Vía de profilaxis con antibióticos para la prevención de la infección de la derivación del líquido cefalorraquídeo
Resumen
Antecedentes
La complicación principal de la cirugía de derivación del líquido cefalorraquídeo (LCR) es la infección de la derivación. La prevención de estas infecciones de derivación consiste en el uso perioperatorio de antibióticos que pueden administrarse de cinco maneras diferentes: por vía oral, intravenosa, intratecal, tópica y mediante la implantación de catéteres de derivación impregnados con antibióticos.
Objetivos
Determinar el efecto de diferentes vías de profilaxis con antibióticos (es decir vía oral, intravenosa, intratecal, tópica y con catéteres de derivación impregnados con antibióticos) en las infecciones de la derivación del LCR en pacientes tratados por hidrocefalia mediante derivaciones internalizadas del LCR.
Métodos de búsqueda
Se realizó una búsqueda electrónica sistemática sin restricciones de idioma, fecha o tipo de publicación. Se realizó la búsqueda en el Registro Cochrane Central de Ensayos Controlados (CENTRAL) en la Biblioteca Cochrane, MEDLINE y Embase, con la ayuda del Especialista en Información del Grupo Cochrane de Esclerosis Múltiple y Enfermedades Raras del SNC. La búsqueda se realizó en enero 2018.
Criterios de selección
Todos los ensayos controlados aleatorizados y cuasialeatorizados que estudiaran el efecto de la profilaxis con antibióticos, en cualquier dosis o vía de administración, para prevenir la infección de la derivación del LCR en pacientes que fueron tratados con una derivación interna del líquido cefalorraquídeo. Los pacientes con derivaciones externas no fueron aptos.
Obtención y análisis de los datos
Dos autores de la revisión extrajeron de forma independiente los datos de los estudios incluidos. Los desacuerdos se resolvieron mediante discusión o mediante la derivación a un investigador independiente dentro del departamento cuando fue necesario. Los análisis también fueron realizados por al menos dos autores de la revisión.
Resultados principales
Esta revisión sistemática incluyó un total de 11 ensayos controlados aleatorizados pequeños con 1109 participantes. Tres de estos estudios incluían exclusivamente a niños, y los ocho restantes incluían a participantes de todas las edades. La mayoría de los estudios estuvieron limitados a la evaluación de derivaciones ventriculoperitoneales. Sin embargo, cinco estudios incluyeron a participantes con derivación ventriculoatrial, de los cuales un estudio incluyó a cuatro participantes con una derivación subduro‐peritoneal. Cuatro de cada 11 ensayos (36%) se consideraron en riesgo incierto de sesgo, mientras que los siete ensayos restantes (64%) se calificaron como en alto riesgo de sesgo en uno o más de los componentes evaluados.
Se analizaron todos los estudios incluidos para calcular el efecto de la profilaxis con antibióticos sobre la proporción de infecciones de la derivación de forma independiente de la vía de administración. Aunque la calidad de la evidencia en estos estudios fue baja, puede haber un efecto positivo de la profilaxis con antibióticos en la cantidad de participantes con infecciones de la derivación (RR 0,55; IC del 95%: 0,36 a 0,84), lo cual significa una reducción del 55% en la cantidad de participantes con infección de la derivación en comparación con atención estándar o placebo.
Dentro de las diferentes vías de administración, sólo en el contexto de la administración intravenosa de la profilaxis con antibióticos puede haber evidencia de un efecto sobre el riesgo de infecciones de la derivación (RR 0,55; IC del 95%: 0,33 a 0,90). Sin embargo, esta vía era la única que contenía más de dos estudios (ocho estudios; 797 participantes). La evidencia fue incierta tanto para la administración intratecal de antibióticos (RR 0,73; IC del 95%: 0,28 a 1,93; dos estudios; 797 participantes; evidencia de baja calidad) como para los catéteres impregnados con antibióticos (RR 0,36; IC del 95%: 0,10 a 1,24; un estudio; 110 participantes; evidencia de muy baja calidad)
Conclusiones de los autores
La profilaxis con antibióticos puede tener un efecto positivo sobre la disminución de la cantidad de participantes que presentaron infecciones de la derivación. Sin embargo, la calidad de los estudios incluidos fue baja y el efecto no es consistente dentro de las diferentes vías de administración que se han analizado. Por lo tanto, no está claro si la prevención de la infección de la derivación varía según los diferentes agentes antibióticos, las diferentes vías de administración, el momento y las dosis, o según las características de los pacientes, por ejemplo, niños y adultos. Los resultados de la revisión deben observarse como generadores de hipótesis en lugar de definitivos, y los resultados deben confirmarse en ensayos con el poder estadístico adecuado o en estudios multicéntricos grandes para obtener evidencia de alta calidad en el área de la prevención de la infección de la derivación ventricular.
PICO
Resumen en términos sencillos
Vía de profilaxis con antibióticos para la prevención de la infección de la derivación del líquido cefalorraquídeo
Pregunta de la revisión
Se examinó la evidencia acerca del efecto de diferentes vías de administración de los antibióticos para prevenir la infección de la derivación en pacientes sometidos a una derivación del líquido cefalorraquídeo.
Antecedentes
Los pacientes con hidrocefalia (una cantidad excesiva de líquido cefalorraquídeo dentro del cerebro, debido a un bloqueo dentro de los cavidades cerebrales o en el sistema de reabsorción) pueden ser tratados mediante el implante de una derivación del líquido cefalorraquídeo. Esta derivación es un tubo que va desde el cerebro al corazón o al abdomen para drenar la cantidad excesiva de líquido cefalorraquídeo a otros compartimientos corporales donde se reabsorberá. Uno de los problemas más frecuentes después de la implantación es la infección de la derivación. Los pacientes se enferman y en la mayoría de los casos la derivación debe retirarse y debe implantarse una nueva después de que el paciente se haya recuperado. Para reducir la cantidad de infecciones, los cirujanos administran antibióticos antes, durante o después de la cirugía, o en diversas combinaciones, para proteger al paciente contra las bacterias que pueden infectar la derivación. Estos antibióticos pueden administrarse de diferentes maneras: por vía oral, intravenosa, directamente en las cavidades cerebrales, directamente en la derivación y mediante la implantación de catéteres en derivación impregnados con antibióticos.
Resultados
Se incluyeron 11 estudios hasta enero de 2018 en esta revisión, que comprenden a un total de 1109 participantes que habían sido sometidos a una derivación del líquido cefalorraquídeo para la hidrocefalia. La mayoría de los estudios incluidos fueron pequeños y de duración variable (desde ocho semanas hasta más de un año). Se encontró que la administración de antibióticos es efectiva para la prevención de las infecciones de la derivación (evidencia de muy baja calidad). Debido a que los estudios incluidos son pocos, las intervenciones usadas difirieron notablemente y la certeza de la evidencia para los resultados fue muy baja, los resultados impidieron una conclusión clara en cuanto a qué tipo de antibiótico y vía de administración son más efectivos para prevenir la infección de la derivación.
Authors' conclusions
Summary of findings
| Antibiotic prophylaxis versus no antibiotic prophylaxis / standard care for CSF‐shunt infections | ||||||
| Patient or population: CSF‐shunt infections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with no antibiotic prophylaxis / standard care | Risk with antibiotic prophylaxis | |||||
| Number of participants experiencing shunt infection after administration of antibiotic prophylaxis | Study population | RR 0.55 | 1109 | ⊕⊕⊝⊝ | ||
| 118 per 1.000 | 65 per 1.000 | |||||
| Number of participants experiencing shunt infection after administration of intravenous antibiotic prophylaxis | Study population | RR 0.55 | 797 | ⊕⊕⊝⊝ | ||
| 116 per 1.000 | 64 per 1.000 | |||||
| Number of participants experiencing shunt infection after administration of intrathecal antibiotic prophylaxis | Study population | RR 0.73 | 202 | ⊕⊕⊝⊝ | ||
| 100 per 1.000 | 73 per 1.000 | |||||
| Number of participants experiencing shunt infection after implantation of antibiotic impregnated catheters | Study population | RR 0.36 | 110 | ⊕⊝⊝⊝ | ||
| 167 per 1.000 | 60 per 1.000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to indirectness of evidence, since control groups differed a lot. For example, one study compared the used of antibiotic prophylaxis to current practice in a multicenter design. 2 Downgraded one level because four studies were considered at serious risk of bias at at least three forms of bias 3 Downgraded one level due to indirectness of evidence, since control groups differed a lot. 4 Downgraded one level because two studies were considered at serious risk of bias at at least three forms of bias 5 Downgraded two levels because both studies were considered at serious risk of bias at at least three forms of bias 6 Downgraded two levels because the study was considered at serious or unclear risk of bias at five out of seven items 7 Downgraded one level, since only one study was included in this subgroup and the total number of participants in this study was low | ||||||
Background
Description of the condition
Hydrocephalus is a condition in which cerebrospinal fluid (CSF) accumulates in the cerebral ventricles and subarachnoid spaces, resulting in dilatation of the ventricular system and an increase in intracranial pressure (Rekate 1999). There are two basic forms of hydrocephalus: non‐communicating and communicating. Non‐communicating hydrocephalus is caused by structural blockage of the CSF within the ventricular system and is the most common form of hydrocephalus in children. Communicating hydrocephalus can be caused either by the excessive production of CSF by the plexus choroideus or by inadequate resorption of CSF by the subarachnoid villi. Treatment of hydrocephalus comprises the drainage of excessive CSF through the implantation of shunts (a tube to drain cerebrospinal fluid from the brain to a body cavity, usually to the abdominal cavity), external drains (a tube to drain cerebrospinal fluid out of the body) or an endoscopic third ventriculostomy (a procedure in which cerebrospinal fluid circulation is restored by making a passage through the floor of the third ventricle). The choice of treatment and its efficacy differs according to the individual's age and the aetiology of the condition (Fu 2002; Hebb 2001; Limbrick 2014). The main complication of CSF‐shunt surgery is the incidence of CSF‐shunt infection (average incidence 3% to 20%) (Borgbjerg 1995; Drake 1998; Greenberg 2010; James 2014; Kestle 2011; Kestle 2016; Konstantelias 2015; Simon 2009); the highest proportion of infections is seen in infants (Bondurant 1995; Casey 1997). The symptoms associated with a shunt infection can be non‐specific, such as fever, nausea, lethargy (a lower level of consciousness), anorexia (a lack of appetite for food) or irritability. Symptoms of shunt infections in children tend to be more distinctive, such as high fever with or without concomitant meningitis, and rapid neurological deterioration. Up to 29% of individuals presenting with shunt malfunction have been shown to have a shunt infection, as confirmed by positive CSF cultures (a method to multiply micro‐organisms in order to determine the type of organism) (Greenberg 2010). Shunt infections can be treated by long‐term administration of antibiotics, but in most cases shunt revision is required (Greenberg 2010; Simon 2010). Both antibiotics and shunt revision can lead to longer hospital stays, additional complications and greater associated costs (Attenello 2010; Sciubba 2007); hence minimising shunt infections would be beneficial to both patients and to the healthcare system.
Description of the intervention
Currently, antibiotics used for the prevention of shunt infections can be administered in five ways: orally; intravenously; intrathecally; topically; and via the implantation of antibiotic‐impregnated shunt catheters. Antibiotics given via the oral route are used as add‐on therapy in the treatment of CSF‐shunt infections, but are rarely used to prevent CSF‐shunt infections (Frame 1984).
Intravenous pre‐operative antibiotics are widely used as shunt infection prophylaxis, and appear to lower the risk of such infections (Klimo 2014); however, these systemic antibiotics infiltrate the central nervous system poorly, and so intravenous antibiotics are often combined with antibiotics administered via one of the other routes. Ragel 2006 found that the addition of intrathecal gentamicin and vancomycin to intravenous cefazolin reduced the shunt infection rate to 0.42% (from 5.4% in the intrathecal gentamicin plus intravenous cefazolin administered to the control group).
Intrathecal antibiotics are usually administered intraoperatively; however, Moussa 2016 used a shunt containing a reservoir in which a prophylactic antibiotic was injected. They showed that an additional administration of antibiotics one week after surgery resulted in a lower shunt infection rate than intra‐operative administration alone. Another option is the administration of topical antibiotics. This route is partly similar to intrathecal administration but provides the opportunity of covering the entire drainage route (including the extracranial pathway), since the drain is drenched in an antibiotic agent.
A relatively new technique in the field of shunt infection prevention is the antibiotic‐impregnated catheter. These catheters, which are impregnated with two antibiotic agents, slowly release antibiotics over a period of days, and they have been shown to significantly reduce the rate of shunt infections (Konstantelias 2015). However, such catheters are relatively expensive when compared with the previously mentioned administration routes: for example the incremental cost of topical vancomycin are USD 10 per surgery versus USD 400 for antibiotic‐impregnated catheters (van Lindert 2018). In addition, Konstantelias 2015 found that antibiotic‐impregnated shunt catheters had a higher probability of colonisation by strains of bacteria that are more virulent than coagulase‐negative staphylococci (CoNS), which can result in a more severe infection. Another concern regarding the use of antibiotic‐impregnated shunt catheters was noted by James 2014, who found that individuals who needed shunt replacement after the implantation of an antibiotic‐impregnated shunt catheter were more prone to infections than those who were initially treated with other types of shunt.
How the intervention might work
Foreign materials, such as a shunt or an external drain, are prone to infection when placed in the body, and once infected, the management can be challenging. Antibiotic agents have a bactericide or bacteriostatic function that helps to eliminate bacterial infections. These functions are also useful when the prevention of bacterial colonisation is the aim. Although a ventriculoperitoneal shunt can be in situ for years, most shunt infections occur within two months of surgery (Greenberg 2010). The source of the infection is usually bacteria from the individual’s own skin (Yogev 1985). Hence contamination of the shunt takes place during, or early after, surgery, which suggests that the perioperative administration of antibiotic prophylaxis could be effective in the prevention of shunt infections.
Why it is important to do this review
CSF‐shunt infection is a major problem in individuals (including children) with hydrocephalus, with a reported proportion of shunt infection rates of 3% to 20% (Greenberg 2010). Shunt infections have a very high impact, both clinically (repeat surgery, prolonged hospitalisation, neurological deterioration) and economically (we estimated that each infection is associated with incremental costs equating to EUR 30,000). Hence a reduction in the shunt infection rate is urgently needed. Prophylactic antibiotics are currently the main prevention strategy in use; however, the best route of administration for the prevention of shunt infection remains to be determined (Ratilal 2008). This Cochrane Review has the potential to establish whether antibiotic prophylaxis has a positive effect on the proportion of CSF‐shunt infections and could direct which route of administration is the most effective. Some other aspects of antibiotic treatment (duration of treatment, dose and intervals between doses) are also unknown, but due to the potentially wide variety in these three factors, we did not include them in our review.
Objectives
To determine the effect of different routes of antibiotic prophylaxis (i.e. oral, intravenous, intrathecal, topical and via antibiotic‐impregnated catheters) on CSF‐shunt infections in persons treated for hydrocephalus using internalised CSF shunts.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised and quasi‐randomised controlled trials that studied the effect of antibiotic prophylaxis for the prevention of CSF‐shunt infection. We considered cluster randomised trials as eligible for inclusion but cross‐over trials were not taken into account since these are technically impossible to perform within the research question of this review. We excluded studies in which participants received more than one shunt simultaneously.
Types of participants
All individuals, of any age and gender, who underwent any type of internalised CSF‐shunt placement for the treatment of hydrocephalus. We imposed no restrictions with respect to the aetiology of hydrocephalus. We included studies that enrolled only a subset of relevant participants; we presented the data from these studies only for the relevant subset. If data on the subset of relevant participants could not be obtained, we excluded the study. We excluded individuals treated with external drains or temporary shunts.
Types of interventions
We included all types of antibiotics compared with standard care, placebo, or other active antibiotics. We included regimens as defined in primary studies irrespective of their dose, frequency and intensity and for any duration of therapy. We included studies investigating any of the following administration routes of antibiotic prophylaxis: oral; intravenous; topical; intrathecal; and via antibiotic‐impregnated shunt catheters.
Types of outcome measures
Primary outcomes
Number of participants experiencing shunt infection after administration of antibiotics.
We defined shunt infection as 'clinical and biochemical signs of infection in combination with a positive CSF culture'. We reported the proportion of participants who experienced shunt infection as counts and percentages.
All outcomes needed to occur within two years after shunt placement. We reported outcomes in three time frames: short term (< 30 days), medium term (30 days to 6 months), long term (> 6 months).
Secondary outcomes
No secondary outcomes were taken into account in this review.
Search methods for identification of studies
We conducted a systematic electronic search without restrictions on language, date or publication type, in line with the advice given in Chapter 6 of the Cochrane Handbook for Systematic Reviews of Interventions (Lefevbre 2011). If we identified studies published in a language other than English, we asked a professional translator to translate the text.
Electronic searches
We searched the following databases.
-
Cochrane Central Register of Controlled Trials (CENTRAL) (2017; Issue 12) in the Cochrane Library; (Appendix 1).
-
MEDLINE (PubMed) (1966 to 23 January 2018); (Appendix 2).
-
Embase (Embase.com) (1974 to 23 January 2018); (Appendix 3).
We searched for the terms in the title, abstract, keywords and controlled vocabularies.
Searching other resources
We then searched the following other resources.
-
The reference lists of all retrieved articles, texts and other reviews on the topic.
-
The ISRCTN registry (isrctn.com/), to identify any unpublished data.
-
Web of Science (webofscience.com), for forward citation search.
We also attempted to contact authors of included studies to obtain key missing data as needed.
Data collection and analysis
Selection of studies
Three review authors (SA, HB and EvL) independently screened titles and abstracts for inclusion with regard to our eligibility criteria, which were stored in a reference management software system. We obtained full‐text versions of the articles that met the eligibility criteria. The three review authors (SA, HB and EvL) again independently read these articles and listed those that did not meet the inclusion criteria in a 'Characteristics of excluded studies' table. We resolved disagreements by discussion or by referral to an independent researcher within our department when necessary.
Data extraction and management
Two review authors (SA and EvL) independently extracted the following data from included studies.
-
Date and country of study.
-
Study design.
-
Number of participants.
-
Demographic data.
-
Inclusion and exclusion criteria.
-
Antibiotic prophylaxis (type, administration route, frequency and doses of antibiotics).
-
Primary and secondary outcomes.
-
Methodological quality of the study.
We summarised all studies that met the inclusion criteria in a ’Characteristics of included studies’ table provided in Review Manager 5 (RevMan 5) and included details related to design, participants, interventions and outcomes (Review Manager 2014).
Assessment of risk of bias in included studies
Two review authors (SA and EvL) independently assessed the risk of bias of the included studies using the Cochrane 'Risk of bias' tool, as described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed the following domains of bias.
-
Random sequence generation (selection bias).
-
Allocation concealment (selection bias).
-
Blinding of participants and personnel (performance bias).
-
Blinding of outcome assessment (detection bias).
-
Incomplete outcome data (attrition bias).
-
Selective reporting (reporting bias).
-
Other biases.
After this independent risk of bias assessment, we resolved any disagreements by discussion with a third review author (HB).
We judged the overall risk of bias of each included study according to the following criteria.
-
Low risk of bias (plausible bias unlikely to seriously alter the results) if all the above items were met.
-
Unclear risk of bias (plausible bias that raises some doubt about the results) if one or more items were assessed as unclear.
-
High risk of bias (plausible bias that seriously weakens confidence in the results) if one or more items were not met.
Measures of treatment effect
We planned to perform meta‐analyses using the risk ratio as described in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011).
Unit of analysis issues
We considered any of the following designs of included studies as having high potential for 'unit of analysis' issues.
-
Cluster‐randomisation.
-
Simultaneous multiple routes of antibiotic administration on each individual.
-
Repeated measurements (i.e. recurring infections in the same participant).
When appropriate controls were not used, we requested additional information from the authors and re‐analysed the data according to Chapters 9 (Deeks 2011) and 16 (Higgins 2011b) in the Cochrane Handbook for Systematic Reviews of Interventions. Hence for cluster‐randomised trials, we first checked whether an effective sample size could be calculated. If this was not possible, we excluded the study from the analysis. If a proper calculation of the effective sample size could be performed, we included the study in the meta‐analysis using the generic inverse‐variance method. We obtained the data for individual participants of studies that included repeated measurements. After obtaining such data, we carried out an analysis that included the whole follow‐up period for each participant (e.g. a time‐to‐event analysis).
Dealing with missing data
In the case of missing data we first contacted the authors and requested their database. In this way we hoped to receive missing data or determine whether the data are randomly or structurally missing. We then performed sensitivity analyses to assess how sensitive the results were to reasonable changes in the assumptions that were made. If the data were missing at random we analysed only the available data. If data were not missing at random we considered one of the following options.
-
Imputing the missing data with replacement values and treating these as if they were observed.
-
Imputing the missing data and accounting for the fact that these were imputed with uncertainty.
-
Using statistical models to allow for missing data and making assumptions about their relationship with the available data.
Assessment of heterogeneity
We assessed clinical and methodological heterogeneity by critically appraising the included studies. When clinical and methodological heterogeneity was unlikely, we assessed statistical heterogeneity by performing a meta‐analysis. First, we assessed heterogeneity using the confidence interval of the forest plot; thereafter we took the Chi² test and I² statistic into account (Higgins 2011c).
We interpreted the I² statistic as follows.
-
0% to 40%: might not be important.
-
30% to 60%: may represent moderate heterogeneity.
-
50% to 90%: may represent substantial heterogeneity.
-
75% to 100%: represents considerable heterogeneity.
When statistical heterogeneity was detected, we re‐assessed the individual studies in order to find the origin of the heterogeneity.
Assessment of reporting biases
We built a funnel plot in order to assess publication bias. We assessed selective outcome reporting bias by critically appraising the included studies.
Data synthesis
Since our research question was broad we used a random‐effects model in our meta‐analyses, though we used a fixed‐effect model in subgroup analysis when heterogeneity was low. If substantial statistical heterogeneity was present and the directon of effect was inconsistent across studies, we did not combine data in meta‐analysis but presented a narrative summary. We used RevMan 5 to perform analyses (Review Manager 2014).
Subgroup analysis and investigation of heterogeneity
We performed subgroup analyses on:
-
administration route of antibiotics (i.e. oral; intravenous; intrathecal; topical; and via antibiotic‐impregnated catheters);
-
children and adults;
-
ventriculoperitoneal shunts and ventriculoatrial shunts;
-
individual type of antibiotic agent used.
When sufficient data were available we performed subgroup analyses according to the aetiology of hydrocephalus and gender. We conducted an indirect comparison analysis.
When heterogeneity was detected, we assessed the individual studies in order to find the origin of the heterogeneity. Furthermore, when moderate heterogeneity was detected we performed both a fixed‐effect and random‐effects model meta‐analysis.
Sensitivity analysis
If statistical heterogeneity was detected or if the eligibility of some studies in the meta‐analysis was dubious because they did not contain full details, we planned to conduct sensitivity analyses in order to check whether particular decisions or missing information that significantly influences the outcomes of this review can be identified, as described in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011c). For example: we analysed if the results of studies in which deficiencies were not likely to invalidate results, based on the study assessment in this review ('trials at low risk of overall bias'), were likely to show other results than studies that contained deficiencies that were more likely to invalidate results ('trials at uncertain and high risk of overall bias'). 'Trials at low risk of overall bias' were defined as studies in which risk of bias was considered low for at least five 'risk of bias' items. 'Trials at uncertain and high risk of bias' were defined as studies in which risk of bias was considered low for less than five 'risk of bias' items.
Overall quality of the body of evidence: 'Summary of findings' table
We reported the number of participants experiencing shunt infection after administration of antibiotics and judged the overall quality of the treatment effect according to the GRADE approach (GRADEpro GDT; Guyatt 2008). Two review authors (SA and EvL) rated the quality of evidence as 'high', 'moderate', 'low', or 'very low' using GRADEpro GDT. We resolved any disagreements by discussion or by referral to a third review author (HB) when necessary.
Results
Description of studies
Results of the search
A total of 1117 records were identified (CENTRAL: 19; MEDLINE: 411; Embase: 681; CINAHL: 1; ISRCTN registry (isrctn.com): 1; and clinicaltrials.gov: 4). After the removal of duplicates we screened titles and abstracts of 1106 records, which resulted in 21 records that met the eligibility criteria. Full text could not be obtained for three records, despite our emails to the corresponding authors and extensive library and database search. We excluded ten studies. In addition, we identified three articles through handsearch of reference lists. These three studies met eligibility criteria and were therefore included. Ultimately, 11 randomised controlled trials that were eligible according to the inclusion criteria provided data for the review (Figure 1).
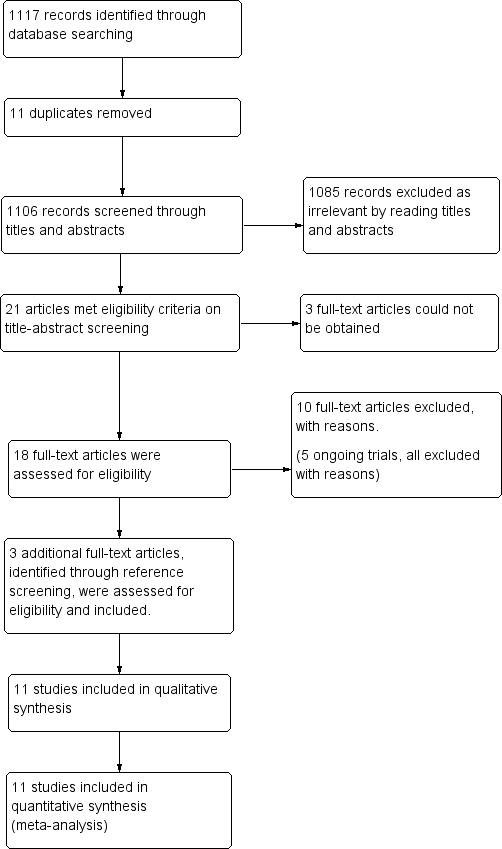
Study flow diagram.
In addition, we identified five ongoing trials through the clinical trials registries of which three were already completed (ISRCTN29451493; NCT00280904a; NCT00286104). All of them were excluded; two (ISRCTN29451493; NCT00286104) were excluded since only external drains were evaluated. NCT02600793 did not randomise and used external drains. NCT00280904a was not a randomised study and NCT01936272 did not include the use of antibiotic prophylaxis in their intervention. See Characteristics of excluded studies.
Included studies
We have presented the list and details of the included studies under Characteristics of included studies.
Participants
Of the 11 included trials, three included solely children (Blum 1989; Haines 1982; Rieder 1987). The other studies included participant of all ages.
Type of shunts
Most studies were limited to the evaluation of ventriculoperitoneal shunts. However five studies included participants with ventriculoatrial shunts (Blomstedt 1985; Blum 1989; Djindjian 1986; Lambert 1984; Schmidt 1985); of which one study contained four participants with a subduroperitoneal shunt (Blum 1989).
Intervention
The most commonly used administration route of antibiotic prophylaxis was intravenously (Blomstedt 1985; Blum 1989; Bullock 1988; Djindjian 1986; Haines 1982; Rieder 1987; Schmidt 1985; Wang 1984), followed by intrathecally (Bayston 1990; Lambert 1984). Administration via antibiotic impregnated catheters were used in one study (Govender 2003;). No studies regarding topical or oral administration of antibiotics were eligible for inclusion.
Controls
Generally, two varieties of control groups were used: standard care (Bayston 1990; Djindjian 1986; Govender 2003; Schmidt 1985); and placebo (Blomstedt 1985; Blum 1989; Bullock 1988; Haines 1982; Rieder 1987; Wang 1984). In the study of Blomstedt 1985 the placebo contained ethanol and propylene glycol. The Bayston 1990 trial did not define their standard of care because of high variability due to the multicentre design. One study compared povidone iodine to antibiotics (Lambert 1984).
Follow‐up
Follow‐up in included studies ranged from eight weeks to more than one year. The time at which shunt infections occurred was only reported in a few studies (Blomstedt 1985; Bullock 1988; Govender 2003; Schmidt 1985; Wang 1984).
Country
Three studies were performed in the UK (Bayston 1990; Govender 2003; Rieder 1987), two in Germany (Blum 1989; Lambert 1984). The other studies were performed in France (Djindjian 1986), the USA (Govender 2003; Haines 1982), Denmark (Schmidt 1985), Canada (Wang 1984), Finland (Blomstedt 1985), and South Africa (Bullock 1988; Govender 2003). Three studies were multicentre (Bayston 1990; Govender 2003; Schmidt 1985).
Defenition of infection
In all studies shunt infection was diagnosed based on clinical assessment, biochemistry and positive CSF culture.
Excluded studies
We have presented the list and descriptions of excluded studies under Characteristics of excluded studies. On the basis of full‐text assessment, we excluded ten publications, two of which were observational studies (Eymann 2008; Kumar 2016). We excluded an additional five studies as they did not report on shunt infections in internal shunts (Odio 1984; Tacconelli 2008; Walters 1992; Young 1987; Zentner 1995). Moreover, three studies were excluded since antibiotic prophylaxis were used in the control group (Moussa 2017; Nejat 2008; Theophilus 2011).
Risk of bias in included studies
All included studies were randomised controlled trials as described in the table of Characteristics of included studies. We have presented details of risk of bias of included studies in Figure 2 and Figure 3. Considering our predefined criteria (Assessment of risk of bias in included studies) we judged four out of 11 (36%) trials at unclear risk of bias (Blomstedt 1985; Bullock 1988; Rieder 1987; Wang 1984), while the remaining seven trials (64%) were scored at high risk of bias in one or more of the components assessed.
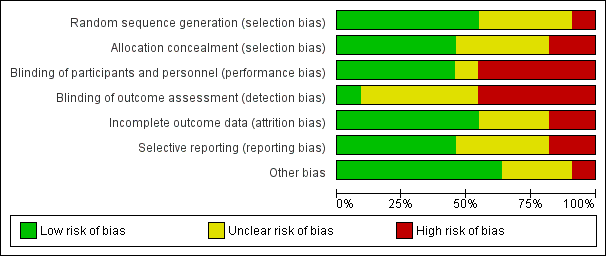
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We considered allocation concealment inappropriate in two studies (Blum 1989; Djindjian 1986). In the Blum 1989 trial they randomised their participants on date of birth; since a date of birth can be checked we considered the allocation concealment as inappropriate. Djindjian 1986 performed an open trial. We scored four studies as unclear risk of bias (Govender 2003; Lambert 1984; Schmidt 1985; Wang 1984).
Blinding
The risk of bias in blinding of participants and personnel remained unclear in one study (Govender 2003) and we considered it to be inadequately performed in five studies (Bayston 1990; Blum 1989; Djindjian 1986; Lambert 1984; Schmidt 1985). Blum 1989 performed a single blinded trial. In the Djindjian 1986 trial they performed an open trial. In the studies of Bayston 1990 and Schmidt 1985 no blinding on participants and personnel or outcome assessment was performed. We considered all five studies that had inadequate blinding on participants and personnel to be at high risk of bias for blinding their outcome assessment. One study scored low risk on blinding their outcome assessment (Blomstedt 1985), but most studies reported inadequately on their method of blinding their outcome assessment.
Incomplete outcome data
In five studies we had concerns about missing data. In three of these five studies the reason for exclusion was described (Blomstedt 1985; Bullock 1988; Wang 1984). Bullock 1988 presented a Chi² test to prove that missing participants were equally distributed between the comparison groups. Two studies reported that participants were lost to follow‐up (Govender 2003; Haines 1982). We were not able to track if these participants were allocated to the intervention group or the control group. Both studies reported that these participants did not suffer from infection at time of their latest follow‐up. We were not able to get the raw data and could therefore not determine whether data were missing at random.
Selective reporting
We considered selective reporting as a potential high risk of bias in two studies (Bayston 1990; Lambert 1984). They had no clear study protocol and no statistical analysis was performed. In four studies it was unclear whether reporting bias was likely to occur (Bullock 1988; Djindjian 1986; Rieder 1987; Schmidt 1985). We considered the remaining studies to have a low risk for reporting bias.
Other potential sources of bias
We considered one study to be at high risk for potential other sources of bias (Lambert 1984), since the dose of gentamicin was increased to 10 mg when ventricles were dilated while no cut‐off point for dilatation was described. We could find no documentation about the number of participants with dilatated ventricles. We scored three studies as unclear (Blum 1989; Bullock 1988; Djindjian 1986). A funnel plot was established in order to check for publication bias (Figure 4). As expected, precise studies were located near the average, but all less precise studies showed a positive effect of antibiotic prophylaxis on the proportion of participants that experienced CSF‐shunt infection. It is unlikely that the asymmetry in this funnel plot is due to publication bias, but rather the result of systematic difference between studies of higher and lower precision, also known as small‐study effects.

Funnel plot of comparison: 1 Treatment benefit of antibiotic prophylaxis versus no antibiotic prophylaxis, outcome: 1.1 Number of participants experiencing shunt infection after administration of antibiotics.
Effects of interventions
summary of findings Table for the main comparison provides a summary of the risk estimates for shunt infection and the grading of the evidence.
We performed four separate meta‐analyses in order to compare the effect of antibiotic prophylaxis on the number of participants who had shunt infections. We compared different administration routes (11 RCTs, 1109 participants), children and adults (6 RCTs, 561 participants), ventriculoperitoneal and ventriculoatrial shunt systems (8 RCTs, 905 participants) and different antibiotic agents (11 RCTs, 1109 participants).
We assessed heterogeneity between trials in three steps: forest plot assessment; using the I² statistic; and using the Chi² test. Based on the combination of these three parameters we used a fixed‐effect model in Analysis 1.1 and Analysis 2.1 and a random‐effects model in Analysis 3.1 and Analysis 4.1.
Number of participants experiencing CSF‐shunt infection
Eleven studies with 1109 participants were available. This analysis showed a positive effect of antibiotic prophylaxis on the number of shunt infections (RR 0.55, 95% CI 0.36 to 0.84), meaning a 45% reduction in the number of participants who had shunt infection compared with standard care or placebo. The heterogeneity I² for this meta‐analysis was 0%, which is low. Using GRADE criteria, we considered the evidence of very low certainty, downgrading two levels because we judged it at serious risk of suffering from selection bias, performance bias, and detection bias. We downgraded an additional level for indirectness.
Subgroup analysis: route of antibiotic prophylaxis (Analysis 1.1)
Intravenous
After analysing the eight included trials (797 participants) that used intravenous antibiotic prophylaxis, we found an overall statistical significance effect on the risk of shunt infections (RR 0.55, 95% CI 0.33 to 0.90). Looking at the individual trials, one trial was significant and favoured the intervention group (Blomstedt 1985). One trial favoured the control group (Schmidt 1985); and the other trials favoured the intervention group, though without significance. The heterogeneity I² for this meta‐analysis was 3%. This result indicates that the relative risk of shunt infection is reduced by 45%.
Intrathecal
We analysed two studies that used intrathecal antibiotics as intervention in this subgroup (Bayston 1990; Lambert 1984). We found no significant effect of intrathecal antibiotic prophylaxis on the risk of shunt infection (RR 0.73, 95% CI 0.28 to 1.93). One of the infections in the trial of Bayston 1990 occurred after trans‐labyrinthine removal of an acoustic neuroma one month after shunt placement. It is not described in which comparison group this participant was included. Heterogeneity between these studies was moderate (I² = 42%). Both studies included ventriculoperitoneal shunts without age limitations, but in Lambert 1984 both ventriculoperitoneal and ventriculoatrial shunts were included. Lambert 1984 and Bayston 1990 were considered at high risk of bias. These uncertainties and varying factors contributed to the moderate level of heterogeneity between these studies.
Topical
No studies regarding the use of topical antibiotic prophylaxis in the prevention of shunt infection met the eligibility criteria.
Oral
No studies regarding the use of oral antibiotic prophylaxis in the prevention of shunt infection met the eligibility criteria.
Antibiotic‐impregnated catheters
One study used antibiotic‐impregnated catheters (Govender 2003). This study did not demonstrate a significant effect on the number of participants that experienced CSF‐shunt infection (RR 0.36, 95% CI 0.10 to 1.24)
Subgroup analysis: adults and children (Analysis 2.1)
Since children seem to be more prone to infection, we analysed differences in the effect of antibiotic prophylaxis between children and adults. Most included trials enrolled participants of any age and therefore we checked if we could find any differentiation on age within the trial results. If this was the case we extracted data on both subgroups and recalculated the proportion of participants who had shunt infections when necessary. We included three studies that reported on the number of children that experienced shunt infection (Djindjian 1986; Govender 2003; Schmidt 1985) . I² was 22%. In the trial of Djindjian 1986 participants were divided into age groups (< 6 years old; 6 to 60 years old; and over 60 years old). It was unclear how many children were included in the '6 to 60' age group; however since the only two infections occurred in adults we added this group to the adult section in the analysis. Six studies reported on the number of adults that experienced shunt infection (338 participants). The test for subgroup differences showed no evidence of a difference (p = 0.33) between children (RR 0.94, 95% CI 0.57 to 1.54) and adults (RR 0.49, 95% CI 0.14 to 1.65).
Subgroup analysis: ventriculoperitoneal shunt and ventriculoatrial shunt (Analysis 3.1)
We included 7 studies (706 participants) in the ventriculoperitoneal shunt group. There was no heterogeneity in this meta‐analysis (I² = 0%). The ventriculoatrial shunt group consisted of two studies (Blomstedt 1985; Schmidt 1985). Heterogeneity in these two studies was substantial (I² = 69%). Schmidt 1985 was not blinded and included both ventriculoatrial shunts and external ventriculostomies. In Blomstedt 1985 only participants older than 12 years were included. The test for subgroup differences showed no evidence of a difference (p = 0.87) between the ventriculoperitoneal shunt group (RR 0.71, 95% CI 0.41 to 1.21) and the ventriculoatrial shunt group (RR 0.61, 95% CI 0.11 to 3.47).
Subgroup analysis: kind of antibiotic used (Analysis 4.1)
Penicillin
We included four studies in this analysis (Bullock 1988; Djindjian 1986; Haines 1982; Schmidt 1985). We found no combined statistically significant effect (RR 0.59, 95% CI 0.22 to 1.63).
Trimethoprim‐sulphamethoxazole
We evaluated two studies in this subgroup (Blomstedt 1985; Wang 1984), of which one showed a statistically significant result (Blomstedt 1985). However, the overall 95% confidence interval was not significant (RR 0.49, 95% CI 0.15 to 1.61).
Vancomycin
We analysed one study that used vancomycin as antibiotic prophylaxis in this subgroup (Bayston 1990), this study did not show a significant effect (RR 1.03, 95% CI 0.35 to 3.04). The risk of bias in this study was high.
Cephalosporin
We analysed two studies in this subgroup (Blum 1989; Rieder 1987). Neither of these showed a statistically significant effect and no overall statistical effect could be demonstrated (RR 0.51, 95% CI 0.18 to 1.43).
Gentamicin
Lambert 1984 used gentamicin as antibiotic agent. Results of this study were not statistically significant (RR 0.21, 95% CI 0.03 to 1.72).
Antibiotic impregnated catheters
Govender 2003 used antibiotic‐impregnated catheters (RR 0.36, 95% CI 0.10 to 1.24).
Other results
Only three studies reported — briefly — on the aetiology of hydrocephalus (Blomstedt 1985; Bullock 1988; Rieder 1987). The other 8 studies reported either nothing (Djindjian 1986; Haines 1982; Lambert 1984; Wang 1984), or that all aetiologies were eligible for inclusion (Bayston 1990; Blum 1989; Govender 2003; Schmidt 1985;). Data on gender were not presented in four studies (Bayston 1990; Blomstedt 1985; Djindjian 1986; Lambert 1984). Although the other studies did report on gender, only two studies reported the proportion of participants that experienced CSF‐shunt infection related to gender (Rieder 1987; Schmidt 1985). Blum 1989 reported that no significant correlation was found between shunt infection and gender.
The onset of shunt infection was mentioned in five studies (Blomstedt 1985; Bullock 1988; Govender 2003; Schmidt 1985; Wang 1984). In these studies it was seen that participants who did not receive antibiotic prophylaxis were more likely to develop shunt infections within 30 days after surgery. In the antibiotic prophylaxis group infections were more distributed between the different time frames. Exact numbers or means are not displayed here since a wide variety of presentation forms were used among the articles.
Sensitivity analysis
The first sensitivity analysis that was performed contained all studies divided into low risk of bias and uncertain or high risk of bias. In the low risk of bias group four studies used intravenous administration and one used topical administration. A significant effect in favour of antibiotic prophylaxis was found (RR 0.48, 95% CI 0.25 to 0.94). Blomstedt 1985 had considerable impact on the overall significance level in the low risk of bias group, since no significant effect was found after excluding this trial from the analysis (RR 0.70, 95% CI 0.30 to 1.65). Studies in the uncertain and high risk of bias group were just without range of a statistically significant result (RR 0.58, 95% CI 0.32 to 1.05). A second sensitivity analysis was performed regarding the difference in control groups. It showed that only studies that used placebo as control had a significant effect of antibiotics prophylaxis on shunt infection (RR 0.44, 95% CI 0.24 to 0.81).
Point estimate of the control group
We performed a point estimate of the control group to check whether the incidence of shunt infections has declined over time without intervention. Possible causes for this decline could be the improvement of hygiene protocols or improved instrumentation. This decline over time would cause an underestimation of the effect of studies that are performed more recently compared to older studies. However, no decline of infections in the control group over time was found (data not shown).
Discussion
Summary of main results
It is uncertain whether antibiotic prophylaxis had a positive effect on reducing the number of participants who experienced CSF‐shunt infection, since the results of this review are equivocal and based on low quality evidence. Most of the included studies compared intravenous administration of antibiotics versus placebo or standard care; two studies used intrathecal antibiotics in the intervention group and one antibiotic‐impregnated catheters. Only intravenous administration of antibiotic prophylaxis showed a significant effect on the number of participants who had shunt infections. However, this was the only subgroup that contained more than two studies. The antibiotic‐impregnated catheter group contained one study that showed no significant effect on the number of shunt infections.
Looking at the difference in effect between adults and children, no evidence of a significant effect in studies regarding adults and those regarding children was found. Although the result in adults was not statistically significant, probably due to the low number of included participants in the analysis, a risk reduction of 51% might be clinically important. Reporting on timing of onset of infection was low. However, participants who did not receive antibiotic prophylaxis were more likely to develop shunt infections within 30 days after surgery which suggest that antibiotic prophylaxis might have an effect on shunt infection that occur within a short period after surgery. Since data on the onset of infection in this review is very low, more research needs to be performed in order to adequately analyse the effect of antibiotic prophylaxis on the timing of onset of infection.
Due to the limited number of RCTs or limited description of outcomes, not every subgroup analysis contained a sufficient number of studies to draw grounded conclusions. In the evaluation of different administration routes only one study on antibiotic‐impregnated catheters (Govender 2003) was available. In the meta‐analysis on ventriculoperitoneal and ventriculoatrial shunts only two studies were eligible for evaluation of ventriculoatrial shunts (Blomstedt 1985; Schmidt 1985), since other studies did not report on shunt infections in ventriculoatrial shunts separately. In the final meta‐analysis regarding the kind of antibiotic agent, only one subgroup — penicillin — comprised more than two studies. Due to the variety of antibiotic agents in combination with the low number of included studies per antibiotic agent, no conclusions could be drawn on which is the most effective in preventing shunt infections.
Overall completeness and applicability of evidence
This review shows a significant effect for the use of antibiotic prophylaxis for the prevention of CSF‐shunt infections. However, the methods in the included studies were very heterogenic on, for example, the age of participants and aetiology of hydrocephalus, the kind and dosage of antibiotic agents, and the time of follow‐up. Moreover, most study methods were marginally described, especially in the older studies. Due to the limited description of methods and results, some parameters could not be studied. Although the overall effect was statistically significant, the only subgroup that showed a statistically significant effect was intravenous administration. A lot of clinically important aspects remain unclear, i.e. in which age group antibiotic prophylaxis are recommended, what kind of antibiotic agent is most effective and for what kind of drains antibiotic prophylaxis might work.
Overall, antibiotic prophylaxis are effective in preventing CSF‐shunt infection. It remains unclear what administration route or agent is best for preventing shunt infections. Heterogeneity between studies limits the external validity and therefore no generalised conclusion can be drawn.
In this review, we performed a thorough search in various databases. However, most included studies were published before the year 2000 and they may not reflect the change in practice over time.
Quality of the evidence
The quality of evidence for the overall effect of the administration of antibiotic prophylaxis, presented in the 'Summary of findings' table, was low. We downgraded the level of evidence two levels. Downgrading of one level was due to indirectness of evidence, since control groups differed a lot. For example, Bayston 1990 compared the used of antibiotic prophylaxis to current practice in a multicentre design, it was not formulated whether one of the centre's standard care enclosed the use of antibiotics. We downgraded another two levels because we considered studies at serious or unclear risk of bias. The quality of evidence for the administration of intravenous antibiotic prophylaxis was downgraded two levels, since the control groups of included studies differed a lot and again the risk of bias was considerably. Also in the studies that administered antibiotic prophylaxis intrathecally, the risk of bias was considered at serious risk, therefore the quality of evidence was downgraded by two levels. One study implanted antibiotic impregnated catheters (Govender 2003). This study was was considered at serious or unclear risk of bias at five out of seven items, therefore the level of evidence was downgraded two levels. Besides the risk of bias, the level of evidence was downgraded one more level, since the subgroup consisted of only one study and this study consisted of only 110 inclusions.
Potential biases in the review process
This review was based on a systematic review of 11 small trials of antibiotic prophylaxis for prevention of shunt infection. There was a statistically significant reduction in shunt infection in participants who received antibiotic prophylaxis. Although we performed a comprehensive search without any restrictions in a variety of databases and performed a comprehensive search for additional records in the reference lists of included studies in order to minimize publication bias, some data could have been missed. Besides, no full text could be obtained in three records. All stages and decisions were performed by at least two of three authors in order to minimize bias in the review process. When disagreement occurred it was discussed in detail within the team. Since we were not able to get raw data, some results may be biased due to incomplete outcome data.
Moreover, randomised controlled trials are rare in the field of CSF‐shunt surgery. Most of the studies performed on CSF‐shunt infection are cohort studies that either focus on incidence or risk factors. This means that valuable data could be missed in a systematic review focusing on RCTs.
Agreements and disagreements with other studies or reviews
Ratilal 2008 reviewed the use of intravenous antibiotic prophylaxis and antibiotic‐impregnated catheters in both internal and external ventricular drainage. They found a significant effect of intravenous antibiotic prophylaxis for internal ventricular shunts but no significant effect was found for external ventricular drains. Besides, no conclusions can be drawn on the use of antibiotic‐impregnated internal shunts, since only one randomised controlled trial was included. Although the objective of the Ratilal 2008 review differs, the sub‐conclusion that the use of antibiotic prophylaxis has a positive effect on lowering CSF‐shunt infection is shared.

Study flow diagram.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
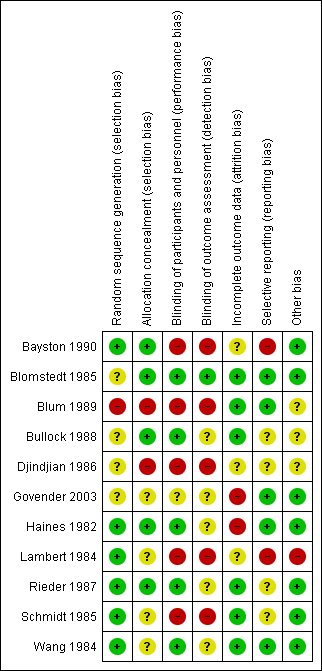
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Funnel plot of comparison: 1 Treatment benefit of antibiotic prophylaxis versus no antibiotic prophylaxis, outcome: 1.1 Number of participants experiencing shunt infection after administration of antibiotics.

Comparison 1 Treatment benefit of antibiotic prophylaxis versus no antibiotic prophylaxis, Outcome 1 Number of participants experiencing shunt infection after administration of antibiotics.

Comparison 2 Treatment benefit of antibiotic prophylaxis in adults and children, Outcome 1 Number of adults and children experiencing shunt infection after administration of antibiotic prophylaxis.
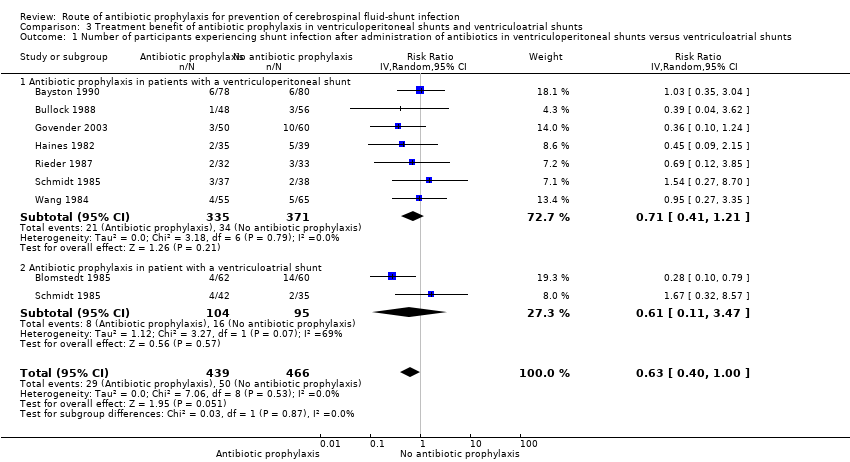
Comparison 3 Treatment benefit of antibiotic prophylaxis in ventriculoperitoneal shunts and ventriculoatrial shunts, Outcome 1 Number of participants experiencing shunt infection after administration of antibiotics in ventriculoperitoneal shunts versus ventriculoatrial shunts.
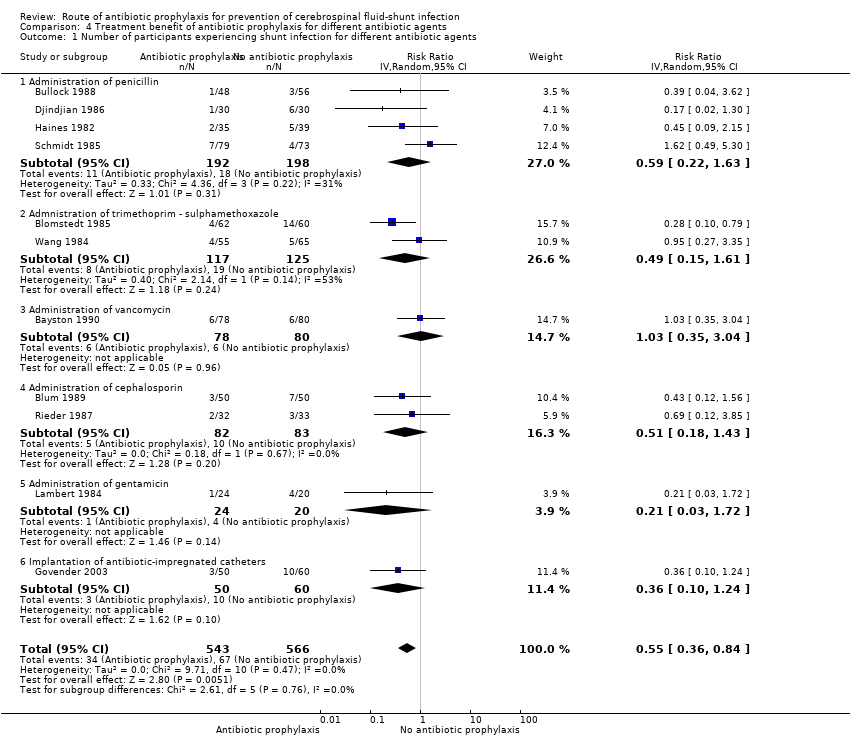
Comparison 4 Treatment benefit of antibiotic prophylaxis for different antibiotic agents, Outcome 1 Number of participants experiencing shunt infection for different antibiotic agents.

Comparison 5 Sensitivity analysis (Trials at low risk of bias and trials at uncertain and high risk of bias), Outcome 1 Number of participants experiencing shunt infection after administration of antibiotics.

Comparison 6 Sensitivity analysis on differences in control groups between studies (placebo, common care, no antibiotics), Outcome 1 Sensitivity analysis on differences in control groups between studies.
| Antibiotic prophylaxis versus no antibiotic prophylaxis / standard care for CSF‐shunt infections | ||||||
| Patient or population: CSF‐shunt infections | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | № of participants | Certainty of the evidence | Comments | |
| Risk with no antibiotic prophylaxis / standard care | Risk with antibiotic prophylaxis | |||||
| Number of participants experiencing shunt infection after administration of antibiotic prophylaxis | Study population | RR 0.55 | 1109 | ⊕⊕⊝⊝ | ||
| 118 per 1.000 | 65 per 1.000 | |||||
| Number of participants experiencing shunt infection after administration of intravenous antibiotic prophylaxis | Study population | RR 0.55 | 797 | ⊕⊕⊝⊝ | ||
| 116 per 1.000 | 64 per 1.000 | |||||
| Number of participants experiencing shunt infection after administration of intrathecal antibiotic prophylaxis | Study population | RR 0.73 | 202 | ⊕⊕⊝⊝ | ||
| 100 per 1.000 | 73 per 1.000 | |||||
| Number of participants experiencing shunt infection after implantation of antibiotic impregnated catheters | Study population | RR 0.36 | 110 | ⊕⊝⊝⊝ | ||
| 167 per 1.000 | 60 per 1.000 | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence | ||||||
| 1 Downgraded one level due to indirectness of evidence, since control groups differed a lot. For example, one study compared the used of antibiotic prophylaxis to current practice in a multicenter design. 2 Downgraded one level because four studies were considered at serious risk of bias at at least three forms of bias 3 Downgraded one level due to indirectness of evidence, since control groups differed a lot. 4 Downgraded one level because two studies were considered at serious risk of bias at at least three forms of bias 5 Downgraded two levels because both studies were considered at serious risk of bias at at least three forms of bias 6 Downgraded two levels because the study was considered at serious or unclear risk of bias at five out of seven items 7 Downgraded one level, since only one study was included in this subgroup and the total number of participants in this study was low | ||||||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants experiencing shunt infection after administration of antibiotics Show forest plot | 11 | 1109 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.36, 0.84] |
| 1.1 Intravenous administration of antibiotics | 8 | 797 | Risk Ratio (IV, Fixed, 95% CI) | 0.55 [0.33, 0.90] |
| 1.2 Intrathecal administration of antibiotics | 2 | 202 | Risk Ratio (IV, Fixed, 95% CI) | 0.73 [0.28, 1.93] |
| 1.3 Antibiotic Impregnated Catheters | 1 | 110 | Risk Ratio (IV, Fixed, 95% CI) | 0.36 [0.10, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of adults and children experiencing shunt infection after administration of antibiotic prophylaxis Show forest plot | 6 | 561 | Risk Ratio (IV, Fixed, 95% CI) | 0.85 [0.54, 1.35] |
| 1.1 Antibiotic prophylaxis in adults | 3 | 223 | Risk Ratio (IV, Fixed, 95% CI) | 0.49 [0.14, 1.65] |
| 1.2 Antibiotic prophylaxis in children | 6 | 338 | Risk Ratio (IV, Fixed, 95% CI) | 0.94 [0.57, 1.54] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants experiencing shunt infection after administration of antibiotics in ventriculoperitoneal shunts versus ventriculoatrial shunts Show forest plot | 8 | 905 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.40, 1.00] |
| 1.1 Antibiotic prophylaxis in patients with a ventriculoperitoneal shunt | 7 | 706 | Risk Ratio (IV, Random, 95% CI) | 0.71 [0.41, 1.21] |
| 1.2 Antibiotic prophylaxis in patient with a ventriculoatrial shunt | 2 | 199 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.11, 3.47] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants experiencing shunt infection for different antibiotic agents Show forest plot | 11 | 1109 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.36, 0.84] |
| 1.1 Administration of penicillin | 4 | 390 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.22, 1.63] |
| 1.2 Admnistration of trimethoprim ‐ sulphamethoxazole | 2 | 242 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.15, 1.61] |
| 1.3 Administration of vancomycin | 1 | 158 | Risk Ratio (IV, Random, 95% CI) | 1.03 [0.35, 3.04] |
| 1.4 Administration of cephalosporin | 2 | 165 | Risk Ratio (IV, Random, 95% CI) | 0.51 [0.18, 1.43] |
| 1.5 Administration of gentamicin | 1 | 44 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.03, 1.72] |
| 1.6 Implantation of antibiotic‐impregnated catheters | 1 | 110 | Risk Ratio (IV, Random, 95% CI) | 0.36 [0.10, 1.24] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Number of participants experiencing shunt infection after administration of antibiotics Show forest plot | 11 | 1109 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.36, 0.84] |
| 1.1 Trials at low risk of bias | 4 | 381 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.25, 0.94] |
| 1.2 Trials at uncertain and high risk of bias | 7 | 728 | Risk Ratio (IV, Random, 95% CI) | 0.58 [0.32, 1.05] |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1 Sensitivity analysis on differences in control groups between studies Show forest plot | 11 | 1109 | Risk Ratio (IV, Random, 95% CI) | 0.55 [0.36, 0.84] |
| 1.1 Studies that used a placebo in the control group | 5 | 520 | Risk Ratio (IV, Random, 95% CI) | 0.44 [0.24, 0.81] |
| 1.2 Studies that applied common care in the control group | 3 | 333 | Risk Ratio (IV, Random, 95% CI) | 0.66 [0.31, 1.37] |
| 1.3 Studies that used neither placebo nor antibiotics in the control group | 2 | 212 | Risk Ratio (IV, Random, 95% CI) | 0.61 [0.07, 5.53] |
| 1.4 Studies that used disinfectant in the control group | 1 | 44 | Risk Ratio (IV, Random, 95% CI) | 0.21 [0.03, 1.72] |

