal detachment (Janjua 2009).
Quality of Life
Quality of life was measured in one of the trials (Shen 2010). Supplementation of 500 mg green tea polyphenols (GTP) daily to postmenopausal osteopenic women for 24 weeks had no influence on quality of life (as assessed by SF‐36 questionnaires).
Costs
None of the included studies provided data on costs.
Discussion
Summary of main results
Eleven trials that randomised 821 participants in studies of three or more months duration were identified from the 1735 papers screened. Four of these examined black tea interventions and seven examined green tea interventions.
None of the trials measured clinical events or mortality as they were relatively short term and conducted in mainly healthy participants. The review showed that black tea produced a statistically significant reduction in LDL‐cholesterol and systolic and diastolic blood pressure (stable to sensitivity analyses). For green tea statistically significant reductions were found in total cholesterol, LDL cholesterol and systolic and diastolic blood pressure but the studies contributing to these analyses were removed in sensitivity analyses. However, only a small number of trials contributed to these analyses, and most trials were very small. Only one trial looked at the effects of tea on health‐related quality of life. Few adverse events were measured and none directly attributable to the intervention.
Overall completeness and applicability of evidence
This review included adult participants who were at varying levels of CVD risk and included both free‐living men and women. Most of the trials were conducted in developed countries. None of the 11 included studies examined our primary outcomes as trials were relatively short term in follow‐up and participants were predominantly healthy. We were also not able to examine the effects of “dose” or duration of the intervention, or the effects of caffeine intake due to the limited number of included trials. Furthermore, due to the varying doses of extracts/tea consumed between the included studies, we could not draw any conclusions about the number of cups of tea per day that would be required to reduce CVD risk factors.
The effectiveness of green tea could not be rigorously assessed as only four trials (447 participants) (Bogdanski 2012; Maron 2003; Nantz 2009; Smith 2010) examined cardiovascular risk factors at three months and one study at six months (39 participants) (Stendell‐Hollis 2010). The remaining two trials examining green tea evaluated quality of life and adverse events over six months (Shen 2010) or adverse events over two years (Janjua 2009). Similarly, few trials were identified that examined the effectiveness of black tea. Four trials were found that measured cardiovascular risk factors (279 participants). Two of these had three months follow‐up (Bahorun 2012; Fujita 2008) and two had six months follow‐up (Hodgson 2012; Mukamal 2007).
One study examining black tea stratified lipid outcomes by gender (Bahorun 2012) and found differences in responses between men and women. Whilst there is well‐established literature on differences in cardio‐metabolic risk profiles between men and women (Mosca 2011), we cannot make any conclusions based on only one study. However, if sufficient evidence accrues, future updates of this review will examine the influence of gender on the outcomes of interest.
There was considerable variability in the interventions, participants recruited and outcomes measured in the included trials. The one ongoing trial will add to the evidence base but more trials are needed.
Quality of the evidence
The studies included in this review were at some risk of bias and as such, the results should be treated with caution. In five of the included trials the methods of random sequence generation were not stated or unclear while in seven trials the details of allocation concealment were not stated. Nine of the 11 included studies stated that they were double blind and in one trial, participants were not blinded to treatment allocation but outcome assessors were. Risk of bias related to incomplete outcome data was high in four studies, low in four studies and unclear in three studies. Bias due to selective outcome reporting was considered unclear in four studies and low in the remaining seven. For all studies there was insufficient information to judge the risk of other biases.
In addition, small study bias is a risk in this review as most trials were very small. We were unable to examine the effects of publication bias in funnel plots due to the limited number of included studies. However, small studies are often less methodologically robust, more likely to be conducted in selected populations and have been shown to report larger beneficial effects than larger trials (Nüesch 2010; Sterne 2000; Sterne 2001). The results of the review need to be interpreted with this in mind.
Potential biases in the review process
A comprehensive search was conducted across major databases for interventions involving black or green tea or tea extract. Systematic review reference lists were also screened and authors contacted when necessary. All screening, inclusion and exclusion and data abstraction were carried out independently by two review authors. Data entry and analysis were also conducted by two review authors.
Our decision to restrict this review to interventions only investigating black or green tea avoided the potential confounding effects of other behavioural interventions on our outcomes e.g. those involving exercise, different dietary interventions or interventions that focused on weight loss. However, this limited the number of trials eligible for inclusion. In addition, the small number of trials on which this review is based, limitations in reporting methodological quality, an unclear risk of bias in most trials and little or no data for primary or secondary outcomes mean that caution should be used when interpreting the results of this review.
Agreements and disagreements with other studies or reviews
To our knowledge, no other systematic review including only randomised controlled trials has been carried out solely to examine the effects of black and green tea in adults for the primary prevention of CVD. Other systematic reviews have looked at flavonoid consumption on cardiovascular risk, which include tea, but also other flavonoid‐rich foods (Hooper 2008). As in our review, the review by Hooper et al (2008) (Hooper 2008) found no studies examining tea that assessed mortality or cardiovascular events.
Other systematic reviews have solely concentrated on the antioxidant effects of green tea consumption (Ellinger 2011) with findings providing some evidence that regular intake of green tea (at least 0.6 to 1.5L/day) reduced lipid peroxidation and increased antioxidant capacity. However, we cannot directly compare the effects on CVD risk factors between the two reviews as Ellinger et al (2011) did not examine CVD risk factors and included very short‐term studies which did not meet our inclusion criteria.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
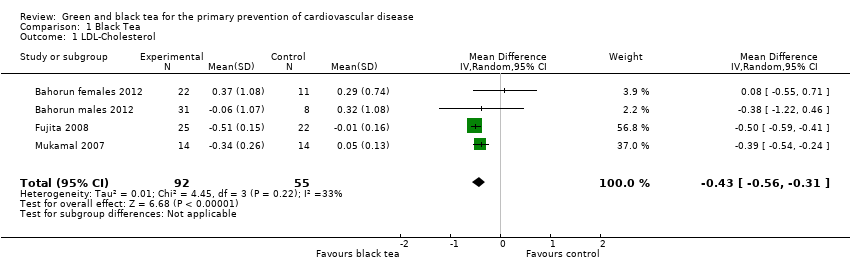
Comparison 1 Black Tea, Outcome 1 LDL‐Cholesterol.
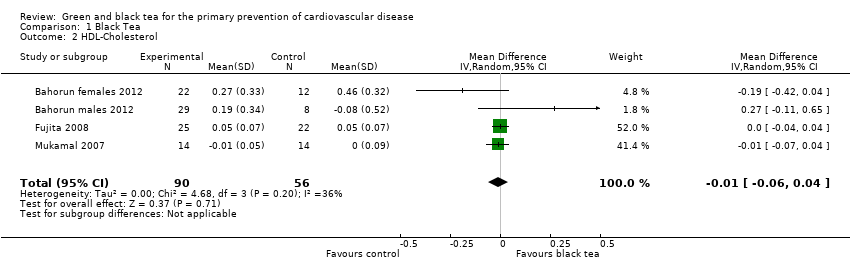
Comparison 1 Black Tea, Outcome 2 HDL‐Cholesterol.

Comparison 1 Black Tea, Outcome 3 Triglycerides.

Comparison 1 Black Tea, Outcome 4 Total Cholesterol.

Comparison 1 Black Tea, Outcome 5 Systolic blood pressure.
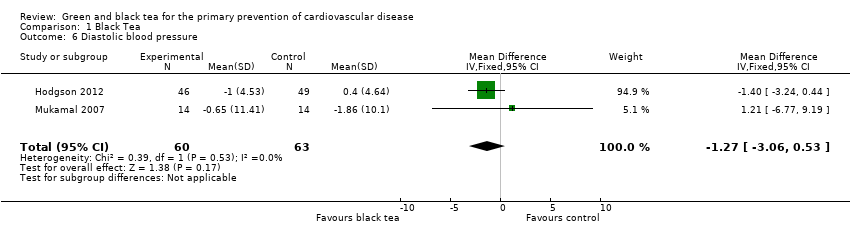
Comparison 1 Black Tea, Outcome 6 Diastolic blood pressure.

Comparison 2 Green Tea, Outcome 1 Total Cholesterol.

Comparison 2 Green Tea, Outcome 2 LDL Cholesterol.

Comparison 2 Green Tea, Outcome 3 Triglycerides.
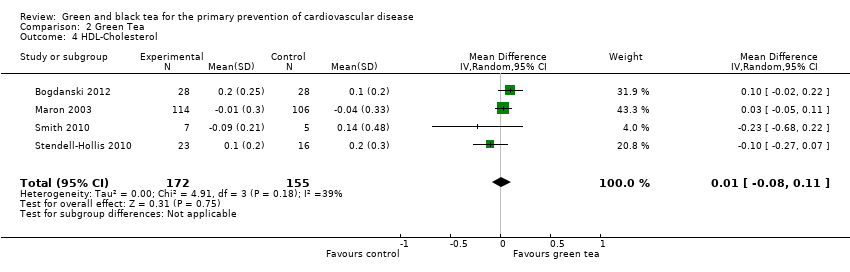
Comparison 2 Green Tea, Outcome 4 HDL‐Cholesterol.
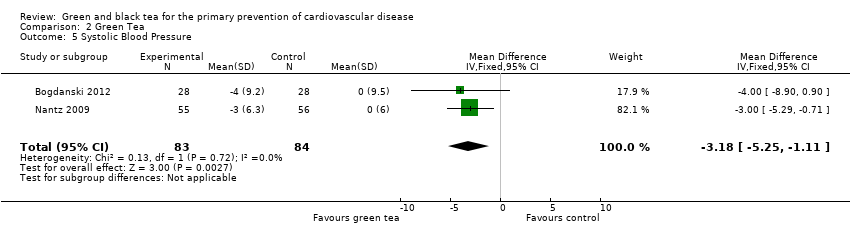
Comparison 2 Green Tea, Outcome 5 Systolic Blood Pressure.
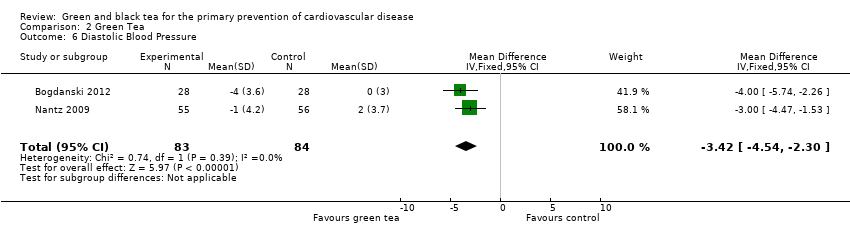
Comparison 2 Green Tea, Outcome 6 Diastolic Blood Pressure.
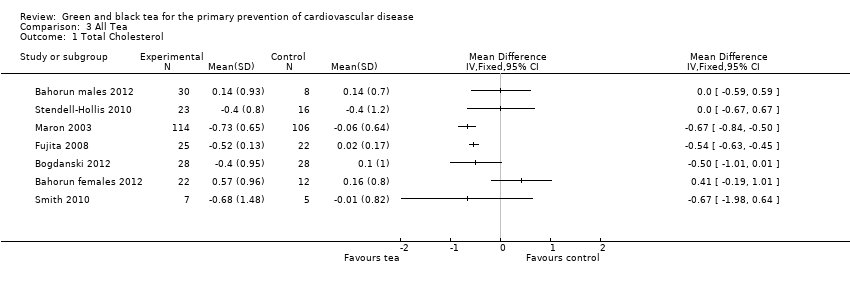
Comparison 3 All Tea, Outcome 1 Total Cholesterol.
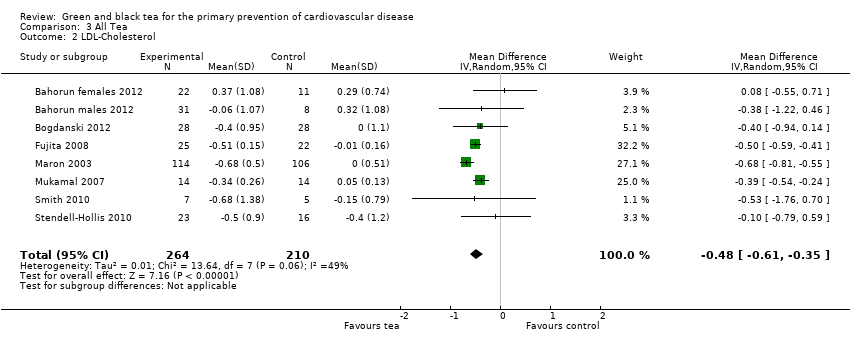
Comparison 3 All Tea, Outcome 2 LDL‐Cholesterol.
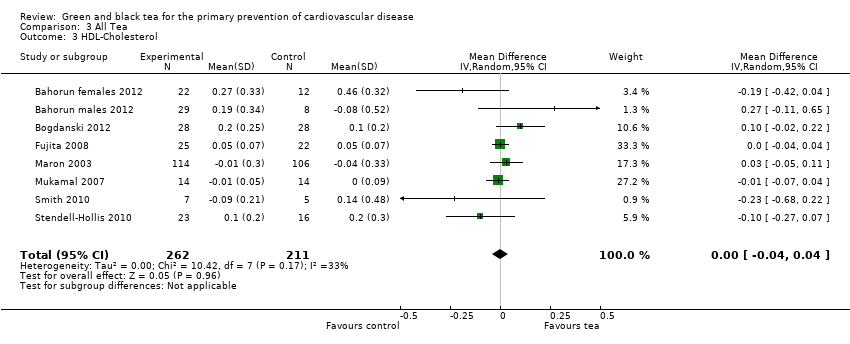
Comparison 3 All Tea, Outcome 3 HDL‐Cholesterol.
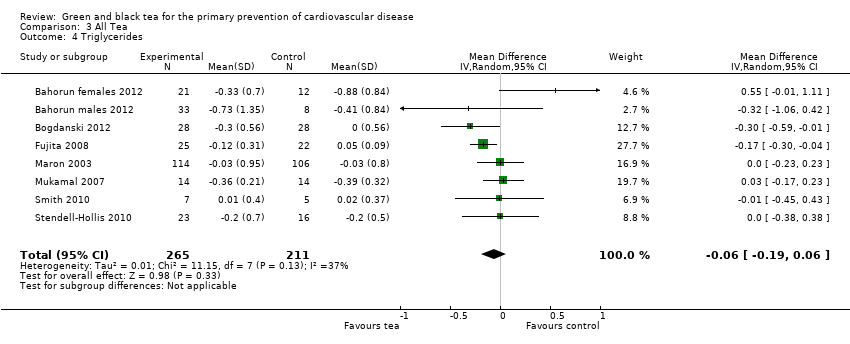
Comparison 3 All Tea, Outcome 4 Triglycerides.
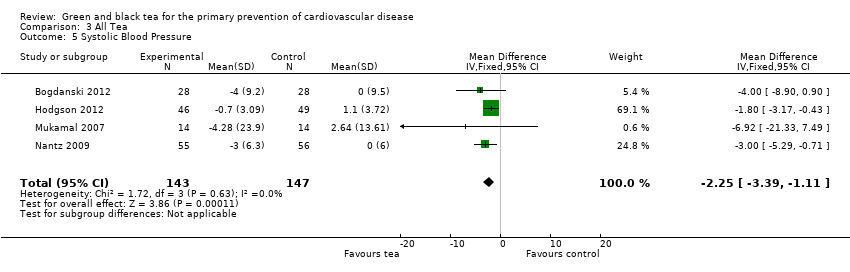
Comparison 3 All Tea, Outcome 5 Systolic Blood Pressure.

Comparison 3 All Tea, Outcome 6 Diastolic Blood Pressure.
| Study | Green/Black or extracts? | Dose | Duration |
| Black and Green tea | 6 g/d | 2 wks | |
| Green tea and Green tea extract | 4 cups/d or 2 capsules and 4 cups of water /d | 8 wks | |
| Green tea and Green tea extract | 4 cups/d or 2 capsules and 4 cups of water /d | 8 wks | |
| Green tea extract | 250 mg/d | 8 wks | |
| Green tea extract | 500 mg | 4 hrs | |
| Black tea | 6 mugs/d | 4 wks | |
| Green tea extract | 530 mg twice a day | 6 wks | |
| Black tea | 5 servings a day | 3 wks | |
| Black tea, Green tea and Green tea extract | 6 cups (150 mL)/day or 6 x 4 capsules/day with 6 x 150 mL of control beverage | 4 wks | |
| Green tea extract | In a beverage consumed once a day | 21 days | |
| Black tea | 5 servings (200 mL)/d | 6 wks | |
| Green tea extract | 6 capsules/d | 3 wks | |
| Green tea extract | 3 g a day | 4 wks | |
| Black tea | 0mg, 100 mg, 200 mg, 400 mg or 800 mg twice a day | 1 wk | |
| Black tea | 450 mL | 2hrs | |
| Black tea | 1250 mL/d | 4 wks | |
| Black or Green tea | 5 cups/d | 7 d | |
| Black tea | 5 cups/d | 4 wks | |
| Green tea extract | 500 mg | 4 wks | |
| Black tea | 5 cups/d (750 mL) | 4 wks | |
| Black tea | > 200 mL twice a day | 8 wks | |
| Green tea | 400ml | 2 hrs | |
| Green tea or Green tea extract | 4 cups a day or 2 capsules and 4 cups of water a day | 8 wks | |
| Black or Green tea | 6 cups/d of Black or Green tea or 3,6g tablet of Green tea polyphenols/day | 4 wks | |
| Black tea | 400 mL | 60 mins | |
| Black tea | 300 mL | 105 mins | |
| Black tea | 5 cups per day | 2 weeks | |
| Black tea | 1 litre | 7 hours | |
| Black tea extract | one capsule/d | 11 wks | |
| Black or Green tea | 6 gm | 3 hrs | |
| Black and Green tea | 1000 mL/d or 250 mL/d | 7 d or 4 wks | |
| Black tea | one cup | 4 hrs | |
| Green tea extract | 1.06 g | 90 mins | |
| Green tea | 2 cups | 42 days | |
| Green tea extract | 400 mg or 800 mg per day | 2 mths | |
| Green tea | 6 g | 120 mins | |
| Black tea | 1 cup | 6 hours | |
| Tea | 10, 224 or 674 mg of tea catechins | 6 hours | |
| Tea | 1069 mg/day of total catechins | 1 wk | |
| Black tea | 4 sachets a day | 6 wks | |
| d:day | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 LDL‐Cholesterol Show forest plot | 4 | 147 | Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.56, ‐0.31] |
| 2 HDL‐Cholesterol Show forest plot | 4 | 146 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.06, 0.04] |
| 3 Triglycerides Show forest plot | 4 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Total Cholesterol Show forest plot | 3 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Systolic blood pressure Show forest plot | 2 | 123 | Mean Difference (IV, Fixed, 95% CI) | ‐1.85 [‐3.21, ‐0.48] |
| 6 Diastolic blood pressure Show forest plot | 2 | 123 | Mean Difference (IV, Fixed, 95% CI) | ‐1.27 [‐3.06, 0.53] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total Cholesterol Show forest plot | 4 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐0.62 [‐0.77, ‐0.46] |
| 2 LDL Cholesterol Show forest plot | 4 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐0.77, ‐0.52] |
| 3 Triglycerides Show forest plot | 4 | 327 | Mean Difference (IV, Fixed, 95% CI) | ‐0.08 [‐0.24, 0.07] |
| 4 HDL‐Cholesterol Show forest plot | 4 | 327 | Mean Difference (IV, Random, 95% CI) | 0.01 [‐0.08, 0.11] |
| 5 Systolic Blood Pressure Show forest plot | 2 | 167 | Mean Difference (IV, Fixed, 95% CI) | ‐3.18 [‐5.25, ‐1.11] |
| 6 Diastolic Blood Pressure Show forest plot | 2 | 167 | Mean Difference (IV, Fixed, 95% CI) | ‐3.42 [‐4.54, ‐2.30] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Total Cholesterol Show forest plot | 7 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2 LDL‐Cholesterol Show forest plot | 8 | 474 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐0.61, ‐0.35] |
| 3 HDL‐Cholesterol Show forest plot | 8 | 473 | Mean Difference (IV, Random, 95% CI) | 0.00 [‐0.04, 0.04] |
| 4 Triglycerides Show forest plot | 8 | 476 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.19, 0.06] |
| 5 Systolic Blood Pressure Show forest plot | 4 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐2.25 [‐3.39, ‐1.11] |
| 6 Diastolic Blood Pressure Show forest plot | 4 | 290 | Mean Difference (IV, Fixed, 95% CI) | ‐2.81 [‐3.77, ‐1.86] |

