Sealants for preventing dental caries in primary teeth
Abstract
This is a protocol for a Cochrane Review (Intervention). The objectives are as follows:
To evaluate the effects of sealants in preventing pit and fissure caries in primary molars.
Background
Description of the condition
Dental caries is a multifactorial disease of the teeth that results in the localised destruction of tooth structure. Once considered solely an infectious disease, caries is currently defined as "a complex disease caused by an imbalance in physiologic equilibrium between tooth mineral and biofilm fluid" (Fejerskov 2003). Caries is caused by an interplay between the tooth substrate, carbohydrates in the diet and cariogenic bacteria in the dental biofilm. The bacteria metabolise refined carbohydrates (sugars) and produce acid, causing fluctuations in the pH of the biofilm and disturbances in the physiologic equilibrium between the tooth and biofilm, resulting in mineral loss (demineralisation) (Herald 2013; Kidd 2011). Under favourable conditions, the mineral loss is reversible (remineralisation); however, if the cariogenic challenge persists, it will lead to the further dissolution of dental hard tissues and possibly visible caries (Figure 1). In the absence of timely treatment, caries can spread through the hard tissues of the tooth to the soft tissue (pulp), leading to pain, inflammation and loss of function (Ten Cate 1999). If left untreated, caries can result in difficulty in chewing, tooth loss, weight loss, changes in behaviour ), and poor academic performance and cognitive development in young children (Acs 1992; Abanto 2011; Ayhan 1996; Miller 1992). It can negatively impact quality of life (Filstrup 2003). Besides personal and public health implications, the management of dental caries can have a substantial economic impact. In some resource‐poor settings, the cost of treating dental caries exceeds the entire allocated national healthcare budget (Yee 2002).
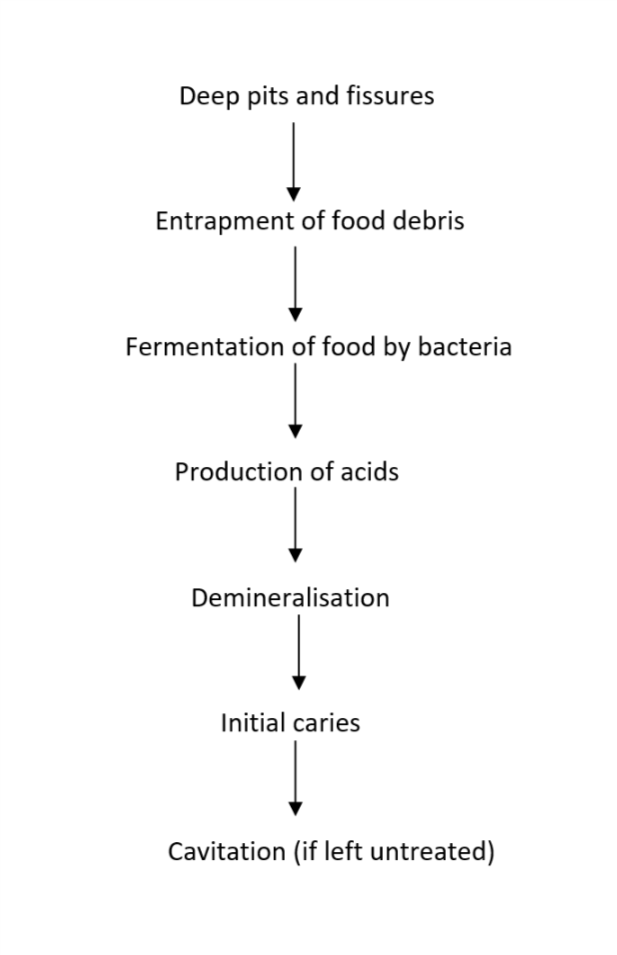
Aetiopathogenesis of pit and fissure caries
As the most common dental disease affecting people of all ages, caries is a significant health problem in children. Untreated dental caries in primary teeth is considered the 10th most prevalent condition, affecting about 621 million children globally (Kassebaum 2015). The prevalence and burden of caries are higher among children in low‐ and middle‐income countries than among those in high‐income countries (WHO 2014). Susceptibility to caries is highly variable among individuals and teeth. Teeth are marked with pits and fissures: a pit is a small pinpoint depression located at the junction of developmental grooves or at the terminals of those grooves, whereas a fissure is a deep cleft between adjoining cusps (Tandon 2009). Within the mouth, pits and fissures on the occlusal (chewing) surfaces of posterior (back) teeth are more prone to the development of dental caries than those of other teeth surfaces due to increased plaque retention, permeable immature enamel structure and the reduced effectiveness of fluoride on pits and fissures (Beauchamp 2008). Pit and fissure caries account for 90% of all dental caries in permanent molars even though occlusal surfaces represent only 12.5% of the total surfaces of the teeth (CDC and National Center for Health Statistics 2005). Caries is also prevalent in the primary molars with about 44% of all caries seen in pits and fissures (Dye 2007), even though the occlusal morphology of primary molars is flatter and less fissured than that of permanent molars (Carol 2015).
Grading the severity of carious lesions is complex, due in part to a lack of consistency among contemporary assessment criteria. However, the recently introduced International Caries Detection and Assessment System (ICDAS) integrates several new criteria into one standard system, which simplifies caries assessment (Ismail 2007). With ICDAS, the codes for assessment range from 0 to 6 depending on the severity of the carious lesion. A code 0, 1 or 2 represents an assessment ranging from sound tooth surface to caries in enamel without cavitation. At this level of severity, teeth have a greater potential for remineralisation than teeth with higher severity caries (ICDAS codes 3 to 6, which represent assessments ranging from cavitated caries in enamel to caries in dentin) (ICDAS II 2008).
Prevention of caries in primary molars is important as the progression of caries is faster here than in permanent molars, owing to thinner enamel and greater porosity (Mortimer 1970; Low 2008).
Description of the intervention
Pit and fissure sealants are applied to the pit and fissure surfaces of teeth that are highly susceptible to dental caries and resistant to other therapeutic approaches such as fluorides and mechanical plaque control (Wright 2016). They can be categorised broadly as resin‐based sealants, glass ionomer sealants and hybrid sealants (Figure 2). The first materials used as pit and fissure sealants were methyl methacrylate or cyanoacrylate cements (Cueto 1967; Herald 2013). With the invention of bisphenol A‐glycidyl methacrylate (BIS‐GMA), resin‐based sealants were introduced (Bowen 1982).
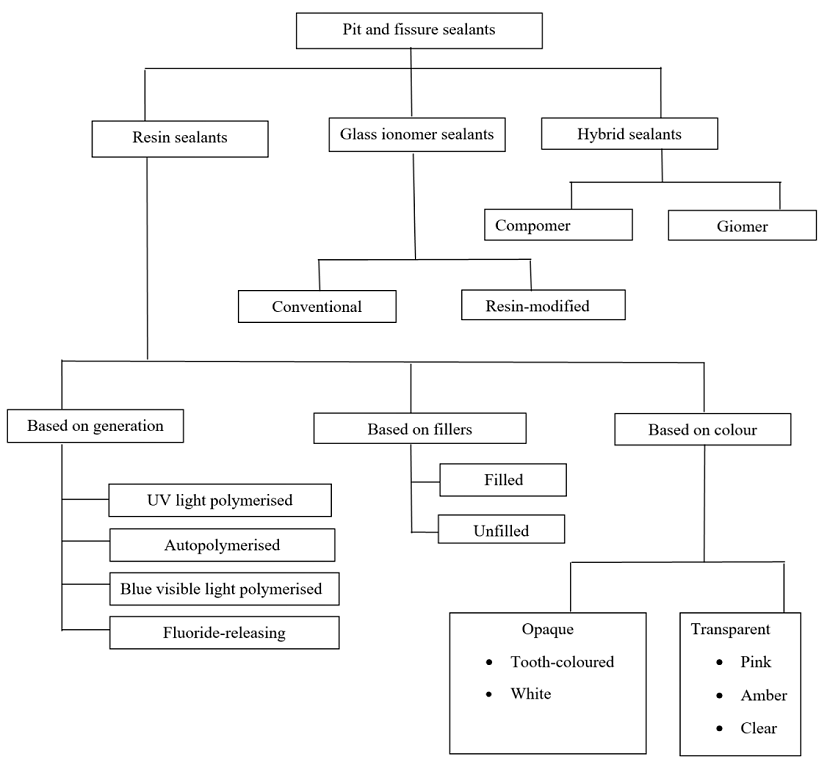
Classification of sealants
-
Resin‐based sealants can be classified into four generations based on their content and method of polymerisation. First‐generation sealants were cyanoacrylates activated using an ultraviolet light source of 365 nm. Due to observed degradation in the oral cavity over time, these sealants are no longer available (Pinkham 2005). Second‐generation resin sealants contain BIS‐GMA or urethane dimethacrylate‐based products, which are autopolymerising or chemically cured (Donly 2002; Pinkham 2005). Third‐generation sealants contain a di‐ketone initiator and a reducing agent to initiate polymerisation, and are visible light‐activated (Sanders 2015). Fourth‐generation sealants are fluoride‐releasing resin‐based products, which have an additional potential benefit in terms of caries prevention (Donly 2002).
-
Glass ionomer sealants are made from glass ionomer cements and can bond chemically to the tooth structure. These sealants are used widely due to their fluoride‐releasing properties. They have the advantage of being less sensitive to moisture, making them a potential alternative to resin‐based sealants when moisture control is an issue. However, glass ionomer sealants have poor retention rates on teeth compared with resin‐based sealants (Simonsen 2002). Glass ionomer sealants can be conventional (chemically cured) or resin modified, in which conventional GICs are combined with resin components that are light cured (Anusavice 2013; Arrondo 2009).
-
Hybrid sealants, such as compomers and giomers, are a combination of resin and GICs. Compomers are polyacid‐modified composite resins and giomers are fluoride‐releasing materials made of urethane resins containing surface prereacted glass ionomer filler particles (Carol 2015). These are relatively newer materials and data on their caries‐preventive effects are limited.
How the intervention might work
The anatomy of the pit and fissure surfaces makes them difficult to clean, and they are thus at higher risk for caries development. If the morphology of fissures is deep and complex, it can lead to the entrapment of food debris, which in turn acts as a niche for plaque formation and bacterial growth (Figure 1). Cleaning deep and complex fissures is difficult as a toothbrush bristle cannot reach into the depth of the fissure. Thus, even excellent home care may not be successful in cleaning a deep fissure (Vann 1999).
Sealants applied to sound occlusal teeth surfaces occlude these pits and fissures forming a physical barrier that helps to prevent caries development. The physical barrier may block the carbohydrates from reaching the bacteria at the base of these structures, as well as making the surfaces easier to clean (Herald 2013; Vann 1999). While resin‐based sealants prevent caries by forming a physical barrier (Mertz‐Fairhurst 1984), GIC sealants bond chemically to dental tissues and have anticariogenic effect by releasing fluoride (McLean 1992).
Why it is important to do this review
The use of sealants in preventing caries in permanent teeth in children and adolescents is well established. A Cochrane systematic review found moderate‐quality evidence that resin‐based sealants were more effective than no sealant for preventing tooth decay in the permanent dentition, reducing it by between 11% and 51% more than in children without sealant when measured two years after sealant application (Ahovuo‐Saloranta 2017). However, results were inconclusive when glass ionomer‐based sealants were compared with no sealant and when one type of sealant material was compared with another. In the four included studies that assessed possible problems from the use of sealants, no adverse effects were reported. Use of sealants for the prevention of caries in permanent teeth have been recommended in clinical guidelines from professional bodies like the American Dental Association, the American Association of Pediatric Dentistry and the British Society of Paediatric Dentistry (Beauchamp 2008; AAPD 2013; BSPD 2000). When it comes to primary teeth, however, empirical data and systematic reviews on the effectiveness of sealants exclusively in primary molars are lacking. The clinical recommendations for the management of deep pits and fissures on primary teeth have been extrapolated from the findings of sealant effectiveness in permanent teeth (AAPD 2013). The lack of synthesised evidence from trials in the primary dentition is a concern as sealants in primary teeth are increasingly being recommended as part of preventive programmes for young children (AAPD 2013; Gooch 2009).
There is uncertainty regarding the use of sealants in primary molars. Opponents of the placement of sealants in primary molars believe that the flatter fissures of primary molars do not support long‐term sealant retention (Horowitz 1982). Apprehension about sealing over incipient (white spot) and non‐cavitated carious lesions is another concern (Ripa 1976). However, this concern may be unfounded. A report from the American Dental Association indicated that children with sealed sound or non‐cavitated pit and fissures in primary molars had a 76% lower risk of developing new caries than children without sealants; retention levels in primary molars ranged from 74% to 93% (Beauchamp 2008).
This review is intended to provide healthcare policymakers, practitioners and consumers with evidence about the effectiveness of pit and fissure sealants for preventing dental caries in primary teeth. It will complement the existing Cochrane Review on sealant use in permanent teeth (Ahovuo‐Saloranta 2017).
Objectives
To evaluate the effects of sealants in preventing pit and fissure caries in primary molars.
Methods
Criteria for considering studies for this review
Types of studies
We will include randomised controlled trials (RCTs) of parallel‐group and split‐mouth study designs that have investigated the prevention of caries in primary molars, with a follow‐up period of any time interval after sealant application. We will include studies in which sealants were placed on the occlusal surfaces of primary molar teeth (ICDAS codes 0, 1 and 2 (ICDAS II 2008) for the purpose of preventing caries, regardless of who undertook the application. The unit of randomisation can be the tooth or teeth, the individual or a group (e.g. school, class).
Types of participants
Children with sound primary molars or with non‐cavitated enamel caries on primary molars.
Types of interventions
We will include studies comparing sealants with no sealant or a different type of sealant for the prevention of caries on primary molars. We will also include studies in which additional caries prevention treatments were used concurrently with sealants, providing that the same adjunct was used with the intervention and comparator (the use of sealant was the only systematic difference between the trial arms).
We will exclude studies of complex interventions for the prevention of dental caries in primary teeth, such as preventive resin restorations, or studies that have used sealants in cavitated lesions.
Types of outcome measures
Primary outcomes
-
Incidence of new dental caries on the treated occlusal surface(s) of sound surfaces of primary molar(s) (dichotomous outcome ‐ absence or presence of a new carious lesion)
-
Progression of non‐cavitated enamel caries (dichotomous outcome ‐ progression into enamel/dentine or no progression)
-
Mean caries increment, measured continuously as change in decayed, missing and filled teeth/surfaces (dmft/s) at the occlusal surface
Secondary outcomes
-
Duration of retention of sealant
-
Adverse events (any type) and safety of sealant
Search methods for identification of studies
Electronic searches
Cochrane Oral Health’s Information Specialist will conduct systematic searches for randomised controlled trials and controlled clinical trials. Due to the Cochrane Centralised Search project to identify all clinical trials on the database and add them to CENTRAL, only recent months of the Embase database will be searched. Please see the searching page on the Cochrane Oral Health website for more information. No other restrictions will be placed on the language or date of publication when searching the electronic databases.
The Information Specialist will search the following databases for relevant trials:
-
Cochrane Oral Health Trials Register;
-
the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Register of Studies;
-
MEDLINE Ovid (from 1946 onwards);
-
Embase Ovid (previous 6 months to present).
The subject strategies for databases will be modelled on the search strategy designed for MEDLINE Ovid (Appendix 1). Where appropriate, this will be combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.c. (Lefebvre 2011)).
Searching other resources
We will search the following trial registries:
-
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov/));
-
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch).
We will check the bibliographies of included studies and any relevant systematic reviews identified for further references to relevant trials.
We will not perform a separate search for adverse effects of this intervention. We will consider adverse effects described in included studies only.
Data collection and analysis
Selection of studies
Two review authors will independently screen the titles and abstracts retrieved from the electronic searches, and exclude studies that clearly do not meet the eligibility criteria. We will retrieve and independently assess full‐text articles for eligibility in duplicate. We will resolve any disagreement through discussion or, if required, by consultation with a third review author. We will record all studies excluded at the full‐text stage that do not meet the inclusion criteria, along with reasons for exclusion, in 'Characteristics of excluded studies' tables. We will present a summary of the study selection process in a PRISMA flow diagram (PRISMA 2009).
Data extraction and management
Two review authors will independently extract data from each included study using a specially designed data extraction form, which we will first pilot on a small sample of studies. We will resolve disagreements through discussion, consulting a third review author to achieve a consensus when necessary. We will contact study authors for clarification or missing data where necessary and feasible. We will record the following data for each included study in a 'Characteristics of included studies' table.
-
Trial characteristics ‐ author, title, source, date of publication, country and language, trial design, location, number of centres, recruitment period, study duration, number of participants at the start of the study, method of allocation, inclusion and exclusion criteria, number of participants randomised and analysed, masking of participants, outcome assessors and personnel, exclusion of participants after randomisation, proportion of follow‐up losses
-
Participant characteristics – age, sex, dmft/s, stage of caries, comparability of baseline characteristics
-
Intervention characteristics – detailed description of the intervention and comparator, including timing and duration, information on compliance with the intervention (type of sealant, type and number of operators, instruments used)
-
Comparator characteristics – detailed description of the comparator, type of control (placebo, no sealant, different sealant type)
-
Outcome characteristics ‐ details of the outcomes reported, including method of assessment and time(s) assessed
-
Other characteristics – adverse events, contact address of authors, funding sources, declarations or conflicts of interest
Assessment of risk of bias in included studies
Two review authors will independently assess the risk of bias in each included study using the Cochrane domain‐based, two‐part tool as described in Chapter 8 of the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011). We will contact study authors for clarification or missing information where necessary and feasible. We will resolve any disagreements through discussion, consulting a third review author to achieve a consensus when necessary.
We will complete a 'Risk of bias' table for each included study. For each 'Risk of bias' domain, we will first describe what was reported to have happened in the study. This will provide the rationale for our judgement of that domain as at low, high or unclear risk of bias.
We will assess the following domains:
-
random sequence generation (selection bias);
-
allocation concealment (selection bias);
-
blinding of participants and personnel (performance bias);
-
incomplete outcome data (attrition bias);
-
selective outcome reporting (reporting bias);
-
other bias.
We will categorise the overall risk of bias of individual studies as being at low, high or unclear risk according to the following criteria (Higgins 2011):
-
low risk of bias (plausible bias unlikely to seriously alter the results) if all domains are at a low risk of bias;
-
high risk of bias (plausible bias that seriously weakens confidence in the results) if one or more domains are at high risk of bias;
-
unclear risk of bias (plausible bias that raises some doubt about the results) if one or more domains are at unclear risk of bias but none are at high risk of bias.
We will also present a 'Risk of bias' summary graphically.
Measures of treatment effect
For continuous outcomes measured on the same scale, we will use the means and standard deviations (SDs) to obtain the difference in means and 95% confidence intervals (CIs). Where the same outcomes are measured on different scales, we will use the standardised mean difference with 95% CIs. For dichotomous outcomes, we will express the estimate of effect as odds ratios (OR) with 95% CIs.
Unit of analysis issues
For parallel‐group and cluster‐randomised studies, we will use the individual as the unit of analysis. If clustered data are provided (e.g. several measurements per individual, more than one tooth or surface, clustering of children at school or class level), we will adjust the standard errors of the estimates to take clustering into account (as outlined in Section 16.3.4 of the Cochrane Handbook for Systemic Reviews of Interventions (Higgins 2011).
For split‐mouth studies, we will use the tooth pair within an individual as a unit of analysis.
For studies that have used a split‐mouth design but reported the data as a parallel‐group study, we will calculate the OR using the Becker‐Balagtas method, as outlined in (Curtin 2002), using Stata software version 14.
Multiple‐armed trials
We will include multiarmed trials and combine the relevant intervention groups to create a single pair‐wise comparison or select one pair of relevant interventions and exclude the others in our meta‐analysis.
Dealing with missing data
We will attempt to contact the author(s) of all included studies, where feasible, for clarification of missing data and details of any outcomes that may have been measured but not reported. We will use the methods described in Section 7.7.3 of the Cochrane Handbook for Systemic Reviews of Interventions to estimate missing SDs (Higgins 2011). We will not use any other statistical methods or perform any further imputation to account for missing data.
Assessment of heterogeneity
If a sufficient number of studies is included in a meta‐analysis, we will assess clinical heterogeneity in the included studies by examining the similarity between the types of participants, interventions and outcomes. We will also assess heterogeneity statistically using the Chi2 test, where a P‐value < 0.1 indicates statistically significant heterogeneity. We will quantify heterogeneity using the I2 statistic. A guide to interpretation of the I2 statistic, given in Section 9.5.2 of the Cochrane Handbook for Systemic Reviews of Interventions, is as follows: 0% to 40% heterogeneity might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% may represent substantial heterogeneity; 75% to 100% represents considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
If at least 10 studies are included in a meta‐analysis, we will assess publication bias according to the recommendations on testing for funnel plot asymmetry provided in the Cochrane Handbook for Systemic Reviews of Interventions (Sterne 2011). If asymmetry is identified, we will examine possible causes.
Data synthesis
We will carry out meta‐analyses using Review Manager 5.3 (RevMan 2014). For each comparison, we will pool the results of studies with similar characteristics in terms of participants, interventions and outcome measures. We will group and analyse studies according to whether they have evaluated the effects of different sealant types, or compared a sealant with placebo or no sealant.
Our approach will be to use a random‐effects model. With this approach, the CIs for the pooled intervention effect will be wider than those obtained using a fixed‐effect approach, leading to a more conservative interpretation. Where feasible, we will pool the results of parallel‐group and split‐mouth studies using the generic inverse variance method.
We will provide an additional table reporting the results from studies not suitable for inclusion in meta‐analysis.
Subgroup analysis and investigation of heterogeneity
If data are available, we will perform subgroup analyses based on the following characteristics:
-
type of sealant used;
-
duration of follow‐up (short duration (12 months or less) versus long duration (more than 12 months));
-
grade of caries (sound tooth versus non‐cavitated enamel caries).
Sensitivity analysis
We will perform sensitivity analyses to assess the impact of excluding studies with unclear or high risk of bias from the analyses. In meta‐analyses that include several small studies and a single very large study, we will undertake a sensitivity analysis comparing the effect estimates from both random‐effects and fixed‐effect models. If these are different, we will report on both analyses in the results section, and consider the possible interpretation of such findings.
Presentation of main results
We will produce a 'Summary of findings' table for each comparison and for the main outcomes (caries incidence; caries progression into enamel, dentine or both; retention of sealant; and adverse events) using GRADE methods and software (GRADE 2004; GRADEpro 2014). We will assess the quality of the body of evidence for each comparison and outcome by considering study design limitations (i.e. the overall risk of bias of the included studies, in particular, which, if any, domains are assessed as being at high risk of bias), the directness of the evidence, the consistency of the results, the precision of the estimates and the risk of publication bias. We will categorise the quality of each body of evidence as high, moderate, low or very low.

Aetiopathogenesis of pit and fissure caries

Classification of sealants

