Lavado ótico (limpieza de oído) para la otitis media supurativa crónica
Resumen
Antecedentes
La otitis media supurativa crónica (OMSC), a veces denominada otitis media crónica (OMC), es una inflamación crónica y a menudo una infección polimicrobiana (que involucra más de un microorganismo) del oído medio y la cavidad mastoidea, caracterizada por la secreción del oído (otorrea) a través de una membrana timpánica perforada. Los síntomas predominantes de la OMSC son la secreción del oído y la pérdida de audición.
El lavado ótico es un término que describe una serie de procesos para la limpieza manual del oído. Las técnicas utilizadas pueden incluir la limpieza en seco (con algodón o pañuelo de papel), la eliminación mediante aspiración (típicamente bajo el microscopio) o la irrigación (utilizando jeringas manuales o automatizadas). La limpieza en seco puede ser eficaz para eliminar la secreción mucopurulenta. Comparada con la irrigación o la microaspiración, es menos eficaz para eliminar los restos epiteliales o el pus espeso. El lavado ótico se pueden utilizar solo o además de otros tratamientos para la OMSC, como los antibióticos o antisépticos tópicos.
Objetivos
Evaluar los efectos de los procedimientos de lavado ótico en personas con OMSC.
Métodos de búsqueda
El documentalista del Grupo Cochrane de Enfermedades de oído, nariz y garganta (ENT) realizó búsquedas en el registro de ensayos de este grupo; el Registro Cochrane de Ensayos Controlados (a través del Registro de Estudios Cochrane [Cochrane Register of Studies]); Ovid MEDLINE; Ovid Embase; CINAHL; Web of Science; ClinicalTrials.gov; ICTRP y fuentes adicionales para obtener ensayos publicados y no publicados. La fecha de la búsqueda fue el 16 de marzo de 2020.
Criterios de selección
Se incluyeron ensayos controlados aleatorizados (ECA) con al menos una semana de seguimiento que incluían a personas (adultos y niños) con secreción ótica crónica de causa desconocida u OMSC, en los que la secreción del oído había continuado durante más de dos semanas.
Se incluyó como intervención cualquier método de lavado ótico, con cualquier frecuencia y duración. Las comparaciones fueron lavado ótico comparado con a) placebo o ninguna intervención y b) cualquier otro método de lavado ótico. Se analizaron los ensayos en los que los tratamientos de base se utilizaron en ambos grupos del estudio (por ejemplo, antisépticos o antibióticos tópicos) por separado.
Obtención y análisis de los datos
Se utilizaron los procedimientos metodológicos Cochrane estándar. Se utilizaron los criterios GRADE para evaluar la certeza de la evidencia de cada desenlace.
Los desenlaces principales fueron: resolución de la secreción del oído u «oído seco» (confirmada o no otoscópicamente), medida entre una y dos semanas, entre dos y cuatro semanas y después de cuatro semanas; calidad de vida relacionada con la salud mediante un instrumento validado; y dolor de oído (otalgia) o malestar o irritación local. Los desenlaces secundarios fueron la audición, las complicaciones graves y los episodios adversos de sangrado de los oídos y mareos/vértigo/problemas de equilibrio.
Resultados principales
Se incluyeron tres estudios con un total de 431 participantes (465 oídos) que informaron de dos comparaciones. Dos estudios, incluyeron solamente a niños con OMSC en la comunidad (351 participantes) y el otro estudio (80 participantes) incluyó a niños y adultos con secreción de oído crónica durante al menos seis semanas. Ninguno de los estudios incluidos informó de los desenlaces de calidad de vida relacionada con la salud, la otalgia ni los episodio adverso de sangrado del oído.
Lavado ótico diario (limpieza en seco) versus ningún tratamiento
Dos estudios (351 niños; 370 oídos) compararon la limpieza en seco diaria con ningún tratamiento. Ninguno de los dos presentaron resultados de resolución de la secreción entre una y dos semanas o entre dos y cuatro semanas. En cuanto a la resolución de la secreción del oído después de cuatro semanas, un estudio comunicó los resultados por persona. Existe muy poca seguridad acerca de si hay una diferencia a las 16 semanas (riesgo relativo [RR] 1,01; intervalo de confianza (IC) del 95% 0,60 a 1,72; un estudio; 217 participantes) porque la certeza de la evidencia es muy baja.
No se comunicaron resultados para los episodios adversos de mareos, vértigo o problemas de equilibrio. Solo un estudio comunicó complicaciones graves, pero no estuvo claro de qué grupo procedían esos pacientes ni si las complicaciones se produjeron antes o después del tratamiento. Un estudio informó sobre la audición, pero los resultados se presentaron por desenlace del tratamiento y no por grupo de tratamiento, por lo que no es posible determinar si hay una diferencia entre los dos grupos.
Lavado ótico diario versus un único lavado ótico además de ciprofloxacino tópico
Un estudio (80 participantes; 95 oídos) comparó el lavado ótico diario (aspiración) con la administración de gotas de antibiótico tópico (ciprofloxacino) en un centro sanitario, con un único episodio de lavado ótico (succión) seguido de gotas de antibiótico tópico autoadministradas diariamente, en participantes de todas las edades. No se sabe si hay una diferencia en la resolución de la secreción tras entre una y dos semanas (RR 1,09; IC del 95%: 0,91 a 1,30; un estudio; 80 participantes) porque la certeza de la evidencia es muy baja. No se comunicaron resultados de resolución de la secreción del oído tras entre dos y cuatro semanas. Los resultados de la resolución de la secreción del oído después de cuatro semanas se presentaron por oído, no por persona, y no pudieron ajustarse por persona. Un paciente del grupo con lavado ótico único y autoadministración de gotas antibióticas tópicas informó del episodio adverso de mareos, que los autores atribuyeron al uso de ciprofloxacino tópico frío. Existe mucha incertidumbre acerca de si hay una diferencia entre los grupos (RR 0,33; IC del 95%: 0,01 a 7,95; un estudio; 80 participantes, certeza muy baja). No se comunicaron resultados para los otros episodios adversos de vértigo o problemas de equilibrio, ni para complicaciones graves. Los autores solo informaron de manera cualitativa de que no hubo diferencias entre los dos grupos en cuanto a los resultados de audición (certeza muy baja).
Conclusiones de los autores
Existe mucha incertidumbre acerca de si el tratamiento de lavado ótico es eficaz para resolver la secreción del oído en personas con OMSC, debido a la falta de datos y a la mala calidad de la evidencia disponible. Tampoco se tiene certeza de los otros desenlaces, incluidos los episodios adversos, ya que no se comunicaron de forma adecuada. Del mismo modo, existe mucha incertidumbre de si la eliminación diaria por aspiración, seguida de gotas antibióticas para los oídos administradas en un centro sanitario, es mejor que una sola sesión de eliminación por aspiración seguida de la autoadministración de gotas antibióticas tópicas para los oídos.
PICO
Resumen en términos sencillos
Beneficios y riesgos de la limpieza del oído para personas con otitis media supurativa crónica (infección de oído persistente o recurrente con secreción de pus)
¿Por qué es esto importante?
La otitis media supurativa crónica (OMSC), también llamada otitis media crónica (OMC), es una inflamación e infección del oído medio que dura dos semanas o más. Las personas con OMSC suelen experimentar una secreción recurrente o persistente del oído (pus que se filtra por un agujero en el tímpano) y pérdida de audición.
Se pueden utilizar diferentes enfoques para limpiar el oído afectado y eliminar las secreciones. Estas medidas incluyen:
‐ usar algodón o pañuelo de papel (limpieza en seco);
‐ aspirar el material que bloquea el oído con un pequeño dispositivo (normalmente se hace bajo el microscopio); o
‐ lavar el oído (irrigación).
Para determinar la efectividad de la limpieza de oídos en personas con OMSC, y si causa efectos no deseados, se revisó la evidencia de los estudios de investigación. En concreto, se quiso saber si la limpieza de los oídos detenía la secreción del oído, y si afectaba a la calidad de vida relacionada con la salud, o a la audición. También se quería saber si causaba dolor, molestias o irritación en el oído, efectos indeseables como mareos o sangrado de oído o alguna complicación grave.
Cómo se identificó y evaluó la evidencia
Primero, se buscaron todos los estudios relevantes en la literatura médica. Luego se compararon los resultados y se resumió la evidencia de todos los estudios. Finalmente se evaluó la certeza de la evidencia. Se consideraron factores como la forma en que se realizaron los estudios, el tamaño de los mismos y la consistencia de los hallazgos entre los estudios. Según las evaluaciones, la evidencia se calificó como de certeza muy baja, baja, moderada o alta.
Datos encontrados
Se encontraron tres estudios con más de 431 personas con OMSC. Las personas tuvieron un seguimiento de entre seis semanas y seis meses después del tratamiento.
Los estudios compararon:
‐ limpieza en seco diario con ningún tratamiento (dos estudios, 351 personas);
‐ aspiración diaria combinada con gotas antibióticas para los oídos administradas en una centro sanitario, frente a una única aspiración (en un centro sanitario) seguida de gotas antibióticas para los oídos autoadministradas diariamente (un estudio, 80 personas).
Limpieza diaria en seco comparada con ningún tratamiento
‐ No se sabe si la limpieza en seco detiene la secreción del oído, porque la evidencia sobre si las personas experimentan secreciones después de cuatro semanas fue de muy baja certeza, y ningún estudio examinó la presencia de secreción antes.
‐ Un estudio (50 participantes) comunicó complicaciones graves, pero no estuvo claro si las personas que comunicaron complicaciones se habían limpiado los oídos en seco o no, ni si las complicaciones se produjeron antes o después del tratamiento. Por lo tanto, no fue posible saber si la limpieza en seco causaba complicaciones graves, ni con qué frecuencia se produjeron.
‐ Un estudio examinó la audición, pero no informó de los resultados de manera que se supiera si la limpieza en seco afecta a la audición o no.
‐ Ningún estudio investigó el efecto de la limpieza en seco sobre la calidad de vida relacionada con la salud, el dolor de oído, el mareo o sobre el sangrado del oído.
Aspiración diaria comparada con una sola aspiración, además de gotas antibióticas para los oídos
‐ No se sabe si la aspiración detiene la secreción del oído, porque la evidencia de entre una y dos semanas después del tratamiento fue de muy baja certeza, y los resultados de secreción después de cuatro semanas no pudieron interpretarse.
‐ No se sabe si la aspiración afecta a la audición o al mareo, ya que la evidencia fue de certeza muy baja.
‐ Ningún estudio investigó el efecto de la aspiración sobre la calidad de vida relacionada con la salud, el dolor de oído, las complicaciones graves o sobre el sangrado del oído.
¿Qué significa esto?
No se sabe cómo de efectiva es la limpieza de oídos en personas con OMSC, ni si causa efectos no deseados. Existen muy pocos estudios en esta área y, los que existen proporcionan evidencia de certeza muy baja. Los efectos no deseados no estaban bien informados en los estudios encontrados. Es necesario que los investigadores lleven a cabo futuros estudios que comparen la limpieza del oído a la ausencia de limpieza y que comparen diferentes técnicas y frecuencias de limpieza para poder evaluar los riesgos y beneficios de la limpieza de oído para personas con OMSC.
¿Cuál es el grado de actualización de esta revisión?
La evidencia de esta revisión Cochrane está actualizada hasta marzo de 2020.
Authors' conclusions
Summary of findings
| Aural toileting compared to no aural toileting for chronic suppurative otitis media | |||||||
| Patient or population: children with chronic suppurative otitis media | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without aural toileting | With aural toileting | Difference | |||||
| Resolution of ear discharge ‐ 1 to 2 weeks | No study reported this outcome at this time point. | ||||||
| Resolution of ear discharge ‐ 4 weeks or more Assessed by: otoscopically confirmed Follow‐up: 16 weeks | RR 1.01 (0.60 to 1.72) | 217 | Study population | ⊕⊝⊝⊝ | We are uncertain about the effect of aural toileting on resolution of ear discharge (at 4 weeks or more) compared with no treatment. | ||
| 22.2% | 22.4% | 0.2% more | |||||
| Health‐related quality of life | No study reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | ||||||
| Hearing | Hearing was measured in one study but the results were presented by treatment outcome rather than by treatment group, so it is not possible to determine whether there is a difference between the two groups. | ||||||
| Serious complications | — | 48 (1 RCT) | One study reported one case of mastoiditis and one case of meningitis with focal encephalitis. It is not clear which group these patients were from (the study was a five‐arm trial of which only two arms are presented here), or whether the complications occurred pre‐ or post‐treatment. | ⊕⊝⊝⊝ | We are very uncertain about the effect of aural toileting on serious complications compared with no treatment. | ||
| Adverse events: dizziness/vertigo/balance problems | No study reported this outcome. | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because there was unclear allocation concealment, attrition bias and selective reporting bias. Downgraded by one level for indirectness (only children were included in the study). Downgraded by two levels for imprecision (as the results are based on one small study with wide confidence intervals). Downgraded by one level for suspected publication bias (this area has a known issue with trials not being published in peer‐reviewed journals). 2Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because it was at high risk of bias for randomisation and was at unclear risk of bias for allocation concealment, attrition bias and selective reporting bias. The study was unblinded. Downgraded by one level for indirectness (only children were included in the study). Downgraded by two levels for imprecision as it was not clear to which group the events could be attributed. Downgraded by one level for suspected publication bias (this area has a known issue with trials not being published in peer‐reviewed journals). | |||||||
| Daily aural toileting compared to single aural toileting episode for chronic suppurative otitis media | |||||||
| Patient or population: people (of any age) with otorrhoea for a duration of at least 6 weeks | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Single aural toileting | Daily aural toileting | Difference | |||||
| Resolution of ear discharge ‐ 1 to 2 weeks Assessed by: unknown ‐ unclear if otoscopically confirmed | RR 1.09, (0.91 to 1.30) | 80 (1 RCT) | Study population | ⊕⊝⊝⊝ | We are uncertain about the effect of daily aural toileting on resolution of ear discharge (at 1 to 2 weeks) compared with single episode of aural toileting. | ||
| 82.5% | 89.9% (75.1 to 100) | 7.4% more (7.4% fewer to 17.5% more) | |||||
| Resolution of ear discharge ‐ 4 weeks or more Assessed by: unknown ‐ unclear if otoscopically confirmed Follow‐up: 3 months | Kiris 1998 provided results for this outcome by ear, but the results could not be adjusted to provide results per person. | ||||||
| Health‐related quality of life | No study reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | ||||||
| Hearing | — | 80 (1 RCT) | Results were only reported qualitatively, the report stating that "there were no differences in pre‐ and post audiographic results or bone conduction in either group ..." | ⊕⊝⊝⊝ | We are uncertain about the effect of daily aural toileting on hearing compared with a single episode of aural toileting. | ||
| Serious complications | The study did not report that any participant died or had any intracranial or extracranial complications. | ||||||
| Adverse events: dizziness Assessed by: self reported Follow‐up: 15 days | RR 0.33, (0.01 to 7.95) | 80 (1 RCT) | Study population | ⊕⊝⊝⊝ | We are uncertain about the effect of daily aural toileting on dizziness compared with a single episode of aural toileting. | ||
| 2.5% | 0.8% (0% to 19.9%) | 1.7% less (2.5% fewer to 17.4% more) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low certainty: downgraded by two levels due to risk of bias (unclear randomisation, allocation concealment, unblinded trial and possible selective reporting). Downgraded by one level due to indirectness: the population is people with otorrhoea for more than six weeks so it is unclear if all included patients had CSOM. Downgraded by one level due to imprecision: the results are from one small study so the confidence intervals are wide. 2Downgraded to very low certainty: downgraded by two levels due to risk of bias (unclear randomisation, allocation concealment, unblinded trial and possible selective reporting). Downgraded by one level due to indirectness: the population is those with otorrhoea so it is unclear if all included patients had CSOM. Downgraded by two levels due to imprecision: the results are from one small study and only reported qualitatively. 3Downgraded to very low certainty: downgraded by two levels due to risk of bias (unclear randomisation, allocation concealment, unblinded trial and possible selective reporting). Downgraded by one level due to indirectness: the population is those with otorrhoea so it is unclear if all included patients had CSOM. Downgraded by two levels due to imprecision: the results are from one small study and only one event was reported resulting in very wide confidence intervals. | |||||||
Background
This is one of a suite of Cochrane Reviews evaluating the comparative effectiveness of non‐surgical interventions for CSOM using topical antibiotics, topical antibiotics with corticosteroids, systemic antibiotics, topical antiseptics and aural toileting (ear cleaning) methods (Table 1).
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
|---|---|---|---|---|---|
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2020).
CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a).
CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b).
CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018b).
CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2020a).
CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2020b).
CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018).
This review compares the effectiveness of aural toileting (ear cleaning) against other methods of aural toileting or placebo/no treatment for CSOM.
Description of the condition
Chronic suppurative otitis media (CSOM), which is also often referred to as chronic otitis media (COM), is a chronic inflammation and infection of the middle ear and mastoid cavity, characterised by ear discharge (otorrhoea) through a perforated tympanic membrane.
The predominant symptoms of CSOM are ear discharge and hearing loss. Ear discharge can be persistent or intermittent, and many sufferers find it socially embarrassing (Orji 2013). Some patients also experience discomfort or earache. Most patients with CSOM experience temporary or permanent hearing loss with average hearing levels typically between 10 and 40 decibels (Jensen 2013). The hearing loss can be disabling, and it can have an impact on speech and language skills, employment prospects, and on children’s psychosocial and cognitive development, including academic performance (Elemraid 2010; Olatoke 2008; WHO 2004). Consequently, quality of life can be affected. CSOM can also progress to serious complications in rare cases (and more often when cholesteatoma is present): both extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy) and intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) have been reported (Dubey 2007; Yorgancılar 2013).
CSOM is estimated to have a global incidence of 31 million episodes per year, or 4.8 new episodes per 1000 people (all ages), with 22% of cases affecting children under five years of age (Monasta 2012; Schilder 2016). The prevalence of CSOM varies widely between countries, but it disproportionately affects people at socio‐economic disadvantage. It is rare in high‐income countries, but common in many low‐ and middle‐income countries (Mahadevan 2012; Monasta 2012; Schilder 2016; WHO 2004).
Definition of disease
There is no universally accepted definition of CSOM. Some define CSOM in patients with a duration of otorrhoea of more than two weeks but others may consider this an insufficient duration, preferring a minimum duration of six weeks or more than three months (Verhoeff 2006). Some include diseases of the tympanic membrane within the definition of CSOM, such as tympanic perforation without a history of recent ear discharge, or the disease cholesteatoma (a growth of the squamous epithelium of the tympanic membrane).
In accordance with a consensus statement, here we use CSOM only to refer to tympanic membrane perforation, with intermittent or continuous ear discharge (Gates 2002). We have used a duration of otorrhoea of two weeks as an inclusion criterion, in accordance with the definition used by the World Health Organization, but we have used subgroup analyses to explore whether this is a factor that affects observed treatment effectiveness (WHO 2004).
Many people affected by CSOM do not have good access to modern primary healthcare, let alone specialised ear and hearing care, and in such settings, health workers may be unable to view the tympanic membrane to definitively diagnose CSOM. It can also be difficult to view the tympanic membrane when the ear discharge is profuse. Therefore, we have also included, as a subset for analysis, studies where participants have had chronic ear discharge for at least two weeks, but where the diagnosis is unknown.
At‐risk populations
Some populations are considered to be at high risk of CSOM. There is a high prevalence of disease among Indigenous people such as the Aboriginal and Torres Strait Islander Australian, Native American and Inuit populations. This is likely due to an interplay of factors, including socio‐economic deprivation and possibly differences resulting from population genetics (Bhutta 2016). Those with primary or secondary immunodeficiency are also susceptible to CSOM. Children with craniofacial malformation (including cleft palate) or chromosomal mutations such as Down syndrome are prone to chronic non‐suppurative otitis media ('glue ear'), and by extrapolation may also be at greater risk of suppurative otitis media. The reasons for this association with craniofacial malformation are not well understood, but may include altered function of the Eustachian tube, coexistent immunodeficiency, or both. These populations may be less responsive to treatment and more likely to develop CSOM, recurrence or complications.
Children who have a grommet (ventilation tube) in the tympanic membrane to treat glue ear or recurrent acute otitis media may be more prone to develop CSOM; however, their pathway to CSOM may differ and therefore they may respond differently to treatment. Children with grommets who have chronic ear discharge meeting the CSOM criteria are therefore considered to be a separate high‐risk subgroup (van der Veen 2006).
Treatment
Treatments for CSOM may include topical antibiotics (administered into the ear) with or without steroids, systemic antibiotics (given either by mouth or by injection), topical antiseptics and ear cleaning (aural toileting), all of which can be used on their own or in various combinations. Whereas primary healthcare workers or patients themselves can deliver some treatments (for example, some aural toileting and antiseptic washouts), in most countries antibiotic therapy requires prescription by a doctor. Surgical interventions are an option in cases where complications arise or in patients who have not responded to pharmacological treatment; however, there is a range of practice in terms of the type of surgical intervention that should be considered and the timing of the intervention. In addition, access to or availability of surgical interventions is setting‐dependent. This series of Cochrane Reviews therefore focuses on non‐surgical interventions. In addition, most clinicians consider cholesteatoma to be a variant of CSOM, but acknowledge that it will not respond to non‐surgical treatment (or will only respond temporarily) (Bhutta 2011). Therefore, studies in which more than half of the participants were identified as having cholesteatoma are not included in these reviews.
Description of the intervention
Aural toileting is an umbrella term used to describe the process of manually cleaning the ear. Techniques used may include dry mopping ('wicking', with cotton wool or tissue paper), suction clearance (typically under a microscope) or irrigation (using manual or automated syringing). Dry mopping may be effective in removing mucopurulent discharge, but less effective in removing epithelial debris or thick pus compared to irrigation or microsuction. Aural toileting can be used alone or in addition to other treatments for CSOM, such as antibiotics or topical antiseptics.
The technique and frequency of toileting may have an impact on its effectiveness. It is possible that dry mopping in the community is more effective because it can be delivered frequently, or it may be that less frequent suctioning by a specialist using a microscope is more effective because debris and pus are comprehensively removed. For these reasons, we considered the different aural toileting methods as separate subgroups and pooling only occurred if there was no evidence of a difference in effect.
How the intervention might work
In aural toileting the ear canal is manually cleaned to remove the pathogenic bacteria and inflammatory mediators that contribute to inflammation, which allows the tympanic membrane to be visualised for diagnosis and facilitates the delivery of topical interventions such as antibiotics or antiseptics to the target area to improve their effectiveness.
There have been reports of pain, bleeding and dizziness and/or vertigo with aural toileting (Addams‐Williams 2010; Gray 1988). With suction techniques there is also the potential for noise‐induced hearing loss, although no lasting effects have been observed (Addams‐Williams 2010).
Why it is important to do this review
Aural toileting is often used prior to other interventions such as topical antiseptics or antibiotics, but it is not known what role aural toileting alone plays in disease resolution or whether there are important differences in the effectiveness of different techniques. In addition, the use of aural toileting may influence clinical decisions regarding which other treatments to use (for example, systemic or topical treatment). Therefore the effectiveness of aural toilet as an adjunctive treatment is also an important question. Aural toileting is a potentially low‐cost treatment that is accessible in most settings; it is even possible to perform some forms of aural toileting such as dry mopping without medical support. The effectiveness of such interventions thus has implications for how and where treatment for CSOM is provided.
Objectives
To assess the effects of aural toilet procedures for people with chronic suppurative otitis media.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
-
Randomised controlled trials (including cluster‐randomised trials where the unit of randomisation is the setting or operator) and quasi‐randomised trials.
-
Patients were followed up for at least one week.
We excluded studies with the following design characteristics:
-
Cross‐over trials, because CSOM is not expected to be a stable chronic condition. Unless data from the first phase were available, we excluded such studies.
Types of participants
We included studies with patients (adults and children) who had:
-
chronic ear discharge of unknown cause; or
-
chronic suppurative otitis media.
We defined patients with chronic ear discharge as patients with at least two weeks of ear discharge, where the cause of the discharge was unknown.
We defined patients with chronic suppurative otitis media (CSOM) as patients with:
-
chronic or persistent ear discharge for at least two weeks; and
-
a perforated tympanic membrane.
We did not exclude any populations based on age, risk factors (cleft palate, Down syndrome), ethnicity (e.g. Australian Aboriginal or Torres Strait Islanders) or the presence of ventilation tubes (grommets). Where available, we recorded these factors in the patient characteristics section during data extraction from the studies. If any of the included studies recruited these patients as a majority (80% or more), we analysed them in a subgroup analysis (see Subgroup analysis and investigation of heterogeneity).
We excluded studies where the majority (more than 50%) of participants:
-
had an alternative diagnosis to CSOM (e.g. otitis externa);
-
had underlying cholesteatoma;
-
had ear surgery within the last six weeks.
We did not include studies designed to evaluate interventions in the immediate peri‐surgical period, which were focused on assessing the impact of the intervention on the surgical procedure or outcomes.
Types of interventions
Intervention
All aural toileting methods, frequencies and durations, including but not limited to the following:
-
Dry mopping ('wicking'): with cotton bud; Jobson‐Horne or other ear probe wrapped in cotton wool; or tissue spears (rolled up tissue papers).
-
Irrigation of the external auditory canal using a syringe or similar device. Different solutions (antiseptics versus normal water/saline) and types of irrigation instrument (e.g. manual syringe versus automated irrigation) have been described. Irrigation may be followed by dry mopping or vice versa.
-
Microsuction of the external auditory canal to remove discharge.
Comparisons
The following were the comparators:
-
Placebo, no treatment.
-
Another method of aural toileting.
There were three potential scenarios for analysis:
-
Aural toileting as a stand‐alone treatment: studies where all participants received no additional treatment or another form of aural toileting (e.g. a study comparing microsuction plus daily dry mopping versus daily dry mopping).
-
Aural toileting as an add‐on to antiseptics: this included studies where all participants also used a daily antiseptic, with or without any other form of aural toileting procedure different to the aural toileting procedure under investigation (for example, daily microsuction plus boric acid ear drops plus dry mopping versus daily boric acid ear drops plus dry mopping)
-
Aural toileting as an add‐on to topical/systemic antibiotics: studies where all participants received topical or systemic antibiotics, with or without another form of aural toileting or antiseptics which was a different type to the aural toileting procedure under investigation (for example, daily microsuction plus topical ciprofloxacin ear drops plus dry mopping versus daily topical ciprofloxacin plus dry mopping)
Many comparison pairs were possible in this review. The main comparisons of interest that we have summarised and presented in the 'Summary of findings' tables are:
-
aural toileting as a main (single) therapy versus placebo or no intervention;
-
aural toileting versus placebo or no intervention, where both arms also received topical antibiotics and/or systemic antibiotics as an add‐on therapy; and
-
aural toileting versus placebo or no intervention, where both arms also received topical antiseptics as an add‐on therapy.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
We extracted and reported data from the longest available follow‐up for all outcomes.
Primary outcomes
-
Resolution of ear discharge or 'dry ear' (whether otoscopically confirmed or not), measured at:
-
between one week and up to two weeks;
-
two weeks to up to four weeks; and
-
after four weeks.
-
-
Health‐related quality of life using a validated instrument for CSOM (e.g. Chronic Otitis Media Outcome Test (COMOT)‐12 (Phillips 2014a; Phillips 2014b; van Dinther 2015), Chronic Otitis Media Outcome Test (COMOT)‐15 (Baumann 2011), Chronic Ear Survey (CES) (Nadol 2000).
-
Ear pain (otalgia) or discomfort or local irritation.
Secondary outcomes
-
Hearing, measured as the pure‐tone average of air conduction thresholds across four frequencies tested (500 Hz, 1000 Hz, 2000 Hz and 4000 Hz) of the affected ear. If this was not available, we reported the pure‐tone average of the thresholds measured.
-
Serious complications, including intracranial complications (such as otitic meningitis, lateral sinus thrombosis and cerebellar abscess) and extracranial complications (such as mastoid abscess, postauricular fistula and facial palsy), and death.
-
Adverse events: dizziness/vertigo/balance problems.
-
Adverse events: ear bleeding.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 16 March 2020.
Electronic searches
The Information Specialist searched:
-
the Cochrane ENT Register (searched via the Cochrane Register of Studies to 16 March 2020);
-
the Cochrane Central Register of Controlled Trials (CENTRAL) (searched via the Cochrane Register of Studies Web to 16 March 2020);
-
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) (1946 to 16 March 2020);
-
Ovid EMBASE (1974 to 16 March 2020);
-
EBSCO CINAHL (1982 to 16 March 2020);
-
LILACS (Latin American and Caribbean Health Science Information database), lilacs.bvsalud.org (search to 16 March 2020);
-
Web of Knowledge, Web of Science (1945 to 16 March 2020);
-
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies to 16 March 2020);
-
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (search to 16 March 2020).
We also searched:
-
IndMed (search to 22 March 2018);
-
African Index Medicus (search to 22 March 2018).
The search strategies for major databases are detailed in Appendix 1. The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. The strategies were designed to identify all relevant studies for a suite of reviews on various interventions for chronic suppurative otitis media (Bhutta 2018; Brennan‐Jones 2020; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2020a; Head 2020b). A supplementary search of the major databases was performed for this review. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011).
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched Ovid MEDLINE to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials. The Information Specialist also ran non‐systematic searches of Google Scholar to retrieve grey literature and other sources of potential trials.
We did not perform a separate search for adverse effects. We considered adverse effects described in the included studies only.
We contacted original authors for clarification and further data if trial reports were unclear and we arranged translations of papers where necessary.
Data collection and analysis
Selection of studies
At least two review authors (KH/LYC) independently screened all titles and abstracts of the references obtained from the database searches to identify potentially relevant studies. At least two review authors (KH/LYC) evaluated the full text of each potentially relevant study to determine whether it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and methodological input where necessary.
Data extraction and management
At least two review authors (KH/LYC/CBJ/MB) independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved any differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the included studies, such as study design, setting (including location), year of study, sample size, age and sex of participants, and how outcomes were defined or collected in the studies. In addition, we have also collected baseline information on prognostic factors or effect modifiers (see Appendix 2). For this review, this included the following information whenever available:
-
duration of ear discharge at entry to the study;
-
diagnosis of ear discharge (where known);
-
number people who may have been at higher risk of CSOM, including those with cleft palate or Down syndrome;
-
ethnicity of participants including the number who were from Indigenous populations;
-
number who had previously had ventilation tubes (grommets) inserted (and, where known, the number who had tubes still in place);
-
number who had previous ear surgery;
-
number who had previous treatments for CSOM (non‐responders, recurrent versus new cases).
We recorded concurrent treatments alongside the details of the interventions used. See the 'Data extraction form' in Appendix 2 for more details.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis, i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
-
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from disease‐specific quality of life scales such as COMOT‐12, COMOT‐15 and CES as continuous data.
-
For binary data: the number of participants who experienced an event and the number of patients assessed at the time point.
-
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we converted it into binary data.
-
Time‐to‐event outcomes: we did not expect any outcomes to be measured as time‐to‐event data. However, if outcomes such as resolution of ear discharge were measured in this way, we reported the hazard ratios.
For resolution of ear discharge, we extracted the longest available data within the time frame of interest, defined as from one week up to (and including) two weeks (7 days to 14 days), from two weeks up to (and including) four weeks (15 to 28 days), and after four weeks (28 days or one month).
For other outcomes, we reported the results from the longest available follow‐up period.
Extracting data for pain/discomfort and adverse effects
For these outcomes, there were potential variations in how studies had reported them. For example, some studies may have reported both 'pain' and 'discomfort' separately whereas others may not. Prior to the commencement of data extraction, we agreed and specified a data extraction algorithm for how data should be extracted.
We extracted data for serious complications as a composite outcome. If a study reported more than one complication and we could not distinguish whether these occurred in one or more patients, we extracted the data with the highest incidence to prevent double counting.
Extracting data from figures
Where values for primary or secondary outcomes were shown as figures within the paper, we attempted to contact the study authors to try to obtain the raw values. When the raw values were not provided, we extracted information from the graphs using an online data extraction tool, using the best quality version of the relevant figures available.
Assessment of risk of bias in included studies
At least two review authors (KH/LYC/CBJ/MB) independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), using the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
-
sequence generation;
-
allocation concealment;
-
blinding of participants, personnel and outcome assessment;
-
incomplete outcome data;
-
selective reporting;
-
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with complete resolution of ear discharge) as risk ratios (RR) with confidence intervals (CIs). For the key outcomes that are presented in the 'Summary of findings' table, we expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also had planned to calculate the number needed to treat to benefit (NNTB) using the pooled results, where the results were moderate or high certainty. The assumed baseline risk would have been either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium‐risk population' or, alternatively, (b) the average risk of the control groups in the included studies, which is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we would have also presented additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
Had we had any continuous outcomes, we would have expressed treatment effects as a mean difference (MD) with standard deviation (SD). If different scales were used to measure the same outcome we would have used the standardised mean difference (SMD) and provided a clinical interpretation of the SMD values.
Unit of analysis issues
Cross‐over studies
This review did not use data from phase II of cross‐over studies.
The ear as the unit of randomisation: within‐patient randomisation in patients with bilateral ear disease
For data from studies where 'within‐patient' randomisation was used (i.e. studies where both ears (right versus left) were randomised) we adjusted the analyses for the paired nature of the data (Elbourne 2002; Stedman 2011), as outlined in section 16.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
The ear as the unit of randomisation: non‐paired randomisation in patients with bilateral ear disease
Some patients with bilateral disease may have received the same treatment in both ears, whereas others received a different treatment in each ear. We did not exclude these studies but we only reported the data if specific pairwise adjustments were completed or if sufficient data were obtained to be able to make the adjustments.
The patient as the unit of randomisation
Some studies randomise by patient and those with bilateral CSOM received the same intervention for both ears. In some studies the results may be reported as a separate outcome for each ear (the total number of ears is used as the denominator in the analysis). The correlation of response between the left ear and right ear when given the same treatment was expected to be very high, and if both ears were counted in the analysis this was effectively a form of double counting, which may be especially problematic in smaller studies if the number of people with bilateral CSOM was unequal. We did not exclude these studies, but we only reported the results if the paper presented the data in such a way that we could include the data from each participant only once (one data point per participant) or if we had enough information to reliably estimate the effective sample size or inflated standard errors as presented in chapter 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If this was not possible, we attempted to contact the authors for more information. If there was no response from the authors, then we did not include data from these studies in the analysis.
If we found cluster‐randomised trials by setting or operator, we analysed these according to the methods in section 16.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We attempted to contact study authors via email whenever the outcome of interest was not reported but the methods of the study had suggested that the outcome had been measured. We did the same if not all of the data required for the meta‐analysis were reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these were reported, as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). Where it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we did not conduct any other imputations. We extracted and analysed data for all outcomes using the available case analysis method.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included studies for potential differences in the types of participants recruited, interventions or controls used, and the outcomes measured. We did not pool studies where the clinical heterogeneity made it unreasonable to do so.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculated the percentage of variability that was due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as within‐study outcome reporting bias and between‐study publication bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results are mentioned but not reported adequately in a way that allowed analysis (e.g. the report only mentioned whether the results were statistically significant or not), bias in a meta‐analysis is likely to occur. We tried to find further information from the study authors, but if no further information could be obtained, we noted this as being a high risk of bias. Where there was insufficient information to judge the risk of bias, we noted this as an unclear risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We intended to create funnel plots if sufficient studies (more than 10) were available for an outcome. If we observed asymmetry of the funnel plot, we would have conducted a more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we analysed treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data were from the same scale, we would have pooled the mean values obtained at follow‐up with change outcomes and reported this as a MD. However, if the SMD had to be used as an effect measurement, we would not have not pooled change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We subgrouped studies where most participants (80% or more) met the criteria stated below in order to determine whether the effect of the intervention was different compared to other patients. Due to the risks of reporting and publication bias with unplanned subgroup analyses of trials, we only analysed subgroups reported in studies if these were prespecified and stratified at randomisation.
We planned to conduct subgroup analyses regardless of whether statistical heterogeneity was observed for studies that included patients identified as high risk (i.e. thought to be less responsive to treatment and more likely to develop CSOM, recurrence or complications) and patients with ventilation tubes (grommets). 'High risk' patients include Indigenous populations (e.g. Australian Aboriginal and Torres Strait Islanders, Native Americans and Inuit populations of Alaska, Canada and Greenland), people with craniofacial malformation (e.g. cleft palate), Down syndrome and people with known immunodeficiency.
We planned to present the main analyses of this review in the form of forest plots based on this main subgroup analysis.
-
For the high‐risk group, this applied to the outcomes resolution of ear discharge (dry ear), quality of life, pain/discomfort, development of complications and hearing loss.
For patients with ventilation tubes, this applied to the outcome resolution of ear discharge (dry ear) for the time point of four weeks or more because this group was perceived to be at lower risk of treatment failure and recurrence than other patient groups. If statistical heterogeneity was observed, we also conducted subgroup analysis for the effect modifiers below. If there were statistically significant subgroup effects, we presented these subgroup analysis results as forest plots.
For this review, effect modifiers include:
-
Diagnosis of CSOM: it was likely that some studies would include patients with chronic ear discharge but who had not had a diagnosis of CSOM. Therefore, we subgrouped studies where most patients (80% or more) met the criteria for CSOM diagnosis in order to determine whether the effect of the intervention was different compared to patients where the precise diagnosis was unknown and inclusion into the study was based purely on chronic ear discharge symptoms.
-
Duration of ear discharge: there is uncertainty about whether the duration of ear discharge prior to treatment has an impact on the effectiveness of treatment and whether more established disease (i.e. discharge for more than six weeks) is more refractory to treatment compared with discharge of a shorter duration (i.e. less than six weeks).
-
Patient age: patients who were younger than two years old versus patients up to six years old versus adults. Patients under two years are widely considered to be more difficult to treat.
We presented the results as subgroups regardless of the presence of statistical heterogeneity based on the main types of aural toileting methods as follows:
-
dry mopping;
-
irrigation;
-
microsuction.
This was because the different methods of aural toileting were expected to have different treatment effects and possible adverse effects due to their intensity (e.g. microsuction is thought to be a more intense method than dry mopping).
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
-
Impact of model chosen: fixed‐effect versus random‐effects model.
-
Risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that have a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed)).
-
Where there was statistical heterogeneity, studies that only recruited patients who had previously not responded to one of the treatments under investigation in the RCT. Studies that specifically recruited patients who did not respond to a treatment could potentially have reduced the relative effectiveness of an agent.
If any of these investigations found a difference in the size of the effect or heterogeneity, we would have mentioned this in the 'Effects of interventions' section and/or presented the findings in a table.
Summary of findings and assessment of the certainty of the evidence
Using the GRADE approach, at least two review authors (KH/LYC) independently rated the overall certainty of evidence using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The certainty of evidence reflects the extent to which we were confident that an estimate of effect was correct and we applied this in the interpretation of results. There are four possible ratings: 'high', 'moderate', 'low' and 'very low' (Handbook 2011). A rating of 'high' certainty evidence implies that we were confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' certainty implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high certainty. However, several factors could lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading was determined by the seriousness of these factors:
-
study limitations (risk of bias);
-
inconsistency;
-
indirectness of evidence;
-
imprecision;
-
publication bias.
The 'Summary of findings' table presents the following outcomes:
-
resolution of ear discharge or 'dry ear':
-
at between one week and up to two weeks;
-
after four weeks;
-
-
health‐related quality of life;
-
ear pain (otalgia) or discomfort or local irritation;
-
hearing;
-
serious complications;
-
adverse events: dizziness/vertigo/balance problems.
Results
Description of studies
Results of the search
The searches retrieved a total of 8900 references, which reduced to 3447 after removal of duplicates. We identified five additional references from other sources. We screened the titles and abstracts and subsequently removed 3218 references. We assessed 229 full texts for eligibility of which we excluded 225 references; we excluded 21 of these references (13 studies) with reasons recorded in the review (see Excluded studies). We included four references (three studies). A flow chart of study retrieval and selection is provided in Figure 1.
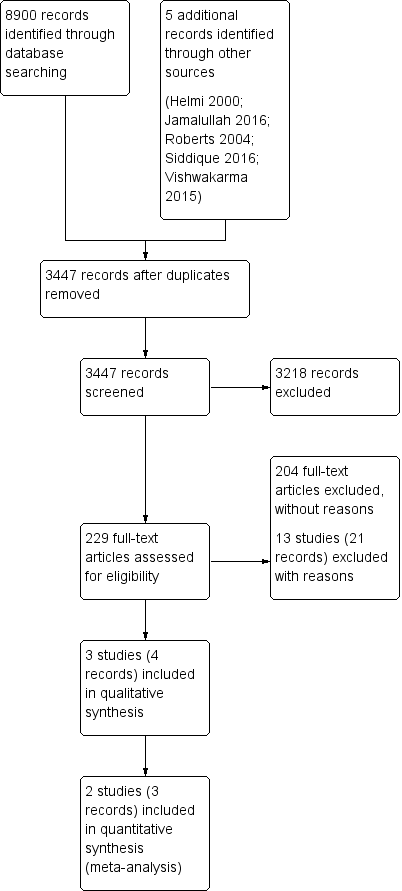
Process for sifting search results and selecting studies for inclusion.
Included studies
We included three studies (Eason 1986; Kiris 1998; Smith 1996). Table 2 and the Characteristics of included studies table provide a summary of the included studies.
| Ref ID (no. of participants) | Setting | Population | Intervention | Comparison | Treatment | Follow‐up | Background treatment | Notes |
|---|---|---|---|---|---|---|---|---|
| Daily dry mopping versus no specific treatment | ||||||||
| (n = 48 people, 67 ears) | Solomon Islands, villages (community) | Children with CSOM for more than 3 months Mean age 5.4 years | 4 times daily aural toilet (dry mopping) | No treatment | 3 to 6 weeks | 6 weeks | None | Part of a 5‐arm trial |
| (n = 303 people) | Kenya (school) | Children with CSOM for more than 2 weeks Mean age not given. 80% of children were between 5 and 14 years. | Twice daily dry mopping (except weekends) | No specific treatment | Up to 16 weeks | Up to 16 weeks | None | Part of a 3‐arm trial |
| Daily suction cleaning PLUS topical antibiotics versus single suction cleaning PLUS topical antibiotics | ||||||||
| (n = 80 people, 95 ears) | Turkey (ENT clinic) | Otorrhoea with at least 6 weeks duration Mean: 26.5 years (range 21 months to 70 years) | Daily external ear channel aspiration | Single external ear channel aspiration at first visit | 15 days | 3 to 6 months | Topical ciprofloxacin | — |
CSOM: chronic suppurative otitis media
Study design and sample size
One study was a two‐arm trial (Kiris 1998), one was a three‐arm trial (Smith 1996) and one was a five‐arm trial (Eason 1986). In all cases only two study arms were relevant to this review. Details of the other study arms can be found in the Characteristics of included studies table.
All studies indicated that they were randomised, controlled, parallel‐arm studies.
Sample sizes
The total sample size was 431 (465 ears) and ranged from 48 to 303 participants. Eason 1986 reported the outcome only by number of ears, while the others reported the results by participant (Smith 1996) or gave enough information to determine the number of participants (Kiris 1998) (Table 3).
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
|---|---|---|---|---|---|---|
| Person | Ear | "dry" or "not discharging" | Unclear | 2 to 4 weeks (3 weeks) 4+ weeks (6 weeks) | Although the results were presented by ear, sensitivity analysis based on converting the results to people did not affect the outcome so we used the results in this review. | |
| Person | Ear, person could be determined | Resolution of otorrhoea | Unclear | 1 to 2 weeks (between 3 to 12 days of treatment) | The results are presented by ear but sufficient data existed to provide the data by person. The base case assumption is that most of the cases were unilateral disease, which provides the most conservative estimate of effect size. | |
| School | Person | "resolution": absence of otorrhoea at 2 successive visits "healed": complete repair of the | Otoscopically confirmed | 4+ weeks (16 weeks) | — |
Unit of randomisation
The unit of randomisation for each study is presented in Table 3. Two studies used the individual as the unit of randomisation (Eason 1986; Kiris 1998); both of these studies presented results for resolution of ear discharge by ear.
Smith 1996 randomised schools, meaning that all of the participants from the school would receive the same treatment. Only one ear from each of the children participating was reported for this trial. Where bilateral disease was present, the ear on the school's allocated side was recruited although the opposite ear was treated in the same way and was monitored (but not reported). The analysis within the study adjusted for the effect of intra‐cluster correlations due to the randomisation by school.
Location
The studies were conducted in Kenya (Smith 1996), Turkey (Kiris 1998) and the Solomon Islands (Eason 1986).
Setting of trials
One study was conducted in a community setting (Eason 1986), one in primary schools (Smith 1996), and the third was in an ENT department of a university hospital (Kiris 1998). Two studies included participants after they had been screened through a community‐based screening programme to identify patients with CSOM (Eason 1986; Smith 1996).
Population
Age and sex
Two of the studies included only children (Eason 1986; Smith 1996), while the third included both children and adults (mean 26.5 years; Kiris 1998). All studies included males and females. The percentage of males in the studies ranged from 45% to 69%.
High‐risk populations
Eason 1986 recruited participants from the Solomon Islands, who were considered to be a 'high‐risk' Indigenous group. The paper stated that incidence of CSOM in the population was 3.8% for under 15‐year olds. None of the other studies reported the inclusion of any of the 'high‐risk' populations as defined by our inclusion criteria (cleft palate, Down syndrome, Indigenous groups, immunocompromised patients).
Diagnosis (confirmed tympanic membrane perforation/presence of mucopurulent discharge)
In two studies the patients had chronic suppurative otitis media, which was confirmed by otoscopy (Eason 1986; Smith 1996). Although the title and abstract in Kiris 1998 indicated CSOM, the methods section used the criteria of otorrhoea for at least six weeks duration and did not indicate if perforation of the membrane was confirmed.
Duration of ear discharge
All participants in Smith 1996 had mucopurulent ear discharge. This was not reported for Eason 1986 or Kiris 1998. The duration of discharge at entry into the study was at least two weeks (Smith 1996), at least six weeks (Kiris 1998) and at least three months (Eason 1986).
Other important effect modifiers
Kiris 1998 confirmed alternative diagnoses after the treatment for some patients but it is unclear how many. This included five ears (5/80 = 6%) identified with cholesteatomas and polypoid hypertrophy in two ears.
None of the other studies identified other important effect modifiers such as the history of, or presence of, ventilation tubes or previous ear surgery.
Interventions
Details of the interventions, background treatments and treatment durations for each of the included studies are summarised in Table 2.
Two forms of aural toileting are described in the studies. Two studies describe 'dry mopping' with cotton wool wisps twisted around sticks, performed either four times daily by parents (Eason 1986) or on weekdays only by trained 'ear monitors' who were older children (Smith 1996). The third study describes daily external ear channel aspiration performed in clinic, in addition to topical ciprofloxacin (Kiris 1998).
Comparisons
The three studies on two comparisons:
-
Aural toileting by cotton wool (dry mopping) versus no intervention (Eason 1986; Smith 1996).
-
Daily aural toileting by aspiration in clinic versus single aural toileting by aspiration, with both groups receiving topical ciprofloxacin (Kiris 1998).
Outcomes
Resolution of ear discharge
All three studies reported resolution of ear discharge or 'dry ear'. However, the length of follow‐up for all three studies differed, ranging from six weeks to six months (Table 3).
Health‐related quality of life using a validated instrument
No studies measured this outcome.
Ear pain (otalgia) or discomfort or local irritation
No studies reported this outcome.
Hearing
All three studies reported that hearing status was measured but none of the studies present quantitative results:
-
Eason 1986 reports pre‐intervention hearing status but does not report post‐intervention hearing outcomes.
-
Smith 1996 measured pure‐tone hearing thresholds, recorded at 0.5 kHz, 1 kHz, 2 kHz, 4 kHz and 8 kHz. The 'post‐treatment' results were reported by CSOM status rather than by treatment group.
-
Kiris 1998 measured hearing with audiography tests pre‐ and post‐treatment. The results section only provides a narrative statement of no difference between groups.
Serious complications (including intracranial complications, extracranial complications and death)
Serious complications such as mastoiditis and encephalitis were specifically mentioned in one study (Eason 1986), although it is not clear whether the complications occurred pre‐ or post‐treatment. The other two studies did not specifically mention any evaluation of serious complications.
Adverse events: dizziness/vertigo/balance problems
One study mentioned a patient with dizziness (Kiris 1998), while the others did not specifically mention any evaluation of dizziness, vertigo and balance problems.
Adverse events: ear bleeding
No studies reported this outcome.
Excluded studies
We excluded 21 papers (13 studies) after reviewing the full text. We excluded 20 papers (12 studies) because the comparisons were not appropriate for this review, but were relevant to another Cochrane Review in this suite, and one paper (one study) due to the population characteristics included in their study. Further details for the reasons for exclusion can be found in the Characteristics of excluded studies table.
Risk of bias in included studies
See Figure 2 for the 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for the 'Risk of bias' summary (our judgements about each risk of bias item for each included study).

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We judged one study to be at high risk of randomisation bias as it did not provide information about the sequence generation and there were unexplained imbalances between the groups (Eason 1986). There were 1.6 times as many patients in the largest group than the smallest group, with the larger number of patients in the more effective treatment groups. We assessed one study as at unclear risk of bias due to inadequate information (Kiris 1998), whilst we assessed the remaining study as low risk (Smith 1996).
Allocation concealment
We assessed all three studies as being at unclear risk of allocation concealment bias. Two were due to lack of details provided (Eason 1986; Kiris 1998), while the other was due to the possibility that researchers could have influenced the allocation to treatment group (Smith 1996).
Performance bias
We assessed all three studies as at high risk of performance bias as no blinding of participants was attempted.
Detection bias
We assessed two studies as at high risk of detection bias (Eason 1986; Kiris 1998), as neither study indicated that the outcome assessment was blinded. We assessed Smith 1996 as at unclear risk as although outcome assessors were not blinded to treatment group, study investigators tried to minimise the risk of bias by randomly assigning outcome assessors and changing the composition of assessor teams daily.
Incomplete outcome data
We assessed two studies as being at an unclear risk of attrition bias. Smith 1996 identified that 28% of participants did not attend some of the follow‐up appointments, but the reasons for non‐attendance were not noted. For Eason 1986 it was not possible to determine if there were any participants lost to follow‐up. We assessed the remaining study to be at a low risk of bias (Kiris 1998).
Selective reporting
We assessed one study as at high risk of selective reporting bias as the outcomes were not clear in the methods section and so it is difficult to know if there were outcomes measured but not reported (Kiris 1998). We assessed the remaining two studies as at unclear risk of selective reporting due to a lack of information (Eason 1986; Smith 1996).
None of the three studies had protocols identified through searches of clinical trials registries.
Other potential sources of bias
Funding
Eason 1986 stated "this study was made possible by a research grant from the Medical Research Council of New Zealand." Smith 1996 stated "the study was supported by the Overseas Development Administration (UK), the Gatsby Charitable Foundation (UK), and the Thrasher Research Fund (USA)". Kiris 1998 did not provide any information.
Declarations of interest
None of the studies provided information about conflicts of interest (Eason 1986; Kiris 1998; Smith 1996).
Effects of interventions
See: Summary of findings 1 Aural toileting compared to no aural toileting for chronic suppurative otitis media; Summary of findings 2 Daily aural toileting compared to single aural toileting episode for chronic suppurative otitis media
Comparison 1: Aural toileting versus no intervention
Two studies (351 participants; 370 ears) were included in this comparison with chronic suppurative otitis media:
-
Eason 1986 (48 children, 67 ears) compared dry mopping four times per day with no specific treatment; and
-
Smith 1996 (303 children, 303 ears) compared dry mopping two times per day (except weekends) with no specific treatment.
See also summary of findings Table 1.
Resolution of ear discharge
Eason 1986 presented the results of ear discharge by ear. Although the number of bilateral cases for each study arm was presented, sufficient data were not presented to allow adjustment of the results by person.
Between one week and up to two weeks
No study reported this outcome at this time point.
Two weeks to up to four weeks
Eason 1986 did not report the resolution of ear discharge at between two to four weeks in the group that did not receive any treatment, although results were reported for the other study arms.
After four weeks
Although two studies reported resolution of ear discharge after four weeks (Eason 1986 reported results at six weeks and Smith 1996 reported results at 16 weeks), Eason 1986 presented the results by ear and adjustment of the results to 'by person' was not possible, so results are presented as a narrative only.
For Smith 1996, it is very uncertain whether there is a difference in resolution of ear discharge with dry mopping compared with no specific treatment (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.60 to 1.72; 1 study; 217 participants; very low‐certainty; Analysis 1.1).
In Eason 1986, the authors reported that at the follow‐up point of six weeks after treatment in the group with no treatment, 18% of 41 ears had resolution of ear discharge and in the group with dry mopping, 50% of 26 ears had resolution of ear discharge.
Health‐related quality of life using a validated instrument
No study reported this outcome.
Ear pain (otalgia) or discomfort or local irritation
No study reported this outcome.
Hearing
Although Smith 1996 measured hearing, they presented the data with regards to the improvements for those who had resolution of ear discharge (irrespective of treatment group) and those who did not. Therefore it was not possible to identify whether there was a difference in hearing between the two groups (Smith 1996).
Eason 1986 did not evaluate post‐intervention hearing.
Serious complications (including intracranial complications, extracranial complications or death)
Eason 1986 reports one case of mastoiditis and one case of meningitis with focal encephalitis. It is not clear which group these patients were from, or whether the complications occurred pre‐ or post‐treatment.
Adverse events (dizziness/vertigo/balance problems)
No study reported this outcome.
Adverse events (ear bleeding)
No study reported this outcome.
Subgroup analysis
We could not undertake any subgroup analysis because there were only two studies in this comparison and for the only outcome where meta‐analysis was possible (resolution of ear discharge after four weeks) the results were heterogeneous. Possible reasons for heterogeneity are already discussed in the results section.
Comparison 2: Daily aural toileting versus single aural toileting on top of topical ciprofloxacin
Kiris 1998 included 80 participants (95 ears) with chronic ear discharge for more than six weeks. The investigators randomised one group to receive daily aural toileting with aspiration (suction) in the clinic followed by administration of daily ciprofloxacin ear drops, whereas the comparison group underwent initial aspiration in the clinic but then self‐administered ciprofloxacin ear drops at home without further aural toileting. Although this study reported the results by ear, there was sufficient information provided for the resolution of ear discharge at between one and up to two weeks for us to be able to convert the results to 'per person' results.
See also summary of findings Table 2.
Resolution of ear discharge
Between one week and up to two weeks
It is very uncertain if there is a difference between daily suction cleaning with administration of topical antibiotic ear drops (ciprofloxacin) in a clinic and a single aural toileting episode followed by daily self‐administered topical antibiotic drops (RR 1.09, 95% CI 0.91 to 1.30; 80 participants; Analysis 2.1; very low‐certainty evidence).
Two weeks to up to four weeks
The study did not report this outcome at this time point.
After four weeks
Kiris 1998 provided results for this outcome by ear but it was not possible to adjust the results by person. The study authors reported that at three to six months "relapse occurred in six ears in the clinic treated group (12.8%) and in five ears in the self treated group (10.4%)."
Health‐related quality of life using a validated instrument
The study did not report this outcome.
Ear pain (otalgia) or discomfort or local irritation
The study did not report this outcome.
Hearing
Kiris 1998 measured hearing but only reported the results qualitatively, stating that "there were no differences in pre‐and post audiographic results or bone conduction in either group". We are very uncertain if there is a difference between the two groups (very low‐certainty evidence).
Serious complications (including intracranial complications, extracranial complications or death)
The study did not report that any participant died or had any intracranial or extracranial complications.
Adverse events (dizziness/vertigo/balance problems)
One patient in the group with single aural toileting and self‐administration of topical antibiotic ear drops reported dizziness, which the authors attributed to the use of cold topical ciprofloxacin. Due to the very low number of events (one event) it is very uncertain if there is a difference between the groups (RR 0.33, 95% CI 0.01 to 7.95; 80 participants; very‐low certainty evidence, Analysis 2.2).
Adverse events (ear bleeding)
The study did not report this outcome.
Subgroup analysis
Although we had planned to complete subgroup analyses, as only one study was included in this comparison this was not possible.
Discussion
Summary of main results
We identified three studies for this review (Eason 1986; Kiris 1998; Smith 1996). Due to the limited number of studies, the methods used, the choice of outcome measures and the poor reporting of results there was a scarcity of evidence that we could include in the review.
See also summary of findings Table 1 and summary of findings Table 2.
Daily aural toilet (dry mopping) versus no treatment
Two studies including 351 children (370 ears) with chronic suppurative otitis media (CSOM) compared daily dry mopping with no treatment (Eason 1986; Smith 1996). In Eason 1986 parents were asked to dry mop their children's ear(s) four times per day for six weeks whereas Smith 1996 trained older school children to act as 'ear monitors' and dry mop younger children's ear(s) twice daily on week days for 16 weeks. Only the results from Smith 1996 could be used in the analysis as Eason 1986 presented results by ear and adjustments could not be made to provide results per person. It is unclear if there is a difference in resolution of ear discharge with dry mopping compared with no treatment measured at 16 weeks (risk ratio (RR) 1.01, 95% confidence interval (CI) 0.60 to 1.72; 217 participants). We assessed the evidence to be very uncertain (very low‐certainty) due to risk of bias in the study, the imprecision of the result and indirectness as the study only included children. Only one study reported serious complications, but it was not clear which group these patients were from (this was a five‐arm study), or whether the complications occurred pre‐ or post‐treatment. One study reported hearing, but the results were presented by treatment outcome rather than by treatment group so it is not possible to determine whether there is a difference between the two groups. Neither study reported results for resolution of ear discharge at any other time points, health‐related quality of life, ear pain or adverse events (dizziness/vertigo/balance problems/ear bleeding).
Daily aural toileting (suction cleaning) versus single aural toileting (suction cleaning) on top of topical ciprofloxacin
Kiris 1998 (80 participants, 95 ears) compared a single episode of suction cleaning with daily suction cleaning by clinical personnel for people with ear discharge for six weeks or more. All patients also used daily ciprofloxacin ear drops. It is uncertain if there is a difference in resolution of ear discharge at between one and up to two weeks when comparing the two methods (RR 1.09, 95% CI 0.91 to 1.30, 80 participants, very low‐certainty evidence). We assessed the results as being very uncertain due to risk of bias in the study, imprecision in the study and indirectness (by including people with 'ear discharge' rather than the diagnosis of CSOM). The study did not provide results for resolution of ear discharge at between two to four weeks and the results for 'relapse' after four weeks (between three to six months) were presented by ear; it was not possible to make adjustments to present the results by person. One event of dizziness was reported in the group that had a single suction cleaning episode, but there is not enough information to determine if there is a difference between the groups (RR 0.33, 95% CI 0.01 to 7.95; 80 participants, very low‐certainty evidence). Hearing was measured but the study only reported the results qualitatively, stating that "there were no differences in pre‐ and post audiographic results or bone conduction in either group". The study did not report results for health‐related quality of life, ear pain, serious complications or any other adverse events (vertigo/balance problems/ear bleeding).
Overall completeness and applicability of evidence
-
Only three studies were available and there were many differences between the studies making comparisons difficult.
-
There were very few data for outcomes other than resolution of ear discharge. No studies reported health‐related quality of life. Adverse events, hearing and serious complications were all poorly reported.
-
The length of follow‐up in studies ranged from six weeks to six months, meaning that there was limited evidence regarding the long‐term effectiveness of aural toileting for the resolution of discharge or healing of the tympanic membrane in people with CSOM.
-
Two studies included children with CSOM (Eason 1986; Smith 1996), but the inclusion criteria varied from discharge of two weeks (Smith 1996) to three months (Eason 1986). Kiris 1998 used the term 'CSOM' in the title but in the methods section the inclusion criteria appeared to be only those who had ear discharge for six weeks. No information about membrane perforation was given.
-
Two studies were conducted in community settings (Eason 1986; Smith 1996) and one in secondary care (Kiris 1998).
-
Even where 'dry mopping' was used the interventions varied from twice daily mopping on school days conducted by trained 'ear monitors' who were older students (Smith 1996), to dry mopping four times per day completed by trained parents (Eason 1986).
Quality of the evidence
Generally the included studies were small and not well reported. There were questions over whether the randomisation was adequate in all except one study. Studies were unblinded and suffered from possible selective reporting bias. This limits our ability to draw firm conclusions.
Of the three included studies, only one clearly described the use of otoscopic confirmation of resolution of discharge. This may have impacted on the accuracy of the diagnostic outcome and therefore the response to treatment.
Potential biases in the review process
Across our suite of Cochrane CSOM reviews (Brennan‐Jones 2020; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2020a; Head 2020b), we have identified studies that may have been relevant to the reviews but were only published as abstracts or internal non peer‐reviewed reports, or were included in systematic reviews but the full data were not published. This raises a concern that there may have been other studies conducted where the results have not been published, or where the results have been published but would not have been identified through our searches. We reviewed some regional medical databases (such as IndMed and the African Index Medicus) but there is still a concern that unpublished data may be an issue for this review.
Agreements and disagreements with other studies or reviews
This review is part of a series of reviews on CSOM (Bhutta 2018; Brennan‐Jones 2020; Brennan‐Jones 2018b; Chong 2018a; Chong 2018b; Head 2020a; Head 2020b).
There are few previous reviews or guidelines for CSOM. The World Health Organization (WHO) in 2004 suggested that first‐line treatment of CSOM should comprise aural toilet and topical antibiotic drops, with second‐line treatment comprising an alternative topical antibiotic (guided by the results of microbiological culture) or parenteral antibiotics (WHO 2004). The Australian government recommendations from 2010 for the treatment of Aboriginal and Torres Strait Islanders gave similar recommendations, with first‐line treatment comprising aural toilet (or antiseptic washout) followed by topical antibiotics, and second‐line treatment with parenteral antibiotics (Morris 2010). An expert panel of the American Academy of Otolaryngologists in 2000 came to a similar conclusion (Hannley 2000).

Process for sifting search results and selecting studies for inclusion.

'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Aural toileting versus no aural toileting, Outcome 1: Resolution of ear discharge (4 weeks +)

Comparison 2: Daily aural toileting versus single aural toileting, Outcome 1: Resolution of ear discharge (1 to 2 weeks)

Comparison 2: Daily aural toileting versus single aural toileting, Outcome 2: Vertigo/dizziness/tinnitus
| Aural toileting compared to no aural toileting for chronic suppurative otitis media | |||||||
| Patient or population: children with chronic suppurative otitis media | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Without aural toileting | With aural toileting | Difference | |||||
| Resolution of ear discharge ‐ 1 to 2 weeks | No study reported this outcome at this time point. | ||||||
| Resolution of ear discharge ‐ 4 weeks or more Assessed by: otoscopically confirmed Follow‐up: 16 weeks | RR 1.01 (0.60 to 1.72) | 217 | Study population | ⊕⊝⊝⊝ | We are uncertain about the effect of aural toileting on resolution of ear discharge (at 4 weeks or more) compared with no treatment. | ||
| 22.2% | 22.4% | 0.2% more | |||||
| Health‐related quality of life | No study reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | ||||||
| Hearing | Hearing was measured in one study but the results were presented by treatment outcome rather than by treatment group, so it is not possible to determine whether there is a difference between the two groups. | ||||||
| Serious complications | — | 48 (1 RCT) | One study reported one case of mastoiditis and one case of meningitis with focal encephalitis. It is not clear which group these patients were from (the study was a five‐arm trial of which only two arms are presented here), or whether the complications occurred pre‐ or post‐treatment. | ⊕⊝⊝⊝ | We are very uncertain about the effect of aural toileting on serious complications compared with no treatment. | ||
| Adverse events: dizziness/vertigo/balance problems | No study reported this outcome. | ||||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because there was unclear allocation concealment, attrition bias and selective reporting bias. Downgraded by one level for indirectness (only children were included in the study). Downgraded by two levels for imprecision (as the results are based on one small study with wide confidence intervals). Downgraded by one level for suspected publication bias (this area has a known issue with trials not being published in peer‐reviewed journals). 2Downgraded to very low certainty: downgraded by one level due to study limitations (risk of bias) because it was at high risk of bias for randomisation and was at unclear risk of bias for allocation concealment, attrition bias and selective reporting bias. The study was unblinded. Downgraded by one level for indirectness (only children were included in the study). Downgraded by two levels for imprecision as it was not clear to which group the events could be attributed. Downgraded by one level for suspected publication bias (this area has a known issue with trials not being published in peer‐reviewed journals). | |||||||
| Daily aural toileting compared to single aural toileting episode for chronic suppurative otitis media | |||||||
| Patient or population: people (of any age) with otorrhoea for a duration of at least 6 weeks | |||||||
| Outcomes | Relative effect | Number of participants (studies) | Anticipated absolute effects* (95% CI) | Certainty of the evidence | What happens | ||
|---|---|---|---|---|---|---|---|
| Single aural toileting | Daily aural toileting | Difference | |||||
| Resolution of ear discharge ‐ 1 to 2 weeks Assessed by: unknown ‐ unclear if otoscopically confirmed | RR 1.09, (0.91 to 1.30) | 80 (1 RCT) | Study population | ⊕⊝⊝⊝ | We are uncertain about the effect of daily aural toileting on resolution of ear discharge (at 1 to 2 weeks) compared with single episode of aural toileting. | ||
| 82.5% | 89.9% (75.1 to 100) | 7.4% more (7.4% fewer to 17.5% more) | |||||
| Resolution of ear discharge ‐ 4 weeks or more Assessed by: unknown ‐ unclear if otoscopically confirmed Follow‐up: 3 months | Kiris 1998 provided results for this outcome by ear, but the results could not be adjusted to provide results per person. | ||||||
| Health‐related quality of life | No study reported this outcome. | ||||||
| Ear pain (otalgia) or discomfort or local irritation | No study reported this outcome. | ||||||
| Hearing | — | 80 (1 RCT) | Results were only reported qualitatively, the report stating that "there were no differences in pre‐ and post audiographic results or bone conduction in either group ..." | ⊕⊝⊝⊝ | We are uncertain about the effect of daily aural toileting on hearing compared with a single episode of aural toileting. | ||
| Serious complications | The study did not report that any participant died or had any intracranial or extracranial complications. | ||||||
| Adverse events: dizziness Assessed by: self reported Follow‐up: 15 days | RR 0.33, (0.01 to 7.95) | 80 (1 RCT) | Study population | ⊕⊝⊝⊝ | We are uncertain about the effect of daily aural toileting on dizziness compared with a single episode of aural toileting. | ||
| 2.5% | 0.8% (0% to 19.9%) | 1.7% less (2.5% fewer to 17.4% more) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | |||||||
| GRADE Working Group grades of evidence | |||||||
| 1Downgraded to very low certainty: downgraded by two levels due to risk of bias (unclear randomisation, allocation concealment, unblinded trial and possible selective reporting). Downgraded by one level due to indirectness: the population is people with otorrhoea for more than six weeks so it is unclear if all included patients had CSOM. Downgraded by one level due to imprecision: the results are from one small study so the confidence intervals are wide. 2Downgraded to very low certainty: downgraded by two levels due to risk of bias (unclear randomisation, allocation concealment, unblinded trial and possible selective reporting). Downgraded by one level due to indirectness: the population is those with otorrhoea so it is unclear if all included patients had CSOM. Downgraded by two levels due to imprecision: the results are from one small study and only reported qualitatively. 3Downgraded to very low certainty: downgraded by two levels due to risk of bias (unclear randomisation, allocation concealment, unblinded trial and possible selective reporting). Downgraded by one level due to indirectness: the population is those with otorrhoea so it is unclear if all included patients had CSOM. Downgraded by two levels due to imprecision: the results are from one small study and only one event was reported resulting in very wide confidence intervals. | |||||||
| Topical antibiotics with steroids | Topical antibiotics | Systemic antibiotics | Topical antiseptics | Aural toileting (ear cleaning) | |
|---|---|---|---|---|---|
| Topical antibiotics with steroids | Review CSOM‐4 | ||||
| Topical antibiotics | Review CSOM‐4 | Review CSOM‐1 | |||
| Systemic antibiotics | Review CSOM‐4 | Review CSOM‐3 | Review CSOM‐2 | ||
| Topical antiseptics | Review CSOM‐4 | Review CSOM‐6 | Review CSOM‐6 | Review CSOM‐5 | |
| Aural toileting | Review CSOM‐4 | Not reviewed | Not reviewed | Not reviewed | Review CSOM‐7 |
| Placebo (or no intervention) | Review CSOM‐4 | Review CSOM‐1 | Review CSOM‐2 | Review CSOM‐5 | Review CSOM‐7 |
| CSOM‐1: Topical antibiotics for chronic suppurative otitis media (Brennan‐Jones 2020). CSOM‐2: Systemic antibiotics for chronic suppurative otitis media (Chong 2018a). CSOM‐3: Topical versus systemic antibiotics for chronic suppurative otitis media (Chong 2018b). CSOM‐4: Topical antibiotics with steroids for chronic suppurative otitis media (Brennan‐Jones 2018b). CSOM‐5: Topical antiseptics for chronic suppurative otitis media (Head 2020a). CSOM‐6: Antibiotics versus topical antiseptics for chronic suppurative otitis media (Head 2020b). CSOM‐7: Aural toilet (ear cleaning) for chronic suppurative otitis media (Bhutta 2018). | |||||
| Ref ID (no. of participants) | Setting | Population | Intervention | Comparison | Treatment | Follow‐up | Background treatment | Notes |
|---|---|---|---|---|---|---|---|---|
| Daily dry mopping versus no specific treatment | ||||||||
| (n = 48 people, 67 ears) | Solomon Islands, villages (community) | Children with CSOM for more than 3 months Mean age 5.4 years | 4 times daily aural toilet (dry mopping) | No treatment | 3 to 6 weeks | 6 weeks | None | Part of a 5‐arm trial |
| (n = 303 people) | Kenya (school) | Children with CSOM for more than 2 weeks Mean age not given. 80% of children were between 5 and 14 years. | Twice daily dry mopping (except weekends) | No specific treatment | Up to 16 weeks | Up to 16 weeks | None | Part of a 3‐arm trial |
| Daily suction cleaning PLUS topical antibiotics versus single suction cleaning PLUS topical antibiotics | ||||||||
| (n = 80 people, 95 ears) | Turkey (ENT clinic) | Otorrhoea with at least 6 weeks duration Mean: 26.5 years (range 21 months to 70 years) | Daily external ear channel aspiration | Single external ear channel aspiration at first visit | 15 days | 3 to 6 months | Topical ciprofloxacin | — |
| CSOM: chronic suppurative otitis media | ||||||||
| Reference | Unit of randomisation | Reported | Definition | Otoscopically confirmed? | Time points | Notes |
|---|---|---|---|---|---|---|
| Person | Ear | "dry" or "not discharging" | Unclear | 2 to 4 weeks (3 weeks) 4+ weeks (6 weeks) | Although the results were presented by ear, sensitivity analysis based on converting the results to people did not affect the outcome so we used the results in this review. | |
| Person | Ear, person could be determined | Resolution of otorrhoea | Unclear | 1 to 2 weeks (between 3 to 12 days of treatment) | The results are presented by ear but sufficient data existed to provide the data by person. The base case assumption is that most of the cases were unilateral disease, which provides the most conservative estimate of effect size. | |
| School | Person | "resolution": absence of otorrhoea at 2 successive visits "healed": complete repair of the | Otoscopically confirmed | 4+ weeks (16 weeks) | — |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Resolution of ear discharge (4 weeks +) Show forest plot | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.60, 1.72] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Resolution of ear discharge (1 to 2 weeks) Show forest plot | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.91, 1.30] |
| 2.2 Vertigo/dizziness/tinnitus Show forest plot | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.95] |

