Reparación híbrida versus reparación abierta convencional para la disección del arco aórtico
Referencias
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
Ir a:
| Study | Reason for exclusion |
| Did not meet review indication (descending thoracic dissection) | |
| Did not meet review intervention (endovascular) | |
| Study withdrawn. The study was never started as was unable to obtain ethical approval. | |
| Did not meet review study design (registry) | |
| Did not meet review indication (patients with acute complicated type B aortic dissections) | |
| Did not meet review indication (abdominal aortic aneurysm) | |
| Did not meet review indication (Stanford type B, deBakey type III) | |
| Did not meet review intervention (endovascular) | |
| Did not meet review indication (patients with acute, complicated type B aortic dissection) | |
| Did not meet review study design (single arm study) | |
| Did not meet review intervention (TEVAR) | |
| Specific population cannot be extracted (mixture of lesion type and extent) |
TEVAR: thoracic endovascular aortic repair
Characteristics of ongoing studies [ordered by study ID]
Ir a:
| Study name | Therapeutic strategy of aortic arch for acute type A aortic dissection |
| Methods | Randomised parallel controlled |
| Participants | Participants diagnosed with type A aortic dissection by CTA imaging, onset time less than 2 weeks Age minimum: 18; age maximum: 70 Gender: both |
| Interventions | TAR group: total arch replacement ARP group: arch reserved procedure Hybrid group: hybrid procedure TBSG group: triple‐branched stent graft |
| Outcomes | Primary outcomes: postoperative 30‐day mortality; postoperative 30‐day major adverse cardiocerebral events (MACCE); postoperative 1‐year mortality; postoperative 1‐year major adverse cardiocerebral events (MACCE) Secondary outcomes: acute kidney injury; renal replacement therapy; hypoxaemia; liver failure; paraplegia; mediastinal infection; reopening for bleeding; ICU length of stay; postoperative hospital length of stay; hospitalisation expense; follow‐up CTA imaging |
| Starting date | 11 January 2017 |
| Contact information | Name: Song Xue |
| Notes | The study investigators have been contacted for clarification, and possible results. No response has been received |
| Study name | The contrast of the outcome between replacing ascending aorta + reconstructing aortic arch with triple‐branched stent graft and replacing ascending aorta + replacing half aortic arch to treat the aortic dissection (the contrast of the outcome of the two different operational methods to treat the aortic dissection) |
| Methods | Randomised parallel controlled trial |
| Participants | Participants diagnosed with aortic dissection; type A; onset time < 2 weeks |
| Interventions | Test group: place triple‐branched stent graft into aortic arch to reconstruct |
| Outcomes | Primary outcomes: operation time; length of stay; aortic angiography CT |
| Starting date | 01 February 2009 |
| Contact information | Name: Chen Liang‐Wan |
| Notes | The study investigators have been contacted for clarification, and possible results. No response has been received |
| Study name | Evaluate the safety and efficacy of Xuper open surgery stent graft system for the surgical of type A aortic dissection: a prospective, multi‐center clinical trial |
| Methods | Randomised parallel controlled trial |
| Participants | Inclusion criteria
Exclusion criteria:
Age minimum: 18 |
| Interventions | Test group: Xuper Open Surgery Stent Graft System (Lifetech Scientific, Shenzhen, China) Control group: open surgical repair using the Intergard artificial graft (Getinge AB, Stockholm, Sweden) |
| Outcomes | Primary outcomes: duration of circulatory arrest Secondary outcomes: incidence of major adverse events (death, paraplegia, brain complications); stent implantation successful (stent in place and successfully released); operation time; cardiopulmonary bypass time; arterial anastomosis time; aortic occlusion time; intraoperative blood loss and blood transfusion volume; treatment success (12 months after operation) |
| Starting date | 31 May 2013 |
| Contact information | Name: Zhiyun Xu Name: Xiangman Zhang |
| Notes | The study investigators have been contacted for clarification, and possible results. No response has been received |
ARP: arch reserved procedure; CT: Computed tomography; CTA: Computed tomography angiography; MACCE: major adverse cardiocerebral events; TAR: total arch replacement; TBSG: triple‐branched stent graft
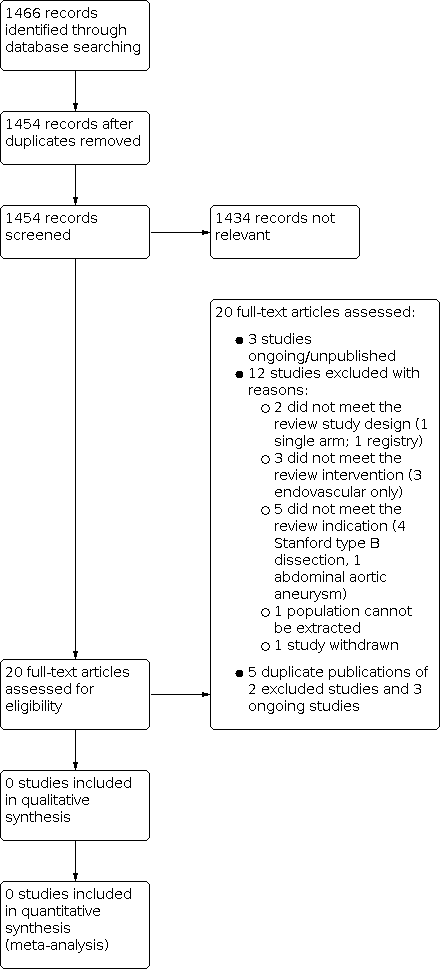
Study flow diagram.
| Types of outcome measures | Defined by | Including |
|---|---|---|
| Primary outcomes | ||
| Mortality | Dissection related and all causes | (Grade V) All deaths at 30 days and 12 months |
| Neurological deficit | Global events | (Grade I ‐ IV) Postoperative agitation, delirium, obtundation, or myoclonic movements, without localised cerebral neurological signs |
| Focal events | (Grade I ‐ IV) Lateralising sensory or motor deficit or focal seizure activity | |
| Spinal neurological events | (Grade I ‐ IV) Paraplegia, paraparesis | |
| Cardiac injury | Myocardial ischaemia | (Grade I ‐ IV) |
| Low cardiac output syndrome | (Grade I ‐ IV) | |
| Arrhythmia | (Grade I ‐ IV) | |
| Pericardial effusion | (Grade I ‐ IV) | |
| Respiratory compromise | Parenchymal complications | (Grade I ‐ IV) Atelectasis, pneumonia, pulmonary oedema, and acute respiratory distress syndrome |
| Pleural complications | (Grade I ‐ IV) Pneumothorax, pleural effusion | |
| Renal ischaemia | Modified RIFLE classification (Bellomo 2004): Risk (I), Injury (II), Failure (III), Loss/End‐Stage Kidney Dysfunction (IV) | (Grade I ‐ IV) Serum creatinine increase, glomerular filtration rate (GFR) decrease, anuria, haemodialysis |
| Secondary outcomes | ||
| False lumen thrombosis | Partial or complete thrombosis | ‐ |
| Mesenteric ischaemia | Gut complications | (Grade I ‐ IV) Ileus or gastric paresis, gut ischaemia manifested as metabolic acidosis or increased lactate |
| Grades as defined by Yan 2014: Grade I: any deviation from the normal postoperative course but self‐limiting or requiring simple therapeutic regimens (including antiemetics, antipyretics, analgesics, electrolytes, and physiotherapy); Grade II: complications requiring pharmacological treatment for resolution; Grade III: complications requiring surgical, endoscopic, or radiological intervention but not requiring regional or general anaesthesia or requiring interdisciplinary intervention; Grade IV: complications requiring surgical, endoscopic, or radiological intervention under regional or general anaesthesia, or requiring new intensive care unit (ICU) admission or ongoing ICU management for > 7 days or hospitalisation for > 30 days, or causing secondary organ failure; Grade V: death caused by a complication. | ||
| Summary of findings for the main comparison: Hybrid repair versus conventional open repair for aortic arch dissection | ||||||
| Patient or population: patients with a diagnosis of aortic arch dissection Settings: hospital Intervention: hybrid repair Comparison: open repair | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | Number of participants | Certainty of the evidence | Comments | |
|---|---|---|---|---|---|---|
| Risk with open repair | Risk with hybrid repair | |||||
| Mortality, Follow‐up: median N (months) | Study population | HR N (N to N) | N | ⊕⊝⊝⊝ ⊕⊕⊝⊝ ⊕⊕⊕⊝ ⊕⊕⊕⊕ | ||
| N per 1000 | N per 1000 | |||||
| Neurological deficit, Follow‐up: median N (months) | Study population | RR N (N to N) | N | ⊕⊝⊝⊝ ⊕⊕⊝⊝ ⊕⊕⊕⊝ ⊕⊕⊕⊕ | ||
| N per 1000 | N per 1000 | |||||
| Cardiac injury, Follow‐up: median N (months) | Study population | RR N (N to N) | N | ⊕⊝⊝⊝ ⊕⊕⊝⊝ ⊕⊕⊕⊝ ⊕⊕⊕⊕ | ||
| N per 1000 | N per 1000 | |||||
| Respiratory compromise, Follow‐up: median N (months) | Study population | RR N (N to N) | N | ⊕⊝⊝⊝ ⊕⊕⊝⊝ ⊕⊕⊕⊝ ⊕⊕⊕⊕ | ||
| N per 1000 | N per 1000 | |||||
| Renal ischaemia, Follow‐up: median N (months) | Study population | RR N (N to N) | N | ⊕⊝⊝⊝ ⊕⊕⊝⊝ ⊕⊕⊕⊝ ⊕⊕⊕⊕ | ||
| N per 1000 | N per 1000 | |||||
| False lumen thrombosis, Follow‐up: median N (months) | Study population | RR N (N to N) | N | ⊕⊝⊝⊝ ⊕⊕⊝⊝ ⊕⊕⊕⊝ ⊕⊕⊕⊕ | ||
| N per 1000 | N per 1000 | |||||
| Mesenteric ischaemia, Follow‐up: median N (months) | Study population | RR N (N to N) | N | ⊕⊝⊝⊝ ⊕⊕⊝⊝ ⊕⊕⊕⊝ ⊕⊕⊕⊕ | ||
| N per 1000 | N per 1000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||

