Antiinflamatorios no esteroideos (AINE) preoperatorios y preventivos para el dolor posoperatorio en adultos sometidos a todo tipo de cirugía
Resumen
Antecedentes
El dolor posoperatorio es una consecuencia común de la cirugía y puede tener muchos efectos negativos perioperatorios. Se ha indicado que la administración de analgesia antes de un estímulo doloroso puede mejorar el control del dolor. Los antiinflamatorios no esteroideos (AINE) preoperatorios se definieron como los que se administran antes de la cirugía pero no se continúan después y los AINE preventivos como los que se administran antes de la cirugía y se continúan después. Estos se compararon con un grupo control al que se le administraron AINE después de la cirugía en lugar de antes de la misma.
Objetivos
Evaluar la eficacia de los AINE preoperatorios y preventivos para reducir el dolor posoperatorio en adultos sometidos a todo tipo de cirugía.
Métodos de búsqueda
Se hicieron búsquedas en las siguientes bases de datos electrónicas: CENTRAL, MEDLINE, Embase, AMED y CINAHL (hasta junio de 2020). Además, se buscaron estudios no publicados en tres bases de datos de ensayos clínicos, resúmenes de congresos, bases de datos de literatura gris y listas de referencias de artículos identificados. No se aplicaron restricciones de idioma ni de fecha de publicación.
Criterios de selección
Sólo se incluyeron ensayos controlados aleatorizados (ECA) de grupos paralelos. Se incluyeron participantes adultos sometidos a cualquier tipo de cirugía. Los AINE preoperatorios se definieron como aquellos que se administran antes de la cirugía pero no se mantienen después y los AINE preventivos como aquellos que se administran antes de la cirugía y se mantienen después. Estos se compararon con un grupo control al que se le administraron AINE después de la cirugía en lugar de antes de la misma. Se incluyeron los estudios que administraron la medicación por cualquier vía, pero no la tópica.
Obtención y análisis de los datos
Se utilizaron los métodos estándar previstos por Cochrane, así como una novedosa prueba de sesgo de publicación creada por el grupo de investigación. Se utilizó el método GRADE para evaluar la certeza de la evidencia de cada desenlace. Los desenlaces incluyeron el dolor posoperatorio agudo (diferencia mínima clínicamente importante [DMCI]: 1,5 en una escala de 0 a 10), los efectos adversos de los AINE, las náuseas y los vómitos, el consumo de morfina en 24 horas (DMCI: 10 mg de reducción), el tiempo hasta la solicitud de analgésicos (DMCI: una hora), el prurito, la sedación, la satisfacción del paciente, el dolor crónico y el tiempo hasta la primera deposición (DMCI: 12 horas).
Resultados principales
Se incluyeron 71 ECA. Hay siete estudios pendientes de clasificación. Se incluyeron 45 estudios que evaluaron los AINE preoperatorios y 26 estudios que evaluaron los AINE preventivos. Se consideró que sólo cuatro estudios tenían un bajo riesgo de sesgo para la mayoría de los dominios. Las cirugías y los AINE utilizados variaron, aunque la mayoría de los estudios se realizaron en cirugía abdominal, ortopédica y dental. La mayoría de los estudios se realizaron en la atención secundaria y en participantes de bajo riesgo. Las exclusiones más frecuentes fueron los participantes que tomaban fármacos analgésicos antes de la cirugía y aquellos con dolor crónico.
AINE preoperatorios en comparación con AINE después de la incisión
En el caso de los AINE preoperatorios, es probable que haya una disminución del dolor posoperatorio agudo temprano (DM ‐0,69; IC del 95%: ‐0,97 a ‐0,41; estudios = 36; participantes = 2032; I2 = 96%; evidencia de certeza moderada). Ninguno de los estudios incluidos que informaron sobre el dolor posoperatorio agudo informó los eventos adversos como un desenlace. Podría haber poca o ninguna diferencia entre los grupos en las náuseas y los vómitos a corto plazo (RR 1,00; IC del 95%: 0,34 a 2,94; estudios = 2; participantes = 100; I2= 0%; evidencia de certeza baja) o a largo plazo (RR 0,85; IC del 95%: 0,52 a 1,38; estudios = 5; participantes = 228; I2 = 29%; evidencia de certeza baja). Podría haber una reducción del dolor posoperatorio agudo tardío (DM ‐0,22; IC del 95%: ‐0,44 a 0,00; estudios = 28; participantes = 1645; I2 = 97%; evidencia de certeza baja). Podría haber una reducción del consumo de morfina en 24 horas con los AINE preoperatorios (DM ‐5,62 mg; IC del 95%: ‐9,00 mg a ‐2,24 mg; estudios = 16; participantes = 854; I2 = 99%; evidencia de certeza baja) y un aumento del tiempo hasta la solicitud de analgésicos (DM 17,04 minutos; IC del 95%: 3,77 minutos a 30,31 minutos; estudios = 18; participantes = 975; I2 = 95%; evidencia de certeza baja). Podría haber poca o ninguna diferencia en los eventos adversos de los opiáceos como el prurito (RR 0,40; IC del 95%: 0,09 a 1,76; estudios = 4; participantes = 254; I2 = 0%; evidencia de certeza baja) o la sedación (RR 0,51; IC del 95%: 0,16 a 1,68; estudios = 4; participantes = 281; I2 = 0%; evidencia de certeza baja), aunque el número de estudios incluidos para estos desenlaces fue pequeño. Ningún estudio informó sobre la satisfacción de los pacientes, el dolor crónico ni el tiempo hasta la primera deposición en el caso de los AINE preoperatorios.
AINE preventivos en comparación con AINE después de la incisión
Para los AINE preventivos, podría haber poca o ninguna diferencia en el dolor posoperatorio agudo temprano (DM ‐0,14; IC del 95%: ‐0,39 a 0,12; estudios = 18; participantes = 1140; I2 = 75%; evidencia de certeza baja). Un estudio informó sobre los eventos adversos de los AINE (reintervención por hemorragia), aunque los eventos fueron escasos, lo que no permitió establecer conclusiones significativas (RR 1,95; IC del 95%: 0,18 a 20,68). Podría haber poca o ninguna diferencia en las tasas de náuseas y vómitos a corto plazo (RR 1,26; IC del 95%: 0,49 a 3,30; estudios = 1; participantes = 76; evidencia de certeza baja) o a largo plazo (RR 0,85; IC del 95%: 0,52 a 1,38; estudios = 5; participantes = 456; I2 = 29%; evidencia de certeza baja). Podría haber una reducción del dolor posoperatorio agudo tardío (DM ‐0,33; IC del 95%: ‐0,59 a ‐0,07; estudios = 21; participantes = 1441; I2 = 81%; evidencia de certeza baja). Probablemente haya una reducción del consumo de morfina en 24 horas (DM ‐1,93 mg; IC del 95%: ‐3,55 mg a ‐0,32 mg; estudios = 16; participantes = 1323; I2 = 49%; evidencia de certeza moderada). No se sabe si hay alguna diferencia en el tiempo hasta la solicitud de analgésicos (DM 8,51 minutos; IC del 95%: ‐31,24 minutos a 48,27 minutos; estudios = 8; participantes = 410; I2 = 98%; evidencia de certeza muy baja). Al igual que con los AINE preoperatorios, podría haber poca o ninguna diferencia en otros eventos adversos de los opiáceos como el prurito (RR 0,56; IC del 95%: 0,09 a 3,35; estudios = 3; participantes = 211; I2 = 0%; evidencia de certeza baja) y la sedación (RR 0,84; IC del 95%: 0,44 a 1,63; estudios = 5; participantes = 497; I2= 0%; evidencia de certeza baja). Probablemente haya poca o ninguna diferencia en la satisfacción de los pacientes (DM ‐0,42; IC del 95%: ‐1,09 a 0,25; estudios = 1; participantes = 72; evidencia de certeza moderada). Ningún estudio informó sobre el dolor crónico. Probablemente haya poca o ninguna diferencia en el tiempo hasta la primera deposición (DM 0,00; IC del 95%: ‐15,99 a 15,99; estudios = 1; participantes = 76; evidencia de certeza moderada).
Conclusiones de los autores
Hubo alguna evidencia de que los AINE preoperatorios y preventivos reducen el dolor y el consumo de morfina, aunque esto no fue unánime para todos los desenlaces de dolor y consumo de morfina. Las diferencias encontradas no fueron clínicamente significativas, aunque no se pueden excluir en las cirugías más dolorosas. Además, sin una evidencia de reducción de los efectos adversos de los opiáceos, la significación clínica de estos resultados es cuestionable, aunque pocos estudios informaron sobre estos desenlaces. Sólo un estudio informó de eventos adversos clínicamente significativos de los AINE administrados antes de la cirugía y, por lo tanto, se tienen muy pocos datos para evaluar la seguridad de los AINE preoperatorios o preventivos. Por lo tanto, los estudios de investigación futuros deben tener como objetivo adherirse a la metodología más alta y tener una potencia estadística suficiente para evaluar los eventos adversos graves de los AINE y las reducciones de los eventos adversos de los opiáceos.
PICO
Resumen en términos sencillos
Analgésicos como el ibuprofeno administrados antes de la operación en comparación con los administrados después de la operación en adultos sometidos a todo tipo de cirugía
El objetivo fue evaluar el efecto en la reducción del dolor en adultos de dar una única dosis de un antiinflamatorio no esteroideo (AINE: por ejemplo, ibuprofeno) antes de realizar el primer corte durante la cirugía (AINE preoperatorios) o antes del primer corte y continuado después de la cirugía (AINE preventivos).
Pregunta de la revisión
Se revisó la evidencia de los analgésicos AINE administrados antes de la cirugía en comparación con el mismo analgésico administrado sólo después de que el cirujano haya cortado la piel en adultos sometidos a todos los tipos de cirugía.
Antecedentes
La mayoría de las personas experimentan dolor después de una cirugía, el cual requiere analgésicos opiáceos fuertes (similares a la morfina). Estos medicamentos se asocian con una serie de efectos secundarios, como la reducción de la respiración, la disminución del ritmo cardíaco y la presión arterial baja, así como vómitos, somnolencia, picores y estreñimiento. Reducir la cantidad de opiáceos necesarios después de la cirugía puede limitar estos efectos secundarios y mejorar la experiencia y los desenlaces de los pacientes. En comparación con empezar a tomar analgésicos más tarde, empezar a tomarlos antes de hacer el primer corte para la cirugía podría reducir la sensibilidad al dolor y, por tanto, disminuir el dolor experimentado. Se quería determinar si la administración de analgésicos AINE antes de la cirugía era más eficaz que la administración del mismo analgésico, en la misma dosis, después de la cirugía.
Características de los estudios
En la literatura médica se buscaron ensayos controlados aleatorizados (un tipo de estudio en el que los participantes se asignan a un grupo de tratamiento
mediante un método aleatorio). La evidencia está actualizada hasta junio de 2020. Los participantes se asignaron al azar a uno de dos grupos. Un grupo fue tratado con AINE antes de que el cirujano cortara la piel, mientras que el otro grupo recibió la misma medicación después de la incisión. Se encontraron 71 ensayos con pacientes de 18 años o más a los que se realizaron muchas operaciones diferentes. Casi todos los pacientes estaban en buena forma y sanos y se sometían a procedimientos en hospitales de todo el mundo.
Resultados clave
En 36 ensayos (2032 pacientes), el uso de AINE preoperatorios dio lugar a una pequeña reducción del dolor experimentado en las primeras seis horas después de la cirugía. Ningún estudio incluyó como desenlace los efectos secundarios graves de los AINE (hemorragias, infartos o insuficiencia renal). No hubo diferencias en las náuseas y los vómitos después de la cirugía. En 28 estudios (1645 pacientes), no hubo diferencias en el dolor entre las 24 y 48 horas posteriores a la cirugía. En 16 estudios (854 pacientes) hubo una reducción de la cantidad de analgésicos fuertes utilizados después de la cirugía y un aumento del tiempo hasta que los pacientes necesitaron estos analgésicos fuertes. A pesar de ello, no se encontró una reducción de los efectos secundarios de estos analgésicos fuertes (picor o somnolencia). Ningún estudio informó sobre la satisfacción de los pacientes, el dolor a largo plazo después de la cirugía o el tiempo hasta que los pacientes defecar.
En cuanto a los AINE preventivos, en 18 estudios (1140 pacientes), no hubo diferencias en el dolor experimentado en las primeras seis horas después de la cirugía. Un estudio informó sobre un sangrado después de la cirugía que requirió otra operación y no encontró diferencias, aunque no hubo eventos suficientes para tener seguridad sobre este resultado. No hubo diferencias en cuanto a las náuseas y los vómitos. En 21 estudios (1441 pacientes), hubo una reducción del dolor entre las 24 y 48 horas después de la cirugía y en 16 estudios (1323 pacientes) una reducción de la cantidad de analgésicos fuertes utilizados después de la cirugía. No hubo diferencias en el tiempo hasta la solicitud de analgésicos fuertes. No hubo diferencias en cuanto al picor, la somnolencia ni la satisfacción del paciente. Ningún estudio informó sobre el dolor a largo plazo. No hubo diferencias en el tiempo hasta la primera defecación.
Certeza de la evidencia
Aunque se encontraron algunas diferencias en el dolor y el uso de analgésicos, la certeza de la evidencia varió entre muy baja y moderada. Además, las diferencias encontradas no eran lo suficientemente grandes como para que los pacientes las consideraran importantes. Esto se debió a las deficiencias en cómo se realizaron los estudios, el escaso número de pacientes reclutados para algunos desenlaces y los diferentes resultados entre los estudios, lo que significa que no existe seguridad en que las diferencias que se encontraron sean reales y, por lo tanto, se requieren estudios de investigación futuros.
Authors' conclusions
Summary of findings
| Pre‐emptive NSAIDs compared with post‐incision NSAIDs for postoperative pain | ||||||
| Patient or population: adults undergoing surgery Settings: secondary care and dental clinics Intervention: pre‐emptive NSAIDs Comparison: post‐incision NSAIDs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Post‐incision NSAIDs | Pre‐emptive NSAIDs | |||||
| Early acute postoperative pain (within 6 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.32 to 6.37 | Overall MD ‐0.69 (95% CI ‐0.97 to ‐0.41) Mild pain MD ‐0.24 (95% CI ‐0.51 to 0.03) Moderate pain MD ‐1.19 (95% CI ‐1.52 to ‐0.86) Severe pain MD ‐1.44 (95% CI ‐2.28 to ‐0.59) | 2032 | ⊕⊕⊕⊝ | Results not clinically significant | |
| Adverse events | N/A | N/A | N/A | N/A | N/A | No study reported this |
| Nausea and vomiting (1‐6 hours postoperatively) | 120 per 1000 | 120 per 1000 (41 to 353) | RR 1.00 (0.34 to 2.94) | 100 | ⊕⊕⊝⊝ | |
| Nausea and vomiting (6‐48 hours postoperatively) | 336 per 1000 | 278 per 1000 (175 to 464) | RR 0.85 (0.52 to 1.38) | 228 | ⊕⊕⊝⊝ | |
| Late acute postoperative pain (24‐48 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.25 to 3.49 | Overall MD ‐0.22 (95% CI ‐0.44 to 0.00) Mild pain MD ‐0.14 (95% CI ‐0.37 to 0.10) Moderate pain MD ‐0.77 (95% CI ‐1.08 to 0.47) | 1645 | ⊕⊕⊝⊝ | Results not clinically significant | |
| 24‐hour morphine consumption (mg) | The mean morphine consumption ranged across post‐incision groups from 1.97 mg to 122.75 mg | Overall MD ‐5.62 mg (95% CI ‐9.00 mg to ‐2.24 mg) Low consumption MD ‐2.66 mg (95% CI ‐4.54 mg to ‐0.79 mg) Medium consumption MD ‐5.46 mg (95% CI ‐10.73 mg to ‐0.19 mg) High consumption MD ‐15.22 mg (95% CI ‐29.67 mg to ‐0.77 mg) | 854 | ⊕⊕⊝⊝ | Results not clinically significant | |
| Time to first analgesic request (mean time in minutes) | The mean time ranged across post‐incision groups from 29.6 minutes to 1146 minutes | MD 17.04 minutes (95% CI 3.77 minutes to 30.31 minutes) | 975 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded owing to concerns over risk of bias (one level) 2Downgraded owing to concerns over risk of bias (one level) and imprecision (one level) 3Downgraded owing to concerns over risk of bias (one level) and unexplained heterogeneity (one level) Abbreviations: | ||||||
| Preventive NSAIDs compared with post‐incision NSAIDs for postoperative pain | ||||||
| Patient or population: adults undergoing surgery Settings: secondary care and dental clinics Intervention: preventive NSAIDs Comparison: post‐incision NSAIDs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Post‐incision NSAIDs | Preventive NSAIDs | |||||
| Early acute postoperative pain (within 6 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.18 to 6.42 | Overall MD ‐0.14 (95% CI ‐0.39 to 0.12) Mild pain MD ‐0.04 (95% CI ‐0.31 to 0.24) Moderate pain MD ‐0.24 (95% CI ‐0.92 to 0.45) Severe pain MD ‐0.80 (95% CI ‐1.23 to ‐0.37) | 1140 | ⊕⊕⊝⊝ | ||
| Adverse events (Re‐operation for bleeding (reoperation for major bleeding within 30 days (yes/no))) | 25 per 1000 | 49 per 1000 (5 to 517) | RR 1.95 (0.18 to 20.68) | 81 | ⊕⊕⊝⊝ | Study performed in tonsillectomy so unclear for other operations. Other adverse events not reported |
| Nausea and vomiting (1‐6 hours postoperatively) | 162 per 1000 | 205 per 1000 (79 to 535) | RR 1.26 (0.49 to 3.30) | 76 | ⊕⊕⊕⊝ | |
| Nausea and vomiting (6‐48 hours postoperatively) | 245 per 1000 | 282 per 1000 (159 to 299) | RR 0.89 (0.65 to 1.22) | 456 | ⊕⊕⊝⊝ | |
| Late acute postoperative pain (24‐48 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.42 to 4.6 | Overall MD ‐0.33 (95% CI ‐0.59 to ‐0.07) Mild pain MD ‐0.33 (95% CI ‐0.62 to ‐0.05) Moderate pain MD ‐0.23 (95% CI ‐0.65 to 0.19) | 1441 | ⊕⊕⊝⊝ | Results not clinically significant | |
| 24‐hour morphine consumption (mg) | The mean morphine consumption ranged across post‐incision groups from 1.08 mg to 42.31 mg | Overall MD ‐1.93 mg (95% CI ‐3.55 mg to ‐0.32 mg) Low consumption MD ‐0.32 mg (95% CI ‐0.40 mg to ‐0.24 mg) Medium consumption MD ‐3.77 mg (95% CI ‐7.27 mg to ‐0.26 mg) | 1323 | ⊕⊕⊕⊝ | Results not clinically significant | |
| Time to first analgesic request (mean time in minutes) | The mean time ranged across post‐incision groups from 3.15 minutes to 523.1 minutes | MD 8.51 minutes (95% CI ‐31.24 minutes to 48.27 minutes) | 410 | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded owing to concerns over risk of bias (one level) and unexplained heterogeneity (one level) 2Downgraded owing to concerns over imprecision (two levels) and indirectness of evidence (one level) 3Downgraded owing to concerns over imprecision (one level) and indirectness of evidence (one level) 4Downgraded owing to concerns over risk of bias (one level) and imprecision (one level) 5Downgraded owing to concerns over risk of bias (one level) 6Downgradedowing to concerns over imprecision (one level), risk of bias (one level) and unexplained heterogeneity (one level) Abbreviations: | ||||||
Background
This review contains text from a previous Cochrane protocol and review on pre‐emptive and preventive opioids (Doleman 2017a; Doleman 2018b). Throughout the review, we have used nonsteroidal anti‐inflammatory drugs (NSAIDs) as an umbrella term which consists of non‐selective NSAIDs (no specific enzyme activity) and cyclooxygenase‐2 (COX‐2) inhibitors (specific for COX‐2 enzyme).
Description of the condition
Postoperative pain is a common consequence of surgery that affects around 80% of patients. The severity of postoperative pain is variable, with 18% to 25% of patients suffering extreme pain (Apfelbaum 2003; Gerbershagen 2014). Pain can have deleterious effects during the postoperative period, including patient dissatisfaction (Myles 2000), interference with daily activities (Strassels 2002), pulmonary complications (Desai 1999), increases in the stress response to surgery (Desborough 2000), and an increased risk of chronic postsurgical pain (Kehlet 2006). Risk factors for severe postoperative pain include gender (Gerbershagen 2014), age (Gerbershagen 2014), the presence of preoperative pain (Gerbershagen 2014), preoperative anxiety and the type of surgery (Ip 2009). Intravenous opioids are commonly used to treat pain in the postoperative period (Benhamou 2008), however, their use is associated with many side effects such as vomiting, pruritus (itching), sedation (sleepiness) and patient concerns over addiction (Apfelbaum 2003). Therefore, alternative strategies to manage both postoperative pain and reduce postoperative opioid consumption may have important benefits for patients undergoing surgery (Frauenknecht 2019; Zhao 2004).
Description of the intervention
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are a commonly used analgesic during the peri‐operative period and include as examples: ibuprofen, naproxen, diclofenac and ketorolac. The mechanism of action of NSAIDs involves inhibition of cyclooxygenase (COX) enzymes, which are involved in the formation of hyperalgesic compounds called prostaglandins (Burian 2005). NSAIDs are effective in reducing postoperative pain, even when added to standard regimens including paracetamol (Ong 2010; Thybo 2019). Adverse events around the peri‐operative period include possible increases in bleeding (Warltier 2003), acute kidney injury and gastrointestinal ulceration (Gilron 2003). However, newer COX‐2‐specific agents that do not target gastrointestinal COX‐1 may offer lower occurrence of gastrointestinal ulceration compared with traditional NSAIDs (Jüni 2002), although studies have suggested an increased risk of cardiac events in high‐risk patients (Nussmeier 2005). Examples of these agents include celecoxib, parecoxib and rofecoxib.
Pre‐emptive analgesia involves the initiation of an analgesic agent (painkiller) prior to surgical incision (before the surgeon cuts the skin). It is thought that by initiating analgesic interventions before surgical injury, the analgesic can provide reductions in intra‐operative nociception to the central nervous system and, therefore, provide superior pain relief compared with the same analgesic given post‐incision (after the surgeon has cut the skin) (Kissin 2000). Preventive analgesia extends this definition to include increasing the intensity and duration of pre‐emptive analgesic interventions until final wound healing (Dahl 2011).
How the intervention might work
Surgical incision promotes changes in both the central and peripheral nervous system, called sensitization. Such sensitization can cause biochemical changes which manifest as hyperalgesia (the same pain stimulus causing increased pain), and allodynia (normal sensations causing pain). It is thought that by initiating analgesia before surgical incision, both peripheral and central sensitization can be reduced, resulting in reductions in intra‐operative nociception, and later, both acute and chronic postoperative pain. Preventive analgesia extends this reduction in sensitization to include the postoperative period. This enhanced definition came from an increased understanding of the development of persistent postsurgical pain, which is associated with postoperative sensitization. Postoperative sensitization may only be reduced by continuing analgesia longer into the postoperative period (Dahl 2011). As opioids are commonly used to treat pain postoperatively (Benhamou 2008), any reductions in opioid use may also result in a reduction in opioid adverse events (Doleman 2015b; Zhao 2004), and improve the patient experience.
Why it is important to do this review
Due to both its common occurrence (Apfelbaum 2003; Gerbershagen 2014), and potential deleterious effects during the postoperative period, reducing postoperative pain is an important clinical issue. A simple change in clinical practice, such as changing the timing of administration of analgesics, could have important implications for postoperative pain management. Moreover, such a change is cost‐neutral and therefore may benefit both anaesthetists in low‐income countries and those working within healthcare systems with finite resources (such as the National Health Service (NHS) in the United Kingdom).
The first review to examine the clinical effects of pre‐emptive analgesia showed pre‐emptive NSAIDs were ineffective in reducing pain scores or analgesic consumption in most of the included trials when compared to post‐incision NSAIDs (Møiniche 2002). A second review, published a few years later, demonstrated a lower analgesic consumption and delayed time to first analgesic request with pre‐emptive NSAIDs (Ong 2005). However, these reviews are now outdated and, importantly, did not evaluate reductions in opioid side effects (from reduced postoperative opioid consumption) and potential NSAID adverse events. This mandates an updated review of the evidence.
Objectives
To evaluate in adult participants undergoing all types of surgery, the effects of pre‐emptive and preventive nonsteroidal anti‐inflammatory drugs (NSAIDs) compared with post‐incision NSAIDs for reducing postoperative pain and opioid consumption.
Methods
Criteria for considering studies for this review
Types of studies
We included parallel‐group, randomized controlled trials (RCTs) only. We considered studies that did and did not use a double‐dummy placebo (for example, intervention group receives active drug before incision and placebo after incision; control group receives placebo before incision and active drug after incision). We excluded studies that included paediatric participants and pharmacokinetic studies not reporting any clinical outcomes.
Types of participants
Adult patients (18 years and above), both male and female, undergoing any type of surgery. We did not include studies that included both adult and paediatric participants.
Types of interventions
We compared both pre‐emptive nonsteroidal anti‐inflammatory drugs (NSAIDs) and preventive NSAIDs (intervention groups) with post‐incision NSAIDs (control group). We defined:
-
pre‐emptive NSAIDs as NSAIDs initiated before incision but not continued postoperatively;
-
preventive NSAIDs as NSAIDs initiated before surgical incision and continued postoperatively; and
-
post‐incision NSAIDs as the same analgesic intervention initiated after surgical incision, whether single dose (as comparator with pre‐emptive analgesia) or continued postoperatively (as comparator with preventive analgesia) (control group).
We only compared interventions if identical analgesics with identical dosages were used. In addition, we only included studies if concurrent use of other multimodal analgesic agents during the peri‐operative period were identical, in order to avoid confounding. If the studies reported multiple intervention subgroups that had comparable control groups (identical interventions), we combined these into one group using recommended methods (Higgins 2011a). We included all types of non‐selective NSAIDs and COX‐2 inhibitors, at any dose, via any route of systemic administration (oral and parenteral but not topical administration) and all types of regimen (pre‐emptive or preventive) in the analysis.
Types of outcome measures
Primary outcomes
-
Early acute postoperative pain (measured within six hours postoperatively using a validated pain scale; converted to a 0 to 10 scale where a 0 to 100 scale was used; and where multiple time points were reported, we included the earliest time point reported after post‐incision dosing).
-
Adverse events (reoperation for major bleeding within 30 days (yes/no)); acute kidney injury within 48 hours (defined using published criteria (Mehta 2007) (yes/no)); gastrointestinal ulceration or bleeding requiring endoscopy within 30 days (yes/no); myocardial infarction within 30 days (defined as two of three of the following: chest pain, electrocardiogram (ECG) changes indicating ischaemia, or > 20% rise in high‐sensitivity troponin (yes/no)). We reported these adverse events separately.
Secondary outcomes
-
Nausea and vomiting (self‐reported by the patient or requirement for anti‐emetic; we reported nausea and vomiting aggregated (yes/no)).
-
Late acute postoperative pain (measured at 24 to 48 hours postoperatively using a validated pain scale; converted to a 0 to 10 scale where a 0 to 100 scale was used; and where multiple time points were reported, we included the earliest time point reported).
-
24‐hour morphine consumption (mg) (if alternative opioids were used, we converted these to morphine‐equivalents using standard conversion factors (Doleman 2018a)).
-
Time to first analgesic request (minutes).
-
Pruritus (self‐reported by the patient (yes/no)).
-
Sedation (as defined in the individual studies (yes/no)).
-
Patient satisfaction (overall satisfaction self‐reported by the patient within 24 hours; converted to a 0 to 10 scale where a 0 to 100 scale was used).
-
Chronic pain (yes/no, measured three to six months postoperatively using a validated scale, such as the Visual Analogue Scale or the McGill Pain Questionnaire; we included the earliest time point closest to three months). We reported this outcome as a separate dichotomous and continuous outcome.
-
Time to first bowel movement (hours).
For the secondary outcomes where time points were not specified, we used the end point closest to two hours (one to six hours) to assess immediate short‐term effects, and the end point closest to 24 hours (six to 48 hours) to assess longer‐term effects. Outcomes did not form part of the study eligibility assessment, and so we included studies that met the participant, intervention and comparison criteria for inclusion in the review even if they reported no relevant outcomes. For the continuous outcomes, we considered the following to be minimal clinically important differences:
• Acute postoperative pain: 1.5 on a 0‐10 scale (Gallagher 2001; Myles 2017)
• 24‐hour morphine consumption: 10 mg reduction (Doleman 2015a)
• Time to analgesic request: one hour
• Time to first bowel movement: 12 hours.
Search methods for identification of studies
Electronic searches
We identified RCTs through literature searching designed to identify relevant trials as outlined in Chapter 4 of the Cochrane Handbook of Systematic Reviews of Interventions (Lefebvre 2019). We searched relevant systematic reviews for further trials (Møiniche 2002; Ong 2005). We did not apply restrictions due to language, publication status or publication year. We searched the following databases for relevant trials (Figure 1).
Study flow diagram.
-
Cochrane Central Register of Controlled Trials (CENTRAL, latest Issue) in the Cochrane Library.
-
MEDLINE (Ovid SP, 1946 to June 2020).
-
Embase (Ovid SP, 1974 to June 2020).
-
CINAHL (1982 to June 2020).
-
AMED (1985 to June 2020).
We developed a draft search strategy for MEDLINE. We used this as the basis for the search strategies in the other databases listed (Appendix 1).
We scanned the following trials registries for ongoing and unpublished trials.
-
World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en);
-
ClinicalTrials.gov (clinicaltrials.gov).
Searching other resources
We conducted a search of the OpenSIGLE database to identify grey literature sources. We scanned the reference lists and citations of included trials and systematic reviews identified for further references to additional trials. When necessary, we contacted trial authors for additional information. In addition, we searched the following conference proceedings to identify further unpublished studies (all years considered).
-
World Congress on Pain (International Association for the Study of Pain).
-
Anaesthetic Research Society Meetings.
-
Association of Anaesthetists of Great Britain and Ireland Winter Symposium and Annual Congress.
-
American Society of Anesthesiologists Annual Meeting.
-
European Society of Anaesthesiologists Euroanaesthesia Conference.
The search strategy was developed in consultation with the Cochrane Anaesthesia Information Specialist.
Data collection and analysis
Selection of studies
We used two review authors (BD and JPW) to independently screen the identified studies using the inclusion criteria to assess eligibility. BD and JPW resolved any disagreements by consensus. If disagreement still existed following discussion, we consulted a third review author (JLB). BD and JPW used the information from the retrieved reports to help identify any duplicate publications, such as author name, study centre, type and dose of interventions used, and study dates. We linked any duplicate publications. We inputted details of all potentially eligible studies into PubMed to identify any retracted publications and we excluded these (Eisenach 2009).
Data extraction and management
We extracted data onto an electronic database using standardized data extraction forms (Appendix 2). We performed this independently using two review authors (BD and TH/HBC/LC), and resolved any disagreements by consensus. If disagreement still existed, we consulted a third review author (JPW). We performed the analysis using one review author (BD). Where possible, we translated non‐English language studies and extracted data following translation. If data were not contained within the original research report, we contacted the corresponding author, irrespective of the age of publication. We extracted the following information:
-
Bibliographic data, including date of completion/publication.
-
Country.
-
Publication status.
-
Source of funding.
-
Trial design, e.g. parallel‐group.
-
Study setting.
-
Number of participants randomized to each trial arm and number included in final analysis.
-
Eligibility criteria and key baseline participant data, including sex and age.
-
Details of treatment regimen received by each group.
-
Details of any co‐interventions.
-
Primary and secondary outcome(s) (with definitions and, where applicable, time points).
-
Outcome data for primary and secondary outcomes (by group).
-
Duration of follow‐up.
-
Number of withdrawals (by group) and number of withdrawals (by group) due to adverse events.
-
Adverse events.
Assessment of risk of bias in included studies
We assessed risk of bias in the included studies using the Cochrane tool for assessing risk of bias (Higgins 2011b). Two review authors (BD and JPW) independently undertook assessment of risk of bias and reached agreement by consensus. We assessed risk of bias for the domains of sequence generation, allocation concealment, blinding of participants, study personnel and outcome assessors, incomplete outcome data, selective outcome reporting and other sources of bias. We assessed each domain as being at low, unclear or high risk of bias (Higgins 2011b). We presented the results in both a risk of bias summary (Figure 2) and a risk of bias graph (Figure 3). We considered a study as being at low risk of bias if it was low risk for all domains (except selective reporting bias, as some studies were published before published protocols and clinical trial databases were standard) with no high‐risk domains and as being at high risk of bias if it was high risk in any domain. Studies were assessed as being at unclear risk if they were not classified into either low or high risk.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Measures of treatment effect
We presented dichotomous outcomes as risk ratios (RRs). For continuous outcomes, we presented these as mean differences (MDs). If non‐comparable scales were used across studies but still presented as continuous data, which we expected may be the case for chronic pain and patient satisfaction, we would have presented these as standardized mean differences (SMDs). We aimed to present the outcomes of time to first analgesic and time to first bowel movement as hazard ratios (HRs) (Tierney 2007). We presented the precision of effect estimates using 95% confidence intervals (CIs).
Unit of analysis issues
As we included parallel‐group RCTs only, unit of analysis issues were not a problem for the main analysis (Higgins 2011c). For the main results, we combined different dose subgroups into one treatment group, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). If it was not possible to combine groups (for example, for continuous outcomes where the combined standard deviation (SD) could not be estimated), we treated these as separate studies and distributed the control group participants between these treatment groups to avoid analysing them twice (Higgins 2011c).
Dealing with missing data
We contacted corresponding authors for any data missing from the original publication, irrespective of publication date. If we did not receive a response, we extracted data from published graphs. If SDs were not reported, we attempted to calculate these from other reported statistics. If this was not possible, we estimated SDs from other studies with similar means. We estimated means from medians (equal if the assumption of normality holds) and SD from interquartile range (IQR)/1.35 (Higgins 2011a) or range (Hozo 2005). We did this as our previous review identified studies where medians were reported, and were more likely to be 'negative' than those reporting means, which could introduce bias into the results (Doleman 2018b). However, we assessed the robustness of our estimates by excluding all studies where means or SDs were estimated in a sensitivity analysis.
Assessment of heterogeneity
We assessed clinical heterogeneity by examining study characteristics, such as the type of population, type of surgery and the intervention used. We assessed statistical heterogeneity using the I2 statistic. We used the following recommended cut‐off values in the interpretation of the I2 statistic (Deeks 2011).
-
> 50% may represent moderate heterogeneity.
-
> 85% considerable heterogeneity.
In addition to the cut‐off values, we examined the direction of the effect in the individual studies. For clinically meaningful magnitudes of the pooled effect, we explored heterogeneity using meta‐regression when the criteria set out in Subgroup analysis and investigation of heterogeneity section were fulfilled.
Assessment of reporting biases
If we included 10 or more studies in the meta‐analysis, we assessed publication bias graphically using funnel plots and quantitatively using an updated publication bias test which uses inverse sample size on the Y axis and performs better than Egger’s linear regression test (Egger 1997) for outcomes dependent on baseline risk, such as pain and morphine consumption (Doleman 2020). This is due to correlation between standard errors and mean differences with these outcomes causing artefactual funnel plot asymmetry with Egger's test, which the use of inverse sample size corrects. Due to the low power of this test, we regarded P < 0.1 as evidence of imprecise study effects and possible publication bias.
Data synthesis
We used Review Manager 5 to aggregate study data (Review Manager 2014). We conducted separate analyses for pre‐emptive and preventive interventions. We aggregated data using the adapted DerSimonian and Laird random‐effects model (for continuous and categorical outcomes), as currently available in Review Manager 5. This is because we expected the treatment effect to vary with respect to the different populations within each study, and, therefore, there was no single underlying effect to estimate, making the random‐effects model more appropriate. We attempted to aggregate reported log hazard ratios and their associated standard errors using the generic inverse variance method, although no study adequately reported this.
Subgroup analysis and investigation of heterogeneity
If there were sufficient included studies, we conducted two separate subgroup analyses for the type of NSAIDs (non‐selective NSAIDs versus COX‐2 inhibitors) and trials with different baseline pain levels (mean pain scores in the control group of < 3 (mild), 3 to 6 (moderate) and > 6 (severe)) (Moore 2013). If we included 10 studies or more in a meta‐analysis and the included studies had a sufficient number of events, we explored reasons for heterogeneity by performing a restricted maximum likelihood, random‐effects meta‐regression where covariates were entered into the model separately based on type of anaesthesia and type of surgery (univariate analysis) (Thompson 2002). For dummy variables, we used the least effective subgroup as the reference category. We presented the R2 analogue with a corresponding P value for each covariate. We used the Knapp‐Hartung method to calculate P values (as this method more appropriately uses the t‐distribution for the between‐study variance). We performed these analyses using the software STATA Version 16.1 (Stata 2020). If there was a low number of studies or events, or both, we only performed traditional subgroup analysis, and reported the P value for subgroup differences.
Sensitivity analysis
We performed a sensitivity analysis by restricting the analysis to studies at low risk of bias (defined as low risk for randomization and allocation concealment). As we judged studies that did not use a double‐dummy design as being at high risk of bias for blinding, we assessed the impact of excluding these from the analysis. We also performed a further sensitivity analysis by excluding studies where means and SDs were estimated (both where SDs were estimated from other studies and where means and/or SDs were estimated from median and IQR). As a further sensitivity analysis, we repeated analysis assuming excluded participants suffered an event to assess the robustness of the findings. Furthermore, we performed sensitivity analyses by excluding studies with a low sample size (< 50 participants).
Summary of findings and assessment of the certainty of the evidence
We presented outcomes in a summary of findings table. We produced two summary of findings tables, one for each comparison.
-
Pre‐emptive NSAIDs versus single dose post‐incision NSAIDs.
-
Preventive NSAIDs versus continuous post‐incision NSAIDs.
The outcomes presented in the summary of findings table for each comparison included: early acute postoperative pain; adverse events; nausea and vomiting; late acute postoperative pain; 24‐hour morphine consumption and time to first analgesic request. We did not include chronic pain as no studies reported this outcome. We presented these outcomes using the GRADE approach (Schünemann 2011). We downgraded the certainty of evidence from high‐certainty to moderate‐, low‐ or very low‐certainty. Downgrading was undertaken independently by two review authors (BD and JPW) and agreement reached by consensus. Characteristics of the evidence that caused downgrading included:
-
limitations in the design and implementation of available studies, suggesting a high likelihood of bias (for example, studies not using a double‐dummy placebo design);
-
indirectness of evidence (indirect population, intervention, control or outcomes);
-
inconsistency of results (considerable heterogeneity not explained by meta‐regression or subgroup analysis);
-
imprecision of results (wide confidence intervals);
-
evidence of publication bias from asymmetry of the funnel plot.
Results
Description of studies
Results of the search
Searching of electronic databases yielded 7749 studies (Figure 1). We found another study from clinical trial databases, a further three studies from conference proceedings and seven from reference list searches. We reviewed 98 full‐text articles and 27 were not included (Excluded studies).
Included studies
Following full‐text review, we included 71 studies (Characteristics of included studies) which satisfied our inclusion criteria.
Participants and surgery
The types of surgery included were diverse. We included 10 studies performed in dental surgery, one study included hand surgery, four in general/colorectal surgery, four in total hip arthroplasty, one in total knee arthroplasty, four in laparoscopic cholecystectomy, three in laparoscopic gynaecological surgery, five in minor orthopaedic surgery, four in laparoscopic or open gynaecological procedures, three in breast surgery, seven in hysterectomy, six in spinal surgery, one in abdominal or thoracic surgery, one in laparoscopic hernia repair, five in joint arthroscopy, one in varicocelectomy, one in laparotomy, one in lung resection, one in thoracotomy, one in mandible open reduction and internal fixation (ORIF), one in diagnostic laparoscopy, two in septo/rhinoplasty, one in spinal, breast and orthopaedic surgery, one in tonsillectomy, one in major plastic surgery and one in thyroid surgery. Seventeen studies included female participants only and one included male participants only. Most studies were conducted in low‐risk participants who were American Society of Anesthesiologists grade (ASA) 1 or 2 and seven included ASA 1‐3 participants, whilst others did not specify any exclusions on the basis of ASA grade.
Settings
Most studies were conducted in secondary care although other settings included dental clinics or dental medical schools. In terms of countries in which the trials were conducted, these included: one in Peru, four in the UK, one in Spain, four in India, 12 in China, three in France, two in Ireland, one in Denmark, six in the USA, three in Italy, one in Mexico, four in Germany, seven in Turkey, two in Romania, three in Poland, three in Austria, one in Australia, two in Japan, one in Iran, one in Canada, one in Northern Ireland, one in Greece, one in Finland, one in Sweden, one in Belgium, one in Indonesia, one in Thailand and two studies where the country was unclear.
Interventions
We included 52 studies which studied non‐selective NSAIDs and 19 which studied COX‐2 inhibitors. We included 45 studies which were pre‐emptive (single‐dose intervention not continued postoperatively) and 26 studies were preventive (intervention continued postoperatively).
Comparators
All included studies gave identical post‐incision doses as those that did not were excluded. In terms of post‐incision dose timing, 53 studies gave post‐incision doses postoperatively or at the end of surgery and 18 studies gave doses intraoperatively.
Funding
Many studies did not report sources of funding or stated no funding; overall, the total was 56 studies. Eleven studies specified that they received non‐industry funding and three studies reported industry funding. One study appeared to have authors who were pharmaceutical company employees.
Postoperative opioids and concurrent analgesia
The included studies used a diverse range of opioids for postoperative analgesia so we used conversion factors to calculate intravenous (IV) morphine equivalents. We included 16 studies which used no postoperative opioids or their use was not reported. Seventeen studies used patient controlled analgesia (PCA) morphine, one used nalbuphine, two used intramuscular (IM)/IV morphine, five used IV or IM pethidine, one used IV fentanyl and morphine, two used IV fentanyl, one used fentanyl PCA, one used buprenorphine, one used an IV morphine infusion, five used a tramadol PCA/IV, one used oral oxycodone, three used IV/PCA piritramide, two used oral or IV tramadol, one used IV and subcutaneous (SC) morphine, one used IV papaveretum, one used IV morphine and pethidine, one used IV fentanyl and codeine, one used cyclimorph and codeine, one used a diamorphine PCA, one used IV/IM oxycodone, one used dextropropoxyphene, one used sufentanil and tramadol, one used fentanyl, hydrocodone and morphine PCA, one used PCA butorphanol, one used a sufentanil PCA and one study used an unspecified analgesic.
In terms of concurrent analgesia, 52 studies reported no concurrent multimodal analgesia or did not mention any in the manuscript. Two studies used paracetamol and NSAIDs, two used NSAIDs which were different NSAIDs from the intervention, one used nurse‐controlled NSAIDs which were the same as the intervention NSAID, one used metamizole, 10 used paracetamol, one used codeine and NSAIDs, one used paracetamol, tramadol and metamizole and one used paracetamol, NSAIDs and codeine.
Excluded studies
We excluded 20 studies (Characteristics of excluded studies) which did not satisfy our inclusion criteria. Three studies were excluded because they were cross‐over trials. Seven studies included different doses of pre‐emptive or preventive versus post‐incision interventions. Two studies were excluded because they studied combination therapy rather than just NSAIDs/COX‐2 inhibitors. Two studies were excluded as they did not have any post‐incision group. Four studies were excluded as they included paediatric participants. Two studies were excluded as interventions had different routes of administration and were, therefore, not comparable.
Studies awaiting classification
There were seven studies (Characteristics of studies awaiting classification) awaiting classification. We were unable to translate two studies. We were unable to obtain the full text of another five studies via the British Library or contacting the study authors.
Risk of bias in included studies
Risk of bias assessments were conducted for the following domains.
Allocation
We included 31 studies which did not report details for randomization so were rated as having unclear risk. Nine studies used a random number chart or table, 27 used computer‐generated randomization, two used a random draw of envelopes or lots, one used block randomization and one used a random number generator so all of these studies were deemed as having low risk of bias. In terms of allocation concealment, 44 studies did not report their method of allocation concealment and four studies did not include enough details so both were deemed as having unclear risk of bias. In addition, a further nine studies reported envelopes but not enough details on envelope safeguards so were also deemed as having unclear risk of bias. Nine studies used third parties for randomization or allocation was pharmacy‐controlled so were deemed as having low risk of bias, whilst five studies reported sealed, sequentially numbered and opaque envelopes so were also deemed as having low risk of bias for allocation concealment.
Blinding
We included 14 studies which did not report the use of a double‐dummy placebo so were regarded as having high risk of bias for blinding. Fifty‐five studies used a double‐dummy placebo so were deemed as having low risk of bias. In two studies, it was unclear if a double‐dummy placebo had been used so they were considered as having unclear risk of bias for this domain.
In terms of blinding of outcome assessment, two studies were regarded as high risk from the lack of blinding of post‐incision dosing. Thirty‐seven studies used blinded outcome assessment and were deemed at low risk. Twenty‐five studies did not mention any outcome assessment blinding and were therefore deemed as having unclear risk of bias for this domain. Seven studies used participant self‐report for outcome assessment or outcomes were likely blinded from the details given, so these were deemed as having low risk for blinding of outcome assessment.
Incomplete outcome data
We included 15 studies which had low dropout rates or dropouts equal in numbers and reasons which were, therefore, regarded as low risk and 30 studies analysed all enrolled participants so were also at low risk of bias. Nine studies had unclear risk as it was unclear which to which group dropouts belonged or the study did not mention dropouts. We included 13 studies which were at high risk of bias due to a high number of dropouts which could bias results or an extreme dropout which could change results. Three studies used intention‐to‐treat analysis so were deemed as having low risk of bias.
Selective reporting
We included 53 studies which did not have a published protocol or clinical trial registration so were regarded as having unclear risk for selective outcome reporting. Ten studies did not report outcomes mentioned in the methods and were therefore deemed at high risk of bias for selective outcome reporting. One study used retrospective registration so was deemed at unclear risk for selective outcome reporting. Two studies pre‐registered the trial on a clinical trials database but did not fully report all prespecified outcomes so were deemed as having high risk of bias. Two studies with a protocol registration number that we could not locate were considered at unclear risk of bias and three studies reported all prespecified outcomes so were considered at low risk of bias.
Other potential sources of bias
We included five studies which had no details on baseline characteristics so were deemed at unclear risk of other bias and one study had industry funding but, because the extent of involvement was unclear, it was considered as having unclear risk of bias. Another 16 studies had disparities in baseline characteristics which could have affected pain so were deemed at high risk. We also judged one study as having high risk as the authors appeared to be pharmaceutical company employees. We included 48 studies which had no differences in baseline characteristics or industry funding so these were deemed at low risk of bias for other sources of bias.
Effects of interventions
See: Summary of findings 1 Summary of findings; Summary of findings 2 Summary of findings
Pre‐emptive NSAIDs versus post‐incision NSAIDs
Primary outcomes
1. Early acute postoperative pain (measured within six hours postoperatively)
Thirty‐six studies reported early acute postoperative pain for pre‐emptive NSAIDs versus post‐incision NSAIDs (Bajaj 2004; Bao 2012; Buggy 1994; Bunemann 1994; Cabell 2000; Chen 2015; Colbert 1998; Demirbas 2019; Esparza‐Villalpando 2016; Fletcher 1995; Gelir 2016; Grifka 2008; Inanoglu 2007; Kaczmarzyk 2010; Karaman 2008; Lee 2008; Likar 1997; Lu 2015; Mojsa 2017; Nezafati 2017; O'Hanlon 1996; Ozer 2012; Ozyilmaz 2005; Peduto 1995; Priya 2002; Sandin 1993; Shuying 2014; Vanlersberghe 1996; Vijayendra 1998; Wang 2010; Yagar 2011; Yamashita 2006; Yan 2004; Zhang 2011; Zhang 2017; Zhou 2019). There is probably a reduction in early postoperative pain with pre‐emptive NSAIDs (MD ‐0.69, 95% CI ‐0.97 to ‐0.41; participants = 2032; I2 = 96%; Analysis 1.1). The certainty of evidence was downgraded to moderate owing to concerns over risk of bias (one level), mainly for allocation concealment. There was no evidence of publication bias both on observation of funnel plots (Figure 4) or quantitative testing (P = 0.27).
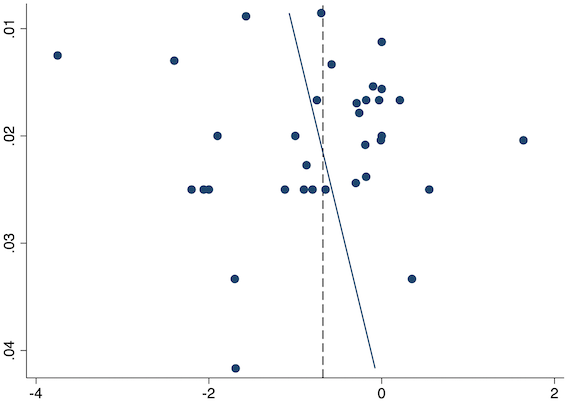
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive early acute postoperative pain
On subgroup analysis of non‐selective NSAIDs versus COX‐2 specific agents, there was no difference between the groups (COX‐2: MD ‐1.14; 95% CI ‐1.78 to ‐0.5 versus NSAIDs: MD ‐0.57; 95% CI ‐0.89 to ‐0.25; P = 0.12; Figure 5). However, on subgroup analysis of baseline pain level (Doleman 2018a), there were greater reductions in pain with higher baseline pain levels (mild: MD ‐0.24; 95% CI ‐0.51 to 0.03, moderate: MD ‐1.19; 95% CI ‐1.52 to ‐0.86 and severe: MD ‐1.44; 95% CI ‐2.28 to ‐0.59; P < 0.001; Figure 6), although statistical heterogeneity was still high in these subgroups (I2 = 86‐90%). On meta‐regression analysis, type of surgery (R2 = 54%; P = 0.003) and type of anaesthesia (R2 = 40%; P = 0.002) explained the majority of the between‐study heterogeneity. On sensitivity analysis, restricting analysis to studies with low risk for randomization and allocation concealment (Esparza‐Villalpando 2016; Kaczmarzyk 2010; Mojsa 2017; Shuying 2014; Zhang 2011) showed a smaller reduction in pain (MD ‐0.20, 95% CI ‐0.43 to 0.04; participants = 318; studies = 5; I2 = 13%). Restricting analysis to studies at low risk for blinding of participants and outcome assessors (Buggy 1994; Chen 2015; Esparza‐Villalpando 2016; Fletcher 1995; Gelir 2016; Grifka 2008; Inanoglu 2007; Kaczmarzyk 2010; Karaman 2008; Lee 2008; Likar 1997; Mojsa 2017; Ozer 2012; Pandazi 2010; Priya 2002; Shuying 2014; Zhang 2011) produced similar results to the main analysis (MD ‐0.60, 95% CI ‐0.99 to ‐0.21; participants = 888; studies = 17; I2 = 85%). Similarly, restricting analysis to studies where standard deviations were not estimated from IQR or other studies, or means were not estimated from medians (excluded studies: Bajaj 2004; Buggy 1994; Bunemann 1994; Cabell 2000; Demirbas 2019; Grifka 2008; Kaczmarzyk 2010; Lee 2008; Ozyilmaz 2005; Peduto 1995; Sandin 1993; Vanlersberghe 1996; Vijayendra 1998; Yagar 2011; Yamashita 2006; Zhang 2011) showed a similar reduction in pain to the main analysis (MD ‐0.62, 95% CI ‐0.98 to ‐0.27; participants = 1167; studies = 20; I2 = 97%). Restricting analysis to studies with more than 50 participants gave similar results to the main analysis (MD ‐0.77, 95% CI ‐1.15 to ‐0.39; participants = 1355; I2 = 97%).
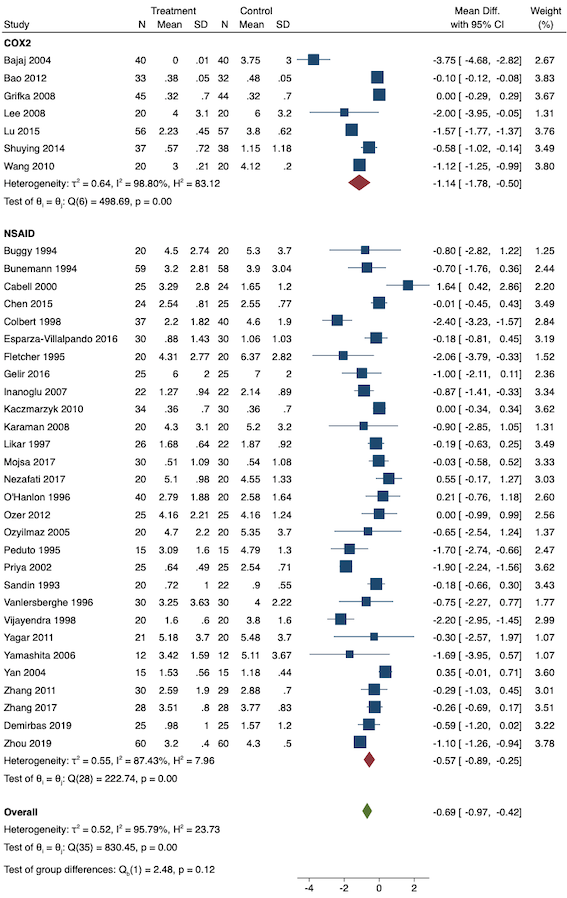
Subgroup analysis for pre‐emptive early postoperative pain (NSAID versus COX‐2 inhibitor)
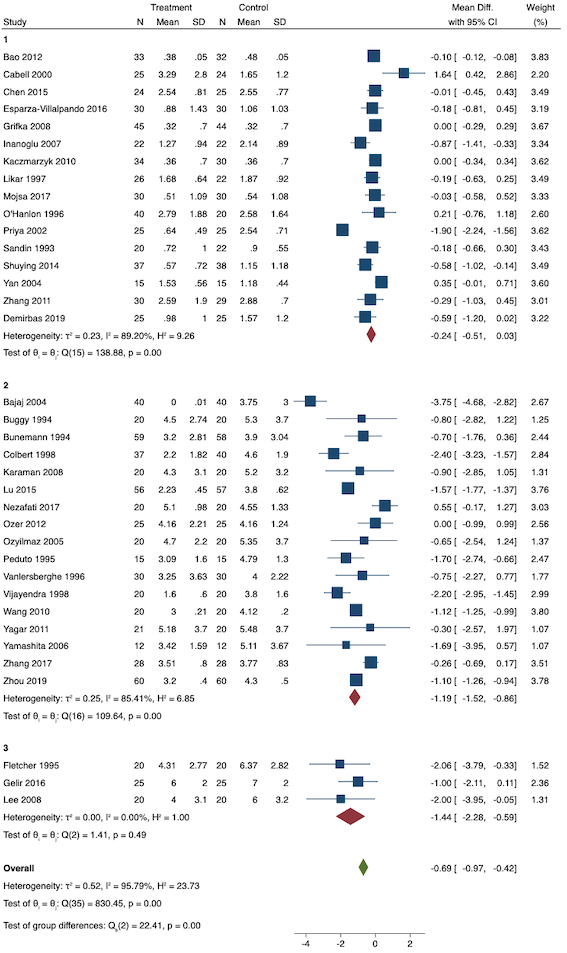
Subgroup analysis for pre‐emptive early postoperative pain (baseline pain level). Subgroups are 1 (mild), 2 (moderate) and 3 (severe)
2. Adverse events (reoperation for major bleeding within 30 days, acute kidney injury within 48 hours, gastrointestinal ulceration or bleeding requiring endoscopy within 30 days, and myocardial infarction within 30 days)
No studies reported any adverse events for pre‐emptive NSAIDs versus post‐incision NSAIDs.
Secondary outcomes
1. Nausea and vomiting (self‐reported by the patient or requirement for anti‐emetic as composite outcome (yes/no))
Short‐term nausea and vomiting
Two studies reported short‐term nausea and vomiting (Lee 2008; Vanlersberghe 1996). Overall, there may be no difference between the pre‐emptive and post‐incision groups (RR 1.00, 95% CI 0.34 to 2.94; participants = 100; studies = 2; I2 = 0%; Analysis 1.2). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly selective outcome reporting (one level), and other bias and imprecision (one level).
We were unable to conduct assessment for publication bias or meta‐regression due to the low number of included studies. On sensitivity analysis, none of the included studies were at low risk for randomization and allocation concealment. One study was at low risk for blinding (Lee 2008) which showed similar results to the main analysis (RR 0.85, 95% CI 0.52 to 1.38). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.75, 95% CI 0.18 to 3.07; participants = 60).
Long‐term nausea and vomiting
For long‐term nausea and vomiting, five studies were included (Fletcher 1995; Karaman 2008; Lee 2008; Priya 2002; Rogers 1995). There may be no difference between the groups in the number of participants suffering from long‐term nausea and vomiting (RR 0.85, 95% CI 0.52 to 1.38; participants = 228; studies = 5; I2 = 29%; Analysis 1.3). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly selective outcome reporting (one level), and other bias and imprecision (one level).
We were unable to conduct assessment for publication bias or meta‐regression due to the low number of included studies. On sensitivity analysis, restricting analysis to one study that was at low risk of bias for randomization and allocation concealment (Rogers 1995) produced similar results to the main analysis (RR 1.28, 95% CI 0.61 to 2.72; participants = 58). All the studies were at low risk of bias for blinding so results were identical to the main analysis. Restricting analysis to studies with more than 50 participants gave similar results (RR 0.69, 95% CI 0.20 to 2.42; participants = 108; I2 = 79%). Only one study (Rogers 1995) excluded participants, although it was unclear when, as each was excluded after receiving analgesia.
2. Late acute postoperative pain (measured at 24 to 48 hours)
Twenty‐eight studies reported early acute postoperative pain for pre‐emptive NSAIDs versus post‐incision NSAIDs (Bajaj 2004; Bao 2012; Bunemann 1994; Cabell 2000; Chen 2015; Demirbas 2019; Flath 1987; Fletcher 1995; Gelir 2016; Grifka 2008; Inanoglu 2007; Karaman 2008; Lee 2008; Likar 1997; Lu 2015; Mojsa 2017; Nezafati 2017; O'Hanlon 1996; Ozer 2012; Parke 1995; Sandin 1993; Shuying 2014; Vijayendra 1998; Wang 2010; Yamashita 2006; Zhang 2011; Zhang 2017; Zhou 2019). There may be a slight difference between the groups (MD ‐0.22, 95% CI ‐0.44 to 0.00; participants = 1645; I2 = 97%; Analysis 1.4). The certainty of evidence was downgraded to low due to concerns over risk of bias, mainly in allocation concealment (one level), and unexplained heterogeneity (one level). There was no evidence of publication bias both on visual inspection of funnel plots (Figure 7) or quantitative testing (P = 0.35).
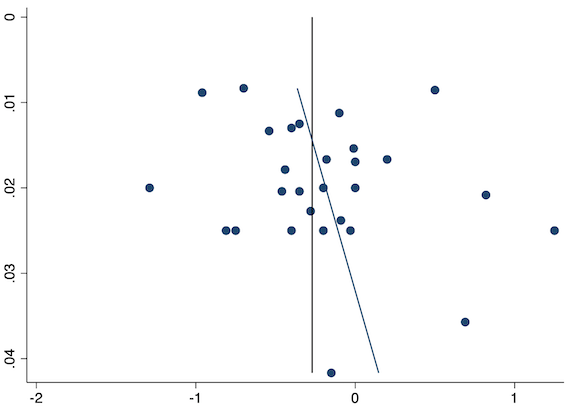
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive late acute postoperative pain
On subgroup analysis of non‐selective NSAIDs versus COX‐2 agents, there was no significant difference between the groups (P = 0.16; Figure 8). When conducting subgroup analysis for different baseline pain levels, there were greater reductions in pain with higher baseline pain levels (Doleman 2018a) (mild: MD ‐0.14; 95% CI ‐0.37 to 0.10, moderate: MD ‐0.77; 95% CI ‐1.08 to ‐0.47; P < 0.001; Figure 9). On meta‐regression analysis, although type of surgery explained some of the between‐study heterogeneity, the result was not statistically significant (R2 = 28%; P = 0.22). Type of anaesthesia did not predict any between‐study heterogeneity (R2 = 0%; P = 0.97). On sensitivity analysis, restricting analysis to the three studies that were at low risk of bias for randomization and allocation concealment (Mojsa 2017; Shuying 2014; Zhang 2011) showed a greater reduction in late acute postoperative pain (MD ‐0.52, 95% CI ‐0.65 to ‐0.40; participants = 194; I2 = 0%). When restricting analysis to studies at low risk for blinding, 14 studies were included (Chen 2015; Flath 1987; Fletcher 1995; Gelir 2016; Grifka 2008; Inanoglu 2007; Karaman 2008; Lee 2008; Likar 1997; Mojsa 2017; Ozer 2012; Parke 1995; Shuying 2014; Zhang 2011) and showed similar results to the main analysis (MD ‐0.14, 95% CI ‐0.39 to 0.11; participants = 749; I2 = 74%). When restricting analysis to studies where standard deviations were not estimated from IQR or other studies, or means were not estimated from medians (excluded studies: Bajaj 2004; Bunemann 1994; Cabell 2000; Demirbas 2019; Flath 1987; Grifka 2008; Lee 2008; Parke 1995; Sandin 1993; Vijayendra 1998; Yamashita 2006; Zhang 2011), 15 studies showed similar results to the overall analysis (MD ‐0.19, 95% CI ‐0.47 to 0.09; participants = 950; I2 = 98%). Restricting analysis to studies with more than 50 participants gave similar results (MD ‐0.16, 95% CI ‐0.41 to 0.08; participants = 999; I2 = 93%).
Subgroup analysis for pre‐emptive late postoperative pain (NSAID versus COX‐2 inhibitor)
Subgroup analysis for pre‐emptive late postoperative pain (baseline pain). Subgroups are 1 (mild) and 2 (moderate)
3. Twenty‐four‐hour morphine consumption (mg)
Sixteen studies reported 24‐hour morphine consumption for pre‐emptive NSAIDs versus post‐incision NSAIDs (Bao 2012; Chen 2015; Coli 1993; Fletcher 1995; Gelir 2016; Karaman 2008; Likar 1997; Munteanu 2016; Ozer 2012; Ozyilmaz 2005; Parke 1995; Shuying 2014; Vijayendra 1998; Wang 2010; Yamashita 2006; Zhang 2017). There may be a reduction in 24‐hour morphine consumption with pre‐emptive versus post‐incision NSAIDs (MD ‐5.62 mg, 95% CI ‐9.00 mg to ‐2.24 mg; participants = 854; studies = 16; I2 = 99%; Analysis 1.5). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly in randomization and allocation concealment (one level), and unexplained heterogeneity (one level). There was no evidence of publication bias both on visual inspection of funnel plots (Figure 10) or quantitative testing (P = 0.61).
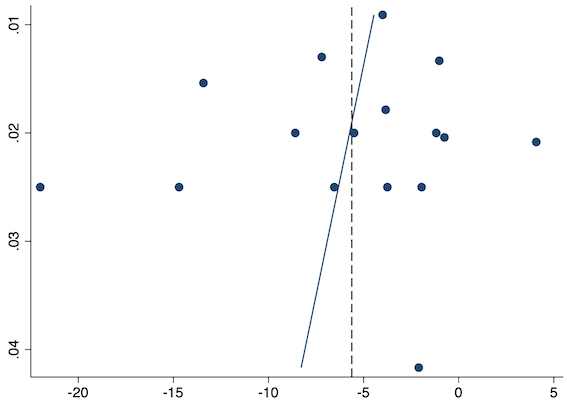
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive 24‐hour morphine consumption
On subgroup analysis of non‐selective NSAIDs versus COX‐2 specific agents, there was no difference between the groups (P = 0.25; Figure 11). When conducting subgroup analysis by baseline consumption of morphine (< 20 mg: low, 20‐50 mg: moderate and > 50 mg: high; Doleman 2018a), there was no evidence of a difference although subgroup results were imprecise (MD ‐2.66 mg; 95% CI ‐4.54 mg to ‐0.79 mg for low and ‐15.22 mg; 95% CI ‐29.67 mg to ‐0.77 mg for moderate; P = 0.16; Figure 12). On meta‐regression, type of surgery did not predict any of the between‐study heterogeneity (R2 = 0%; P = 0.66). Similarly, type of anaesthesia did not predict any between‐study heterogeneity (R2 = 0%; P = 0.72). On sensitivity analysis, restricting analysis to the one study that was at low risk of bias for randomization and allocation concealment (Shuying 2014) (MD ‐1.02 mg, 95% CI ‐2.16 mg to 0.12 mg; participants = 75) demonstrated a lower reduction in morphine consumption. Nine studies were at low risk of bias for blinding (Chen 2015; Fletcher 1995; Gelir 2016; Karaman 2008; Likar 1997; Munteanu 2016; Ozer 2012; Parke 1995; Shuying 2014). The results for these studies showed a smaller reduction in morphine consumption (MD ‐1.14 mg, 95% CI ‐2.29 mg to 0.01 mg; participants = 539; I2 = 62%). When restricting analysis to studies where means and standard deviations were not estimated, 12 studies remained (Bao 2012; Chen 2015; Fletcher 1995; Gelir 2016; Karaman 2008; Likar 1997; Munteanu 2016; Ozer 2012; Ozyilmaz 2005; Parke 1995; Shuying 2014; Zhang 2017). Results were similar to the main analysis (MD ‐4.76 mg, 95% CI ‐8.79 mg to ‐0.73 mg; participants = 700; I2 = 99%). Restricting analysis to studies with more than 50 participants gave similar results (MD ‐5.31 mg, 95% CI ‐8.61 mg to ‐2.02 mg; participants = 533; I2 = 93%).
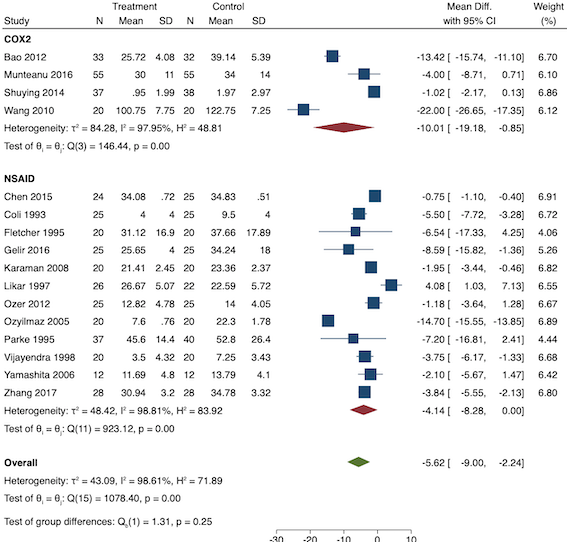
Subgroup analysis for pre‐emptive 24‐hour morphine consumption (NSAID versus COX‐2)
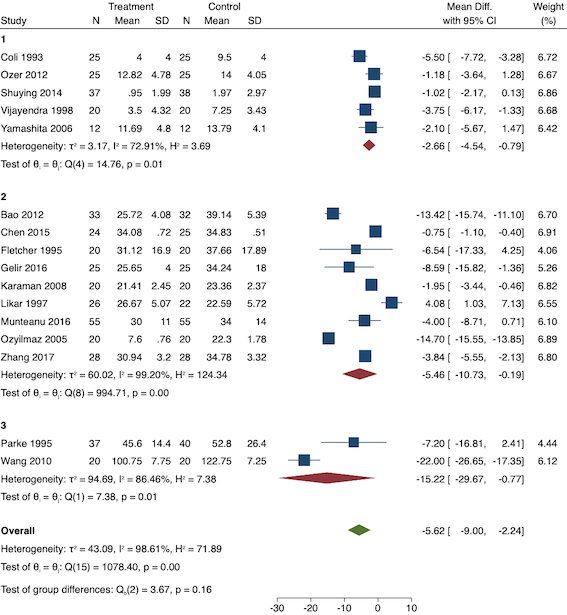
Subgroup analysis for pre‐emptive 24‐hour morphine consumption (baseline consumption). Subgroups are 1 (low), 2 (medium) and 3 (high)
4. Time to first analgesic request (minutes)
Eighteen studies reported time to analgesic request for pre‐emptive NSAIDs versus post‐incision NSAIDs (Buggy 1994; Chen 2015; Colbert 1998; Coli 1993; Esparza‐Villalpando 2016; Gelir 2016; Inanoglu 2007; Kaczmarzyk 2010; Likar 1997; Munteanu 2016; Nezafati 2017; O'Hanlon 1996; Priya 2002; Vanlersberghe 1996; Vijayendra 1998; Vogol 1992; Yagar 2011; Zhang 2017). There may be an increase in the time to analgesic request with pre‐emptive NSAIDs (MD 17.04 minutes, 95% CI 3.77 minutes to 30.31 minutes; participants = 975; studies = 18; I2 = 95%; Analysis 1.6). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly allocation concealment (one level), and unexplained heterogeneity (one level). There was no evidence of publication bias both on visual inspection of funnel plots (Figure 13) or quantitative testing (P = 0.52).
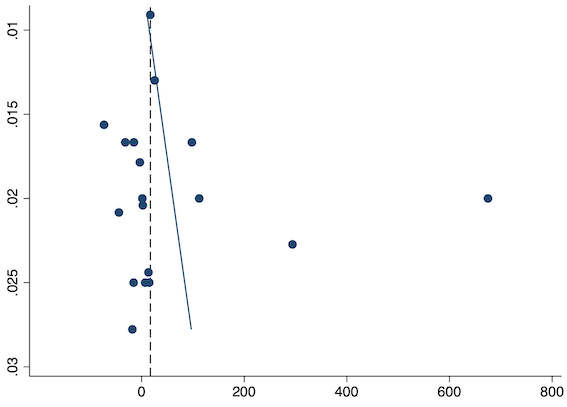
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive time to analgesic request
We did not conduct subgroup analysis as there was only one study in one of the subgroups. On meta‐regression analysis, neither type of surgery (R2 = 28%; P = 0.14) nor type of anaesthesia (R2 = 0%; P = 0.98) was a significant predictor of between‐study heterogeneity. On sensitivity analysis, restricting analysis to the two studies that were at low risk of bias for randomization and allocation concealment (Esparza‐Villalpando 2016; Kaczmarzyk 2010), results were opposite to the main analysis, with a reduction in time to analgesia (MD ‐70.25 minutes, 95% CI ‐101.28 minutes to ‐39.21 minutes; participants = 124; I2 = 0%). Nine studies were at low risk of bias for blinding (Buggy 1994; Chen 2015; Esparza‐Villalpando 2016; Gelir 2016; Inanoglu 2007; Kaczmarzyk 2010; Likar 1997; Munteanu 2016; Priya 2002) which showed a prolonged duration of time to analgesia in the pre‐emptive NSAIDs group (MD 56.84 minutes, 95% CI 13.27 minutes to 100.42 minutes; participants = 515; I2 = 97%). When restricting analysis to the five studies that did not estimate standard deviations or means (Buggy 1994; Coli 1993; Vanlersberghe 1996; Vijayendra 1998; Yagar 2011), the results were similar to the main analysis (MD 17.93 minutes, 95% CI 1.09 minutes to 34.78 minutes; participants = 744; studies = 18; I2 = 96%). Restricting analysis to studies with more than 50 participants gave broadly similar results (MD 33.66 minutes, 95% CI 14.12 minutes to 53.19 minutes; participants = 637; I2 = 97%). One study included data as time to an event (Mojsa 2017), but only reported data with reference to a control group so could not be included in the meta‐analysis.
5. Pruritus (yes/no)
Four studies included data for long‐term pruritus (Lee 2008; Likar 1997; Munteanu 2016; Zhang 2017). Overall, there may be no difference between pre‐emptive and post‐incision NSAIDs (RR 0.40, 95% CI 0.09 to 1.76; participants = 254; I2 = 0%; Analysis 1.7). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly in allocation concealment (one level), and imprecision (one level).
We were unable to assess publication bias or conduct meta‐regression due to the low number of included studies. On subgroup analysis, non‐selective NSAIDs versus COX‐2 agents showed no difference between groups (P = 0.52; Figure 14). On sensitivity analysis, no study was at low risk of bias for randomization and allocation concealment. When restricting analysis to the three studies that were at low risk of bias for blinding (Lee 2008; Likar 1997; Munteanu 2016), results were similar to the main analysis (RR 0.50, 95% CI 0.09 to 2.78; participants = 198; I2 = 0%). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.25, 95% CI 0.03 to 2.25; participants = 166; I2 = 0%). Assuming those participants who dropped out from Zhang 2017 suffered an event gave similar results (RR 0.50, 95% CI 0.15 to 1.62; participants = 258; studies = 4; I2 = 0%).
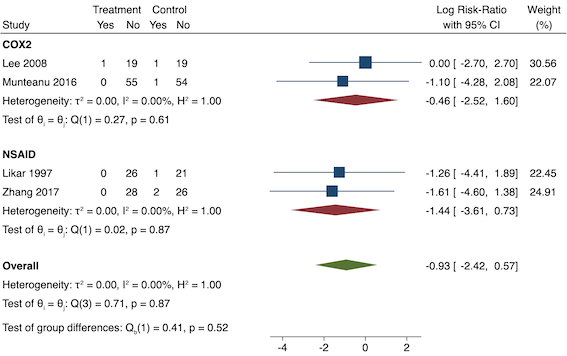
Subgroup analysis for pre‐emptive pruritus (NSAID versus COX‐2). Effect estimate presented as log risk ratio
6. Sedation (yes/no)
Four studies reported sedation (long‐term) (Fletcher 1995; Munteanu 2016; Shuying 2014; Zhang 2017). There may be no difference in sedation between the pre‐emptive and post‐incision NSAID groups (RR 0.51, 95% CI 0.16 to 1.68; participants = 281; I2 = 0%; Analysis 1.8). The certainty of evidence was low owing to concerns over risk of bias, mainly in allocation concealment (one level), and imprecision (one level).
We were unable to assess publication bias or conduct meta‐regression due to the low number of included studies. On subgroup analysis, there was no difference between the non‐selective NSAID and COX‐2 groups (P = 0.66; Figure 15). On sensitivity analysis, restricting analysis to the only study that was at low risk of bias for randomization and allocation concealment (Shuying 2014), results were similar to the main analysis (RR 0.51, 95% CI 0.05 to 5.42; participants = 75). Three studies were at low risk of bias for blinding (Fletcher 1995; Munteanu 2016; Shuying 2014) and results were again similar to the main analysis (RR 0.55, 95% CI 0.15 to 1.98; participants = 225; I2 = 0%). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.55, 95% CI 0.15 to 1.99; participants = 241; I2 = 0%). When assuming participants who dropped out suffered events from two studies (Shuying 2014; Zhang 2017), the results were similar (RR 0.75, 95% CI 0.32 to 1.78; participants = 290; I2 = 0%).
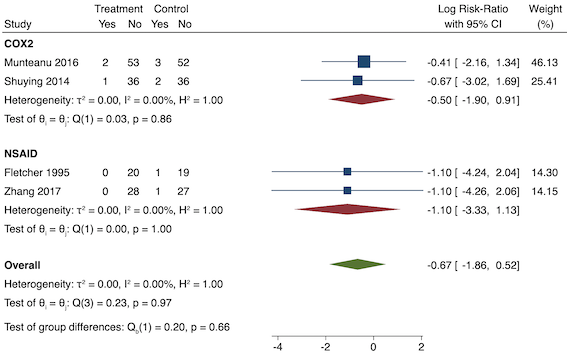
Subgroup analysis for pre‐emptive sedation (NSAID versus COX‐2). Effect estimate is log risk ratio
7. Patient satisfaction (< 24 hours)
No studies reported patient satisfaction within 24 hours.
8. Chronic pain (yes/no)
No studies reported chronic pain.
9. Time to first bowel movement (hours)
No studies reported time to first bowel movement.
Preventive NSAIDs versus post‐incision NSAIDs
Primary outcomes
1. Early acute postoperative pain (measured within six hours postoperatively)
Eighteen studies reported early acute postoperative pain for preventive NSAIDs versus post‐incision NSAIDs (Abanto 2014; Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Giuliani 2015; Gramke 2006; Gunter 2012; Guran 2010; Likar 1998; Martinez 2007; Moonla 2018; Norris 2001; Pandazi 2010; Salonen 2001; Sun 2008; Trampitsch 2003; Wnek 2004; Young 2006). There may be no reduction in early acute postoperative pain with preventive NSAIDs (MD ‐0.14, 95% CI ‐0.39 to 0.12; participants = 1140; I2 = 75%; Analysis 2.1). The certainty of evidence was downgraded to low owing to concerns over risk of bias, mainly in allocation concealment (one level), and unexplained heterogeneity (one level). There was some evidence of publication bias on visual inspection of funnel plots (Figure 16), although quantitative testing was not significant (P = 0.15).
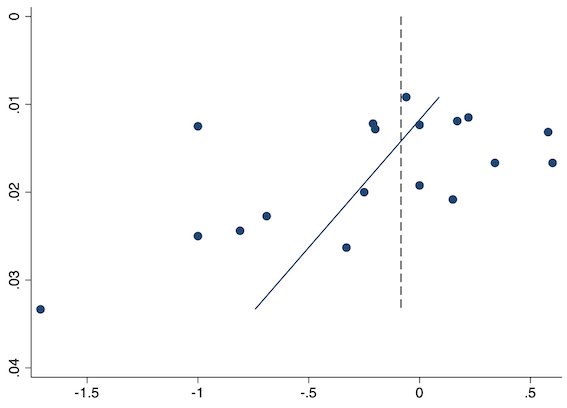
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive early acute postoperative pain
On subgroup analysis of non‐selective NSAIDs versus COX‐2 inhibitors, there was no difference between the groups (P = 0.32; Figure 17). When comparing groups with different baseline pain levels (Doleman 2018a), there was a significant difference between the groups (P = 0.01; Figure 18) with those in the severe group having greater reductions (MD ‐0.80; 95% CI ‐1.23 to ‐0.37) than those in the moderate (MD ‐0.24; 95% CI ‐0.92 to 0.45) and mild group (MD ‐0.04; 95% CI ‐0.31 to 0.24). On meta‐regression analysis, neither type of surgery (P = 0.23) nor type of anaesthesia (P = 0.17) predicted reductions in early postoperative pain. On sensitivity analysis, when restricting analysis to the three studies that were at low risk of bias for randomization and allocation concealment (Martinez 2007; Pandazi 2010; Sun 2008), results were similar to the main analysis (MD ‐0.37, 95% CI ‐1.48 to 0.75; participants = 157; I2 = 94%). Similarly, 11 studies were at low risk of bias for blinding (Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Giuliani 2015; Gramke 2006; Martinez 2007; Norris 2001; Pandazi 2010; Salonen 2001; Sun 2008; Wnek 2004) and showed similar results to the main analysis (MD ‐0.14, 95% CI ‐0.58 to 0.30; participants = 702; I2 = 72%). When restricting analysis to studies where SD and means were not estimated, this left nine studies (Abanto 2014; Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Giuliani 2015; Moonla 2018; Norris 2001; Salonen 2001; Trampitsch 2003) and results were opposite to the main analysis (MD 0.17, 95% CI ‐0.06 to 0.40; participants = 621; I2 = 10%). Restricting analysis to studies with more than 50 participants gave opposite results (MD 0.09, 95% CI ‐0.18 to 0.36; participants = 899; studies = 18; I2 = 69%).
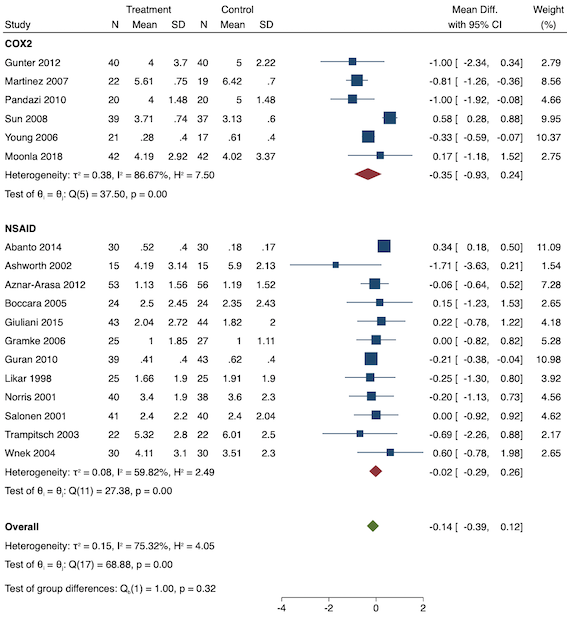
Subgroup analysis for preventive early postoperative pain (NSAID versus COX‐2)
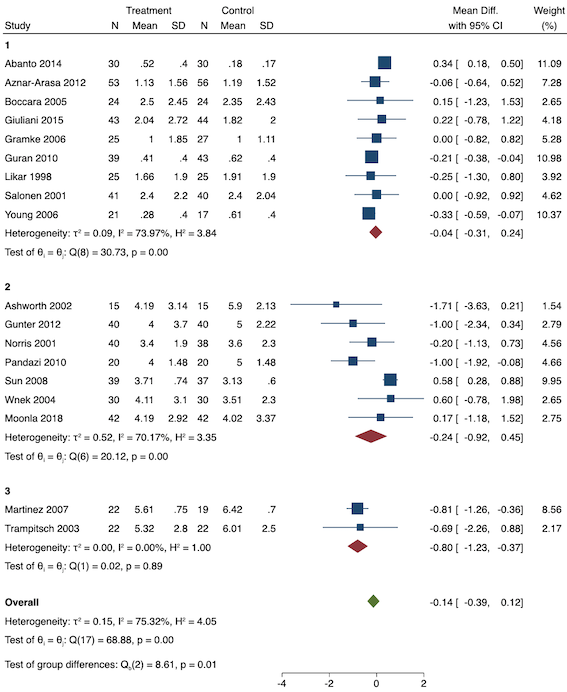
Subgroup analysis for preventive early postoperative pain (baseline pain). Subgroups are 1 (mild), 2 (moderate) and 3 (severe)
2. Adverse events (reoperation for major bleeding within 30 days, acute kidney injury within 48 hours, gastrointestinal ulceration or bleeding requiring endoscopy within 30 days and myocardial infarction within 30 days)
No studies reported acute kidney injury, gastrointestinal ulceration or myocardial infarction for preventive NSAIDs versus post‐incision NSAIDs. One study reported reoperation for bleeding following tonsillectomy (Salonen 2001). There may be no difference between preventive or post‐incision NSAIDs (RR 1.95, 95% CI 0.18 to 20.68; participants = 81). The certainty of evidence was very low owing to concerns over imprecision, due to a low number of events (two levels), and indirectness of evidence (one level), as it was conducted in tonsillectomy only. Due to the inclusion of only one study, we could not conduct analysis for publication bias, investigation of heterogeneity or sensitivity analysis. No participants were excluded from this study (Salonen 2001).
Secondary outcomes
1. Nausea and vomiting (self‐reported by the patient or requirement for anti‐emetic as composite outcome (yes/no))
Short‐term nausea and vomiting
One study reported short‐term nausea and vomiting (Sun 2008). There may be no difference in the number of events between preventive and post‐incision NSAID groups (RR 1.26, 95% CI 0.49 to 3.30; participants = 76). The certainty of evidence was low owing to concerns over imprecision (one level) and indirectness (one level). Due to the inclusion of only one study, we could not conduct analysis for publication bias, investigation of heterogeneity or most sensitivity analyses. When assuming excluded participants suffered an event from Sun 2008, results were similar to the main analysis (RR 1.00, 95% CI 0.44 to 2.26).
Long‐term nausea and vomiting
Eight studies reported long‐term nausea and vomiting (Boccara 2005; Gramke 2006; Gunter 2012; Martinez 2007; Moonla 2018; Nakayama 2001; Pandazi 2010; Salonen 2001). There may be no difference between preventive and post‐incision NSAIDs (RR 0.89, 95% CI 0.65 to 1.22; participants = 456; I2 = 0%; Analysis 2.4). The certainty of evidence was low owing to concerns over risk of bias, mainly in allocation concealment (one level), and imprecision (one level).
There were too few studies to undertake assessment of publication bias or meta‐regression analysis. On subgroup analysis, there was no difference between non‐selective NSAIDs or COX‐2 inhibitors (P = 0.13; Figure 19). On sensitivity analysis, restricting analysis to only studies with low risk for randomization and allocation concealment left two studies (Martinez 2007; Pandazi 2010) which gave similar results to the main analysis (RR 0.76, 95% CI 0.35 to 1.63; participants = 81; I2 = 0%). Restricting analysis to only studies that were low risk for blinding left six studies (Boccara 2005; Gramke 2006; Martinez 2007; Nakayama 2001; Pandazi 2010; Salonen 2001). The results were similar to the main analysis (RR 1.02, 95% CI 0.70 to 1.48; participants = 292; I2 = 0%). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.89, 95% CI 0.54 to 1.46; participants = 297; studies = 8; I2 = 30%). In Gunter 2012, it was unclear if excluded participants were analysed and, in Martinez 2007 and Moonla 2018, it was unclear to which group exclusions belonged. Assuming excluded participants suffered an event gave similar results (RR 0.89, 95% CI 0.64 to 1.24; participants = 376; studies = 7; I2 = 0%).
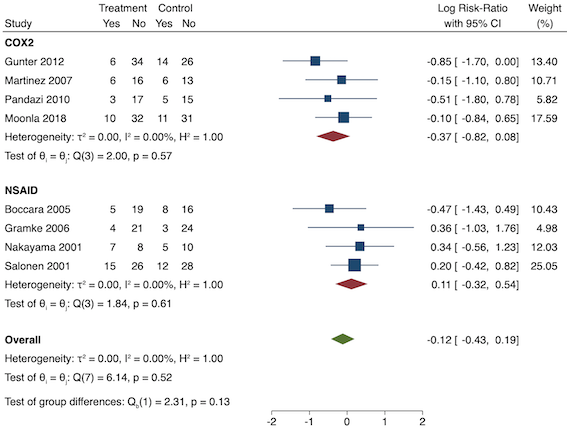
Subgroup analysis for preventive late nausea and vomiting (NSAID versus COX‐2)
2. Late acute postoperative pain (measured at 24 to 48 hours)
Twenty‐one studies reported late acute postoperative pain (Abanto 2014; Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Gramke 2006; Gunter 2012; Guran 2010; Likar 1998; Martinez 2007; Moonla 2018; Murphy 1993; Nakayama 2001; Norris 2001; Pandazi 2010; Salonen 2001; Sun 2008; Trampitsch 2003; Wnek 2004; Young 2006; Yuan 2019; Zhou 2017). There may be a reduction in late acute postoperative pain with preventive NSAIDs versus post‐incision NSAIDs (MD ‐0.33, 95% CI ‐0.59 to ‐0.07; participants = 1441; I2 = 81%; Analysis 2.5). The certainty of evidence was low owing to concerns over risk of bias, mainly in allocation concealment and blinding of outcome assessment (one level), and unexplained heterogeneity (one level). There was no evidence of funnel plot asymmetry on visual inspection (Figure 20) or on quantitative testing (P = 0.23).
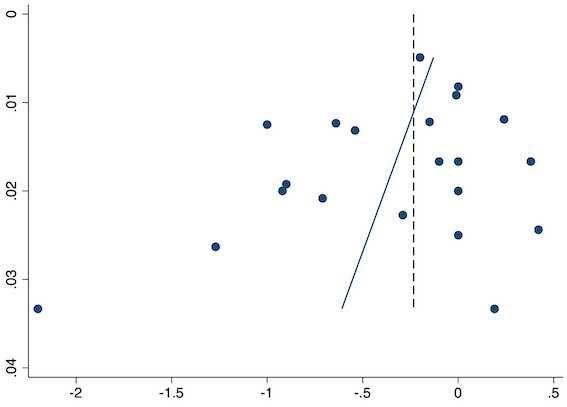
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive late acute postoperative pain
On subgroup analysis of non‐selective NSAIDs versus COX‐2 inhibitors, there was no difference between the groups (P = 0.63; Figure 21). When comparing groups with different baseline pain levels (Doleman 2018a), there was no difference between those with mild or moderate baseline pain levels (P = 0.69; Figure 22). On meta‐regression analysis, type of anaesthesia (R2 = 29%; P = 0.11) or type of surgery (R2 = 0%; P = 0.95) did not predict between‐study heterogeneity. On sensitivity analysis, restricting analysis to only three studies (Pandazi 2010; Sun 2008; Yuan 2019) that were at low risk of bias for randomization and allocation concealment showed similar results, although these were less precise (MD ‐0.28, 95% CI ‐0.63 to 0.07; participants = 320; I2 = 64%). Eleven studies were at low risk of bias for blinding (Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Gramke 2006; Martinez 2007; Nakayama 2001; Norris 2001; Pandazi 2010; Salonen 2001; Sun 2008; Wnek 2004) and showed similar results (MD ‐0.35, 95% CI ‐0.61 to ‐0.09; participants = 627; I2 = 32%). When restricting analysis to studies where SD and means were not estimated, 11 studies remained in the analysis (Abanto 2014; Ashworth 2002; Aznar‐Arasa 2012; Boccara 2005; Moonla 2018; Murphy 1993; Nakayama 2001; Norris 2001; Salonen 2001; Trampitsch 2003; Yuan 2019). They showed similar effects, although these were less precise (MD ‐0.25, 95% CI ‐0.61 to 0.11; participants = 800; I2 = 69%). Restricting analysis to studies with more than 50 participants gave similar results (MD ‐0.28, 95% CI ‐0.56 to ‐0.01; participants = 1170; studies = 21; I2 = 76%).
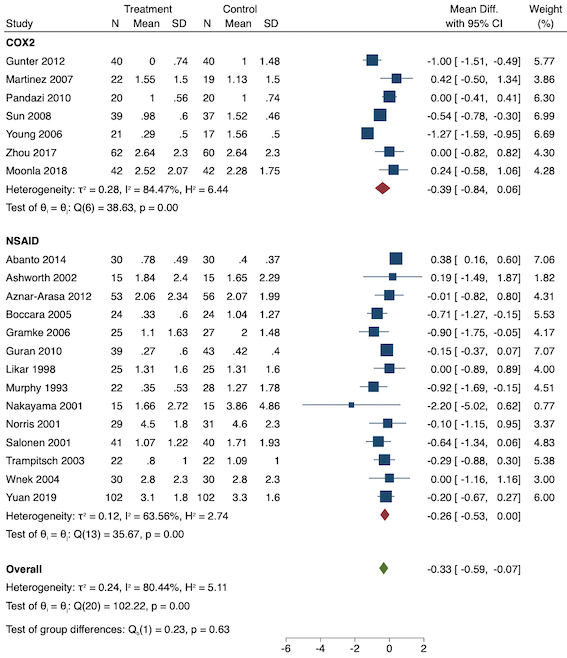
Subgroup analysis for preventive late postoperative pain (NSAID versus COX‐2)
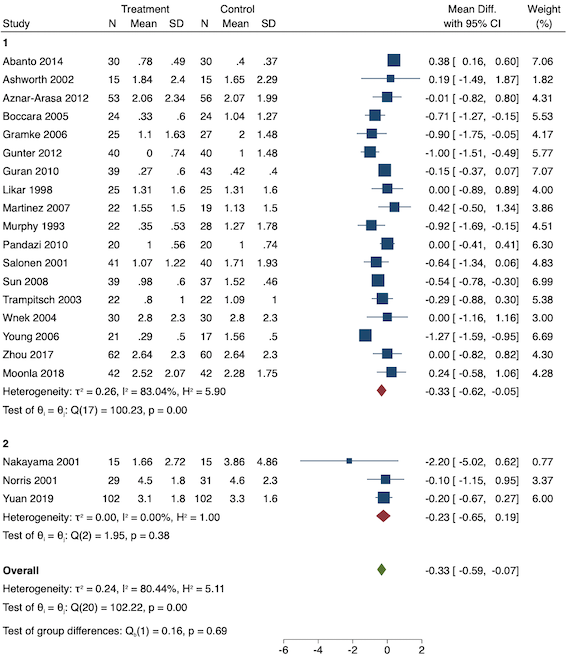
Subgroup analysis for preventive late postoperative pain (baseline pain). Subgroups are 1 (mild) and 2 (moderate)
3. Twenty‐four‐hour morphine consumption (mg)
Sixteen 16 studies reported 24‐hour morphine consumption (Ashworth 2002; Boccara 2005; Fleckenstein 2016; Gramke 2006; Gunter 2012; Guran 2010; Likar 1998; Martinez 2007; Moonla 2018; Murphy 1993; Pandazi 2010; Riest 2006; Riest 2008; Salonen 2001; Sun 2008; Trampitsch 2003). There is probably a reduction in 24‐hour morphine consumption with preventive versus post‐incision NSAIDs (MD ‐1.93 mg, 95% CI ‐3.55 mg to ‐0.32 mg; participants = 1323; I2 = 49%; Analysis 2.6). The certainty of evidence was moderate, downgraded owing to concerns over risk of bias, mainly in allocation concealment (one level), and incomplete outcome data. There was no evidence of publication bias both on visual inspection of funnel plots (Figure 23) or quantitative testing (P = 0.18).
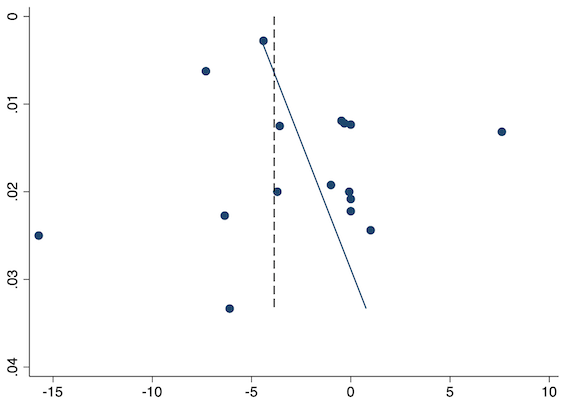
Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive 24‐hour morphine consumption
On subgroup analysis of non‐selective NSAIDs versus COX‐2 inhibitors, there was no difference between the groups (P = 0.06; Figure 24). When conducting subgroup analysis by baseline consumption of morphine (< 20 mg: low, 20‐50 mg: moderate and > 50 mg: high; Doleman 2018a), those with higher baseline consumption had greater reductions in morphine consumption (moderate: MD ‐3.77 mg; 95% CI ‐7.27 mg to ‐0.26 mg versus low: MD ‐0.32 mg; 95% CI ‐0.40 mg to ‐0.24 mg; P = 0.05; Figure 25). On meta‐regression analysis, type of surgery predicted all the between‐study heterogeneity (R2 = 100%; P = 0.1), although the number of studies in each group was limited. We could not conduct analysis for type of anaesthesia as all studies used general anaesthesia. On sensitivity analysis, restricting analysis to four studies (Fleckenstein 2016; Martinez 2007; Pandazi 2010; Riest 2006) that were low risk of bias for randomization and allocation concealment (MD ‐5.31 mg, 95% CI ‐12.36 mg to 1.73 mg; participants = 486; I2 = 68%), the results were more imprecise although differed from the main analysis with a greater reduction in morphine consumption. Nine studies were at low risk for blinding (Ashworth 2002; Boccara 2005; Fleckenstein 2016; Gramke 2006; Martinez 2007; Pandazi 2010; Riest 2006; Salonen 2001; Sun 2008) and results were similar to the main analysis although confidence intervals crossed zero (MD ‐2.13 mg, 95% CI ‐5.55 mg to 1.29 mg; participants = 773; I2 = 55%). When restricting analysis to studies that did not estimate means or SDs, there were eight studies in the analysis (Ashworth 2002; Boccara 2005; Guran 2010; Moonla 2018; Murphy 1993; Riest 2006; Riest 2008; Trampitsch 2003) and results were similar to the main analysis (MD ‐1.30 mg, 95% CI ‐2.80 mg to 0.19 mg; participants = 858; I2 = 34%) although again confidence intervals crossed zero. Restricting analysis to studies with more than 50 participants were similar although less precise (MD ‐1.07 mg, 95% CI ‐2.33 mg to 0.19 mg; participants = 1075; studies = 16; I2 = 28%).
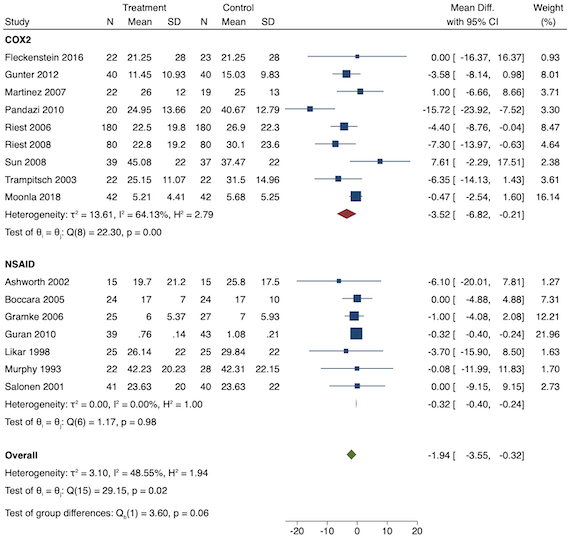
Subgroup analysis for preventive 24‐hour morphine consumption (NSAID versus COX‐2)
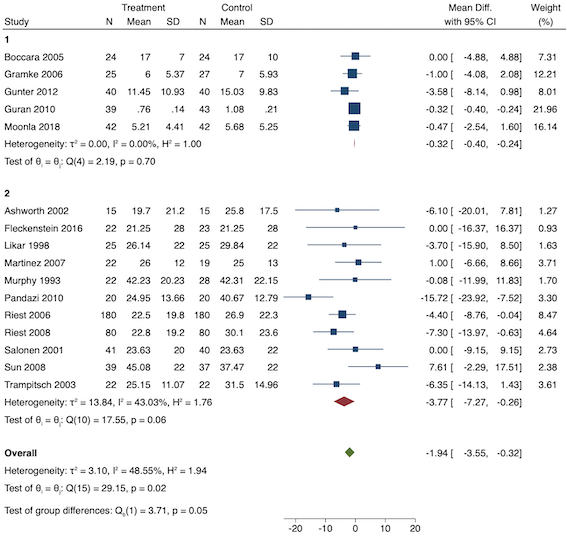
Subgroup analysis for 24‐hour morphine consumption (baseline morphine consumption). Subgroups are 1 (low) and 2 (medium)
4. Time to first analgesic request (minutes)
Eight studies reported time to analgesic request for preventive versus post‐incision NSAIDs (Boccara 2005; Gunter 2012; Likar 1998; Martinez 2007; Mishra 2012; Nakayama 2001; Sun 2008; Wnek 2004). There may be no difference between the groups in time to analgesic request (MD 8.51 minutes, 95% CI ‐31.24 minutes to 48.27 minutes; participants = 410; I2 = 98%; Analysis 2.7). The certainty of evidence was very low owing to concerns over imprecision (one level), risk of bias, mainly in allocation concealment (one level), and other bias and unexplained heterogeneity (one level).
We could not conduct investigation for publication bias or meta‐regression due to the low number of included studies. On subgroup analysis, there was no difference between non‐selective NSAID and COX‐2 groups (P = 0.96; Figure 26). On sensitivity analysis, when restricting analysis to two studies that were at low risk of bias for randomization and allocation concealment (Martinez 2007; Sun 2008), it showed similar effect estimates to the main analysis (MD 7.51 minutes, 95% CI 1.21 minutes to 13.81 minutes; participants = 117; I2 = 42%). Five studies were at low risk of bias for blinding (Boccara 2005; Martinez 2007; Nakayama 2001; Sun 2008; Wnek 2004) which showed opposite effects to the main analysis (MD ‐7.94 minutes, 95% CI ‐57.28 minutes to 41.41 minutes; participants = 255; I2 = 99%). When restricting analysis to studies where means or SDs were not estimated, four studies remained (Boccara 2005; Likar 1998; Nakayama 2001; Wnek 2004) which showed opposite effects to the main analysis (MD ‐2.71 minutes, 95% CI ‐155.26 minutes to 149.83 minutes; participants = 188; I2 = 99%). Restricting analysis to studies with more than 50 participants again found opposite effects to the main analysis (MD ‐17.62 minutes, 95% CI ‐115.94 minutes to 80.70 minutes; participants = 266; I2 = 97%).
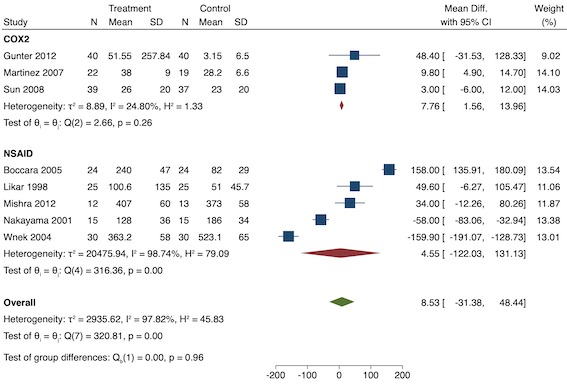
Subgroup analysis for preventive time to analgesic request (NSAID versus COX‐2)
5. Pruritus (yes/no)
No studies reported short‐term pruritus. Three studies reported long‐term pruritus for preventive versus post‐incision NSAIDs (Gunter 2012; Likar 1998; Salonen 2001). There may be no difference between the groups (RR 0.56, 95% CI 0.09 to 3.35; participants = 211; I2 = 0%; Analysis 2.8). The certainty of evidence was low owing to concerns over risk of bias (one level) and imprecision (one level).
We were unable to conduct analysis for publication bias and meta‐regression due to the low number of included studies. We did not conduct subgroup analysis as only one study used non‐selective NSAIDs. On sensitivity analysis, restricting analysis to only one study that was at low risk of bias for randomization, allocation concealment and blinding (Sun 2008), it showed different, more imprecise effects to the main analysis (RR 2.93, 95% CI 0.12 to 69.83; participants = 81). No studies had fewer than 50 participants. In Gunter 2012, it was unclear if excluded participants were analysed. In Likar 1998, it was unclear which group exclusions belonged to. The other study (Salonen 2001) had no participants lost to follow‐up.
6. Sedation (yes/no)
No studies reported short‐term sedation. Five studies reported long‐term sedation for preventive versus post‐incision NSAIDs (Boccara 2005; Guran 2010; Martinez 2007; Yuan 2019; Zhou 2017). There may be no difference between the groups (RR 0.84, 95% CI 0.44 to 1.63; participants = 497; I2 = 0%; Analysis 2.9). The certainty of evidence was low owing to concerns over risk of bias, mainly in allocation concealment (one level), and imprecision (one level).
We were unable to conduct analysis for publication bias and meta‐regression due to the low number of included studies. On subgroup analysis, there was no difference between non‐selective NSAID and COX‐2 agents (P = 0.86; Figure 27). On sensitivity analysis, only two studies were at low risk of bias for randomization and allocation concealment (Martinez 2007; Yuan 2019) which gave similar results to the overall analysis (RR 1.08, 95% CI 0.34 to 3.45; participants = 41). Two studies were at low risk of bias for blinding (Boccara 2005; Martinez 2007) which again gave similar results to the main analysis (RR 0.77, 95% CI 0.30 to 1.98; participants = 245; I2 = 0%). Restricting analysis to studies with more than 50 participants gave similar results (RR 0.67, 95% CI 0.26 to 1.72; participants = 408; studies = 5; I2 = 0%). In Martinez 2007, it was unclear to which group exclusions belonged. Assuming excluded participants suffered an event in the other studies gave similar results (RR 0.95, 95% CI 0.58 to 1.58).
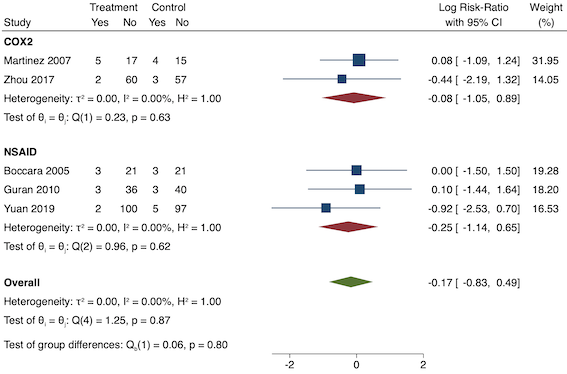
Subgroup analysis for preventive sedation (NSAID versus COX‐2). Effect estimate is log risk ratio
7. Patient satisfaction (< 24 hours)
Only one study reported patient satisfaction for preventive versus post‐incision NSAIDs (Giuliani 2015). There is probably no difference between the groups on a 10‐point scale (MD ‐0.42, 95% CI ‐1.09 to 0.25; participants = 72; Analysis 2.10). The certainty of evidence was moderate owing to concerns over risk of bias (one level), mainly due to attrition bias. There were too few studies to undertake assessment for publication bias, investigation of heterogeneity and sensitivity analysis.
8. Chronic pain (yes/no)
No studies reported chronic pain.
9. Time to first bowel movement (hours)
Only one study reported time to bowel movement for preventive versus post‐incision NSAIDs (Sun 2008). There is probably no difference between the groups (MD 0.00 hours, 95% CI ‐15.99 hours to 15.99 hours; participants = 76; Analysis 2.11). The certainty of evidence was moderate owing to concerns over imprecision (one level). There were too few studies to undertake assessment for publication bias, investigation of heterogeneity and sensitivity analysis.
Discussion
Summary of main results
For pre‐emptive NSAIDs, there is probably a decrease in early acute postoperative pain, although none of the included studies reported adverse events. There may be no difference between the groups in nausea and vomiting (short‐ or long‐term). There may be a reduction in late acute postoperative pain. There may also be a reduction in 24‐hour morphine consumption with pre‐emptive NSAIDs and an increase in time to analgesic request. There may be no difference in opioid adverse events such as pruritus or sedation, although the number of included studies for these outcomes was small. No study reported patient satisfaction, chronic pain or time to first bowel movement. The certainty of evidence ranged from moderate to low mainly owing to concerns over risk of bias. However, investigation of heterogeneity showed that baseline pain or morphine consumption may result in more clinically significant reductions in agreement with previous research (Doleman 2018a).
For preventive NSAIDs, there may be no difference in early acute postoperative pain. One study (Salonen 2001) reported adverse events from NSAIDs (reoperation for bleeding) although the events were low which did not allow any meaningful conclusions to be drawn. There may be no difference in rates of nausea and vomiting (short‐ and long‐term). There may be a reduction in late acute postoperative pain. There is probably a reduction in 24‐hour morphine consumption. We are very uncertain if there is any effect on time to analgesic request. As with pre‐emptive NSAIDs, there may be no difference in other opioid adverse events such as pruritus and sedation. There is probably no difference in patient satisfaction (Giuliani 2015). No study reported chronic pain. There is probably no difference in time to first bowel movement (Sun 2008). The certainty of evidence ranged from moderate to very low and there was some evidence that higher baseline pain or morphine consumption led to larger reductions in effects (Doleman 2018a).
None of the observed differences for both pre‐emptive or preventive NSAIDs were clinically significant as defined in our protocol (1.5 reduction in pain, 10 mg reduction in morphine consumption or delay in analgesic request of one hour). However, these results need to be taken in the context of variable effects with baseline risk, that is, clinically significant effects may be observed at higher baseline risk (10 mg reduction in morphine consumption may be seen in studies with high morphine consumption in the control group; Doleman 2018a).
Overall, there may be some evidence of small, non‐clinically significant reductions in acute postoperative pain and 24‐hour morphine consumption with pre‐emptive and preventive NSAIDs, with five out of six of these outcomes showing possible benefit. In addition, there was some evidence of better efficacy with higher baseline pain levels and morphine consumption (more painful operations or higher‐risk patients; Doleman 2018a). However, only one study reported major adverse events for preventive NSAIDs (Salonen 2001). We found no difference in opioid adverse events from a limited number of included studies, further questioning the clinical significance of any observed differences.
Overall completeness and applicability of evidence
Our review had a wide ranging search strategy including electronic databases, grey literature sources, reference searches and searching of conference proceedings (Doleman 2019). This was important in order to reduce the risk of publication bias (Chong 2016). Indeed, when using more appropriate tests for publication bias, we found no evidence of publication bias (imprecise study effects). We excluded 20 studies mainly for reasons which would bias results, such as different doses in each group and different routes of administration (Characteristics of excluded studies). There are currently seven studies awaiting classification, two that we were unable to translate (Aoki 2002; Bai 1998) and five where we were unable to locate full texts despite contacting authors and the British Library (Beg 2001; Belzarena 1994; De Oliveira 1999; Jia 2011; Nicholson 2014). Of these studies, three found an improvement in pain with pre‐emptive NSAIDs (Aoki 2002; Bai 1998; Jia 2011) while another two found no difference between the groups (De Oliveira 1999; Nicholson 2014). The other two results are unknown (Beg 2001; Belzarena 1994). Depending on their results, inclusion of these studies could potentially alter the conclusions of this review. This would be less likely for outcomes that include many studies (such as pain), due to the similar sample size of the missing studies (see Characteristics of studies awaiting classification) but more likely for outcomes with few included studies (nausea and vomiting).
We chose to include a range of time points for measurement of pain outcomes such as early acute postoperative pain (0‐6 hours) and late acute postoperative pain (24‐48 hours). This is due to the fact that postoperative pain trials tend to report a variety of different time points for pain (Doleman 2018a) and exclude others. If we had included more precise time points, this would lead to the exclusion of many studies for each of these outcomes. However, we recognise that including different studies with different time points within one outcome (e.g. one study measuring pain at 0 hours versus 6 hours) could affect pain levels, as these may improve with time. From our experience, this is less of an issue with opioid consumption (Doleman 2018a). We selected early pain as our primary outcome as this is when pain is likely to be at its maximal severity. It could be argued that opioid consumption may be a more appropriate primary outcome (and opioid adverse events more so) as the aim of multimodal analgesia is to reduce opioid consumption. Also, it may not be ethical to have two groups with differences in pain. We will consider this in future review updates.
A major limitation of our previous review on preventive opioids related to the reporting of central tendency as both means and medians in the included studies (Doleman 2018b). One analysis of 24‐hour morphine consumption showed a difference on meta‐analysis of means although nearly all studies that reported median values showed no significant difference. This raises two important issues, firstly, the likelihood of this leading to false conclusions on the efficacy of preventive opioids by review consumers not appreciating this subtle fact (especially those not reading the whole review) and the issues it raises in giving false positives on publication bias assessment (by excluding a large number of 'negative' studies). The options are to include median results in a narrative synthesis (Doleman 2018b) or estimate means from medians (Higgins 2011a). For the reasons described above, the narrative synthesis option has severe disadvantages. Although estimating means from medians risks making false assumptions about the data, it helps ensure all studies contribute data to the analysis. Moreover, even if studies report data as means and SDs, this does not ensure that the underlying data is not skewed. Therefore, we felt the ideal solution was to estimate means from medians and SD from IQR as stated in the Cochrane Handbook (Higgins 2011a) or the range from published research (Hozo 2005) so that all studies could be included. We then conducted a sensitivity analysis by excluding studies where these values had been estimated or imputed. This allows both scenarios to be compared and reduces the risks described previously. In most cases, results of the sensitivity analysis were similar to the main analysis (Effects of interventions).
In terms of the applicability of the evidence, the range of operations included in this review (Description of studies) were more diverse than in our opioid review (Doleman 2018b). Although this helps with external validity, it limited the meta‐regression analysis as there were few studies in each surgical subgroup. Despite helping external validity, diverse types of surgery may raise issues surrounding clinical heterogeneity. However, our investigation of heterogeneity did not consistently identify type of surgery as a significant predictor of between‐study heterogeneity. Moreover, our previous research has demonstrated that baseline risk models (our subgroup analysis) can account for differences between different surgical subtypes (Doleman 2018a). Another issue with respect to applicability is the inclusion of mainly low‐risk participants, those without prior analgesic intake or chronic pain, and a lack of standardisation of postoperative opioids which may affect opioid consumption outcomes (Description of studies). Furthermore, some of our analyses included few studies from a limited set of surgical subtypes which may raise issues about whether these results can be applied to other operations. This also raises the issue around type of NSAIDs used and whether results from the use of certain NSAIDs can be applied to others. Despite our subgroup analysis suggesting no difference between non‐selective NSAIDs and COX‐2 inhibitors, we cannot rule out differences between agents within these classes, especially if different doses were used.
Quality of the evidence
The certainty of evidence for all outcomes ranged from moderate to very low, most commonly due to issues with risk of bias (Figure 2; Figure 3) although unexplained heterogeneity and imprecision were other common reasons. Issues with risk of bias were mainly due to a lack of reporting on allocation concealment and a lack of published protocols (unclear risk) which may result in selection bias and selective reporting bias, respectively. Moreover, 14 studies did not use a double‐dummy placebo so were at high risk of bias for blinding which may affect a subjective outcome such as pain. Our previous research has identified that bias in domains of trial conduct, such as allocation concealment, can exaggerate effects so results from this review must be treated with caution (Doleman 2018a). However, when we conducted sensitivity analysis, there was no consistent effect of risk of bias and effect estimates were similar when we included only studies that scored low risk for either domains of random allocation or blinding (Risk of bias in included studies).
Similar to our previous research, when we conducted subgroup analysis based on differences in baseline pain/morphine consumption, we found larger effects with higher baseline pain/morphine consumption which may have contributed to the heterogeneity observed with these outcomes (Doleman 2018a). This may also be the mechanism by which subgroup differences observed with type of surgery may manifest. Furthermore, unexplained heterogeneity may have contributed to imprecision as our analysis was conducted using random‐effects models where confidence intervals are wider if statistical heterogeneity exists in analyses. For our meta‐regression analysis, type of surgery and type of anaesthesia were not significant predictors of between‐study heterogeneity in most analyses. However, the limitations of such analysis should be considered when the number of included studies is 40 or less (López‐López 2014).
Potential biases in the review process
Similar to our opioid review (Doleman 2018b), none of our review authors were involved in any of the included studies. However, some authors are involved in an ongoing study on preventive paracetamol (see Declarations of interest). Our previous research has identified issues with traditional meta‐analysis when analysing outcomes dependent on baseline risk (Doleman 2018a), although we accounted for this when performing subgroup analysis and found that on most occasions this supported our previous findings. We have also conducted research that has identified type I errors when using Egger's linear regression test for this type of data using simulated meta‐analyses. With this in mind, we have used our novel test which is based on inverse sample size rather than standard error and has been found to perform better than Egger's test due to lack of dependence between effect estimates and standard errors (Doleman 2020).
Agreements and disagreements with other studies or reviews
Although now over a decade old, the two previous reviews of pre‐emptive and preventive NSAIDs found some similar and some different results to our review (Møiniche 2002; Ong 2005). The first review published in 2002 (Møiniche 2002) found that pre‐incisional NSAIDs had no effect on acute pain (MD 0 mm; 95% CI ‐2 mm to 2 mm) with many studies also showing no benefit in reducing analgesic consumption. In contrast, we found some positive evidence for pre‐emptive and preventive NSAIDs in reducing some measures of acute pain and opioid consumption, although we did find limitations in the certainty of evidence and clinical significance. A later review published in 2005 (Ong 2005) found no difference in acute pain, although it did find that pre‐incisional NSAIDs reduced analgesic consumption and prolonged time to first analgesia. We found similar results with analgesic consumption, although we did find some reductions in acute pain in contrast to this review (Ong 2005).

Study flow diagram.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive early acute postoperative pain

Subgroup analysis for pre‐emptive early postoperative pain (NSAID versus COX‐2 inhibitor)

Subgroup analysis for pre‐emptive early postoperative pain (baseline pain level). Subgroups are 1 (mild), 2 (moderate) and 3 (severe)

Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive late acute postoperative pain

Subgroup analysis for pre‐emptive late postoperative pain (NSAID versus COX‐2 inhibitor)

Subgroup analysis for pre‐emptive late postoperative pain (baseline pain). Subgroups are 1 (mild) and 2 (moderate)

Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive 24‐hour morphine consumption

Subgroup analysis for pre‐emptive 24‐hour morphine consumption (NSAID versus COX‐2)

Subgroup analysis for pre‐emptive 24‐hour morphine consumption (baseline consumption). Subgroups are 1 (low), 2 (medium) and 3 (high)

Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for pre‐emptive time to analgesic request

Subgroup analysis for pre‐emptive pruritus (NSAID versus COX‐2). Effect estimate presented as log risk ratio
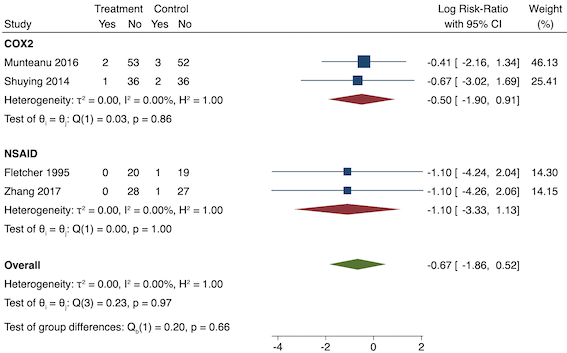
Subgroup analysis for pre‐emptive sedation (NSAID versus COX‐2). Effect estimate is log risk ratio

Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive early acute postoperative pain

Subgroup analysis for preventive early postoperative pain (NSAID versus COX‐2)
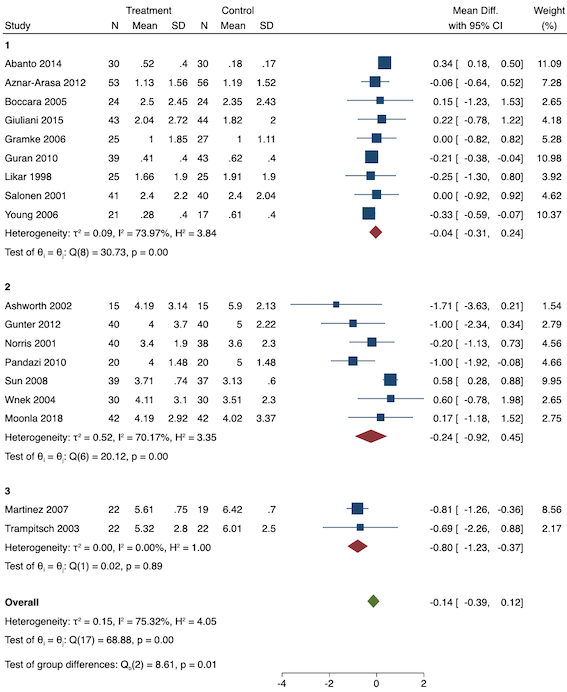
Subgroup analysis for preventive early postoperative pain (baseline pain). Subgroups are 1 (mild), 2 (moderate) and 3 (severe)

Subgroup analysis for preventive late nausea and vomiting (NSAID versus COX‐2)

Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive late acute postoperative pain
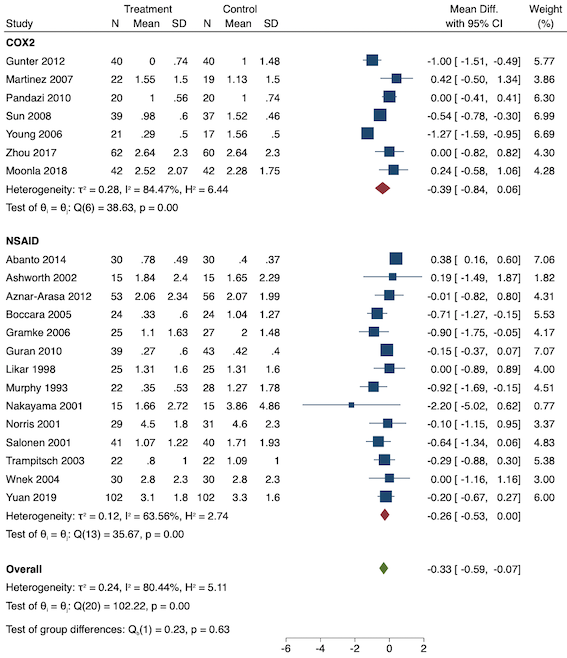
Subgroup analysis for preventive late postoperative pain (NSAID versus COX‐2)

Subgroup analysis for preventive late postoperative pain (baseline pain). Subgroups are 1 (mild) and 2 (moderate)

Funnel plot with mean difference on the X‐axis and inverse sample size on the Y‐axis for preventive 24‐hour morphine consumption

Subgroup analysis for preventive 24‐hour morphine consumption (NSAID versus COX‐2)

Subgroup analysis for 24‐hour morphine consumption (baseline morphine consumption). Subgroups are 1 (low) and 2 (medium)

Subgroup analysis for preventive time to analgesic request (NSAID versus COX‐2)

Subgroup analysis for preventive sedation (NSAID versus COX‐2). Effect estimate is log risk ratio

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 1: Early acute postoperative pain (within 6 hours postoperatively)

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 2: Nausea and vomiting (short‐term)

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 3: Nausea and vomiting (long‐term)

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 4: Late acute postoperative pain (24‐48 hours postoperatively)

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 5: 24‐hour morphine consumption (mg)

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 6: Time to first analgesic request (minutes)

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 7: Pruritus (long‐term)

Comparison 1: Pre‐emptive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 8: Sedation (long‐term)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 1: Early acute postoperative pain (within 6 hours postoperatively)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 2: Re‐operation for bleeding

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 3: Nausea and vomiting (short‐term)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 4: Nausea and vomiting (long‐term)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 5: Late acute postoperative pain (24‐48 hours postoperatively)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 6: 24‐hour morphine consumption (mg)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 7: Time to first analgesic request (minutes)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 8: Pruritus (long‐term)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 9: Sedation (long‐term)

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 10: Patient satisfaction

Comparison 2: Preventive NSAIDS/COX‐2 inhibitors versus post‐incision NSAIDS/COX‐2 inhibitors, Outcome 11: Time to bowel movement (hours)
| Pre‐emptive NSAIDs compared with post‐incision NSAIDs for postoperative pain | ||||||
| Patient or population: adults undergoing surgery Settings: secondary care and dental clinics Intervention: pre‐emptive NSAIDs Comparison: post‐incision NSAIDs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Post‐incision NSAIDs | Pre‐emptive NSAIDs | |||||
| Early acute postoperative pain (within 6 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.32 to 6.37 | Overall MD ‐0.69 (95% CI ‐0.97 to ‐0.41) Mild pain MD ‐0.24 (95% CI ‐0.51 to 0.03) Moderate pain MD ‐1.19 (95% CI ‐1.52 to ‐0.86) Severe pain MD ‐1.44 (95% CI ‐2.28 to ‐0.59) | 2032 | ⊕⊕⊕⊝ | Results not clinically significant | |
| Adverse events | N/A | N/A | N/A | N/A | N/A | No study reported this |
| Nausea and vomiting (1‐6 hours postoperatively) | 120 per 1000 | 120 per 1000 (41 to 353) | RR 1.00 (0.34 to 2.94) | 100 | ⊕⊕⊝⊝ | |
| Nausea and vomiting (6‐48 hours postoperatively) | 336 per 1000 | 278 per 1000 (175 to 464) | RR 0.85 (0.52 to 1.38) | 228 | ⊕⊕⊝⊝ | |
| Late acute postoperative pain (24‐48 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.25 to 3.49 | Overall MD ‐0.22 (95% CI ‐0.44 to 0.00) Mild pain MD ‐0.14 (95% CI ‐0.37 to 0.10) Moderate pain MD ‐0.77 (95% CI ‐1.08 to 0.47) | 1645 | ⊕⊕⊝⊝ | Results not clinically significant | |
| 24‐hour morphine consumption (mg) | The mean morphine consumption ranged across post‐incision groups from 1.97 mg to 122.75 mg | Overall MD ‐5.62 mg (95% CI ‐9.00 mg to ‐2.24 mg) Low consumption MD ‐2.66 mg (95% CI ‐4.54 mg to ‐0.79 mg) Medium consumption MD ‐5.46 mg (95% CI ‐10.73 mg to ‐0.19 mg) High consumption MD ‐15.22 mg (95% CI ‐29.67 mg to ‐0.77 mg) | 854 | ⊕⊕⊝⊝ | Results not clinically significant | |
| Time to first analgesic request (mean time in minutes) | The mean time ranged across post‐incision groups from 29.6 minutes to 1146 minutes | MD 17.04 minutes (95% CI 3.77 minutes to 30.31 minutes) | 975 | ⊕⊕⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded owing to concerns over risk of bias (one level) 2Downgraded owing to concerns over risk of bias (one level) and imprecision (one level) 3Downgraded owing to concerns over risk of bias (one level) and unexplained heterogeneity (one level) Abbreviations: | ||||||
| Preventive NSAIDs compared with post‐incision NSAIDs for postoperative pain | ||||||
| Patient or population: adults undergoing surgery Settings: secondary care and dental clinics Intervention: preventive NSAIDs Comparison: post‐incision NSAIDs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect | No of Participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Assumed risk | Corresponding risk | |||||
| Post‐incision NSAIDs | Preventive NSAIDs | |||||
| Early acute postoperative pain (within 6 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.18 to 6.42 | Overall MD ‐0.14 (95% CI ‐0.39 to 0.12) Mild pain MD ‐0.04 (95% CI ‐0.31 to 0.24) Moderate pain MD ‐0.24 (95% CI ‐0.92 to 0.45) Severe pain MD ‐0.80 (95% CI ‐1.23 to ‐0.37) | 1140 | ⊕⊕⊝⊝ | ||
| Adverse events (Re‐operation for bleeding (reoperation for major bleeding within 30 days (yes/no))) | 25 per 1000 | 49 per 1000 (5 to 517) | RR 1.95 (0.18 to 20.68) | 81 | ⊕⊕⊝⊝ | Study performed in tonsillectomy so unclear for other operations. Other adverse events not reported |
| Nausea and vomiting (1‐6 hours postoperatively) | 162 per 1000 | 205 per 1000 (79 to 535) | RR 1.26 (0.49 to 3.30) | 76 | ⊕⊕⊕⊝ | |
| Nausea and vomiting (6‐48 hours postoperatively) | 245 per 1000 | 282 per 1000 (159 to 299) | RR 0.89 (0.65 to 1.22) | 456 | ⊕⊕⊝⊝ | |
| Late acute postoperative pain (24‐48 hours postoperatively using a validated pain scale: 0, no pain to 10, maximum pain) | The mean pain ranged across post‐incision groups from 0.42 to 4.6 | Overall MD ‐0.33 (95% CI ‐0.59 to ‐0.07) Mild pain MD ‐0.33 (95% CI ‐0.62 to ‐0.05) Moderate pain MD ‐0.23 (95% CI ‐0.65 to 0.19) | 1441 | ⊕⊕⊝⊝ | Results not clinically significant | |
| 24‐hour morphine consumption (mg) | The mean morphine consumption ranged across post‐incision groups from 1.08 mg to 42.31 mg | Overall MD ‐1.93 mg (95% CI ‐3.55 mg to ‐0.32 mg) Low consumption MD ‐0.32 mg (95% CI ‐0.40 mg to ‐0.24 mg) Medium consumption MD ‐3.77 mg (95% CI ‐7.27 mg to ‐0.26 mg) | 1323 | ⊕⊕⊕⊝ | Results not clinically significant | |
| Time to first analgesic request (mean time in minutes) | The mean time ranged across post‐incision groups from 3.15 minutes to 523.1 minutes | MD 8.51 minutes (95% CI ‐31.24 minutes to 48.27 minutes) | 410 | ⊕⊝⊝⊝ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1Downgraded owing to concerns over risk of bias (one level) and unexplained heterogeneity (one level) 2Downgraded owing to concerns over imprecision (two levels) and indirectness of evidence (one level) 3Downgraded owing to concerns over imprecision (one level) and indirectness of evidence (one level) 4Downgraded owing to concerns over risk of bias (one level) and imprecision (one level) 5Downgraded owing to concerns over risk of bias (one level) 6Downgradedowing to concerns over imprecision (one level), risk of bias (one level) and unexplained heterogeneity (one level) Abbreviations: | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Early acute postoperative pain (within 6 hours postoperatively) Show forest plot | 36 | 2032 | Mean Difference (IV, Random, 95% CI) | ‐0.69 [‐0.97, ‐0.41] |
| 1.2 Nausea and vomiting (short‐term) Show forest plot | 2 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.34, 2.94] |
| 1.3 Nausea and vomiting (long‐term) Show forest plot | 5 | 228 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.52, 1.38] |
| 1.4 Late acute postoperative pain (24‐48 hours postoperatively) Show forest plot | 28 | 1645 | Mean Difference (IV, Random, 95% CI) | ‐0.22 [‐0.44, 0.00] |
| 1.5 24‐hour morphine consumption (mg) Show forest plot | 16 | 854 | Mean Difference (IV, Random, 95% CI) | ‐5.62 [‐9.00, ‐2.24] |
| 1.6 Time to first analgesic request (minutes) Show forest plot | 18 | 975 | Mean Difference (IV, Random, 95% CI) | 17.04 [3.77, 30.31] |
| 1.7 Pruritus (long‐term) Show forest plot | 4 | 254 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.09, 1.76] |
| 1.8 Sedation (long‐term) Show forest plot | 4 | 281 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.16, 1.68] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 2.1 Early acute postoperative pain (within 6 hours postoperatively) Show forest plot | 18 | 1140 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.39, 0.12] |
| 2.2 Re‐operation for bleeding Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.3 Nausea and vomiting (short‐term) Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.4 Nausea and vomiting (long‐term) Show forest plot | 8 | 456 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.65, 1.22] |
| 2.5 Late acute postoperative pain (24‐48 hours postoperatively) Show forest plot | 21 | 1441 | Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.59, ‐0.07] |
| 2.6 24‐hour morphine consumption (mg) Show forest plot | 16 | 1323 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.55, ‐0.32] |
| 2.7 Time to first analgesic request (minutes) Show forest plot | 8 | 410 | Mean Difference (IV, Random, 95% CI) | 8.51 [‐31.24, 48.27] |
| 2.8 Pruritus (long‐term) Show forest plot | 3 | 211 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.09, 3.35] |
| 2.9 Sedation (long‐term) Show forest plot | 5 | 497 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.44, 1.63] |
| 2.10 Patient satisfaction Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.11 Time to bowel movement (hours) Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |

