Intervensi terapi fizikal, selain daripada intervensi senaman fizikal umum, pada kanak‐kanak dan remaja sebelum, semasa dan selepas rawatan untuk kanser
Abstract
Background
Children and adolescents diagnosed with cancer are at high risk of experiencing severe side effects from cancer treatment, many of which are amenable to physical therapy. These side effects can negatively impact a child's quality of life and ability to participate in daily activities (e.g. play and attendance at school). Researchers have evaluated physical therapy interventions in children with cancer and childhood cancer survivors. However, factors such as small sample sizes, varying intervention protocols and differences in cancer types among trials make it difficult to draw conclusions about efficacy.
Objectives
The primary aim of this review was to evaluate the efficacy of physical therapy interventions ‐ with a specific focus on symptom relief and compensation of therapy‐related side effects ‐ on the quality of life of children and adolescents diagnosed with cancer. Participants must be between the ages of 0 and 19 years at the time of the physical therapy intervention study. The intervention may occur prior to, during or following cancer treatment. The intervention must be compared to a control group of children receiving standard care, no physical therapy intervention or a comparison intervention. We have excluded general physical exercise studies where the primary aim was to improve physical fitness through aerobic, anaerobic, resistance exercise or combined physical exercise training regimens (i.e. combined aerobic and resistance exercise regimens). We have also intended to record the occurrence of any adverse effects resulting from physical therapy interventions.
The secondary aims were to evaluate the efficacy of physical therapy on impairments of pain, peripheral neuropathy, balance, gait, functional abilities and mobility, motor function and performance, range of motion, strength and fatigue.
Search methods
We searched CENTRAL, MEDLINE, Embase, CINAHL, PEDro, ongoing trial registries, conference proceedings and the reference lists of relevant studies and reviews in March 2020. We also contacted oncology rehabilitation researchers working in paediatrics in March 2020 to identify additional studies.
Selection criteria
The review included randomised controlled trials (RCTs), cross‐over trials, and controlled clinical trials (CCTs) that compared the effects of physical therapy interventions to a control group, and involved children and adolescents diagnosed with cancer between the ages of 0 and 19 years at the time of the intervention. We excluded studies examining general physical exercise interventions where the primary aim was to improve physical fitness through aerobic exercise, resistance exercise or combined physical exercise training regimens (i.e. combined aerobic and resistance exercise regimens).
Data collection and analysis
We used standard methodological procedures expected by Cochrane.
Main results
We found no RCTs, cross‐over trials or CCTs comparing the effects of physical therapy interventions with a focus on symptom relief and compensation of therapy‐related side effects for children and adolescents between the ages of 0 and 19 years.
Authors' conclusions
Results demonstrate that the evidence to date is inadequate to inform clinical practice. Recommendations for future research include the need for large‐scale, high‐quality designs that examine: (1) paediatric populations with same cancer types; (2) similar intervention protocols; (3) long‐term outcomes; (4) physical therapy interventions (e.g. electrophysical modalities and sensory interventions); and (5) outcomes commonly impaired in children with cancer (e.g. peripheral neuropathy and gait deficits).
PICO
Ringkasan bahasa mudah
Apakah kesan intervensi terapi fizikal sebelum, semasa, dan selepas rawatan kanser kanak‐kanak?
Soalan ulasan
Kami mengulas bukti tentang kesan intervensi terapi fizikal, selain daripada intervensi latihan senaman umum, terhadap kualiti hidup kanak‐kanak dan remaja yang didiagnosis dengan kanser berbanding dengan kumpulan kawalan kanak‐kanak yang mendapat penjagaan piawai, tiada intervensi terapi fizikal atau satu intervensi perbandingan. Kami juga mengulas kejadian bahaya (kesan buruk) yang disebabkan oleh intervensi terapi fizikal. Untuk tujuan ulasan ini, intervensi terapi fizikal yang diminati harus memberi tumpuan kepada melegakan gejala atau menangani kesan sampingan berkaitan terapi (gejala dan gangguan). Kami mengecualikan kajian yang memeriksa intervensi senaman fizikal umum di mana tujuan utamanya adalah untuk meningkatkan kecergasan fizikal melalui senaman aerobik, senaman rintangan atau gabungan latihan senaman fizikal (iaitu gabungan rejimen latihan aerobik dan rintangan).
Latar belakang
Kanak‐kanak dan remaja yang menghidap kanser sering mempunyai kesan sampingan dari kanser dan rawatannya. Kesan sampingan ini boleh memberi kesan negatif terhadap kualiti hidup anak dan kemampuan untuk mengambil bahagian dalam aktiviti harian seperti bermain. Penyelidik telah menjalankan kajian yang meneliti intervensi terapi fizikal pada kanak‐kanak dengan kanser. Namun, faedah terapi fizikal adalah tidak jelas.
Ciri‐ciri kajian
Bukti adalah terkini sehingga Mac 2020. Kami tidak mengenal pasti kajian yang sesuai.
Keputusan utama
Kami tidak mengenal pasti kajian yang meneliti kesan intervensi terapi fizikal terhadap kualiti hidup kanak‐kanak dan remaja yang didiagnosis menghidap kanser, berbanding dengan kumpulan kawalan kanak‐kanak yang mendapat penjagaan piawai, tanpa intervensi terapi fizikal atau satu intervensi perbandingan. Oleh itu, tidak ada kesimpulan yang dapat dibuat. Hasil kajian kami menunjukkan bahawa penyelidikan lebih lanjut adalah diperlukan untuk mengkaji kesan intervensi terapi fizikal pada kanak‐kanak dan remaja dengan kanser.
Authors' conclusions
Summary of findings
| Physical therapy compared to usual care in children and adolescents before, during and following cancer treatment | |
| Patient or population: children and adolescents before, during and following cancer treatment | |
| Outcomes | Comments |
|---|---|
| Quality of Life | No studies included |
| Fatigue | No studies included |
| Pain | No studies included |
| Balance | No studies included |
| Range of motion | No studies included |
| Strength | No studies included |
| Adverse events | No studies included |
Background
Description of the condition
It is estimated that, globally, around 300,000 children and adolescents between the ages of 0 and 19 years will be diagnosed with cancer each year (Kids Cancer Care 2020; WHO 2020). Over 175,000 (70%) will be children under the age of 15 years (Ward 2014). Progress in cancer treatments has resulted in improved survival rates for children and adolescents with cancer, now approaching or exceeding 80% for five‐year post‐diagnosis survival (Gatta 2014; Noone 2018). Thus, there is an increased awareness of the need for survivorship care plans, including medical follow‐up and surveillance for long‐term effects of cancer treatment (Buckner 2014; CCS 2020; Robison 2009).
Two‐thirds of children who have been diagnosed with cancer will also develop at least one chronic or long‐term side effect after the cancer treatment (CCS 2020; Skinner 2012). Long‐term and late effects are expected health complications resulting from the cancer or cancer therapy (chemotherapy, radiation therapy, surgery and stem cell transplant), that never resolve or emerge months or years following treatment completion, and impact overall health and quality of life (Green 2012). These effects include impairments such as pain, fatigue, weakness, peripheral neuropathy, limitations in range of motion, and deficits in balance and gait (Baggott 2009; Beulertz 2016b; Gilchrist 2016; Gilchrist 2018; Hartman 2008; Ness 2013; Robison 2009; Skinner 2012; Van Cleve 2012). All of these may negatively affect a child’s overall function, quality of life and ability to participate in age‐appropriate activities including play (Moody 2006; Pruitt 2009).
Cancer treatments can negatively impact the major body systems including musculoskeletal, cardiorespiratory, and neurological systems (Pruitt 2009). The risk of long‐term side effects are dependent on the tumour type and tumour‐related factors (e.g. location within the body and extent of the cancer); the type of cancer treatment administered (e.g. type of surgery, chemotherapy type and dosage, radiation therapy type, location and dosage); as well as patient‐related factors (e.g. the child’s gender, age, overall health pre‐cancer diagnosis and developmental stage at time of diagnosis) (Pruitt 2009; NCI 2020). The focus of this review was on the outcomes associated with physical interventions for musculoskeletal and neurological effects of cancer and cancer treatment.
Musculoskeletal System
Specific long‐term effects from cancer treatment on the musculoskeletal system include effects on muscle and soft tissues (myopathies including proximal muscle weakness, soft tissue contracture and radiation fibrosis syndrome), as well as effects on bone resulting in scoliosis or kyphosis, limb length discrepancies and osteoporosis (NCI 2020; Pruitt 2009). The impact of surgery such as amputation and limb‐salvage intervention may result in chronic pain, gait and balance dysfunction, and impact overall activity (Krivitzky 2015; Pruitt 2009). Effects involving the musculoskeletal system are more likely to occur in cancers such as acute lymphoblastic leukaemia, osteosarcoma, and brain and spinal cord tumours; and in those children who have undergone a stem cell transplant (NCI 2020; Pruitt 2009).
Neurological System
Specific long‐term effects involving the neurological system include motor and sensory deficits (loss of fine motor skills, impairments in coordination and balance, movement disorders and peripheral nerve damage in the hands and feet) (Krivitzky 2015; NCI 2020; Pruitt 2009). Chronic peripheral neuropathy, a condition that may result from the use of neurotoxic agents such as vincristine and cisplatin, is a common long‐term effect experienced by both childhood and especially adult survivors (Kandula 2016; Pruitt 2009; Wickham 2007).
Description of the intervention
Focused physical therapy interventions may help children with the late and long‐term physical effects resulting from cancer treatments, particularly prolonged cancer treatments (Stubblefield 2013). Physical therapists aim to restore and optimise function, mobility and quality of life of individuals of all ages (Punzalan 2009). In oncology rehabilitation, physical therapists work with individuals to manage musculoskeletal and neuromuscular impairments (Punzalan 2009). Rehabilitation for cancer patients include treatments to address acute, late and long‐term effects, as well as those associated with palliative care (Punzalan 2009), with the ultimate aim of improving quality of life.
Physical therapists perform assessments to determine physical function, joint mobility, muscle strength and flexibility. Findings of the assessment are used to inform an appropriate, tailored intervention for the child (Punzalan 2009). Physical therapy for children with cancer aims to regain function through interventions to reduce pain in soft tissues (muscles, tendons and ligaments), increase muscle strength, improve soft tissue and joint flexibility, range of motion and functional mobility. Treatment can be delivered before (prehabilitation), during and after cancer treatment completion (rehabilitation) (Krivitzky 2015). Prehabilitation services include interventions that are administered between the time of diagnosis and cancer treatment initiation. Prehabilitation interventions may be prescribed to enhance a child’s physical functioning and general health status to enable improved tolerance to cancer treatments, overall outcomes and recovery from the prospective cancer treatment. Rehabilitation services delivered during or following cancer treatment are defined as services to decrease adverse effects of treatment and to enhance recovery of functional abilities. Importantly, focused and timely physical therapy intervention may help to prevent the development of late effects and attenuate the severity of long‐term effects (Krivitzky 2015).
The interventions considered in this review included physical therapy techniques such as manual therapy, therapeutic range of motion and strengthening exercises for a joint or muscle region, balance retraining, gait re‐education and electrophysical modalities provided with the aim of addressing impairments related to cancer treatment to ultimately improve quality of life. For this review, physical therapy could have been delivered as prehabilitation or rehabilitation interventions. The children and adolescents participating in the included studies needed to have been between 0 and 19 years old at the time of receipt of the physical therapy intervention in the studies.
Why it is important to do this review
To date, the majority of research trials in cancer rehabilitation have involved adult cancer survivors. Researchers have reported positive results from physical therapy interventions, primarily in the area of breast cancer (Cho 2016; De Groef 2015; McNeely 2010; Nilsen 2015; Pergolotti 2015).
Impairment‐based cancer rehabilitation for children and adolescents with cancer is a growing area of research and clinical practice. Researchers have evaluated physical therapy interventions in children with cancer and childhood cancer survivors. However, factors such as small sample sizes, varying intervention protocols and differences in cancer types among trials make it difficult to draw conclusions about efficacy.
A recent Cochrane Review examined the effects of general exercise training interventions for children and adolescents with cancer (Braam 2016). The review included six studies involving 171 participants, all of whom were being treated for acute lymphoblastic leukaemia. Preliminary findings support benefit from general physical exercise training for body composition, flexibility and cardiorespiratory fitness. To date, however, no systematic reviews have been performed examining the benefits of physical therapy interventions for specific impairments related to cancer treatment. Thus, the main distinctions between this review and that of Braam 2016 were: (1) the type of intervention (physical therapy versus general physical exercise); and (2) the focus of the intervention (amelioration of specific iatrogenic impairments versus physical fitness).
Objectives
Primary objective
To evaluate the efficacy of physical therapy interventions ‐ with a specific focus on symptom relief and compensation of therapy‐related side effects ‐ on the quality of life of children and adolescents who have been diagnosed with cancer. Participants must be between the ages of 0 and 19 years at the time of the physical therapy intervention study. The intervention may occur prior to, during or following cancer treatment. The intervention must be compared to a control group of children receiving standard care, no physical therapy intervention or a comparison intervention. We have excluded general physical exercise studies where the primary aim was to improve physical fitness through aerobic, anaerobic, resistance exercise or combined physical exercise training regimens (i.e. combined aerobic and resistance exercise regimens). We have also intended to record the occurrence of any adverse effects resulting from physical therapy interventions.
Secondary objectives
To evaluate the efficacy of physical therapy interventions on pain, peripheral neuropathy, balance, gait, functional abilities and mobility, motor function and performance, range of motion, strength and fatigue.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), cross‐over trials (when data were available prior to the cross‐over), and controlled clinical trials (CCTs) examining the effects of physical therapy interventions for children and adolescents between the ages of 0 and 19 years.
Types of participants
Children and adolescents aged 0 to 19 years at the time of the physical therapy intervention. All childhood cancer types were eligible for inclusion in the review. We considered studies that also included adults (20 years old or more) with cancer only if the results of the subgroup of children with cancer (0 to 19 years of age) were available or reported separately.
Types of interventions
We included studies comparing physical therapy interventions (such as manual therapy techniques, therapeutic range of motion and strengthening for a specific joint or impaired body region, balance and gait retraining), and electrophysical modalities to address a specific symptom (e.g. pain), impairment (e.g. gait dysfunction) or body region (e.g. shoulder). For the purpose of this review, physical therapy interventions of interest had to have a focus on symptom relief and compensation of therapy‐related side effects. The intervention may have been delivered before (prehabilitation), during or following cancer treatment. The intervention had to be compared to a control group receiving standard care, no intervention or a comparison intervention (assuming the effect of the physical therapy intervention can be isolated).
The physical therapy intervention had to be delivered or supervised by a physical therapist or healthcare professional (e.g. nurse or occupational therapist). The programme could have been offered as an individualised treatment or a group intervention, and performed in any setting or location (e.g. hospital, outpatient hospital or physical therapy clinic, home, or elsewhere). The duration of the physical therapy intervention period had to be at least four weeks. The time spent per physical therapy session had to be reported or sufficiently described, and delivery of the intervention had to last at least 15 minutes (Hanson 2015; Ospina 2019).
Exclusion criteria
-
Studies where the primary focus of the intervention was aerobic capacity or general physical fitness alone.
-
Studies where the intervention comprised a general exercise or physical activity prescription, described in terms of frequency, intensity, type and time.
Types of outcome measures
Primary and secondary outcomes listed below were not used as criteria for including studies, but rather as a list of outcomes of interest within the included studies.
Primary outcomes
The primary outcomes of this review were quality of life and adverse events.
-
Quality of life: measured by scales such as the Pediatric Quality of Life Inventory (PedsQL), Pediatric Quality of Life (PedsQL Core), Child Health Questionnaire (CHQ), and DISABKIDS or other validated questionnaires.
-
Adverse events related to the physical therapy intervention such as falls, fractures, soft tissue injuries, or any worsening of impairments (e.g. pain) requiring withdrawal from the study.
Secondary outcomes
Secondary outcomes of the review are as follows.
-
Pain measured by Visual Analog Scale (VAS), or other valid instruments.
-
Peripheral neuropathy measured by a validated scale such as the Pediatric Modified Total Peripheral Neuropathy Score (ped‐mTNS), Total Neuropathy Score‐Pediatric Vincristine (TNS‐PV), or Total Neuropathy Score (TNS).
-
Balance assessed by a validated scale such as the Bruininks Osteretsky Test of Motor Proficiency (BOTMP) Balance Subtest, Bruininks Osteretsky Test of Motor Proficiency Second Edition (BOT‐2) Balance Subtest, Pediatric Berg Balance Scale (BBS), Movement Assessment Battery for Children (Movement ABC‐2), the Flamingo Balance Test or equivalent.
-
Motor development and performance measured by a validated scale such as Bruininks Osteretsky Test of Motor Proficiency (BOTMP), Bruininks Osteretsky Test of Motor Proficiency Second Edition (BOT‐2), Alberta Infant Motor Scale (AIMS), Movement Assessment Battery for Children (Movement ABC‐2), Peabody Developmental Motor Scales (PDMS‐2), Miller Function and Participation Scales (MFUN‐PS), Gross Motor Function Measure (GMFM), or another valid instrument.
-
Functional abilities and mobility assessed by a validated scale such as the Functional Mobility Assessment (FMA) tool, Timed Up and Down Stairs (TUDS), Timed Up and Go (TUG), Functional Independence Measure for Children (WeeFIM), Pediatric Evaluation of Disability Inventory (PEDI), Vineland Adaptive Behaviour Scale, or another valid instrument.
-
Gait assessed descriptively or by use of a computerised or electronic gait analysis.
-
Fatigue assessed by a validated scale such as the PedsQL Multidimensional Fatigue Scale, Childhood Cancer Fatigue Scale (CCFS), or the Fatigue Scale for a child (FS‐C), the same scale for adolescents (FS‐A), and for parents (FS‐P), or equivalent valid instrument.
-
Range of motion measured by goniometry, or another valid instrument.
-
Strength assessed with a hand‐held dynamometer, use of a Biodex/Cybex, spring scale, lateral step‐up test, sit‐to‐stand test, up‐and‐down stairs test, minimum chair height test, incremental shuttle walking test, or another valid instrument.
Search methods for identification of studies
In 2018, Cochrane Childhood Cancer ran the searches in CENTRAL, MEDLINE and Embase. In 2020, the review authors ran all searches (CENTRAL, MEDLINE, Embase, CINAHL, PEDro, ongoing trial registries, conference proceedings, reference lists of relevant articles, and personal communication with researchers). No language restrictions were applied.
Electronic searches
We searched the following electronic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) in the Cochrane Library — we used the latest issue; MEDLINE Ovid (from 1946 to 15 March 2020); Embase Ovid (from 1947 to 15 March 2020); CINAHL/EBSCO (1937 to 15 March 2020); and Physiotherapy Evidence Database PEDro (from 1929 to 15 March 2020) (www.pedro.org.au). We modified electronic searches from the recommended Cochrane Childhood Cancer methods used in reviews (Kremer 2016).
The search strategies for the different electronic databases (using a combination of controlled vocabulary and text words) are shown in the appendices (Appendix 1; Appendix 2; Appendix 3; Appendix 4; Appendix 5).
Searching other resources
Bibliographic searching
We searched for trials not registered in CENTRAL, MEDLINE, Embase, CINAHL and PEDro, either published or unpublished, by searching the reference lists of relevant articles and review articles. We searched the five latest editions (2015‐2019) of conference proceedings of the International Society for Paediatric Oncology (SIOP), the American Society of Clinical Oncology (ASCO), the American Society of Pediatric Hematology/Oncology (ASPHO), and the Multinational Association for Supportive Care in Cancer (MASCC). We scanned the ISRCTN Registry (www.isrctn.com), the National Institutes of Health (NIH) Register (www.clinicaltrials.gov), and the World Health Organization portal (http://apps.who.int./trialsearch) for ongoing trials on 14 March 2020.
The search strategies for other resources are shown in the appendices (Appendix 6; Appendix 7).
Personal communication
We contacted oncology rehabilitation researchers working in paediatrics to verify details of any outstanding clinical trials and any relevant unpublished data or study information.
Data collection and analysis
Selection of studies
Two authors independently undertook identification of studies meeting the inclusion criteria. In most cases, we resolved discrepancies between authors by consensus. The authors resolved disagreements through discussion, or if necessary, by involving a third review author to reach consensus. We obtained any study seemingly meeting the inclusion criteria on the grounds of the title, abstract, or both, in full for closer inspection. We clearly stated details of reasons for exclusion of any study considered for review. We would have noted duplicate publications of the same study, and the study would have been counted only once.
We provided a flow diagram for the selection of studies in our review (Figure 1).

Study flow diagram
Data extraction and management
Two review authors would have extracted the characteristics for each included trial using a data extraction form. If disagreements had arisen and were not resolved by consensus, a third review author would have resolved the discrepancies. In case of missing data or relevant information, we would have contacted study authors.
Should we have had more than one publication for a study, we would have used the primary publication and referenced the other publication, if used, for supplementary information.
Items that we would have included on the data extraction form are reported in the protocol of this review (Ospina 2018).
Assessment of risk of bias in included studies
If studies had met our eligibility criteria, two review authors would have independently assessed risk of bias in the RCTs, cross‐over trials and CCTs, rating each risk of bias item as 'low', 'unclear' or 'high' risk of bias. We would have used the 'risk of bias' criteria as described in the module of Cochrane Childhood Cancer (Kremer 2016), and based on recommendations in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The two review authors would have resolved any disagreements through discussion, or if necessary, by involving a third review author to reach consensus. Further details can be found in the protocol of this review (Ospina 2018).
Measures of treatment effect
If studies had met our eligibility criteria, we would have analyzed continuous data (QoL, adverse events, fatigue, pain, gait, peripheral neuropathy, balance, range of motion, strength) as mean differences either weighted or standardised using a random‐effects model. We would use difference in means (MD) for continuous variables when data are provided using the same units, measurement methods or outcome measure. We would use the standardised mean difference (SMD) for continuous variables when trials report results using different measurement units, measurement methods or outcome measures. We would analyze dichotomous outcomes (e.g. adverse event rates, outcomes reported as dichotomous variables) using risk ratio (RR). All results would be presented with corresponding 95% confidence intervals (CIs).
Unit of analysis issues
If studies had met our eligibility criteria, the only unit of analysis issue we anticipated is with cross‐over designs, in which we would use only first‐cycle data (prior to cross‐over).
Dealing with missing data
If studies had met our eligibility criteria, when information relevant to study selection, data extraction or assessment of risk of bias was missing, we would attempt to contact the authors to obtain the missing data. When applicable, we would extract data by allocated intervention, irrespective of compliance with the allocated intervention, to allow an intention‐to‐treat analysis. We would state if this is not possible and will perform an 'as treated' analysis.
Assessment of heterogeneity
If studies had met our eligibility criteria, we would assess heterogeneity by visual inspection of the forest plots and by the use of the statistical test for heterogeneity I² statistic (Higgins 2011). I² values ranging from 0% (homogeneity) to 100% (heterogeneity) will be calculated to quantify variability in study effect. An I² value greater than 50% would be considered the cutpoint for significant heterogeneity (Higgins 2011). Where possible, subgroup analyses would be performed to explore and explain heterogeneity among studies.
Assessment of reporting biases
In addition to the evaluation of reporting bias as described in the Assessment of risk of bias in included studiessection, we planned to assess reporting bias by constructing a funnel plot if there are a sufficient number of included studies (at least 10 studies included in a meta‐analysis), otherwise the power of the tests is too low to distinguish chance from real asymmetry (Higgins 2011).
To minimise the effect of publication bias, we searched the grey literature and contacted authors of trials.
Data synthesis
We did not identify any eligible studies. As a result, data analyses could not be performed. If studies had met our eligibility criteria, we would enter data of the included studies into Review Manager 5 software (Review Manager 2014) and undertake analyses according to the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We would pool the results of studies, (1) if there are at least three studies with the same outcome measure (or measurement) for the given primary or secondary outcome; and (2) if appropriate, after consideration of heterogeneity between the trials. We will pool outcomes when sufficient data are available in the papers or from the trialists' data sets using the random‐effects model. We will describe outcomes that we cannot pool in narrative form in the Results section. We would create a 'Summary of findings' table, including post‐intervention results as well as short‐term follow‐up results (3 to 6 months after the intervention completion) and long‐term follow‐up results (1 year or more after the intervention completion).
In a multi‐arm study we would include the intervention groups as separate comparisons if each arm meets the criteria for inclusion, and would split the 'shared' control/comparison group for analyses. We would note all the intervention groups in the table of 'Characteristics of the included studies'. However, we would only describe and analyse the intervention groups relevant to the review (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
If studies had met our eligibility criteria, we would have analyzed, a priori subgroup analyses would include examining the pooled effect estimate by the type of physical therapy intervention, the timing of the intervention (i.e. prior to, during, or following cancer treatment), and cancer type.
Where possible, we would perform subgroup analyses to assess if the observed effect of an intervention is consistent across participants based on subgroups of (1) age of the participant (continuous co‐variate) and (2) the location of the physical therapy intervention (inpatient hospital, outpatient clinic or home), and (3) by study design (RCT, CCT, cross‐over).
Sensitivity analysis
If studies had met our eligibility criteria, for any outcomes for which pooling was possible we would perform sensitivity analyses for 'Risk of bias' criteria separately. We would exclude studies with a high risk of bias and unclear risk of bias in the sensitivity analyses, and compare the results of studies with a low risk of bias with the results of all available studies. Sensitivity analyses would only be done when there remain at least two studies with a low risk of bias in the analyses
Summary of findings and assessment of the certainty of the evidence
For each comparison we prepared a 'Summary of findings' table using the GRADE profiler software (Guyatt 2008), in which we presented the following outcomes: QoL, fatigue, pain, balance, range of motion, strength, adverse events.
We did not identify any eligible studies. As a result, GRADE assessments could not be performed. If studies had met our eligibility criteria, For each outcome two review authors would independently assess the quality of the evidence by using the five GRADE considerations, i.e. study limitations, inconsistency, indirectness, imprecision and publication bias, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Results
Description of studies
Results of the search
We conducted searches of CENTRAL, MEDLINE, Embase, CINAHL, PEDro, ongoing trial registries, conference proceedings (SIOP, ASCO, ASPHO, and MASCC), and reference lists of relevant studies and reviews. We also contacted oncology rehabilitation researchers working in paediatrics for additional studies. These searches resulted in the retrieval of 2013 references (Figure 1).
After removal of duplicates, the search yielded 1927 potentially eligible articles (Figure 1). After initial screening of titles and abstracts, we excluded 1886 references as they did not meet eligibility criteria. We obtained, read and assessed for eligibility the full‐text versions of 41 studies. Thirty‐eight of these studies did not meet eligibility criteria (refer to Characteristics of excluded studies), and we excluded three studies as they were protocols or reported preliminary results of ongoing trials (refer to Characteristics of ongoing studies). No studies were eligible for the review (refer to Characteristics of included studies).
Included studies
We identified no trials that matched our inclusion cirteria.
Excluded studies
Reasons for exclusions can be found in Characteristics of excluded studies.
Risk of bias in included studies
We found no RCTs, cross‐over trials or CCTs comparing the effects of physical therapy interventions, with a focus on symptom relief and compensation of therapy‐related side effects, for children and adolescents between the ages of 0 and 19 years, and risk of bias assessment is thus not applicable.
Effects of interventions
We found no RCTs, cross‐over trials or CCTs comparing the effects of physical therapy interventions for children and adolescents between the ages of 0 and 19 years, and thus the effects of physical therapy interventions on the quality of life of children and adolescents diagnosed with cancer remain unclear.
Discussion
Summary of main results
A growing number of studies have investigated the effects of physical therapy in adults with cancer (Cho 2016; Cormie 2017; De Groef 2015; McNeely 2010; Nilsen 2015; Pergolotti 2015). Conversely, literature examining the effects of physical therapy in childhood cancer is just beginning to emerge. Unfortunately, the vast majority of the research examining the effects of physical therapy in childhood cancer comprises lower‐quality study designs (e.g. single‐subject designs, case‐control studies) not eligible for this review (Ospina 2019). No eligible studies could be included.
Although no studies met our eligibility criteria, it is promising that further research in paediatric oncology physical therapy is underway. Our review identified three ongoing clinical trials that might be eligible for this review upon completion (Tanner 2016; Tanriverdi 2017; Xipell 2019).
Overall completeness and applicability of evidence
As demonstrated in this review, there is a paucity of high‐quality research evidence from studies examining physical therapy interventions with a focus on symptom relief and compensation of therapy‐related side effects in children and adolescents with cancer. Of note, chemotherapy‐induced peripheral neuropathy (CIPN) is one of the most common side effects in children undergoing cancer treatment, causing debilitating consequences such as balance issues, gait impairments, muscle weakness and sensory impairments. Despite the lack of research examining the effects of physical therapy for commonly occurring side effects in childhood cancer, it is promising that clinical trials are currently in progress (Weiner 2019; Wickham 2007).
Quality of the evidence
We identified no eligible studies for inclusion.
Potential biases in the review process
We developed comprehensive search strategies to cover the main databases relevant to our research question, including: CENTRAL, MEDLINE, Embase, CINAHL and PEDro. It is possible that we missed studies. However, our search results retrieved repeats of many of the same studies across databases, suggesting that a comprehensive search was performed. Also, we searched reference lists and conference proceedings to minimise the risk of missing relevant literature. Finally, we excluded studies from this review ‐ even though many incorporated a physical therapy intervention ‐ due to an overlap with the review of Braam 2016.
Agreements and disagreements with other studies or reviews
To our knowledge, this is the first systematic review specifically focused on physical therapy interventions, other than general physical exercise interventions, in children and adolescents before, during and following treatment for cancer. Other reviews have been conducted exploring general exercise interventions and non‐pharmacological interventions in childhood cancer survivors. Braam 2016 published an updated version of their previous systematic review (Braam 2013), examining the effects of physical exercise in childhood cancer. Braam 2016 included six studies (n = 171), of which five were RCTs and one was a controlled clinical trial. None of the studies included in their review ‐ De Macedo 2010, Hartman 2009, Marchese 2004, Moyer‐Mileur 2009, Tanir 2013, and Yeh 2011 ‐ met the eligibility criteria of this review either because (1) physical activity was the primary interest of these studies, or (2) the effect of the physical therapy intervention could not be isolated from the physical activity or exercise intervention. Braam 2016 concluded that overall findings of the benefits of physical exercise interventions were not convincing possibly due to the small number of participants and lack of high quality evidence, but it can also be that this type of intervention is not as effective as in adult cancer patients. However, Braam and colleagues did identify positive intervention effects for body composition, flexibility, cardiorespiratory fitness, muscle strength and health‐related quality of life (cancer‐related items). They also reported that the quality of the evidence was low, suggesting that further research is needed using larger‐scale studies.
Wacker 2017 conducted a systematic review on nonsurgical, nonpharmacologic, rehabilitation interventions on physical impairments and functional mobility limitations for children and adolescents undergoing treatment for non‐central nervous system cancers. They included a total of 22 studies in their review. No restrictions on study design were applied; thus, uncontrolled studies and case‐control studies were included. Their findings comprised a general overview of interventions in the field, but did not consider quality and risk of bias. The authors identified that the majority of the studies were focused on increasing physical activity, with few studies evaluating physiotherapeutic interventions for other outcomes.
A recent systematic review by Morales 2018 investigated RCTs that examined the effects of exercise training on physical capacity‐related endpoints, survival, disease relapse and adverse effects in children after cancer diagnosis. They included a total of eight studies (n = 283) in their review. Results demonstrated that exercise training during childhood cancer treatment significantly improved Timed Up and Down Stairs (TUDS) scores (SMD ‐0.73, P < 0.001). These findings, however, were based on pooling of data that also included exercise‐based studies not eligible for inclusion in our review.
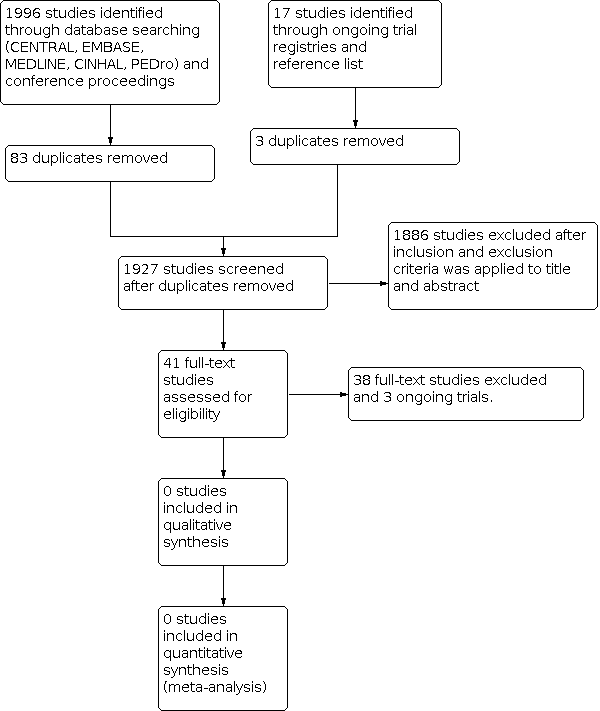
Study flow diagram
| Physical therapy compared to usual care in children and adolescents before, during and following cancer treatment | |
| Patient or population: children and adolescents before, during and following cancer treatment | |
| Outcomes | Comments |
|---|---|
| Quality of Life | No studies included |
| Fatigue | No studies included |
| Pain | No studies included |
| Balance | No studies included |
| Range of motion | No studies included |
| Strength | No studies included |
| Adverse events | No studies included |

