Diálisis peritoneal de inicio urgente versus hemodiálisis para personas con nefropatía crónica
Resumen
Antecedentes
Los pacientes con nefropatía crónica (NC) que requieren comenzar con urgencia la diálisis pero sin tener un acceso permanente de diálisis han comenzado tradicionalmente con hemodiálisis (HD) utilizando un catéter venoso central (CVC). Sin embargo, varios estudios han informado de que el inicio urgente de la diálisis peritoneal (DP) es una opción alternativa viable para estos pacientes.
Objetivos
Esta revisión tuvo como objetivo examinar los efectos beneficiosos y perjudiciales de la DP de inicio urgente en comparación con inicio de HD con CVC en adultos y niños con NC que requieren tratamiento sustitutivo de la función renal a largo plazo.
Métodos de búsqueda
Se realizaron búsquedas de ensayos controlados aleatorizados en el Registro de estudios del Grupo Cochrane de Riñón y trasplante (Cochrane Kidney and Transplant) hasta el 25 de mayo de 2020, mediante contacto con el documentalista y el uso de términos de búsqueda relevantes para esta revisión. Los estudios en el registro se identifican mediante búsquedas en CENTRAL, MEDLINE y EMBASE, en actas de congresos, en el portal de búsqueda de la plataforma de registros internacionales de ensayos clínicos (ICTRP), y en ClinicalTrials.gov.
Se buscaron ensayos controlados no aleatorizados en MEDLINE (OVID) (1946 hasta el 11 de febrero de 2020) y en EMBASE (OVID) (1980 hasta el 11 de febrero de 2020).
Criterios de selección
Ensayos controlados aleatorizados (ECA), ensayos cuasialeatorizados y ensayos controlados no aleatorizados que compararan un inicio urgente de la DP con el inicio con HD mediante CVC.
Obtención y análisis de los datos
Dos autores de la revisión extrajeron los datos y evaluaron la calidad de los estudios de forma independiente. Se obtuvo información adicional a partir de los investigadores principales. Las estimaciones del efecto se analizaron mediante un modelo de efectos aleatorios y los resultados se presentaron como razones de riesgos (RR) con intervalos de confianza (IC) del 95%. Se utilizó el marco GRADE para hacer valoraciones acerca de la evidencia para cada desenlace.
Resultados principales
En general, se incluyeron siete estudios observacionales (991 participantes): tres estudios prospectivos de cohortes y cuatro estudios retrospectivos de cohortes. Todos los desenlaces, excepto uno (bacteriemia), se consideraron con una muy baja certeza de la evidencia, dado que todos los estudios incluidos eran observacionales e informaron de pocos eventos, lo que dio lugar a imprecisiones, y a resultados inconsistentes. La DP de inicio urgente podría reducir la incidencia de bacteriemia relacionada con el catéter en comparación con la HD iniciada con un CVC (dos estudios, 301 participantes: RR 0,13; IC del 95%: 0,04 a 0,41; I2 = 0%; evidencia de certeza baja), que se tradujo en 131 episodios de bacteriemia menos por cada 1000 (IC del 95%: 89 a 145 menos). No existe seguridad acerca de los efectos de la DP sobre el riesgo de peritonitis (dos estudios, 301 participantes: RR 1,78; IC del 95%: 0,23 a 13,62; I2= 0%; evidencia de certeza muy baja), infección de túnel/sitio de salida (un estudio, 419 participantes: RR 3,99; IC del 95%: 1,2 a 12,05; evidencia de certeza muy baja), hemorragia del sitio de salida (un estudio, 178 participantes: RR 0,12; IC del 95%: 0,01 a 2,33; evidencia de certeza muy baja), disfunción del catéter (dos estudios, 597 participantes: RR 0,26; IC del 95%: 0,07 a 0,91; I2 = 66%; evidencia de certeza muy baja), reajuste del catéter (dos estudios, 225 participantes: RR: 0,13; IC del 95%: 0,00 a 18,61; I2 = 92%; evidencia de certeza muy baja), supervivencia técnica (un estudio, 123 participantes: RR: 1,18; IC del 95%: 0,87 a 1,61; evidencia de certeza muy baja) o supervivencia del paciente (cinco estudios, 820 participantes; RR 0,68; IC del 95%: 0,44 a 1,07; I2 = 0%; evidencia de certeza muy baja) en comparación con el inicio con HD con CVC. Dos estudios que utilizaron diferentes métodos de medición de la hospitalización informaron de que la hospitalización fue similar, aunque un estudio informó de tasas de hospitalización más altas en la HD iniciada con catéter en comparación con la DP de inicio urgente.
Conclusiones de los autores
En comparación con la HD iniciada con un CVC, la DP de inicio urgente podría reducir el riesgo de bacteriemia y tuvo efectos inciertos sobre otras complicaciones de la diálisis y la técnica y en la supervivencia del paciente. En resumen, hay muy pocos estudios que comparen directamente los desenlaces de la DP de inicio urgente y de la HD iniciada mediante CVC en pacientes con enfermedad renal crónica que necesitan comenzar la diálisis de forma urgente. Esta falta de evidencia debe abordarse en estudios futuros.
PICO
Resumen en términos sencillos
Diálisis peritoneal de inicio urgente versus hemodiálisis para personas con enfermedad renal crónica
¿Cuál es el problema?
La diálisis peritoneal (DP) es una forma establecida de tratamiento sustitutivo de la función renal que utiliza la propia membrana peritoneal del paciente (revestimiento interno del abdomen) como filtro para la diálisis. Tradicionalmente, el comienzo de la DP se ha retrasado a dos semanas después de la colocación de un catéter de DP para dar tiempo a que cicatrice la herida. Sin embargo, algunos pacientes necesitan diálisis urgentemente y no pueden esperar dos semanas. Con el fin de evitar un procedimiento adicional de inserción de un catéter para hemodiálisis (HD) en los pacientes en DP, ha habido estudios que informan de un comienzo exitoso de la DP de forma urgente en las dos semanas siguientes a la inserción del catéter de DP. La revisión comparó los desenlaces de los pacientes en DP que iniciaron la DP de forma urgente con los pacientes en HD que iniciaron la diálisis con un catéter.
¿Qué se hizo?
Se realizó una revisión sistémica para analizar los efectos beneficiosos y perjudiciales de los pacientes con enfermedad renal crónica que iniciaron DP urgente (generalmente en las dos semanas posteriores a la inserción del catéter de DP) con los que se sometieron a HD mediante un catéter de diálisis.
¿Qué se encontró?
Se identificaron siete estudios (991 participantes) que compararon los riesgos y los beneficios del comienzo urgente de la diálisis peritoneal con la hemodiálisis con catéter. Se encontró que los pacientes que se sometieron a DP urgente podrían tener un menor riesgo de infección sanguínea (presencia de bacterias en la sangre) en comparación con los pacientes que se sometieron a HD utilizando un catéter de diálisis. No existe seguridad acerca de las diferencias en los riesgos de presentar otras complicaciones infecciosas y mecánicas de un catéter de diálisis, o la continuidad del tipo de tratamiento de diálisis de origen (DP o HD) entre los dos modos de diálisis.
Conclusiones
Los pacientes en DP podría tener un menor riesgo de infección sanguínea en comparación con los que están en HD con un catéter. Sin embargo, no está claro si existen diferencias en otras complicaciones relacionadas con la infección o con el catéter, la capacidad de seguir con el mismo tipo de tratamiento de diálisis y la supervivencia del paciente entre la DP urgente y la HD con catéter.
Authors' conclusions
Summary of findings
| Urgent‐start peritoneal dialysis versus haemodialysis initiated with a catheter for patients with chronic kidney disease | |||||
| Patient or population: people with CKD Settings: community Intervention: USPD Comparison: HD initiated with a central venous catheter | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Risk with USHD | Risk with USPD | ||||
| Bacteraemia up to 6 months | 151 per 1,000 | 20 per 1,000 (6 to 62) | RR 0.13 (0.04 to 0.41) | 301 (2) | ⊕⊕⊝⊝ |
| Peritonitis up to 6 months | 7 per 1,000 | 13 per 1,000 (2 to 98) | RR 1.78 (0.23 to 13.62) | 301 (2) | ⊕⊝⊝⊝ |
| Exit‐site or tunnel infection | 18 per 1,000 | 71 per 1,000 (24 to 216) | RR 3.99 (1.32 to 12.05) | 419 (1) | ⊕⊝⊝⊝ |
| Exit‐site bleeding | 37 per 1,000 | 4 per 1,000 (0 to 85) | RR 0.12 (0.01 to 2.33) | 178 (1) | ⊕⊝⊝⊝ |
| Catheter malfunction | 151 per 1,000 | 39 per 1,000 (11 to 137) | RR 0.26 (0.07 to 0.91) | 597 (2) | ⊕⊝⊝⊝ |
| Catheter re‐adjustment up to 60 months | 373 per 1,000 | 48 per 1,000 (0 to 1,000) | RR 0.13 (0.00 to 18.61) | 225 (2) | ⊕⊝⊝⊝ |
| Technique survival up to 6 months | 526 per 1,000 | 621 per 1,000 (458 to 847) | RR 1.18 (0.87 to 1.61) | 123 (1) | ⊕⊝⊝⊝ |
| Home dialysis | ‐ | ‐ | ‐ | No studies | ABSENT |
| Death (any cause) up to 24 months | 204 per 1000 | 139 per 1,000 (90 to 218) | RR 0.68 (0.44 to 1.07) | 820 (5) | ⊕⊝⊝⊝ |
| Hospitalisation up to 6 months | 579 per 1,000 | 683 per 1,000 (515 to 897) | RR 1.18 (0.89 to 1.55) | 123 (1) | ⊕⊝⊝⊝ |
| *The risk in the USPD group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; HD: haemodialysis; USHD: urgent‐start HD; USPD: urgent‐start peritoneal dialysis | |||||
| GRADE Working Group grades of evidence | |||||
| 1downgraded for observational studies 2 downgraded for observational studies, imprecision due to small number of events 3 downgraded for observational studies and imprecision due to small number of events, and inconsistency | |||||
Background
Description of the condition
Chronic kidney disease (CKD) requiring long‐term kidney replacement therapy (KRT) is a common and growing problem affecting over two million people worldwide (AIHW 2016; Couser 2011; Gilg 2016). Even though one of the main predictors of better patient survival is having an established dialysis access at the time of dialysis commencement (Pisoni 2009; Ravani 2013), a large proportion of patients commence treatment via a central venous catheter (CVC) (40% to 80%) (ANZDATA 2015; Moist 2014; Rao 2016; USRDS 2015). In part, this is due to many patients (20% to 30%) presenting ‘late’ to a nephrology service that necessitates commencement of dialysis urgently or in an unplanned manner (Foote 2014). In other cases, it could be the consequence of health system failure such as a lack of an established responsive dialysis access programme with limited access to a surgical or interventional nephrology service. In this setting, most patients start haemodialysis (HD) via a CVC, which then places them at a heightened risk of infection, prolonged hospitalisation, mortality (Perl 2011) as well as future complications from central vascular stenosis (Shingarev 2012). A recent systematic review, that included a total of 586,337 patients, identified that the use of a CVC led to the highest risk of death, fatal infections, and cardiovascular events, compared with other types of vascular access (Ravani 2013). Moreover, these patients were more likely to remain on facility‐based HD (Morton 2010) rather than to transition to home‐based dialysis program such as peritoneal dialysis (PD), which confers an initial survival advantage (Kumar 2014; Masterson 2008).
PD is a type of home‐based dialysis that uses the peritoneum in a person’s abdomen as the membrane through which fluid and dissolved substances are exchanged with the blood. PD solution is introduced through a PD catheter, which is placed in the lower abdomen permanently (Mehrotra 2016). PD has many benefits at the patient‐level, including initial survival advantage compared to HD, easier mastery of the technique, better preservation of residual kidney function, better patient‐level satisfaction, and preservation of vascular access for future use (Tokgoz 2009). PD can also offer annual cost savings of up to 40% compared to facility HD (KHA 2012; KHA 2016). However, uptake of PD remains relatively low and only accounts for approximately 11% of the global dialysis population (Jain 2012). The decision‐making process which leads to undertaking a home therapy is complex and can be influenced by social circumstances, education, and the capacity to undertake training (Machowska 2016). However, one of the contributors to limited growth in PD may relate to the reluctance to utilise PD as the dialysis modality of choice without established dialysis access, which is driven by the practice to delay treatment by at least two weeks from the time of PD catheter insertion to lower the risk of catheter‐related complications, such as leaks (Dombros 2005; Figueiredo 2010). However, these practices are guided by recommendations based on the weak level of evidence (Dombros 2005; Figueiredo 2010).
More recently, urgent‐start PD has been promoted as an alternative form of urgent, unplanned dialysis treatment, which has been reported to be effective and potentially has fewer adverse consequences based on findings from observational studies (Casaretto 2012; Ghaffari 2012; Jo 2007; Koch 2012; Lobbedez 2008; See 2017).
Description of the intervention
Traditionally, new CKD patients who require dialysis urgently but do not have a permanent functional dialysis access are subjected to HD via central venous dialysis catheter (CVC). In order to avoid the CVC and its related complications, urgent‐start PD, has been introduced as an alternative form of KRT for CKD patients who require dialysis urgently or in an unplanned fashion. Currently, there is no universally agreed definition regarding the duration between PD catheter insertion and commencement that qualifies as urgent‐start PD. The International Society for Peritoneal Dialysis (ISPD) recommends the use of PD catheters at least two weeks after its insertion (Figueiredo 2010). The duration between PD catheter insertion and commencement (Ranganathan 2017), fill volume and insertion technique may have an impact on outcomes observed following urgent‐start PD and therefore will be considered as part of subgroup analyses in the present review.
How the intervention might work
Urgent‐start PD is initiated with low fill volumes in the supine position using a cycler to minimise the risk of peri‐catheter leak. Treatment can be delivered in both inpatient and outpatient settings.
Why it is important to do this review
The vast majority of evidence relating to outcomes from urgent‐start PD has been generated from single‐centre observational studies with relatively small patient numbers (Casaretto 2012; Ghaffari 2012; Jo 2007; Koch 2012; Lobbedez 2008), which has resulted in ad hoc implementation rather than a ‘standard’ care across the world.
Objectives
This review aimed to look at the benefits and harms of urgent‐start PD (defined as initiation of PD within 2 weeks of catheter insertion) compared to HD (defined as initiation of HD using a CVC) in adults and children with CKD requiring long‐term KRT.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs), quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods), and non‐RCTs comparing urgent‐start PD to HD treatments via CVC.
Types of participants
Inclusion criteria
Participants included in this review were both adults and children with CKD, who require dialysis treatment. Participants had a PD catheter inserted to undergo PD or a CVC for HD.
Exclusion criteria
The review did not include data obtained from patients with acute kidney injury.
Types of interventions
Studies comparing urgent‐start PD and HD via CVC were included in this review.
-
Intervention: patients commenced on urgent‐start PD, defined as initiation of PD therapy within two weeks of catheter placement.
-
Comparator: patients commenced on urgent‐start HD, defined as initiation of HD therapy using a CVC (cuffed and uncuffed at commencement).
Types of outcome measures
Primary outcomes
-
Catheter‐related infectious complications occurring within 30 days (early complication) and 90 days (late complication)
-
Bacteraemia (defined as blood culture positive for bacteria) after commencement of dialysis (proportion of patients developing bacteraemia)
-
Peritonitis as defined by the ISPD guidelines (Li 2010) after commencement of dialysis (proportion of patients developing peritonitis)
-
Exit site or tunnel tract infection in PD patients was defined by the ISPD guidelines (Li 2010) after commencement of dialysis and CVC exit‐site infection was defined as presence of erythema, induration, and/or tenderness within 2 cm of the catheter exit site; may be associated with fever or purulent drainage from the exit site, with or without concomitant bloodstream infection (Mermel 2009) and tunnel infection, defined as tenderness, erythema, and/or induration > 2 cm from the catheter exit site, along the subcutaneous tract of a tunnelled catheter, with or without concomitant bloodstream infection (Mermel 2009). (proportion of patients developing exit site or tunnel tract infections)
-
-
Catheter‐related non‐infectious complications occurring within 30 days (early complication) and 90 days (late complication)
-
Exit site bleeding requiring intervention (e.g. additional application of suture) after commencement of dialysis (proportion of patients developing exit site bleeding)
-
Catheter malfunction, defined as catheter flow problems requiring intervention (medical (e.g. urokinase) or surgical (e.g. catheter replacement)) or malposition after commencement of dialysis (proportion of patients developing catheter malfunction)
-
Catheter re‐adjustment, defined as catheter malfunction requiring intervention to re‐adjust or replace the catheter (proportion of patients requiring catheter re‐adjustment procedure)
-
-
Home dialysis (proportion of patients on home dialysis (e.g. PD or home HD)).
Secondary outcomes
-
Technique survival (number of patients remaining on the initial mode of KRT at the end of study)
-
Death (any cause)
-
Hospitalisation (average days spent in hospital, episodes of hospitalisation, or number requiring hospitalisation)
-
Pain/discomfort related to dialysis therapy
-
Adverse effects
-
Quality of life
-
Cost of dialysis treatment
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 25 May 2020 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
-
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
-
Weekly searches of MEDLINE OVID SP
-
Hand searching of kidney‐related journals and the proceedings of major kidney conferences
-
Searching of the current year of EMBASE OVID SP
-
Weekly current awareness alerts for selected kidney and transplant journals
-
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies, as well as a list of hand searched journals, conference proceedings and current awareness alerts, are available in the Specialised Register section of information about Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in strategies for this review.
For non‐RCTs, MEDLINE (OVID) (1946 ‐ 11 February 2020) and EMBASE (OVID) (1980 ‐ 11 February 2020), Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov (up to 14 February 2019) were searched.
Searching other resources
-
Reference lists of review articles, relevant studies, and clinical practice guidelines.
-
Letters seeking information about unpublished or incomplete studies to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by two authors, who discarded studies that were not applicable; however, studies and reviews that might have included relevant data or information on studies were retained initially. Two authors independently assessed and retrieved abstracts and, if necessary, the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions, these data were used. Any discrepancies between published versions were highlighted.
Assessment of risk of bias in included studies
Randomised controlled trials
The following items were independently assessed by two authors using the risk of bias assessment tool for RCTs (Higgins 2011) (see Appendix 2).
-
Was there adequate sequence generation (selection bias)?
-
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
-
Participants and personnel (performance bias)
-
Outcome assessors (detection bias)
-
-
Were incomplete outcome data adequately addressed (attrition bias)?
-
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
-
Was the study apparently free of other problems that could put it at a risk of bias?
Non‐randomised controlled trials
The Newcastle‐Ottawa Scale (NOS) (www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf) for assessing quality of non‐randomised studies were used.
-
For case control studies the following items were evaluated.
-
Selection (adequacy of definition, representativeness of the cases, selection of controls, definition of controls)
-
Comparability (comparability of cases and controls on the basis of the design or analysis)
-
Exposure (ascertainment of exposure, same method of ascertainment for cases and controls, non‐response rate).
-
-
For cohort studies the following items were evaluated.
-
Selection (representativeness of the exposed cohort, selection of the non‐exposed cohort, ascertainment of exposure, demonstration that outcome of interest was not present at start of study)
-
Comparability (comparability of cohorts on the basis of the design or analysis)
-
Outcome (assessment of outcome, adequacy of follow‐up and duration of follow‐up).
-
Measures of treatment effect
For dichotomous outcomes (e.g. death, mechanical complications within one month of commencement of PD) results were expressed as risk ratio (RR) with 95% confidence intervals (CI). Where continuous scales of measurement were used to assess the effects of treatment (e.g. duration of hospitalisation, duration of PD training), the mean difference (MD) was used, or the standardised mean difference (SMD) if different scales had been used. Outcomes from RCTs and non‐RCTs were reported separately.
Unit of analysis issues
If the review included cluster RCTs, the unit of analysis was at the same level as the allocation, using a summary measurements from each cluster. All data were collected and analysed according to the type of measure (e.g. hazard ratios, odds ratio).
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing the corresponding author) and any relevant information obtained in this manner was included in the review. Evaluation of important numerical data such as screened, randomised patients, as well as intention‐to‐treat, as‐treated and per‐protocol populations were carefully performed. Attrition rates, for example drop‐outs, losses to follow‐up and withdrawals were evaluated. Issues of missing data and imputation methods (for example, last‐observation‐carried‐forward) were critically appraised (Higgins 2011).
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I2 values was as follows.
-
0% to 40%: might not be important
-
30% to 60%: may represent moderate heterogeneity
-
50% to 90%: may represent substantial heterogeneity
-
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a Cl for I2) (Higgins 2011).
Assessment of reporting biases
If possible, funnel plots were used to assess for the potential existence of small study bias (Higgins 2011).
Data synthesis
We combined data of studies having similar designs and interventions and reporting similar outcomes. A random effects model was used to measure treatment effects for dichotomous outcomes. In sensitivity analyses, adjusted effect estimates and their standard errors were used for pooling studies in meta‐analyses and the generic inverse‐variance method was used whenever adjusted data were available.
Risks of bias of included studies were assessed using the Newcastle‐Ottawa Scale (NOS). Certainty of evidence was evaluated using GRADE recommendations by starting at “low certainty” for observational studies and upgrading based on a large magnitude of effect, lack of concern about confounders or a dose‐response gradient and downgrading based on imprecision, indirectness, inconsistency, and reporting bias (GRADE 2008).
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was used to explore possible sources of heterogeneity (e.g. participants, interventions and study quality including method of PD catheter insertion). Heterogeneity among participants could have been related to age and renal pathology (e.g. children versus adults). Heterogeneity in treatments could have been related to prior agent(s) used and the agent, dose, and duration of therapy (e.g. initial fill volume). Therefore, subgroup analysis was conducted to evaluate the source of heterogeneity according to:
-
Participants
-
Adult versus paediatric patients
-
Incident versus prevalent patients
-
-
Setting
-
Single‐centre versus multi‐centre
-
-
Type of treatment utilised
-
According to initial fill volume
-
Days to PD commencement (e.g. within 24 hours versus 7 days)
-
-
Methodological quality
Adverse effects were tabulated and assessed with descriptive techniques, as they were likely to be different for the various agents used. Where possible, the risk difference with 95% CI was calculated for each adverse effect, either compared to no treatment or to another agent.
Sensitivity analysis
We performed sensitivity analyses in order to explore the influence of the following factors on effect size.
-
Repeating the analysis excluding unpublished studies
-
Repeating the analysis taking account of risk of bias, as specified
-
Repeating the analysis excluding any very long or large studies to establish how much they dominate the results
-
Repeating the analysis excluding studies using the following filters: diagnostic criteria, language of publication, source of funding (industry versus other), and country.
Summary of findings and assessment of the certainty of the evidence
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We planned to present the following outcomes in the 'Summary of findings' tables.
-
Catheter‐related infectious complications within 30 and 90 days of commencement of dialysis
-
Bacteraemia
-
Peritonitis
-
Exit‐site or tunnel infections
-
-
Catheter‐related non‐infectious complications within 30 and 90 days of commencement
-
Exit‐site bleeding
-
Catheter malfunction
-
Catheter re‐adjustment
-
-
Technique survival
-
Home dialysis
-
Death (any cause)
-
Hospitalisation
Results
Description of studies
Results of the search
An electronic search (last search date for RCTs: 25 May 2020; non‐RCTs: 11 February 2020) identified 210 potentially relevant reports in total. After removing duplicates and screening through 196 titles and abstracts, 133 reports were excluded. Full text review was conducted of the remaining 63 records (43 studies). Seven studies (9 records) were included, 37 studies (50 records) and were excluded. Two ongoing studies (2 records) and two studies waiting classification (2 records) will be assessed in a future update of this review (Figure 1).
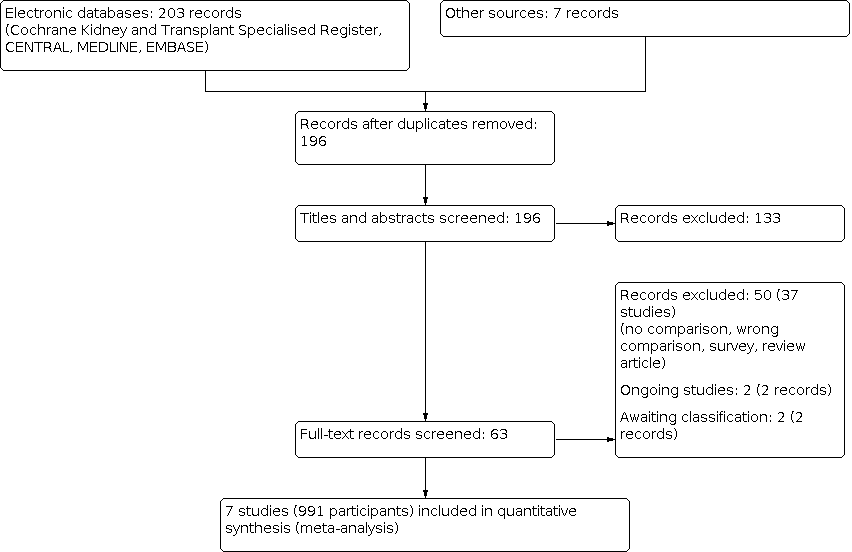
Study flow diagram.
Included studies
Seven studies (991 participants) were included in the review. Studies were conducted in the USA (Bhalla 2017; Ghaffari 2015; Wang 2017), China (Jin 2016), Brazil (Brabo 2018), France (Lobbedez 2008), and Germany (Koch 2012). There were four single‐centre retrospective cohort studies and three single‐centre prospective cohort studies (Table 1). Six out of seven studies compared the clinical outcomes between urgent‐start PD and HD using a CVC and one study (Brabo 2018) compared the cost of dialysis between these two modalities.
| Study | Country | Study design | Time frame | No. of participants | Follow‐up duration |
|---|---|---|---|---|---|
| USA | Retrospective cohort (SC) | 2011‐2014 | 419 | Not reported | |
| Brazil | Prospective cohort (SC) | Not reported | 40 | 6 months | |
| USA | Prospective cohort (SC) | 2010‐2013 | 124 | Average 810 days | |
| China | Retrospective cohort (SC) | 2013‐2014 | 178 | At least 30 days (up to Jan 2016) | |
| Germany | Retrospective cohort (SC) | 2005‐2010 | 123 | 6 months | |
| France | Prospective cohort study (SC) | 2004‐2006 | 60 | Till 31 December 2006 | |
| USA | Retrospective cohort (SC) | 2015‐2016 | 47 | HD: 60 months PD: 46 months |
SC: single‐centre; HD: haemodialysis; PD: peritoneal dialysis
Two studies (301 participants) examined peritonitis (Jin 2016; Koch 2012), two studies (301 participants) examined bacteraemia (Jin 2016; Koch 2012, one study (419 participants) examined the exit‐site infection (Bhalla 2017), one study (178 participants) examined the exit‐site bleeding (Jin 2016), two studies (597 participants) examined catheter malfunction (Bhalla 2017; Jin 2016), two studies (225 participants) examined catheter readjustment (Jin 2016; Wang 2017), one study (123 participants) examined technique survival (Koch 2012), five studies (820 participants) examined death (any cause) (Bhalla 2017; Brabo 2018; Jin 2016; Koch 2012; Lobbedez 2008), one study (123 participants) examined hospitalisation (Koch 2012), and one study (40 participants) examined the cost of dialysis (Brabo 2018).
Excluded studies
In total, 37 studies were excluded. The reasons for exclusion were: review articles; surveys; lack of control group for urgent‐start PD; wrong comparison; or wrong intervention (Figure 1).
Risk of bias in included studies
Risk of bias for all cohort studies is presented in Table 2.
| Study | Selection | Comparability | Outcome | Evidence of quality | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness | Selection of | Ascertainment | Outcomes | Assessment of outcome | Length of follow‐up | Adequacy of follow‐up | |||
| * | * | * | * | * | * | * | ‐‐ | 7 | |
| ‐‐ | ‐‐ | * | * | ‐‐ | ‐‐ | * | * | 4 | |
| * | * | ‐‐ | * | ‐ | ‐‐ | * | * | 5 | |
| * | * | * | * | * | * | * | ‐‐ | 7 | |
| * | * | * | * | * | * | * | ‐‐ | 7 | |
| * | * | * | * | * | * | * | ‐‐ | 7 | |
| ‐‐ | ‐‐ | * | * | ‐‐ | ‐‐ | * | ‐‐ | 3 | |
Allocation of star was based on NEWCASTLE ‐ OTTAWA Quality Assessment Scale (www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf)
Selection
Four included cohort studies (Bhalla 2017; Jin 2016; Koch 2012; Lobbedez 2008) met all criteria of the Newcastle‐Ottawa Scale (NOS) for selection including representativeness of exposed cohort, selection of non‐exposed cohort, ascertainment of exposure and outcomes of interest was not presented at the start of the study. The representativeness of cohort was unable to be assessed in two studies (Brabo 2018; Wang 2017), selection of non‐exposed cohort was unable to be assessed in two studies (Brabo 2018; Wang 2017) and ascertainment of exposure was unable to be assessed in two studies (Brabo 2018; Ghaffari 2015).
Comparability of groups of study
Four cohort studies (Bhalla 2017; Jin 2016; Koch 2012; Lobbedez 2008) met the comparability criteria of NOS. There was insufficient information to assess the comparability between groups for two included studies (Brabo 2018; Ghaffari 2015). One study was assessed as having a high risk of bias due to differences in baseline characteristics between PD and HD groups (Wang 2017).
Outcome
Three cohort studies (Bhalla 2017; Jin 2016; Koch 2012; Lobbedez 2008) met the two domains of outcome including assessment of outcome and length of follow‐up and another two studies (Brabo 2018; Ghaffari 2015) met the criteria of length of follow‐up and adequacy of follow‐up.
Effects of interventions
See: Summary of findings 1 Summary of findings
Infectious complications
Bacteraemia
Urgent‐start PD may reduce the incidence of catheter‐related bacteraemia compared with HD using CVC (Analysis 1.1 (2 studies, 301 participants): RR 0.13, 95% CI 0.04 to 0.41; I2 = 0%; low certainty evidence). This translated into 131 fewer bacteraemia episodes per 1000 (89 to 145 fewer). It was graded as low certainty of evidence. The evidence was downgraded due to the fact that included studies were observational studies (summary of findings Table 1). In a sensitivity analysis that included adjusted data, a similar result was observed (Analysis 1.2 (2 studies, 301 participants): RR 0.13, 95% CI 0.04 to 0.42, I2 = 0%). Ghaffari 2015 also reported a higher risk of catheter‐related bacteraemia in HD initiated with a CVC compared with urgent‐start PD (adjusted incidence risk ratio (IRR) 4.32; 95% CI 1.48 to 12.62).
Peritonitis
It is uncertain whether urgent‐start PD increases the risk of peritonitis compared to HD using a CVC, (Analysis 1.3 (2 studies, 301 participants): RR 1.78, 95% CI 0.23 to 13.62; I2 = 0%; very low certainty evidence). The certainty of evidence was graded as very low given non‐RCT designs and imprecision.
Exit‐site or tunnel infection
It is uncertain whether urgent‐start PD increases the incidence of exit‐site/tunnel infection (Analysis 1.4 (1 study, 419 participants): RR 3.99, 95% CI 1.32 to 12.05; very low certainty evidence) compared with HD initiated using a catheter. The remaining included studies did not report exit‐site/tunnel infection.
Non‐infectious complications
Exit‐site bleeding
It is uncertain whether urgent‐start PD reduces the risk of exit‐site bleeding (Analysis 1.5 (1 study, 178 participants): RR 0.12, 95% CI 0.01 to 2.33; very low certainty evidence) compared with HD initiated using a catheter. The remaining included studies did not report exit‐site bleeding
Catheter malfunction
It is uncertain whether urgent‐start PD reduces catheter malfunction compared with HD using a CVC (Analysis 1.6 (2 studies; 597 participants): RR 0.26, 95% CI 0.07 to 0.91; I2 = 66%; very low certainty evidence). The certainty of evidence was graded as very low as both studies were observational, imprecise, and inconsistent. The subgroup analysis was unable to be meaningfully performed given that a limited number of included studies.
Catheter re‐adjustment
It is uncertain whether urgent‐start PD reduces the risk of catheter re‐adjustment as compared with HD via CVC (Analysis 1.7 (2 studies, 225 participants): RR 0.13, 95% CI 0.00 to 18.61; I2 = 92%; very low certainty evidence). Further sub‐group or sensitivity analyses were unable to be performed meaningfully given that a limited number of studies reported this particular outcome.
Technique survival
It is uncertain whether urgent‐start PD increases technique survival compared with HD using CVC (Analysis 1.8 (1 study, 123 participants): RR 1.18, 95% CI 0.87 to 1.61; very low certainty evidence). The certainty of evidence was graded as very low given that only one non‐RCT was included in the review.
Death (any cause)
Urgent‐start PD had uncertain effect on death (any cause) compared with HD using CVC (Analysis 1.9 (5 studies, 820 participants): RR 0.68, 95% CI 0.44 to 1.07; I2 = 27% ; very low certainty evidence).In a sensitivity analysis that included adjusted data, a similar result was observed (Analysis 1.10 (5 studies, 820 participants); OR 0.64, 95% CI 0.36 to 1.15; I2 = 29%). Sensitivity analyses including all studies with a low risk of bias (Analysis 1.11 (4 studies, 780 participants): RR 0.70, 95% CI 0.44 to 1.12; I2 = 37%) or exclusion of the largest study showed similar results (Analysis 1.12 (4 studies, 401 participants): RR 0.80, 95% CI 0.55 to 1.17; I2 = 0%).
Hospitalisation
Three studies (Ghaffari 2015; Koch 2012; Lobbedez 2008) reported hospitalisation. Lobbedez 2008 reported that the duration of initial hospitalisation was similar between urgent‐start PD and HD groups (median: 16.5 versus 20 days; P = 0.16). The study also reported urgent‐start PD had similar survival free of re‐hospitalisation compared to the HD group (21% versus 36% at one year; P = 0.12). Koch 2012 also reported a similar incidence of re‐hospitalisation between urgent‐start PD and HD groups (Analysis 1.13 (1 study, 123 participants): RR 1.18, 95% CI 0.89 to 1.55). However, Ghaffari 2015 reported a higher adjusted rate of hospitalisation in the HD group compared with the urgent‐start PD group (1 study, 124 participants; adjusted IRR 1.43; 95% CI 1.11 to 1.85). The continuous scale of measurement of effect was unable to be used given the different methods of reporting the duration of hospitalisation between studies and the limited number of studies.
Cost of dialysis
Comparison of the cost of dialysis between urgent‐start PD and HD using a CVC was reported in Brabo 2018 who compared cost/patient over six months and reported similar cost (US$ 6091.7 ± 1289.4 versus 6209.1 ± 1600) between urgent‐start PD and HD using a CVC (Table 3).
| Study | Variables | USPD | USHD |
|---|---|---|---|
| Direct cost/patient over 6 months (US$) | 6092 ± 1289 | 6209 ± 1600 | |
| Dialysis access | 3.7% | 9.3% | |
| Dialysis service | 80.3% | 75.2% | |
| Hospitalisation | 0% | 2.1% | |
| Laboratory tests | 1.7% | 1.6% | |
| Treatment cost for infectious complications | 1.1% | 2.5% | |
| Medication | 9.6% | 12.3% |
USHD ‐ urgent‐start haemodialysis; USPD ‐ urgent‐start peritoneal dialysis
Quality of Life
None of the included studies reported QoL.
Home dialysis
None of the included studies reported the proportion of HD patients on home HD.
Pain/discomfort related to dialysis therapy
None of the included studies reported pain/discomfort related to dialysis therapy.
Adverse events
Jin 2016 reported that there was no catheter thrombosis in PD group but 6 (7.3%) catheter thrombosis in the HD group. There was no leakage or organ rupture in either the PD or HD groups.
Discussion
Summary of main results
The present review demonstrated that, in low certainty evidence, urgent‐start PD may reduce the incidence of catheter‐related bacteraemia compared with HD using a CVC. It is uncertain whether urgent‐start PD increases the risk of peritonitis compared with HD using a CVC. Urgent‐start PD has uncertain effects on exit‐site or tunnel infection, exit‐site bleeding, catheter malfunction, catheter re‐adjustment, technique or patient survival compared with HD using a CVC. There were two studies that reported a comparable risk of hospitalisation although one study reported a higher hospitalisation rate in the urgent‐start HD group compared with the urgent‐start PD group. One study comparing cost over six months reported comparable cost between the two modalities.
Overall completeness and applicability of evidence
The present review only included observational studies (no RCTs were identified) and the majority of included studies failed to adjust for important potential confounders including age, comorbidities such as presence of diabetes mellitus, cardiovascular disease, malignancy, baseline residual kidney function, aetiology of kidney failure, and urgency for dialysis initiation in the HD group who initiated using a CVC. Few studies adjusted for confounders, and the confounders adjusted were varied across studies. In addition, the total number or included studies was very small which resulted in imprecision and further reduced the certainty of evidence. Moreover, subgroup analyses with different break‐in periods, fill volume and insertion techniques in PD patients could not be performed due to insufficient number of studies. Last but not least, this systemic review used a broad definition of urgent‐start (within 14 days of catheter insertion) and was unable to separately identify patients who required to initiate dialysis acutely after catheter insertion compared to an earlier start of planned PD.
The current review included seven observational studies. However, only three studies compared the outcomes of bacteraemia between urgent‐start PD and HD initiated with a CVC. Though certainty of evidence was graded as low to very low due to the observational study design, the results indicated HD initiated using a CVC may increase the risk of catheter‐related bacteraemia compared with urgent‐start PD. This result is biologically plausible as using a CVC for dialysis access has been well established to be independently and strongly associated with bacteraemia in HD patients (Thomson 2007).
The outcome of peritonitis was examined in two observational studies, which found that urgent‐start PD has an uncertain effect on the risk of peritonitis compared with HD group. The event rates were very low in these two studies which resulted in imprecision. The follow‐up duration was also very short in one included study that only reported peritonitis in the first 30 days of dialysis. Future studies with reasonably long follow up durations in excess of one year are required to permit more confident conclusions to be drawn. It is uncertain whether urgent‐start PD increases exit‐site/tunnel infections compared with HD using a catheter as these events were only reported in one retrospective study.
Mechanical catheter complications were examined in the present review. Urgent‐start PD has uncertain effect on catheter malfunction compared with HD initiated using a CVC. The certainty of evidence was graded as very low due to observational study design, impression due to small events, and the presence of inconsistencies between studies. Further subgroup analysis was unable to be performed given the small number of included studies. The certainty of evidence for catheter re‐adjustment was graded as very low due to observational study design the presence of considerable heterogeneity and imprecision. No strong conclusion can be drawn regarding the mechanical complications between urgent‐start PD and HD group based on the current evidence.
Technique survival was compared in one study with relatively short follow‐up durations. Similarly, death (any cause) was reported in five studies with short follow‐up durations and suboptimal methodologic quality, thereby preventing any conclusions being drawn regarding the effect of urgent‐start PD as compared with HD initiated with a catheter on technique and patient survival. In addition, patients who required emergency dialysis were routinely initiated on dialysis using a CVC rather than urgent‐start PD, which potentially could have led to selection bias with sicker patients defaulted to initiate HD using a CVC. Future well‐designed studies with adequate follow up period are needed to permit the valid comparison of technique and patient survival between the two urgent‐start modalities.
Hospitalisation was reported in three observational studies. Lobbedez 2008 reported the length of initial hospitalisation and re‐hospitalisation‐free survival, Koch 2012 reported incidence of re‐hospitalisation, and Ghaffari 2015 reported adjusted hospitalisation rate. Though the first two studies reported similar results between the two forms of urgent‐start dialysis, the last study reported a higher risk of hospitalisation in patients initiating HD using a CVC compared with urgent‐start PD patients. Meta‐analysis was unable to be performed because of the different methods of outcome reporting.
Cost of dialysis was analysed in one study conducted in Brazil (Brabo 2018) over the initial six‐month period and reported similar cost between the two urgent‐start dialysis modalities.
Quality of the evidence
All included studies were observational, of which four were single‐centre retrospective cohorts and three were single‐centre prospective cohorts. Since all included studies were non‐RCTs, the certainty of evidence was graded as low to very low. The number of studies in this area was also relatively small, which further downgraded the certainty of evidence.
The outcome bacteraemia was graded as low certainty of evidence. The evidence was downgraded due to its inclusion of only non‐RCT studies. The research provides some degree of plausible effect because the CVC enters directly into a blood vessel, thereby increasing the probability of bacteraemia in the setting of catheter‐related infection in urgent‐start HD in contrast to PD catheter which is inserted into a peritoneal cavity without direct exposure to the circulatory system.
Most of the included studies did not adjust for the potential confounders in their analyses. Most of the outcomes analysed in the present review used unadjusted data which reduced the certainty of the evidence.
Potential biases in the review process
The review process involved obtaining a comprehensive review of available publications through MEDLINE, EMBASE, and CENTRAL electronic search with the help of an information specialist. Two authors performed data extraction, data analysis and assessment of study quality independently and any discrepancies were resolved by discussion with additional two authors. The additional data from previous publications were obtained by contacting the corresponding authors. There were only few included studies adjusted for potential confounders, and confounders adjusted were different across studies. There might be potential bias of combined studies that adjusted for different confounders or with no adjustment at all.
Agreements and disagreements with other studies or reviews
This is the first systematic review performed to compare the benefits and harms between urgent‐start PD and HD initiated using a CVC.

Study flow diagram.

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 1: Bacteraemia

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 2: Bacteraemia (adjusted data)

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 3: Peritonitis

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 4: Exit‐site or tunnel infection

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 5: Exit‐site bleeding

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 6: Catheter malfunction

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 7: Catheter readjustment

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 8: Technique survival

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 9: Death (any cause)

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 10: Death (any cause): adjusted data

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 11: Death (any cause): studies with low risk of bias

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 12: Death (any cause): sensitivity analysis (excluding large studies)

Comparison 1: Urgent‐start PD versus urgent‐start HD, Outcome 13: Hospitalisation
| Urgent‐start peritoneal dialysis versus haemodialysis initiated with a catheter for patients with chronic kidney disease | |||||
| Patient or population: people with CKD Settings: community Intervention: USPD Comparison: HD initiated with a central venous catheter | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect | No. of participants | Quality of the evidence | |
|---|---|---|---|---|---|
| Risk with USHD | Risk with USPD | ||||
| Bacteraemia up to 6 months | 151 per 1,000 | 20 per 1,000 (6 to 62) | RR 0.13 (0.04 to 0.41) | 301 (2) | ⊕⊕⊝⊝ |
| Peritonitis up to 6 months | 7 per 1,000 | 13 per 1,000 (2 to 98) | RR 1.78 (0.23 to 13.62) | 301 (2) | ⊕⊝⊝⊝ |
| Exit‐site or tunnel infection | 18 per 1,000 | 71 per 1,000 (24 to 216) | RR 3.99 (1.32 to 12.05) | 419 (1) | ⊕⊝⊝⊝ |
| Exit‐site bleeding | 37 per 1,000 | 4 per 1,000 (0 to 85) | RR 0.12 (0.01 to 2.33) | 178 (1) | ⊕⊝⊝⊝ |
| Catheter malfunction | 151 per 1,000 | 39 per 1,000 (11 to 137) | RR 0.26 (0.07 to 0.91) | 597 (2) | ⊕⊝⊝⊝ |
| Catheter re‐adjustment up to 60 months | 373 per 1,000 | 48 per 1,000 (0 to 1,000) | RR 0.13 (0.00 to 18.61) | 225 (2) | ⊕⊝⊝⊝ |
| Technique survival up to 6 months | 526 per 1,000 | 621 per 1,000 (458 to 847) | RR 1.18 (0.87 to 1.61) | 123 (1) | ⊕⊝⊝⊝ |
| Home dialysis | ‐ | ‐ | ‐ | No studies | ABSENT |
| Death (any cause) up to 24 months | 204 per 1000 | 139 per 1,000 (90 to 218) | RR 0.68 (0.44 to 1.07) | 820 (5) | ⊕⊝⊝⊝ |
| Hospitalisation up to 6 months | 579 per 1,000 | 683 per 1,000 (515 to 897) | RR 1.18 (0.89 to 1.55) | 123 (1) | ⊕⊝⊝⊝ |
| *The risk in the USPD group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio; HD: haemodialysis; USHD: urgent‐start HD; USPD: urgent‐start peritoneal dialysis | |||||
| GRADE Working Group grades of evidence | |||||
| 1downgraded for observational studies 2 downgraded for observational studies, imprecision due to small number of events 3 downgraded for observational studies and imprecision due to small number of events, and inconsistency | |||||
| Study | Country | Study design | Time frame | No. of participants | Follow‐up duration |
|---|---|---|---|---|---|
| USA | Retrospective cohort (SC) | 2011‐2014 | 419 | Not reported | |
| Brazil | Prospective cohort (SC) | Not reported | 40 | 6 months | |
| USA | Prospective cohort (SC) | 2010‐2013 | 124 | Average 810 days | |
| China | Retrospective cohort (SC) | 2013‐2014 | 178 | At least 30 days (up to Jan 2016) | |
| Germany | Retrospective cohort (SC) | 2005‐2010 | 123 | 6 months | |
| France | Prospective cohort study (SC) | 2004‐2006 | 60 | Till 31 December 2006 | |
| USA | Retrospective cohort (SC) | 2015‐2016 | 47 | HD: 60 months PD: 46 months | |
| SC: single‐centre; HD: haemodialysis; PD: peritoneal dialysis | |||||
| Study | Selection | Comparability | Outcome | Evidence of quality | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness | Selection of | Ascertainment | Outcomes | Assessment of outcome | Length of follow‐up | Adequacy of follow‐up | |||
| * | * | * | * | * | * | * | ‐‐ | 7 | |
| ‐‐ | ‐‐ | * | * | ‐‐ | ‐‐ | * | * | 4 | |
| * | * | ‐‐ | * | ‐ | ‐‐ | * | * | 5 | |
| * | * | * | * | * | * | * | ‐‐ | 7 | |
| * | * | * | * | * | * | * | ‐‐ | 7 | |
| * | * | * | * | * | * | * | ‐‐ | 7 | |
| ‐‐ | ‐‐ | * | * | ‐‐ | ‐‐ | * | ‐‐ | 3 | |
| Allocation of star was based on NEWCASTLE ‐ OTTAWA Quality Assessment Scale (www.ohri.ca/programs/clinical_epidemiology/nosgen.pdf) | |||||||||
| Study | Variables | USPD | USHD |
|---|---|---|---|
| Direct cost/patient over 6 months (US$) | 6092 ± 1289 | 6209 ± 1600 | |
| Dialysis access | 3.7% | 9.3% | |
| Dialysis service | 80.3% | 75.2% | |
| Hospitalisation | 0% | 2.1% | |
| Laboratory tests | 1.7% | 1.6% | |
| Treatment cost for infectious complications | 1.1% | 2.5% | |
| Medication | 9.6% | 12.3% | |
| USHD ‐ urgent‐start haemodialysis; USPD ‐ urgent‐start peritoneal dialysis | |||
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Bacteraemia Show forest plot | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.04, 0.41] |
| 1.2 Bacteraemia (adjusted data) Show forest plot | 2 | Risk Ratio (IV, Random, 95% CI) | 0.13 [0.04, 0.42] | |
| 1.3 Peritonitis Show forest plot | 2 | 301 | Risk Ratio (M‐H, Random, 95% CI) | 1.78 [0.23, 13.62] |
| 1.4 Exit‐site or tunnel infection Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.5 Exit‐site bleeding Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.6 Catheter malfunction Show forest plot | 2 | 597 | Risk Ratio (M‐H, Random, 95% CI) | 0.26 [0.07, 0.91] |
| 1.7 Catheter readjustment Show forest plot | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.00, 18.61] |
| 1.8 Technique survival Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 1.9 Death (any cause) Show forest plot | 5 | 820 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.44, 1.07] |
| 1.10 Death (any cause): adjusted data Show forest plot | 5 | Odds Ratio (IV, Random, 95% CI) | 0.64 [0.36, 1.15] | |
| 1.11 Death (any cause): studies with low risk of bias Show forest plot | 4 | 780 | Risk Ratio (M‐H, Random, 95% CI) | 0.70 [0.44, 1.12] |
| 1.12 Death (any cause): sensitivity analysis (excluding large studies) Show forest plot | 4 | 401 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.17] |
| 1.13 Hospitalisation Show forest plot | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |

