Probióticos para pacientes con fibrosis quística
Referencias
References to studies included in this review
References to studies excluded from this review
References to studies awaiting assessment
References to ongoing studies
Additional references
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Trial design: RCT. Trial grouping: cross‐over, without a washout period. Randomisation method: randomisation was constructed using the random numbers table with a block design for groups of 10 participants. | |
| Participants | Inclusion criteria
Exclusion criteria
Baseline characteristics
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Secondary outcome measures
Time points for measurements: baseline 6 months for each period (baseline, 6 months, 7 months, 13 months). Length of follow‐up: 56 weeks. | |
| Identification | Country: Italy. Setting: Department of Pediatrics, University of Naples "Federico II". Author's name: Alfredo Guarino. Institution: Department of Pediatrics, University of Naples "Federico II". Email: [email protected]. Address: Department of Pediatrics, University of Naples "Federico II", Via S. Pansini 5, 80131 Naples, Italy. | |
| Funding Source | Fondazione per la ricerca sulla fibrosi cistica (grant). | |
| Declaration of Interest Among the Primary Researchers | No conflicts of interest exists for any author. | |
| Notes | Pretreatment: Group A (started on Lactobacillus GG first then ORS) had higher mean value of FEV1 %, P < 0.05 Population: in text age range (5 ‐ 23 years) does not match table 1 (5 ‐ 18 years); number of males in text (16) does not match table 1 (17), mean FEV1 % predicted = 63.57 (19.5) in Group A and mean FEV1 % predicted = 45.37 (13.3) in Group B. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation was constructed using the random numbers table with a block design for groups of ten patients." Judgement comment: block design for groups of 10 participants used ‐ methods to prevent selection bias not taken as stringent inclusion criteria taken to limit baseline group differences. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: unclear about allocation concealment mechanism. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "LGG or ORS were assumed, blindly, in a single daily dose by the patients." Judgement comment: participants blinded. Investigators (except outcome assessor) not blinded, however, this is unlikely to affect objective outcome measures. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The outcome measures of efficacy were recorded by one of the investigators who was unaware about the administration of LGG/ORS." Judgement comment: outcome assessor blinded. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "Clinical stability was defined as the absence of substantial, acute onset clinical problems that could affect the outcome measures included in the clinical protocol, at the time of enrolment, shift of treatment and/or exit from treatment." Quote: "One of the 5 patients who did not complete the study was unable to receive probiotics because of vomiting, and whereas the other 4 were clinically unstable at the end of probiotic or ORS administration." Quote: "Thirty‐eight of the 43 patients enrolled, completed the study" Judgement comment: 4 clinically unstable participants were not analysed, this may skew outcome measures. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: data not given for not significant results, e.g. mean duration of hospital stay per pulmonary episode, mean duration of pulmonary exacerbations, otherwise reported on everything in methods ‐ no protocol available. |
| Other bias | Low risk | Judgement comment: Fondazione Ricerca Fibrosi Cistica funding. No conflicts declared. |
| Methods | Trial design: RCT. Trial grouping: parallel groups. Randomisation method: not specified ("generated by a statistician not involved in patient management"). | |
| Participants | Inclusion criteria
Exclusion criteria
Baseline characteristics
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Time points for measurements: baseline and 1 month. Length of follow‐up: 4 weeks. | |
| Identification | Country: Italy Setting: Department of Pediatrics, University of Naples "Federico II". Author's name: Alfredo Guarino. Institution: Department of Translational Medical Science, Section of Pediatrics, University Federico II, Naples, Italy. Email: [email protected]. Address: Department of Translational Medical Science, Section of Pediatrics University of Naples Federico II Naples, Italy. | |
| Funding Source | Grants from the Cystic Fibrosis Foundation and Therapeutics (GUARIN10A0/2009) and the Fondazione Italiana Ricerca Fibrosis Cistica (FC 23/2009). | |
| Declaration of Interest Among the Primary Researchers | The authors declared that no competing interests existed. | |
| Notes | Baseline calprotectin, mean (SD): LGG group 164 (70), placebo group 251 (174). This trial recruited healthy controls (without CF) to act as a reference for normal (e.g. inflammatory markers): data from these healthy participants are not included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The randomisation protocol was generated by a statistician not involved in patient management." Judgement comment: not clear what type of randomisation process was used. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly assigned to the treatment or placebo arms." Judgement comment: not clear who performed the allocation and if concealed. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "double‐blind randomized clinical trial" Quote: "LGG and placebo capsules were identical in appearance, taste, and colour, and only a numeric code differentiated the two formulations." Quote: "LGG and placebo formulations were masked and coded appropriately to ensure blindness." Judgement comment: protocol confirms blinding of participants, care providers and investigators. |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: unclear if outcome assessors were blinded but outcome measures are objective. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: 25 participants were assessed for eligibility. All 22 participants randomised were analysed. |
| Selective reporting (reporting bias) | High risk | Quote: "Children with CF who were treated with LGG showed a significant decrease in fecal CLP concentration as compared with those treated with placebo (164 +/‐ 70 vs. 78 +/‐ 54 µg/g, P<0.05; 251 +/‐ 174 vs. 176 +/‐ 125 µg/g, P = 0.3, respectively)." Judgement comment: comparison within groups but not between groups. Adverse events not reported. |
| Other bias | Low risk | Judgement comment: competitive grant funding with no influence on trial design and nil competing interests disclosed. |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: Pocock‐Simon covariate‐adaptive randomisation scheme was adopted with a bias parameter equal to 2/3. The stratification variables considered were sex (2 levels) and age (3 levels). Randomisation was centralised. | |
| Participants | Inclusion criteria
Exclusion criteria
Pretreatment: no overt group differences between those on probiotic and those on placebo. Baseline characteristics Overall
Probiotic
Placebo
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Secondary outcome measures
Time points for measurements: ‐ 6 months (pre‐allocation), 0 months (baseline), 6 months (midway), 12 months (completion). Length of follow‐up: 52 weeks. | |
| Identification | Country: Italy. Setting: multicentre study with 5 CF Regional Centres. Author's name: Alfredo Guarino. Institution: Department of Translational Medicine, Section of Paediatrics, University of Naples. Email: [email protected]. Address: Department of Translational Medicine, Section of Pediatrics, University of Naples“Federico II”, Via Pansini 5, 80131 Naples, Italy. | |
| Funding Source | Grant funding from the Cystic Fibrosis Foundation and Therapeutics (GUARIN10A0). Probiotic and placebo was supplied by DICOFARM. | |
| Declaration of Interest Among the Primary Researchers | Alfredo Guarino received research grant funding from DICOFARM not related to the present trial. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Pocock‐Simon covariate‐adaptive randomisation scheme was adopted". Judgement comment: appropriate randomisation scheme. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was centralised." Quote: "randomised" Judgement comment: appropriate allocation concealment using random sequence. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "LGG and placebo preparations were identical in appearance, taste, and colour to ensure blindness, the numeric code being the only distinctive feature." Judgement comment: double‐blind study (participants and investigators) confirmed in study study protocol. Identical probiotic and placebo formulations. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: blinding of outcome assessors was not described. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: 81 participants randomised, 4 (5%) lost to follow‐up (3 probiotic group and 1 control group). Quote: "Respiratory function tests were performed only in compliant subjects with CF in accordance to the protocol." Judgement comment: unclear how many participants were included in the FEV1 and MMEF analyses. |
| Selective reporting (reporting bias) | High risk | Quote: "Intestinal inflammation" Judgement comment: as outlined in protocol, intestinal inflammation, abdominal pain and intestinal microbiota not reported in study. Adverse events not reported. |
| Other bias | Low risk | Judgement comment: funding sources and Dicofarm did not appear to influence the study design, conduct or results. |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: block randomisation with strata for age and gender. | |
| Participants | Inclusion criteria CF group
Exclusion criteria
Pretreatment: placebo group had significantly worse nutritional status and inflammatory markers at baseline compared with intervention group. Baseline characteristics Overall
Probiotic
Placebo
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Time points for measurements: 0 days (baseline), 90 days (completion). Length of follow‐up: 13 weeks. | |
| Identification | Country: Brasil. Setting: Joana de Gusmão Children’s Hospital. Author's name: Emilia Addison Machado Moreira. Institution: Department of Nutrition, Graduate Program in Nutrition, Federal University of Santa Catarina, Florianópolis, Brazil. Email: [email protected]. | |
| Funding Source | National Council for Scientific and Technological Development. UNIEDU‐Santa Catarina and the CAPES. Farmoquímica. | |
| Declaration of Interest Among the Primary Researchers | No conflicts declared. | |
| Notes | This trial recruited healthy children and adolescents aged between 1 and 16 years without CF to act as a reference for normal (e.g. inflammatory markers). These children were clinically stable for at least 30 days prior to the data collection process. Data from these healthy participants are not included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a block randomisation process and stratified by sex and age". Judgement comment: appropriate randomisation scheme. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The non‐probabilistic, convenience sample was composed of 72 children and adolescents allocated". Judgement comment: allocation concealment mechanism not specified. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "randomized, placebo‐controlled, double‐blind trial" Judgement comment: participants and personnel blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Judegement comment: assessor blinding not specified, however double‐blind study and all outcomes of interest objective. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Five sets of imputed data were created". Judegement comment: 5 sets of missing data; intention‐to‐treat analysis including a principal analysis using multiple imputations. |
| Selective reporting (reporting bias) | High risk | Judegement comment: CRP, lymphocytes and monocytes reported at baseline but not completion. Not all markers in protocol have been measured or recorded. This includes: serum IL‐17A, TGF‐beta, INF‐gamma, faecal calprotectin, intestinal microbiota and handshaking force. Adverse events not reported. |
| Other bias | Low risk | Quote: "The authors declare that they have no competing interests." Judegement comment: no conflicts for authors., competitive grant funding, synbiotic donated. |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: randomisation between 2 probiotic preparations, method not specified. | |
| Participants | Inclusion criteria
Exclusion criteria
Baseline characteristics
| |
| Interventions | Probiotic 1
Probiotic 2
| |
| Outcomes | Primary outcome measures
Time points for measurements: baseline, 6 months. Length of follow‐up: 24 weeks. | |
| Identification | Sponsorship source: no stated. Country: Spain. Setting: Cystic Fibrosis Unit, Hospital. Author's name: Rosa del Campo. Institution: Cystic Fibrosis Unit, Hospital Universitario Ramón y Cajal, Madrid, Spain. Email: [email protected]. Address: Servicio de Microbiología, Hospital Universitario Ramón y Cajal. Ctra. Colmenar Km 9,1. 28034 Madrid, Spain. | |
| Funding Source | Not specified. | |
| Declaration of Interest Among the Primary Researchers | Not specified. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized protocol assigned correlatively" Judgement comment: randomisation not specified. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: no specified allocation concealment method. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "capsule/day of CasenBiotic, (CasenFleet), and group B consumed 2 package/day of VLS3 (Faes Farma)." Judgement comment: no clear blinding process. Both groups consumed probiotics and one intervention was given in a capsule and the other as a powder in a sachet. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: no clear blinding process. |
| Incomplete outcome data (attrition bias) | Unclear risk | Judgement comment: unclear how many were enrolled and how many completed. |
| Selective reporting (reporting bias) | Unclear risk | Judgement comment: specific results not reported in the abstract. Adverse events not reported. |
| Other bias | Unclear risk | Judgement comment: unclear funding source or other sources of bias given abstract. |
| Methods | Trial design: RCT. Trial grouping: cross‐over, without a washout period. Randomisation method: not specified, performed by the Biostatistics Unit of Hospital Ramón y Cajal. | |
| Participants | Incluson criteria
Excluded criteria
Baseline characteristics Overall
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Secondary outcome measures
Time points for measurements: 6 months. Length of follow‐up: 24 weeks. | |
| Identification | Country: Spain. Setting: 2 separate CF‐Units in Spain (Madrid and Granada). Author's name: Rosa del Campo. Institution: Instituto Ramón y Cajal de Investigación Sanitaria (IRYCIS), Madrid, Spain. Email: [email protected]. Address: Servicio de Microbiología, Hospital Universitario Ramón y Cajal. Ctra. Colmenar Km 9,1. 28034 Madrid, Spain. | |
| Funding Source | FP‐7‐HEALTHF3‐2011‐EVOTAR‐282004 project and the PI12/00734 and PI13/00205 projects supported by Instituto de Salud Carlos III (Ministry of Economy and Competitiveness) and co‐financed by the European Development Regional Fund “A Way toAchieve Europe,” ERDF, and the Spanish Network for the Research in Infectious Diseases (REIPI RD12/0015). BioGaia provided the placebo formulation. | |
| Declaration of Interest Among the Primary Researchers | No declaration made. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomized". Judgement comment: Randomisation not specified. Author correspondence: The Biostatistics Unit of Hospital Ramón y Cajal prepared the randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: allocation method not specified. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "Only the manufacturers knew the significance of the product labels and the double blind code was opened only at the end of the study". Quote: "Active and placebo tablets and packaging were identical". Judgement comment: only manufacturer knew labels of probiotic and placebo so all participants and personnel should have been blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: unblinding only at the end of the trial. |
| Incomplete outcome data (attrition bias) | High risk | Quote: "although finally only 25 pairs offered sufficient quality results, due to bad processing or abundance of unspecific sequences". Quote: "Thirty‐nine CF patients were initially enrolled, and 30 completed the entire protocol". Judgement comment: 23% of participants did not complete the entire protocol.16S rRNA analysis only included 25/30 pairs of samples (83%). |
| Selective reporting (reporting bias) | High risk | Quote: "Anthropometric data, clinical status, antibiotic treatments, hospital admittances". Judgement comment: no protocol available, unclear if clinical status data (listed) were collected throughout as not presented. Judgement comment: 3 Spanish hospitals reported in 2013 abstract (Oral presentation in the 36th ECFC, Lisbon, 2013), however only 2 centres reported in this article. |
| Other bias | Unclear risk | Judgement comment: cross‐over study design with no true baseline data collected. Post‐probiotic and post‐placebo data pooled. Competitive grant funding. No conflicts reported. |
| Methods | Trial design: RCT. Trial grouping: cross‐over, without a washout period. Randomisation method: not specified. | |
| Participants | Inclusion criteria
Exclusion criteria
Baseline characteristics
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Time points for measurements: onset, end of treatment administration, and end of subsequent placebo administration. Length of follow‐up: 52 weeks. | |
| Identification | Country: Italy. Setting: CF Centre. Author's name: Alfredo Guarino. Institution: Department of Pediatrics, University of Naples ‘‘Federico II’’, Via S. Pansini 5, 80131 Naples, Italy. Email: [email protected]. | |
| Funding Source | Not specified. | |
| Declaration of Interest Among the Primary Researchers | No declaration. | |
| Notes | Abstract only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly assigned". Judgement comment: not sure method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not specified. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "child's family was blind to the treatment or placebo regimen". Judgement comment: participants blinded to treatment however investigators not. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Judgement comment: outcome assessors not clearly blinded. |
| Incomplete outcome data (attrition bias) | Low risk | Judgement comment: all 24 children included. |
| Selective reporting (reporting bias) | Low risk | Judgement comment: all outcome measures reported in abstract. Adverse events not reported. |
| Other bias | Low risk | Judgement comment: no conflicts reported in abstract. |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: random permuted blocks algorithm. | |
| Participants | Inclusion criteria
Exclusion criteria
Pretreatment: mean (SD) values for FEV1 % predicted for probiotic and placebo groups were 94.7% (15.4) and 97.5% (15.2) respectively (not significant). Baseline characteristics Overall
Probiotic
Placebo
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Secondary outcome measures
Time‐points for measurements: baseline, 6 months. Length of follow‐up: 26 weeks. | |
| Identification | Country: Italy. Setting: Regional Center for CF of the Department of Pediatrics, University of Rome "La Sapienza". Author's name: Laura Stronati. Institution: ENEA, Italian National Agency for New Technologies, Energy and Sustainable Economic Development, Rome, Italy. Email: [email protected]. Address: Section of Toxicology and Biomedical Sciences, Italian National Agency for New Technologies, Energy and Sustainable Economic Development, ENEA – Via Anguillarese 301, 00123 Rome, Italy. | |
| Funding Source | Italchimici (Pomezia, Italy) supplied probiotic and placebo. | |
| Declaration of Interest Among the Primary Researchers | The authors report no conflicts of interest. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "allocation schedule was computer generated, using a random permuted blocks algorithm". Judgement comment: appropriate sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation schedule was fully concealed from the doctors working in the Regional Center for CF who recruited patients to the study". Judgement comment: appropriate allocation concealment. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "The unblinding procedures were performed after the study was completed and the statistical analysis carried out". Quote: "The placebo was packed in identical bottles, had the same colour, weight, smell, and taste of the probiotic formulation". Judgement comment: participants and personnel adequately blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "Outcome measures of efficacy were recorded by investigators totally unaware of LR or placebo administration to patients". Judgement comment: adequate blinding of assessors. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "A premature discontinuation occurred only in 1 of the placebo group for with‐ drawn consent after randomisation". Judgement comment: 61 randomised, 1 (1.6%) withdrew consent after randomisation (placebo). |
| Selective reporting (reporting bias) | Unclear risk | Quote: "The duration in days of each episode corresponded to the duration of the antibiotic therapy". Judgement comment: number of days for treatment of pulmonary exacerbation not presented. |
| Other bias | Low risk | Quote: "Both products were supplied by Italchimici (Pomezia, Italy), who had no role in the conception, design, conduct of the study, or in the analysis and interpretation of the data". Judgement comment: the authors report not conflicts and pharmaceutical company played no role in study. |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: simple randomisation. | |
| Participants | Inclusion criteria
Exclusion criteria
Baseline characteristics Overall
Probiotic
Placebo
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Time points for measurements: baseline, 1 month. Length of follow‐up: 4 weeks. | |
| Identification | Country: Iran. Setting: The Pediatrics Center of Excellence in Iran. Author's name: Nima Rezaei. Institution: Molecular Immunology Research Center, and Department of Immunology, School of Medicine, Tehran University of Medical Sciences, Tehran. Email: [email protected]. Address: Children Medical Center, 62 Gharib St, 14194 Tehran, Iran. | |
| Funding Source | Sponsored by Tehran University of Medical Sciences. | |
| Declaration of Interest Among the Primary Researchers | No conflicts disclosed. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were simply randomly divided into two groups". Judgement comment: unclear about method of randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not specified. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "As a double‐blind study, neither the patients nor the doctors/researchers were aware of the placebo or probiotic packs". Judgement comment: participants and personnel all blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: not specified but outcome measure objective. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "Although 50 patients were initially selected for this study, three patients later decided not to participate in this study and did not bring the stool samples. Therefore, 47 patients were enrolled in the study". Judgement comment: 50 participants enrolled and 47 randomised (all included). |
| Selective reporting (reporting bias) | Low risk | Judgement comment: stool calprotectin only outcome measure reported. Consistent with the protocol. Adverse events not reported. |
| Other bias | Low risk | Judgement comment: sponsored by Tehran University of Medical Sciences. No conflicts disclosed. |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: not specified, "randomly divided". | |
| Participants | Inclusion criteria
Exclusion criteria
Baseline characteristics Overall
Probiotic
Placebo
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Time points for measurements: baseline, 1 month (end intervention), 4 months (3 months post treatment) and 7 months (6 months post treatment). Length of follow‐up: 30 weeks. | |
| Identification | Country: Iran. Setting: Dr. Sheikh Pediatric Hospital CF clinic, Mashhad, Iran. Author's name: Atieh Mehdizadeh‐Hakkak. Institution: Cystic Fibrosis Clinic, Dr. Sheikh Pediatric Hospital, Mashhad University of Medical Sciences, Mashhad, Iran. Email: [email protected]. Address: Number 21, 12th Felestin Street, Mashhad, Iran. | |
| Funding Source | Research Chancellor of Mashhad University of Medical Sciences. | |
| Declaration of Interest Among the Primary Researchers | No conflicts of interest. | |
| Notes | The authors have compared probiotic participants with themselves in the previous year instead of against the placebo cohort; these data were not included in our review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomly divided into two groups of probiotic and placebo". Judgement comment: randomisation process not specified. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not specified. |
| Blinding of participants and personnel (performance bias) | High risk | Quote: "placebo capsules which contained all ingredients of probiotic capsules except the effective substance". Judgement comment: participants were blinded. Protocol states investigators were not blinded. |
| Blinding of outcome assessment (detection bias) | Low risk | Quote: "The questionnaires were filled out by a trained nurse who was unaware about the principles of study". Judgement comment: HRQoL assessment blinded. Pulmonary exacerbation assessment not blinded but defined based on consensus criteria. |
| Incomplete outcome data (attrition bias) | Low risk | Quote: "All patients enrolled in both groups (placebo group: 7 females, 13 males, mean age 5.36±2.66 years; placebo group: 10 females, 7 males, mean age 5.50±2.55 years) completed the study period". Judgement comment: all participants completed. |
| Selective reporting (reporting bias) | High risk | Judgement comment: growth outcomes (weight, height, head circumference and arm circumference) included in protocol (IRCT201205219823N1 ‐ this differs from registration number listed in the article which cannot be found) and not reported in paper. Also unclear why pulmonary exacerbations were not compared between probiotic and placebo groups. Adverse events not reported. |
| Other bias | Unclear risk | Judgement comment: unclear if Probiotics International Company, UK also provided the placebo product and if they had any influence on the study. |
| Methods | Trial design: RCT. Trial grouping: cross‐over design. Randomisation method: not specified. | |
| Participants | Inclusion criteria
Exclusion criteria
Baseline characteristics Overall
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measure
Secondary outcome measures
Time points for measurements: not specified. Length of follow‐up: not specified. | |
| Identification | Country: Israel. Setting: Edmond and Lily Safra Children's Hospital, Sheba Medical Center. Author's name: Dr Batia Weiss. Institution: Pediatric Gastroenterology Unit, Edmond and Lily Safra Children's Hospital, Sheba Medical Center, Israel. Email: [email protected]. Address: Emek Ha’ela 31, Ramat Gan, Israel. | |
| Funding Source | Not specified. | |
| Declaration of Interest Among the Primary Researchers | Not specified. | |
| Notes | Trial was terminated in December 2013 after a severe allergic reaction (severe urticaria) for 1 participant in the probiotic group. 12 participants were enrolled at the time of termination. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information available. |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information available. |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information available. |
| Blinding of outcome assessment (detection bias) | Unclear risk | Insufficient information available. |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient information available. |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information available. |
| Other bias | Unclear risk | Blinding of outcome assessment (detection bias). |
| Methods | Trial design: RCT. Trial grouping: cross‐over, with a 1‐month washout period. Randomisation method: not specified. | |
| Participants | Inclusion criteria
Exclusion criteria
Baseline characteristics Overall
| |
| Interventions | Probiotic
Placebo
| |
| Outcomes | Primary outcome measures
Secondary outcome measures
Time points for measurements: baseline, 4 months (end), 5 months (washout) and 9 months (end). Length of follow‐up: 39 weeks. | |
| Identification | Sponsorship source: not stated. Country: Belgium. Setting: 3 CF centres (Ghent, Antwerp and Brussels). Authors name: S. Van Biervliet. Institution: Ghent University Hospital. Email: [email protected]. Address: Ghent University Hospital, Ghent, Belgium. | |
| Funding Source | A company (Vésale Pharma) provided the probiotic. No funding source(s) declared. | |
| Declaration of Interest Among the Primary Researchers | No conflicts of interest statement declared. | |
| Notes | In the first phase, 17 and 14 participants commenced the probiotic and placebo respectively. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Judgement comment: not specified; randomisation provided by the company who provided the probiotic. |
| Allocation concealment (selection bias) | Unclear risk | Judgement comment: not specified. |
| Blinding of participants and personnel (performance bias) | Low risk | Quote: "A double blind cross over study". Judgement comment: probiotic and placebo vials were differentiated only by a number (personal communication). |
| Blinding of outcome assessment (detection bias) | Low risk | Judgement comment: outcome assessors were blinded (personal communication). |
| Incomplete outcome data (attrition bias) | Unclear risk | Quote: "we didn't reach the acquired number needed to treat to draw firm conclusions". Quote: "31 patients agreed, only 25 finished (3 non‐starters, 1 stopped due to diarrhoea, 2 not compliant)". Judgement comment: 20% of participants did not finish. |
| Selective reporting (reporting bias) | High risk | Quote: "a blood sample was taken for analysis of CRP and total IgG". Judgement comment: most reported outcomes presented but serum CRP and IgG not presented. No published protocol available. |
| Other bias | Low risk | Judgement comment: no conflicts identified. |
B: bifidobacterium
BMI: body mass index
CF: cystic fibrosis
CFU: colony forming units
CRP: C‐reactive protein
FEV1 % predicted: forced expiratory volume in 1 second % predicted
FISH: fluorescence in situ hybridisation
FOS: fructooligosaccharides
FVC: forced vital capacity
GIQLI: Gastrointestinal Quality of Life Index
HRQoL: health‐related quality of life
IQR: interquartile range
IV: intravenous
L: lactobacillus
LGG: Lactobacillus rhamnosus GG
MD: mean difference
MMEF 25 ‐ 75%: maximal (mid‐) expiratory flow
MPO: myeloperoxidase
NIRA: near‐infrared reflectance analysis
NOx: nitric oxide metabolites
ORS: oral rehydration solution
P aeruginosa: Pseudomonas aeruginosa
PedsQLTM 4.0 SF 15: Pediatric Quality of Life Inventory 4.0 Short form questionnaire
RCT: randomised controlled trial
S: streptococcus
SD: standard deviation
vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Ineligible design, case control trial. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Double‐blind RCT. Design: parallel. Duration: 4 weeks. Location: single centre (Children Medical Center, Tehran). |
| Participants | Inclusion criteria: children with CF aged between 4 and 18 years of age; either gender; pancreatic insufficient ; no NSAIDs for 2 weeks prior to enrolment. Exclusion criteria: younger than 4 years of age; pancreatic sufficient; used NSAIDs during 2 weeks prior to enrolment. Target sample size: 25. |
| Interventions | Intervention: protexin 2 sachet per day which can be mixed with yoghurt. Placebo: maltodextrin 2 sachet per day which can be mixed in yoghurt. |
| Outcomes | Change in fecal calprotectin at 1 month. |
| Notes | Funding source: drug and placebo send by manufacturer free of charge and no payment to the Tehran Medical University for running the trial. Contact: A/Professor Dr Farzaneh Motamed, Pediatric Gasteroentrologist, Children Medical Center, 62 Gharib St, 14194 Tehran, Iran ([email protected]). |
| Methods | RCT. Design: parallel. Duration: 1 month. |
| Participants | Inclusion criteria: aged 2 to 12 years old; CF diagnosed by sweat testing, clinical symptoms and common mutations. Exclusion criteria: participant intolerance to the medication; no parental consent; unable to consume probiotic because of severe disease. 37 participants randomised. Age, range: 2 ‐ 12 years. Gender: both. |
| Interventions | Intervention (n = 20): protexin, 2 capsules daily for 1 month. Control (n = 17): usual treatment only. |
| Outcomes | Number of pulmonary exacerbations (at 1 and 3 months post‐intervention) QoL (PedsQL 4.0) (at baseline and 3 and 6 months post‐intervention) Height (at baseline and 1 and 3 months post‐intervention) Weight (at baseline and 1 and 3 months post‐intervention) Head circumference (at baseline and 1 and 3 months post‐intervention) Arm circumference (at baseline and 1 and 3 months post‐intervention) |
| Notes | Funding source: 100% Vice Chancellor of Research, Mashhad University of Medical Sciences, Iran. Contact: Dr. Hamidreza Kianifar, Department of Paediatrics, Ghaem Hospital, Iran ([email protected]). |
CF: cystic fibrosis
NSAIDs: non‐steroidal anti‐inflammatory drugs
QoL: quality of life
RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Probiotics and the EARly Life effects on intestinal bacteria and inflammation in children with Cystic Fibrosis (PEARL‐CF). |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: block randomisation. |
| Participants | Inclusion criteria
Exclusion criteria
Target sample size Probiotic group: n = 32. Placebo group: n = 32. Healthy controls: n = 64. |
| Interventions | Probiotic
Placebo
|
| Outcomes | Primary outcome measures
Secondary outcome measures
Time points for measurements: baseline, 2 months, 4 months, 6 months, 8 months, 10 months, 12 months (end), 15 months (i.e. 3 months post‐intervention), 18 months, 21 months and 24 months (i.e. 12 months post‐intervention). Length of follow‐up: 104 weeks. |
| Starting date | 17 June 2016. |
| Contact information | Associate Professor (Keith) Chee Y. Ooi Sydney Children's Hospital Randwick High Street, Randwick 2031, New South Wales Australia Phone: +61293821752 Fax: +61293821401 Email: [email protected] |
| Notes | Multicentre at 5 sites: Sydney (Randwick and Westmead), Melbourne, Brisbane and Christchurch (New Zealand). This trial is recruiting healthy controls (without CF) to act as a reference for normal (e.g. intestinal microbiota and inflammatory markers): Data from these healthy participants will not be included in the review. |
| Trial name or title | Effect of synbiotic supplementation in comparison with placebo on anthropometric indices, quality of life and clinical outcome in children with cystic fibrosis. |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: table of random numbers. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Probiotic
Placebo
|
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | 14 February 2017. |
| Contact information | Assistant Professor Zeinab Nikniaz Liver and Gastrointestinal Disease Research Center, Tabriz University of Medical Sciences, Tabriz, Islamic Republic of Iran. Phone: +98 41 3336 7473. Email address: [email protected]. |
| Notes |
| Trial name or title | Modulation of Intestinal and Pulmonary Inflammation by Lactobacillus Diet Supplementation in Pediatric Cystic Fibrosis (MoHuM‐1). |
| Methods | Trial design: RCT. Trial grouping: cross‐over, with 1‐week washout period. Randomisation method: not specified. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Probiotic
Placebo
|
| Outcomes | Primary outcome measures
Time points for measurements: baseline, 12 weeks, 24 weeks. Length of follow‐up: 25 weeks. |
| Starting date | March 2013. |
| Contact information | Dr Christian Kahlert, MD Childrens's Hospital of Eastern Switzerland St. Gallen, SG, Switzerland, 9008 Phone: +41714941111 Email address: [email protected] |
| Notes |
| Trial name or title | Effect of supplementation with a synbiotic on markers of the inflammatory response in children and adolescents with cystic fibrosis. |
| Methods | Trial design: RCT. Trial grouping: parallel group. Randomisation method: not specified. |
| Participants | Inclusion criteria CF group
Controls without CF
Exclusion criteria
Target sample size Probiotic group: n = 25. Placebo group: n = 25. |
| Interventions | Probiotic
Placebo
|
| Outcomes | Primary outcome measures
Secondary outcome measures
Time points for measurements: baseline, 90 days. Length of follow‐up: 13 weeks. |
| Starting date | 03 October 2014. |
| Contact information | Emilia Addison Machado Moreira Universidade Federal de Santa Catarina Campus Universitário, Trindade 88.040‐97 Florianópolis Brazil Phone: +55(48) 3721 9784 Email: [email protected] |
| Notes |
B: bifidobacterium
BMI: body mass index
CF: cystic fibrosis
FEV1: forced expiratory volume in 1 second
FISH: fluorescence in situ hybridisation
FVC: forced vital capacity
HRQoL: health‐related quality of life
IV: intravenous
L: lactobacillus
RCT: randomised controlled trial
S: streptococcus
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary exacerbation (mean number per participant) Show forest plot | 4 | 225 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.68, 0.03] |
| Analysis 1.1  Comparison 1 Probiotic versus placebo, Outcome 1 Pulmonary exacerbation (mean number per participant). | ||||
| 1.1 Over 3 months and up to 6 months | 3 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.80, 0.02] |
| 1.2 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.75, 0.95] |
| 2 Pulmonary exacerbation (duration of antibiotic therapy ‐ any route) Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐9.04, 8.14] |
| Analysis 1.2  Comparison 1 Probiotic versus placebo, Outcome 2 Pulmonary exacerbation (duration of antibiotic therapy ‐ any route). | ||||
| 2.1 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐12.89, 8.89] |
| 2.2 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐11.88, 16.08] |
| 3 Faecal calprotectin (µg/g) Show forest plot | 4 | 177 | Mean Difference (IV, Random, 95% CI) | ‐47.41 [‐93.28, ‐1.54] |
| Analysis 1.3  Comparison 1 Probiotic versus placebo, Outcome 3 Faecal calprotectin (µg/g). | ||||
| 3.1 Up to 3 months | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐105.02 [‐205.23, ‐4.81] |
| 3.2 Over 3 months and up to 6 months | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐32.14 [‐83.73, 19.45] |
| 4 Faecal calprotectin (µg/g) ‐ change from baseline ‐ sensitivity analysis Show forest plot | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐31.17 [‐81.56, 19.22] |
| Analysis 1.4  Comparison 1 Probiotic versus placebo, Outcome 4 Faecal calprotectin (µg/g) ‐ change from baseline ‐ sensitivity analysis. | ||||
| 4.1 Up to 3 months | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐11.0 [‐246.06, 224.06] |
| 4.2 Over 3 months and up to 6 months | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐32.14 [‐83.73, 19.45] |
| 5 Faecal calprotectin (µg/g) ‐ post treatment ‐ sensitivity analysis Show forest plot | 3 | 119 | Mean Difference (IV, Random, 95% CI) | ‐45.46 [‐176.25, 85.32] |
| Analysis 1.5  Comparison 1 Probiotic versus placebo, Outcome 5 Faecal calprotectin (µg/g) ‐ post treatment ‐ sensitivity analysis. | ||||
| 5.1 Up to 3 months | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐107.29 [‐171.20, ‐43.38] |
| 5.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 77.0 [3.11, 150.89] |
| 6 Serum cytokines Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.6  Comparison 1 Probiotic versus placebo, Outcome 6 Serum cytokines. | ||||
| 6.1 TNF‐α (pg/ml) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.72, 1.13] |
| 6.2 IL‐8 (pg/ml) | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 26.93 [‐46.25, 100.11] |
| 6.3 IL‐1β (pg/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.28, 0.88] |
| 6.4 IL‐6 (pg/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.68, 0.10] |
| 6.5 IL‐10 (pg/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.43, 0.33] |
| 6.6 NOx (μmol/L) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.26, 0.42] |
| 6.7 IL‐12 (pg/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.10, 0.96] |
| 6.8 MPO (mU/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.14, 0.24] |
| 7 Sputum cytokines ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.7  Comparison 1 Probiotic versus placebo, Outcome 7 Sputum cytokines ‐ change from baseline. | ||||
| 7.1 TNF‐α (pg/ml) | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.94, 0.54] |
| 7.2 IL‐8 (pg/ml) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.92, 0.52] |
| 8 Adverse events Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.8  Comparison 1 Probiotic versus placebo, Outcome 8 Adverse events. | ||||
| 8.1 Mortality (all causes) | 5 | 310 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Serious adverse reaction | 5 | 310 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.13, 67.06] |
| 8.3 Adverse reaction | 5 | 310 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.49, 18.46] |
| 9 Height (z score) ‐ post treatment Show forest plot | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.27, 0.47] |
| Analysis 1.9  Comparison 1 Probiotic versus placebo, Outcome 9 Height (z score) ‐ post treatment. | ||||
| 9.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.62, 1.04] |
| 9.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.34, 0.48] |
| 10 Weight (z score) ‐ post treatment Show forest plot | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.51, 0.05] |
| Analysis 1.10  Comparison 1 Probiotic versus placebo, Outcome 10 Weight (z score) ‐ post treatment. | ||||
| 10.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.4 [‐0.70, ‐0.10] |
| 10.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] |
| 11 Weight (kg) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.11  Comparison 1 Probiotic versus placebo, Outcome 11 Weight (kg) ‐ change from baseline. | ||||
| 11.1 Over 3 months and up to 6 months | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 1.2 [‐0.04, 2.44] |
| 12 BMI (z score) Show forest plot | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.25, 0.22] |
| Analysis 1.12  Comparison 1 Probiotic versus placebo, Outcome 12 BMI (z score). | ||||
| 12.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.54, 0.32] |
| 12.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.26, 0.31] |
| 13 Lung function (FEV1 % predicted) Show forest plot | 5 | 284 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐1.20, 3.91] |
| Analysis 1.13  Comparison 1 Probiotic versus placebo, Outcome 13 Lung function (FEV1 % predicted). | ||||
| 13.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐8.07 [‐19.87, 3.73] |
| 13.2 Over 3 months and up to 6 months | 3 | 166 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐0.51, 4.94] |
| 13.3 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐12.13, 6.53] |
| 14 Lung function (FEV1 % predicted) ‐ change from baseline ‐ sensitivity analysis Show forest plot | 3 | 166 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐0.51, 4.94] |
| Analysis 1.14  Comparison 1 Probiotic versus placebo, Outcome 14 Lung function (FEV1 % predicted) ‐ change from baseline ‐ sensitivity analysis. | ||||
| 14.1 Over 3 months and up to 6 months | 3 | 166 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐0.51, 4.94] |
| 15 Lung function (FEV1 % predicted) ‐ post treatment ‐ sensitivity analysis Show forest plot | 3 | 168 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐7.10, 4.48] |
| Analysis 1.15  Comparison 1 Probiotic versus placebo, Outcome 15 Lung function (FEV1 % predicted) ‐ post treatment ‐ sensitivity analysis. | ||||
| 15.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐14.48, 11.68] |
| 15.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐8.85, 9.05] |
| 15.3 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐12.13, 6.53] |
| 16 Hospitalisations (number ‐ all causes) Show forest plot | 2 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.41, 0.54] |
| Analysis 1.16  Comparison 1 Probiotic versus placebo, Outcome 16 Hospitalisations (number ‐ all causes). | ||||
| 16.1 Over 3 months and up to 6 months | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.74, ‐0.26] |
| 16.2 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.25, 0.25] |
| 17 HRQoL (validated questionnaire) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.17  Comparison 1 Probiotic versus placebo, Outcome 17 HRQoL (validated questionnaire). | ||||
| 17.1 PedsQLTM 4.0 SF 15 ‐ Parent Report (performed 3 months post 1 month intervention period) | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.87 [0.19, 1.55] |
| 17.2 PedsQLTM 4.0 SF 15 ‐ Child Report (performed 3 months post 1 month intervention period) | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [‐0.07, 1.26] |

Study flow diagram.
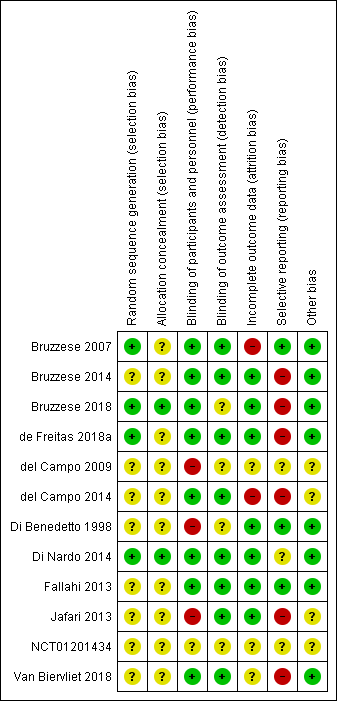
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Comparison 1 Probiotic versus placebo, Outcome 1 Pulmonary exacerbation (mean number per participant).

Comparison 1 Probiotic versus placebo, Outcome 2 Pulmonary exacerbation (duration of antibiotic therapy ‐ any route).
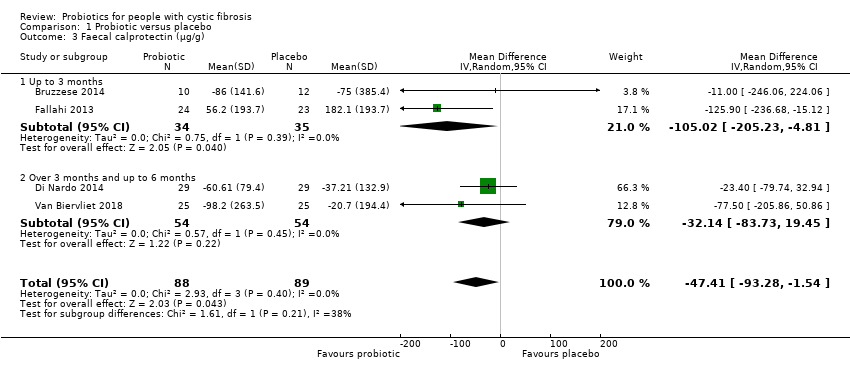
Comparison 1 Probiotic versus placebo, Outcome 3 Faecal calprotectin (µg/g).

Comparison 1 Probiotic versus placebo, Outcome 4 Faecal calprotectin (µg/g) ‐ change from baseline ‐ sensitivity analysis.

Comparison 1 Probiotic versus placebo, Outcome 5 Faecal calprotectin (µg/g) ‐ post treatment ‐ sensitivity analysis.

Comparison 1 Probiotic versus placebo, Outcome 6 Serum cytokines.

Comparison 1 Probiotic versus placebo, Outcome 7 Sputum cytokines ‐ change from baseline.

Comparison 1 Probiotic versus placebo, Outcome 8 Adverse events.

Comparison 1 Probiotic versus placebo, Outcome 9 Height (z score) ‐ post treatment.
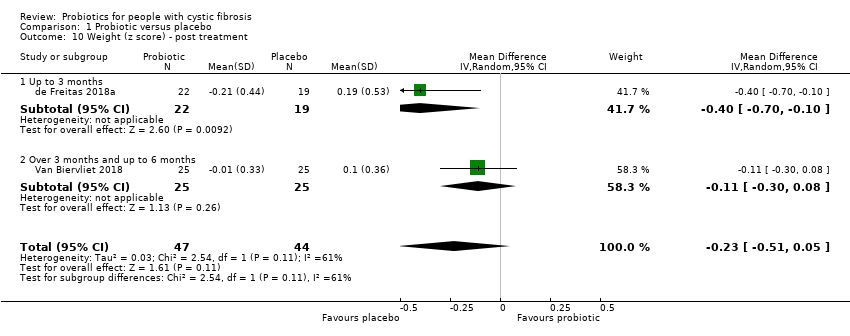
Comparison 1 Probiotic versus placebo, Outcome 10 Weight (z score) ‐ post treatment.
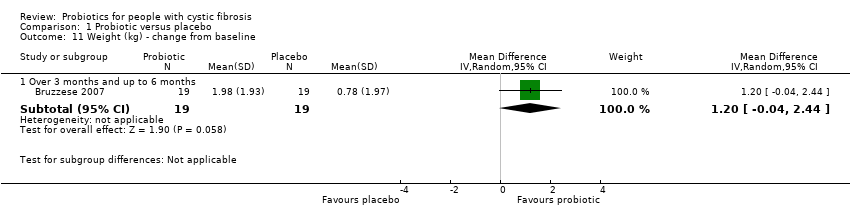
Comparison 1 Probiotic versus placebo, Outcome 11 Weight (kg) ‐ change from baseline.

Comparison 1 Probiotic versus placebo, Outcome 12 BMI (z score).

Comparison 1 Probiotic versus placebo, Outcome 13 Lung function (FEV1 % predicted).

Comparison 1 Probiotic versus placebo, Outcome 14 Lung function (FEV1 % predicted) ‐ change from baseline ‐ sensitivity analysis.

Comparison 1 Probiotic versus placebo, Outcome 15 Lung function (FEV1 % predicted) ‐ post treatment ‐ sensitivity analysis.

Comparison 1 Probiotic versus placebo, Outcome 16 Hospitalisations (number ‐ all causes).

Comparison 1 Probiotic versus placebo, Outcome 17 HRQoL (validated questionnaire).
| Probiotics compared to placebo for children and adults with CF | ||||||
| Patient or population: children and adults with CF | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Certainty of the evidence | Comments | |
| Risk with placebo | Risk with probiotics | |||||
| Number of pulmonary exacerbations | The mean (range) number of pulmonary exacerbations in the placebo group was 1.42 (0.37 to 2.2) episodes per participant. | The mean number of pulmonary exacerbations in the probiotics group was 0.32 episodes per participant lower (0.68 lower to 0.03 higher). | NA | 225 | ⊕⊕⊝⊝ | Probiotics probably reduce pulmonary exacerbations (mean number per participant) slightly. |
| Faecal calprotectin | The mean (range) faecal calprotectin in the placebo group was 132.9 µg/g (67 µg/g to 182.1 µg/g). | The mean faecal calprotectin in the probiotics group was 47.4 µg/g lower (93.28 lower to 1.54 lower). | NA | 177 | ⊕⊕⊝⊝ | Probiotics result in a reduction in faecal calprotectin. |
| Adverse events (serious adverse reaction and adverse reaction) | There were 0 adverse events in the placebo group. | There were 4 adverse events in the probiotics group. | RR 3.00 | 310 | ⊕⊕⊝⊝ | Probiotics result in a small number of adverse events. The terminated RCT reported a serious adverse event (severe urticaria) in 1 participant on probiotics. No mortalities were reported in any included RCTs. |
| Weight (z score) | The mean (range) weight (z score) in the placebo group was ‐1.2 (‐1.79 to ‐0.81). | The mean weight (z score) in the probiotics group was0.24 SD lower (0.52 lower to 0.05 higher). | NA | 91 | ⊕⊕⊝⊝ | Insufficient evidence to determine if probiotics result in little to no difference in weight. A third RCT (n = 38) reported weight in kg and also reported no significant difference in weight. |
| Lung function (FEV1 % predicted) | The mean (range) FEV1 (% predicted) in the placebo group was 84.8% (52.7% to 104%) | The mean FEV1 (% predicted) in the probiotics group was 1.36% higher (1.20 lower to 3.91 higher). | NA | 284 | ⊕⊕⊝⊝ | Insufficient evidence to determine if probiotics result in little to no difference in lung function (FEV1 % predicted). |
| Hospitalisations (all causes) | The mean number of hospitalisations (all causes) in the placebo group was 0.53 admissions per participant. | The mean number of hospitalisations in the probiotics group was 0.44 admissions per participant lower (1.41 lower to 0.54 higher). | NA | 115 | ⊕⊕⊝⊝ | Insufficient evidence to determine if probiotics result in little to no difference in hospitalisation rates. |
| HRQoL (PedsQLTM 4.0 SF 15 (Scale from: 0 to 100)) | The mean HRQoL score from the PedsQLTM 4.0 SF 15 ‐ Parent Report in the placebo group was 81.4. | The standardised mean HRQoL score from the PedsQLTM 4.0 SF 15 ‐ Parent Report in the probiotics group was 0.87 SD higher (0.19 higher to 1.55 higher). | NA | 37 | ⊕⊕⊝⊝ | Insufficient evidence to determine if probiotics result in a small effect in HRQoL. |
| The mean HRQoL score from the PedsQLTM 4.0 SF 15 ‐ Child Report in the placebo group was 85.9. | The standardised mean HRQoL score from the PedsQLTM 4.0 SF 15 ‐ Child Report in the probiotics group was 0.59 SD higher (0.07 lower to 1.26 higher). | NA | 37 | ⊕⊕⊝⊝ | Insufficient evidence to determine if probiotics result in a small effect in HRQoL. | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| a Downgraded once due to high risk of bias due to selective reporting. b Downgraded due to high risk of bias due to incomplete outcome data. c Downgraded due to lack of generalisability as majority of the studies only include children. d Downgraded due to high risk of bias due to blinding. | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Pulmonary exacerbation (mean number per participant) Show forest plot | 4 | 225 | Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.68, 0.03] |
| 1.1 Over 3 months and up to 6 months | 3 | 148 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.80, 0.02] |
| 1.2 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.75, 0.95] |
| 2 Pulmonary exacerbation (duration of antibiotic therapy ‐ any route) Show forest plot | 2 | 127 | Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐9.04, 8.14] |
| 2.1 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐2.0 [‐12.89, 8.89] |
| 2.2 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 2.10 [‐11.88, 16.08] |
| 3 Faecal calprotectin (µg/g) Show forest plot | 4 | 177 | Mean Difference (IV, Random, 95% CI) | ‐47.41 [‐93.28, ‐1.54] |
| 3.1 Up to 3 months | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐105.02 [‐205.23, ‐4.81] |
| 3.2 Over 3 months and up to 6 months | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐32.14 [‐83.73, 19.45] |
| 4 Faecal calprotectin (µg/g) ‐ change from baseline ‐ sensitivity analysis Show forest plot | 3 | 130 | Mean Difference (IV, Random, 95% CI) | ‐31.17 [‐81.56, 19.22] |
| 4.1 Up to 3 months | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐11.0 [‐246.06, 224.06] |
| 4.2 Over 3 months and up to 6 months | 2 | 108 | Mean Difference (IV, Random, 95% CI) | ‐32.14 [‐83.73, 19.45] |
| 5 Faecal calprotectin (µg/g) ‐ post treatment ‐ sensitivity analysis Show forest plot | 3 | 119 | Mean Difference (IV, Random, 95% CI) | ‐45.46 [‐176.25, 85.32] |
| 5.1 Up to 3 months | 2 | 69 | Mean Difference (IV, Random, 95% CI) | ‐107.29 [‐171.20, ‐43.38] |
| 5.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 77.0 [3.11, 150.89] |
| 6 Serum cytokines Show forest plot | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 TNF‐α (pg/ml) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.72, 1.13] |
| 6.2 IL‐8 (pg/ml) | 2 | 95 | Mean Difference (IV, Random, 95% CI) | 26.93 [‐46.25, 100.11] |
| 6.3 IL‐1β (pg/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.28, 0.88] |
| 6.4 IL‐6 (pg/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.68, 0.10] |
| 6.5 IL‐10 (pg/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.43, 0.33] |
| 6.6 NOx (μmol/L) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.08 [‐0.26, 0.42] |
| 6.7 IL‐12 (pg/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.53 [0.10, 0.96] |
| 6.8 MPO (mU/ml) | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.14, 0.24] |
| 7 Sputum cytokines ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 TNF‐α (pg/ml) | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.94, 0.54] |
| 7.2 IL‐8 (pg/ml) | 1 | 54 | Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.92, 0.52] |
| 8 Adverse events Show forest plot | 5 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 8.1 Mortality (all causes) | 5 | 310 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 8.2 Serious adverse reaction | 5 | 310 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.13, 67.06] |
| 8.3 Adverse reaction | 5 | 310 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.49, 18.46] |
| 9 Height (z score) ‐ post treatment Show forest plot | 2 | 91 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.27, 0.47] |
| 9.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.62, 1.04] |
| 9.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.34, 0.48] |
| 10 Weight (z score) ‐ post treatment Show forest plot | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.51, 0.05] |
| 10.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.4 [‐0.70, ‐0.10] |
| 10.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.30, 0.08] |
| 11 Weight (kg) ‐ change from baseline Show forest plot | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 Over 3 months and up to 6 months | 1 | 38 | Mean Difference (IV, Random, 95% CI) | 1.2 [‐0.04, 2.44] |
| 12 BMI (z score) Show forest plot | 2 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.25, 0.22] |
| 12.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.54, 0.32] |
| 12.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.26, 0.31] |
| 13 Lung function (FEV1 % predicted) Show forest plot | 5 | 284 | Mean Difference (IV, Random, 95% CI) | 1.36 [‐1.20, 3.91] |
| 13.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐8.07 [‐19.87, 3.73] |
| 13.2 Over 3 months and up to 6 months | 3 | 166 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐0.51, 4.94] |
| 13.3 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐12.13, 6.53] |
| 14 Lung function (FEV1 % predicted) ‐ change from baseline ‐ sensitivity analysis Show forest plot | 3 | 166 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐0.51, 4.94] |
| 14.1 Over 3 months and up to 6 months | 3 | 166 | Mean Difference (IV, Random, 95% CI) | 2.22 [‐0.51, 4.94] |
| 15 Lung function (FEV1 % predicted) ‐ post treatment ‐ sensitivity analysis Show forest plot | 3 | 168 | Mean Difference (IV, Random, 95% CI) | ‐1.31 [‐7.10, 4.48] |
| 15.1 Up to 3 months | 1 | 41 | Mean Difference (IV, Random, 95% CI) | ‐1.40 [‐14.48, 11.68] |
| 15.2 Over 3 months and up to 6 months | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐8.85, 9.05] |
| 15.3 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | ‐2.80 [‐12.13, 6.53] |
| 16 Hospitalisations (number ‐ all causes) Show forest plot | 2 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.41, 0.54] |
| 16.1 Over 3 months and up to 6 months | 1 | 38 | Mean Difference (IV, Random, 95% CI) | ‐1.0 [‐1.74, ‐0.26] |
| 16.2 Over 9 months and up to 12 months | 1 | 77 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐0.25, 0.25] |
| 17 HRQoL (validated questionnaire) Show forest plot | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 PedsQLTM 4.0 SF 15 ‐ Parent Report (performed 3 months post 1 month intervention period) | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.87 [0.19, 1.55] |
| 17.2 PedsQLTM 4.0 SF 15 ‐ Child Report (performed 3 months post 1 month intervention period) | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.59 [‐0.07, 1.26] |

