Planificación anticipada de la atención para adultos con insuficiencia cardíaca
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Advance Care Planning] explode all trees
#2 advance* care near plan* (Word variations have been searched)
#3 advance* near (medical plan* or statement*) (Word variations have been searched)
#4 "ACP" (Word variations have been searched)
#5 Statement of wishes (Word variations have been searched)
#6 MeSH descriptor: [Terminal Care] explode all trees
#7 terminal care (Word variations have been searched)
#8 ((end of life or EOL) near/5 (care or discuss* or decision* or plan* or preference*)) (Word variations have been searched)
#9 MeSH descriptor: [Treatment Refusal] this term only
#10 MeSH descriptor: [Withholding Treatment] explode all trees
#11 (treatment near/3 (refus* or withhold* or withdraw*)) (Word variations have been searched)
#12 MeSH descriptor: [Living Wills] this term only
#13 living will* (Word variations have been searched)
#14 ((shared or sharing) near/2 decision*) (Word variations have been searched)
#15 SDM (Word variations have been searched)
#16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15
#17 MeSH descriptor: [Heart Failure] explode all trees
#18 (cardi* or heart* or myocard*) near/2 (fail* or incompet* or insufficien* or decomp*) (Word variations have been searched)
#19 #17 or #18
#20 #16 and #19
MEDLINE (Ovid)
1. exp Advance Care Planning/
2. (advance* care adj plan*).tw.
3. (advance* adj (medical plan* or statement*)).tw.
4. acp.tw.
5. Statement of wishes.tw.
6. Terminal Care/
7. terminal care.tw.
8. ((end of life or EOL) adj5 (care or discuss* or decision* or plan* or preference*)).tw.
9. Treatment Refusal/
10. exp Withholding Treatment/
11. (treatment adj3 (refus* or withhold* or withdraw*)).tw.
12. Living Wills/
13. living will*.tw.
14. ((shared or sharing) adj2 decision*).tw.
15. SDM.tw.
16. 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15
17. exp Heart Failure/
18. ((cardi* or heart* or myocard*) adj2 (fail* or incompet* or insufficien* or decomp*)).tw.
19. 17 or 18
20. 16 and 19
21. randomized controlled trial.pt.
22. controlled clinical trial.pt.
23. randomized.ab.
24. placebo.ab.
25. drug therapy.fs.
26. randomly.ab.
27. trial.ab.
28. groups.ab.
29. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28
30. exp animals/ not humans.sh.
31. 29 not 30
32. 20 and 31
Embase (Ovid)
1 terminal care/
2 advance care planning/
3 living will/
4 (advance* care adj plan*).tw.
5 (advance* adj (medical plan* or statement*)).tw.
6 acp.tw.
7 statement of wishes.tw.
8 terminal care.tw.
9 ((end of life or EOL) adj5 (care or discuss* or decision* or plan* or preference*)).tw.
10 treatment refusal/
11 treatment withdrawal/
12 withholding treatment.tw.
13 (treatment adj3 (refus* or withhold* or withdraw*)).tw.
14 "living will*".tw.
15 shared decision making/
16 ((shared or sharing) adj2 decision*).tw.
17 SDM.tw.
18 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19 exp heart failure/
20 ((cardi* or heart* or myocard*) adj2 (fail* or incompet* or insufficien* or decomp*)).tw.
21 19 or 20
22 18 and 21
23 "random*".tw.
24 "factorial*".tw.
25 "crossover*".tw.
26 "cross over*".tw.
27 "cross‐over*".tw.
28 "placebo*".tw.
29 (doubl* adj blind*).tw.
30 (singl* adj blind*).tw.
31 "assign*".tw.
32 "allocat*".tw.
33 "volunteer*".tw.
34 crossover procedure/
35 double blind procedure/
36 randomized controlled trial/
37 single blind procedure/
38 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37
39 (animal/ or nonhuman/) not human/
40 38 not 39
41 22 and 40
CINAHL
S33 S20 AND S32
S32 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31
S31 TX allocat* random*
S30 (MH "Quantitative Studies")
S29 (MH "Placebos")
S28 TX placebo*
S27 TX random* allocat*
S26 (MH "Random Assignment")
S25 TX randomi* control* trial*
S24 TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
S23 TX clinic* n1 trial*
S22 PT Clinical trial
S21 (MH "Clinical Trials+")
S20 S16 AND S19
S19 S17 OR S18
S18 TX ((cardi* or heart* or myocard*) N2 (fail* or incompet* or insufficien* or decomp*))
S17 (MH "Heart Failure+")
S16 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15
S15 TX SDM
S14 TX ((shared or sharing) N2 decision*)
S13 TX living will*
S12 (MH "Living Wills")
S11 TX (treatment N3 (refus* or withhold* or withdraw*))
S10 (MH "Euthanasia, Passive")
S9 (MH "Treatment Refusal")
S8 TX ((end of life or EOL) N5 (care or discuss* or decision* or plan* or preference*))
S7 TX terminal care
S6 (MH "Terminal Care")
S5 TX Statement of wishes
S4 TX acp
S3 TX advance* N1 (medical plan* or statement*)
S2 TX advance* care N1 plan*
S1 (MH "Advance Care Planning")
Social Work Abstracts
heart failure AND ("advance care planning" OR "living will" OR "acp" OR "Statement of wishes" OR "shared decision making" OR "SDM" OR "terminal care")
ClinicalTrials.gov
heart failure AND ("advance care planning" OR "living will" OR "acp" OR "Statement of wishes" OR "shared decision making" OR "SDM" OR "terminal care")
WHO International Clinical Trials Registry Platform (ICTRP) Search Portal
heart failure AND (advance care planning OR living will OR acp OR Statement of wishes OR shared decision making OR SDM OR terminal care)

Study flow diagram.
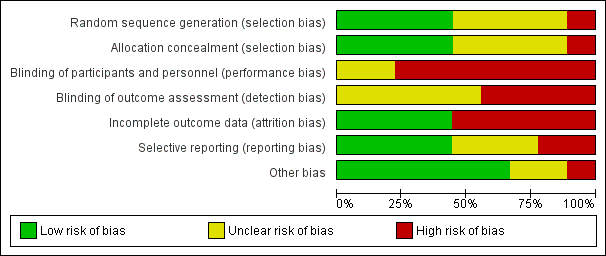
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
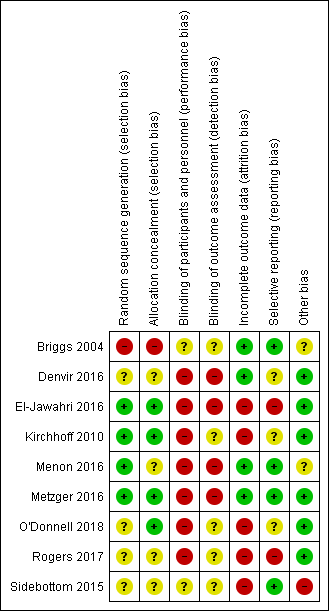
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
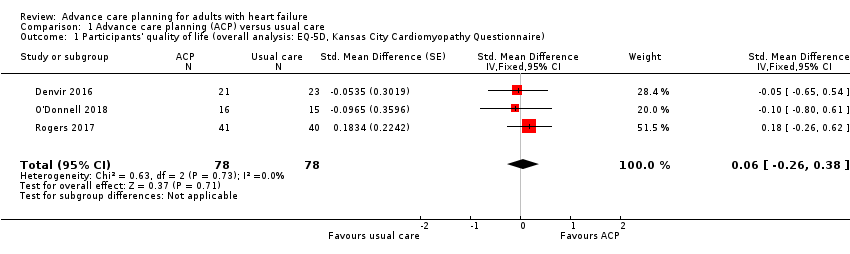
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 1 Participants' quality of life (overall analysis: EQ‐5D, Kansas City Cardiomyopathy Questionnaire).
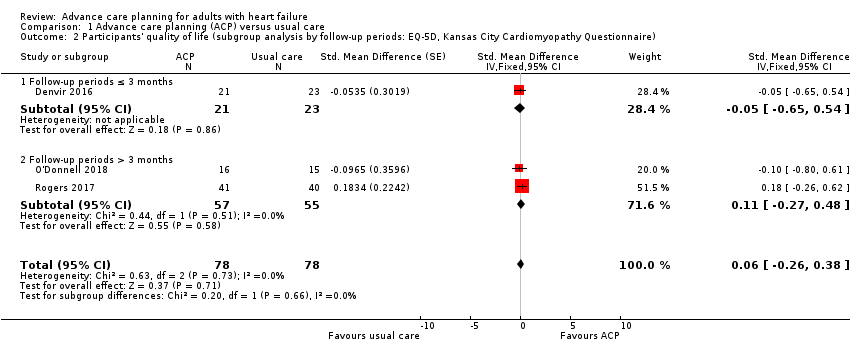
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 2 Participants' quality of life (subgroup analysis by follow‐up periods: EQ‐5D, Kansas City Cardiomyopathy Questionnaire).
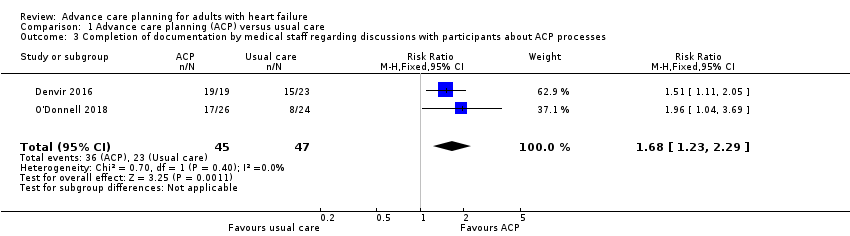
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 3 Completion of documentation by medical staff regarding discussions with participants about ACP processes.

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 4 Participants' depression (overall analysis).
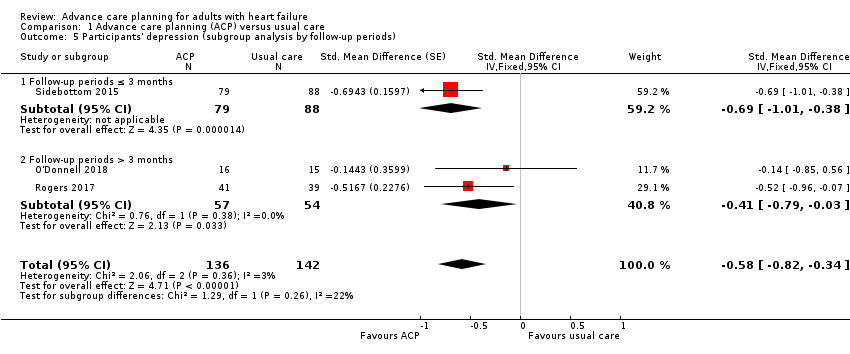
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 5 Participants' depression (subgroup analysis by follow‐up periods).

Comparison 1 Advance care planning (ACP) versus usual care, Outcome 6 Participants' decisional conflict.
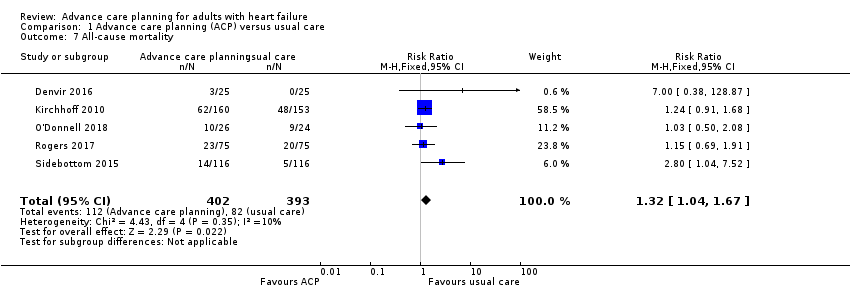
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 7 All‐cause mortality.
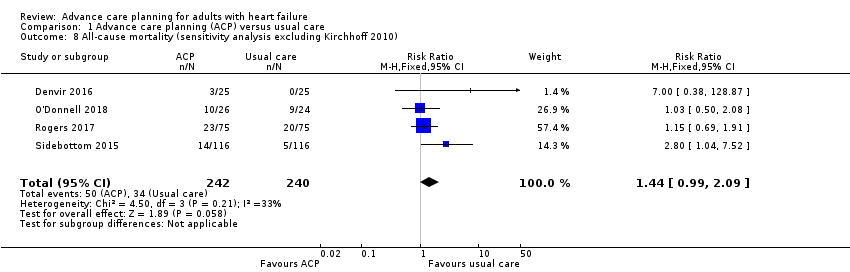
Comparison 1 Advance care planning (ACP) versus usual care, Outcome 8 All‐cause mortality (sensitivity analysis excluding Kirchhoff 2010).
| Advance care planning compared with usual care for patients with heart failure | ||||||
| Patient or population: people with heart failure with or without their surrogate decision‐makers/carers Settings: inpatient and outpatient hospitals and clinics Intervention: ACP Comparison: usual care | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of participants | Quality of the evidence | Comments | |
| Assumed risk | Corresponding risk | |||||
| Usual care | ACP | |||||
| Concordance between participants' preferences and end‐of‐life care (yes/no) Post‐death data: mean days to death, ACP group (388.8 ± 255.7); | 625 per 1000 | 744 per 1000 | RR 1.19 (0.91 to 1.55) | 110 | ⊕⊝⊝⊝ | — |
| Participants' quality of life Measured by EQ‐5D and KCCQ. Higher scores indicate high‐quality of life. Follow‐up: 2 weeks to 6 months | The quality of life score in the ACP groups was on average 0.06 SDs higher (0.26 lower to 0.38 higher) than in the usual care groups. | — | 156 | ⊕⊕⊝⊝ | 1 additional study reported quality of life using the MLHF Questionnaire. The study showed that the quality of life score was improved by 14.86 points in the intervention group compared with 11.80 points in the usual care group at 3 months. Generally, 0.2 SD represents a small difference, 0.5 moderate, and 0.8 large. | |
| Patients' satisfaction with care/treatment (yes/no) | — | — | — | — | — | Outcome not reported. |
| Completion of documentation by medical staff regarding discussions with participants about ACP processes (yes/no) Follow‐up: 3–6 months | 489 per 1000 | 822 per 1000 | RR 1.68 (1.23 to 2.29) | 92 | ⊕⊕⊝⊝ | 1 additional study reported completion of documentation with HR (HR 2.87, 95% CI 1.09 to 7.59; P = 0.033). |
| Participants' depression Measured on PHQ‐8, PHQ‐9, and HADS. Higher scores indicate high depression Follow‐up: 2 weeks to 6 months | The depression score in the ACP groups was on average 0.58 SDs (0.82 to 0.34) lower than in the usual care groups. | — | 278 | ⊕⊕⊝⊝ Lowc,e | Generally, 0.2 SD represents a small difference, 0.5 moderate, and 0.8 large. | |
| Caregivers' satisfaction with care/treatment (yes/no) | — | — | — | — | — | Outcome not reported. |
| Quality of communication Measured on Quality of Patient‐Clinician Communication About End‐of‐Life Care. Higher score indicates high satisfaction with the quality of communication Assessed after intervention | 11.2 ± 0.8 (mean ± SD) | MD 0.4 lower (1.61 lower to 0.81 higher) | — | 9 | ⊕⊝⊝⊝ | — |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACP: advanced care planning; CI: confidence interval; EQ‐5D: EuroQol‐5D; HADS: Hospital Anxiety and Depression Survey; HR: hazard ratio; KCCQ: Kansas City Cardiomyopathy Questionnaire; MD: mean difference; MLHF: Minnesota Living with Heart Failure; PHQ: Patient Health Questionnaire; RCT: randomised controlled trial; RR: risk ratio; SD: standard deviation; SMD: standardised mean difference. | ||||||
| GRADE Working Group grades of evidence | ||||||
| aDowngraded one level for indirectness because the study included participants other than people with heart failure. bSince the outcome included only one study, the sample size was too small, and had wide confidence intervals. Therefore, we downgraded two levels for imprecision. cDowngraded one level for risk of bias because most included studies showed unclear selection bias and high attrition bias. dDowngraded one level for imprecision due to small sample size and wide confidence intervals. eDowngraded one level for imprecision due to small sample size. fDowngraded one level for risk of bias due to high risk of selection bias. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Participants' quality of life (overall analysis: EQ‐5D, Kansas City Cardiomyopathy Questionnaire) Show forest plot | 3 | 156 | Std. Mean Difference (Fixed, 95% CI) | 0.06 [‐0.26, 0.38] |
| 2 Participants' quality of life (subgroup analysis by follow‐up periods: EQ‐5D, Kansas City Cardiomyopathy Questionnaire) Show forest plot | 3 | 156 | Std. Mean Difference (Fixed, 95% CI) | 0.06 [‐0.26, 0.38] |
| 2.1 Follow‐up periods ≤ 3 months | 1 | 44 | Std. Mean Difference (Fixed, 95% CI) | ‐0.05 [‐0.65, 0.54] |
| 2.2 Follow‐up periods > 3 months | 2 | 112 | Std. Mean Difference (Fixed, 95% CI) | 0.11 [‐0.27, 0.48] |
| 3 Completion of documentation by medical staff regarding discussions with participants about ACP processes Show forest plot | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.23, 2.29] |
| 4 Participants' depression (overall analysis) Show forest plot | 3 | 278 | Std. Mean Difference (Fixed, 95% CI) | ‐0.58 [‐0.82, ‐0.34] |
| 5 Participants' depression (subgroup analysis by follow‐up periods) Show forest plot | 3 | 278 | Std. Mean Difference (Fixed, 95% CI) | ‐0.58 [‐0.82, ‐0.34] |
| 5.1 Follow‐up periods ≤ 3 months | 1 | 167 | Std. Mean Difference (Fixed, 95% CI) | ‐0.69 [‐1.01, ‐0.38] |
| 5.2 Follow‐up periods > 3 months | 2 | 111 | Std. Mean Difference (Fixed, 95% CI) | ‐0.41 [‐0.79, ‐0.03] |
| 6 Participants' decisional conflict Show forest plot | 2 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.55, 0.02] |
| 7 All‐cause mortality Show forest plot | 5 | 795 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.04, 1.67] |
| 8 All‐cause mortality (sensitivity analysis excluding Kirchhoff 2010) Show forest plot | 4 | 482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.99, 2.09] |

