Uso de torniquetes para la cirugía de reemplazo de rodilla
Referencias
Referencias de los estudios incluidos en esta revisión
Referencias de los estudios excluidos de esta revisión
Referencias de los estudios en curso
Referencias adicionales
Referencias de otras versiones publicadas de esta revisión
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 2 years Study design: single‐centre randomised controlled trial | |
| Participants | 80 participants in total Male:Female: 17:23; 15:25 Age, years (range): 72 (65 to 80); 74 (64 to 82) Inclusion criteria: non‐diabetic patients who had no previous knee surgery; normal neurovascular supply to the leg (proved by Doppler) Duration of illness: unspecified | |
| Interventions | Group A (n = 50): total knee replacement surgery performed with a tourniquet Group B (n = 50): total knee replacement surgery performed without a tourniquet All operations were performed under general anaesthesia by one surgeon. For all patients, cefuroxime 1.5 g was given intravenously at the time of induction of anaesthesia and two further doses of 750 mg were given postoperatively. Anticoagulant prophylaxis was with Fragmin, started 2 hours preoperatively and continued postoperatively until the patient was fully mobile. A pneumatic tourniquet was placed around the thigh in both groups but was inflated only for patients in group A. The limb was first exsanguinated by elevation for 2 minutes, and the tourniquet was inflated to twice the systolic blood pressure (in group A) | |
| Outcomes |
| |
| Identification | Contact information: A Abdel‐Salem, Consultant orthopaedic surgeon, George Elliot Hospital NHS Trust, Nuneaton, CV10 7DJ | |
| Notes | Country: UK Language: English Study author contacted: no contact details given Trial registry record or protocol available: none found Funding source/declaration of interest: none reported Adverse events: In group A: 5 patients had wound infection; 3 patients had confirmed venous thrombosis In group B: no adverse events were reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Card system |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | HSS, pain, analgesia consumption Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition; no CONSORT diagram |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement; no protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: up to 24 hours post surgery Study design: single‐centre randomised controlled trial | |
| Participants | 20 participants in total Male:Female: 3:10; 4:6 Age, years (SD): 70(8); 68 (4.5) BMI, years (SD): 27.9; 27.3 Inclusion criteria: patients undergoing total knee replacement surgery for osteoarthritis Exclusion criteria: disturbances of coagulation, history of deep vein thrombosis, previous surgery of the knee, neoplastic disease, inflammatory disease, had received anticoagulant therapy or drugs that affected the haemostatic system during the last 2 weeks Duration of illness: unspecified | |
| Interventions | Group I (n = 10): total knee replacement surgery performed with a tourniquet Group II (n = 10): total knee replacement surgery performed without a tourniquet Anaesthetic techniques were standardised. All patients received subarachnoid spinal anaesthesia by injection of 4 mL of 0.5% bupivacaine at the L2‐L3 interspace approximately 1 hour before surgery and were sedated with midazolam and fentanyl intravenously. Ringer’s lactate solution was infused as needed to maintain haernodynamic stability. Patients did not receive blood transfusions during the observation period. Autologous blood was transfused postoperatively as needed after the study was completed. Unilateral primary cemented total knee replacements (M.B.K. prosthesis, Zimmer, Warsaw, IN, USA) were performed on all patients by the same surgeon at approximately the same time of the morning. Patients were assigned randomly to either Group I or Group II. Group I comprised 10 patients who underwent total knee replacement with a tourniquet inflated at the root of the limb. Group II consisted of 10 patients who underwent total knee replacement without the tourniquet. Before the surgical incision was begun in patients in Group I, the limb was exsanguinated with an elastic bandage and the tourniquet inflated at the pressure of 0.8 bar | |
| Outcomes |
| |
| Identification | Contact information: P Aglietti, MD, Second Orthopaedic Clinic, Largo P. Palagi 1, 50139, Florence, Italy | |
| Notes | Country: Italy Language: English Study author contacted: yes, however, received no reply Trial registry record or protocol available: none found Funding source/declaration of interest: none reported Adverse events: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 3 months Study design: single‐centre randomised controlled trial | |
| Participants | 81 participants in total Male:Female: 18:20; 22:21 Age, years (SD): 68 (7.4); 69.7 (6.4) BMI (SD): 28.6 (3.4); 27.9 (3.5) Inclusion criteria: patients between 50 and 80 years of age undergoing total knee replacement surgery for treatment of primary osteoarthritis Exclusion criteria: revision surgery, valgus deformity > 30°, 1‐stage bilateral procedures, rheumatoid arthritis, BMI > 35 Duration of illness: unspecified | |
| Interventions | Group A (n = 38): total knee replacement surgery performed with a tourniquet Group B (n = 41): total knee replacement surgery performed without a tourniquet One group underwent surgery with a tourniquet (34 in., single bladder, dual port, Zimmer) around the thigh that applied pressure of 300 mmHg; the other group underwent surgery without a tourniquet. No femoral nerve block was used. A standard medial parapatellar incision was used | |
| Outcomes | Primary outcome Active range of motion (AROM) in the knee is measured before surgery, at day 3, and at 3‐month control with a goniometer, with the patient lying supine Secondary outcomes:
| |
| Identification | Contact information: Staffan Eriksson, Centre for Clinical Research Sormland, Uppsala University, Kungsgatan 1, 531 88 Eskilstuna, Sweden, [email protected] | |
| Notes | Country: Sweden Language: English Study author contacted: no Trial registry record or protocol available: clinical trial number ISRCTN85166072 Funding source/declaration of interest: none reported Adverse events: In non‐tourniquet group: 1 patient had a urinary tract infection In tourniquet group: 2 patients had a wound infection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Computer‐generated random numbers table |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that a surgeon would alter his or her performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: TUG, VAS, and OKS Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: swelling, quadriceps function, and gait speed Outcome assessors were blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 3 months Study design: single‐centre randomised controlled trial | |
| Participants | 70 participants randomised | |
| Interventions | Intervention: | |
| Outcomes |
| |
| Identification | Contact information: Mehmet Demirel, MD, Department of Orthopaedics and Traumatology, Istanbul University, Istanbul School of Medicine, Istanbul, Turkey, [email protected] | |
| Notes | Country: Turkey | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; surgeons not blinded |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Participants were blinded; therefore low risk of bias Self‐reported outcomes: KSS, pain |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors are blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources identified |
| Study characteristics | ||
| Methods | Three groups: surgery without a tourniquet; surgery with a tourniquet at low pressure (225 mmHg); surgery with a tourniquet at high pressure (350 mmHg) Follow‐up: 7 days Study design: single‐centre randomised controlled trial | |
| Participants | 31 participants in total Male:Female: not reported Age, years (SD): not reported BMI (SD): not reported 31 participants Inclusion criteria: patients undergoing total knee replacement surgery Exclusion criteria: patients with non‐osteoarthritic disease, previous open knee surgery, systemic or local hypoxia, receiving anticoagulant or antiplatelet agents or steroid, with significant varus or valgus deformity or preoperative lateral release Duration of illness: unspecified | |
| Interventions | Group A: surgery without a tourniquet (n = 10) Group B: surgery with a tourniquet at low pressure (225 mmHg) (n = 10) Group C: surgery with a tourniquet at high pressure (350 mmHg) (n = 11) A standard protocol was followed utilising a tourniquet 11.5 cm wide, with an effective pressurising width of 9 cm (DePuy UK Ltd, Leeds, UK), with exsanguination in extension via a Rhys‐Davies device where appropriate. All patients had general (non‐halothane) anaesthesia, a midline skin incision, a medial parapatellar approach, insertion of a cemented Insall‐Burnstein II TKR (Zimmer, Warsaw, IN, USA), and skin closure using continuous Vicryl (Ethicon Ltd, Somerville, NJ, USA) over a single drain. All were mobilised on the second postoperative day. No patient received thromboembolic prophylaxis or used a continuous passive motion machine | |
| Outcomes |
| |
| Identification | Contact information: Mr M.T. Clarke, Box 37, Orthopaedic Surgery, Addenbrookes Hospital, Cambridge, CB2 2QQ | |
| Notes | Country: UK Language: English Study author contacted: yes, no reply from author. Trial registry record or protocol available: none reported Funding source/declaration of interest: funded by grants from the Wishbone Trust and from the research and development fund of West Suffolk Hospital Adverse events: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors are blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | 2 groups: surgery performed with a tourniquet inflated; surgery performed without a tourniquet inflated | |
| Participants | 129 participants randomised | |
| Interventions | Patients in both groups were anaesthetised by general anaesthesia. General anaesthesia induction drugs included midazolam 0.5 mg/kg, propofol 1.5 mg/kg, sulfentanyl 0.5 μg/kg, and rocuronium 0.8 mg/kg by intravenous bolus injection. Intermittent injection of rocuronium 10 mg per 40 to 60 minutes, continuous intravenous infusion of remifentanil 0.1 to 0.3 μg/kg/min, and propofol 2 to 4 mg/kg/h; continuous inhalation of sevoflurane was used for anaesthesia maintenance. All patients underwent TKA via a standardised technique and process | |
| Outcomes |
| |
| Identification | Contact information: Jun Dong, [email protected], Department of Anesthesiology, The First Affiliated Hospital of Chongqing Medical University, No. 1 Youyi Road, Yuzhong District, Chongqing, China | |
| Notes | Country: China | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Insufficient information to permit judgement Self‐reported outcomes: pain, Montreal Cognitive Assessment Scale |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Insufficient information to permit judgement Assessor‐reported outcomes: range of motion, blood loss, thigh swelling, serum creatinine, GFR, CRP |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 12 months Study design: single‐centre randomised controlled trial | |
| Participants | 64 participants in total Male:Female: 18:15; 17:14 Age, years (SD): 68 (8.4); 68 (7.8) BMI (SD): 25 (2.0); 25 (2.5) Inclusion criteria: patients aged 50‐85 undergoing an elective unilateral total knee replacement because of arthritis Exclusion criteria: rheumatoid arthritis, peripheral vascular disease, diabetes, prior knee surgery, use of anticoagulant medication, BMI > 35 Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 33) Group B: surgery performed without a tourniquet (n = 31) All procedures were standardised with regard to preoperative tranexamic acid, spinal anaesthesia, postoperative pain treatment, and rehabilitation regimen. Before surgery, tranexamic acid (1 g) was administered orally, and cefuroxime (1.5 g) was administered intravenously immediately before skin incision. In addition, tranexamic acid (0.5 g) was given 3 hours after surgery, and cefuroxime (750 mg) was given 6 and 12 hours postoperatively. Thrombosis prophylaxis was achieved with the use of rivaroxaban (10 mg/d) throughout the hospital stay. Both groups had an appropriately sized thigh tourniquet applied, but it was inflated only in the Tq group; in the non‐Tq group, it was placed on the thigh but was not inflated, thereby serving as a safety device in case of uncontrollable bleeding. In the Tq group, limb exsanguination was done by elevation for 2 minutes, and the cuff was inflated to 250 mmHg | |
| Outcomes |
| |
| Identification | Contact information: Ashir Ejaz, Department of Orthopaedic Surgery, Aslborg University Hospital, Aalborg, Denmark, [email protected] No source of funding identified | |
| Notes | Country: Denmark Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: clinical trials number NCT1209035 Funding source/declaration of interest: none reported Adverse events: In the tourniquet group: 2 patients had confirmed DVT, and 2 patients required further operations on the index knee In the non‐tourniquet group: 1 patient had confirmed DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: KOOS, pain, analgesia consumption, DVT Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across interventions |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 5 hours Study design: single‐centre randomised controlled trial | |
| Participants | 62 participants in total Male:Female: 16:15; 17:14 Age, years (SD): 68.3 (8.4); 68.2 (7.8) BMI (SD): 25.1(2.0); 25.2 (2.5) Inclusion criteria: patients aged 50 to 85 undergoing elective unilateral cemented total knee replacement because of arthritis Exclusion criteria: rheumatoid arthritis, peripheral vascular disease, diabetes, prior knee surgery, use of anticoagulant medication, BMI > 35 Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 31) Group B: surgery performed without a tourniquet (n = 31) All procedures were standardised with regard to preoperative tranexamic acid, spinal anaesthesia, postoperative pain treatment, and rehabilitation regimen. Before surgery, tranexamic acid (1 g) was administered orally and cefuroxime (1.5 g) was administered intravenously immediately before skin incision. In addition, tranexamic acid (0.5 g) was given 3 hours after surgery, and cefuroxime (750 mg) was given 6 and 12 hours postoperatively. Thrombosis prophylaxis was achieved with the use of rivaroxaban (10 mg/d) throughout the hospital stay. Both groups had an appropriately sized thigh tourniquet applied, but it was inflated only in the Tq group. In the non‐Tq group, it was placed on the thigh but was not inflated, thereby serving as a safety device in case of uncontrollable bleeding. In the Tq group, limb exsanguination was done by elevation for 2 minutes and the cuff was inflated to 250 mmHg | |
| Outcomes |
| |
| Identification | Contact information: Ashir Ejaz, Department of Orthopaedic Surgery, Aslborg University Hospital, Aalborg, Denmark, [email protected] No source of funding identified | |
| Notes | Country: Denmark Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: clinical trials number NCT1209035 Funding source/declaration of interest: none reported Adverse events: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 24 months Study design: single‐centre randomised controlled trial | |
| Participants | 57 participants in total Male:Female: 13:16; 15:13 Age, years (SD): 68.3 (8.0); 68.2 (7.8) BMI (SD): 25.1 (2.0); 25.2 (2.5) Inclusion criteria: patients aged 50 to 85 undergoing elective unilateral total knee replacement because of arthritis Exclusion criteria: rheumatoid arthritis, peripheral vascular disease, diabetes, prior knee surgery, use of anticoagulant medication, BMI > 35 Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 29) Group B: surgery performed without a tourniquet (n = 28) All procedures were standardised with regard to preoperative tranexamic acid, spinal anaesthesia, postoperative pain treatment, and rehabilitation regimen. Before surgery, tranexamic acid (1 g) was administered orally, and cefuroxime (1.5 g) was administered intravenously immediately before skin incision. In addition, tranexamic acid (0.5 g) was given 3 hours after surgery, and cefuroxime (750 mg) was given 6 and 12 hours postoperatively. Thrombosis prophylaxis was achieved with the use of rivaroxaban (10 mg/d) throughout the hospital stay. Both groups had an appropriately sized thigh tourniquet applied, but it was inflated only in the Tq group. In the non‐Tq group, it was placed on the thigh but was not inflated, thereby serving as a safety device in case of uncontrollable bleeding. In the Tq group, limb exsanguination was done by elevation for 2 minutes and the cuff was inflated to 250 mmHg | |
| Outcomes |
| |
| Identification | Contact information: Ashir Ejaz, Department of Orthopaedic Surgery, Aslborg University Hospital, Aalborg, Denmark, [email protected] No source of funding identified | |
| Notes | Country: Denmark Language: English Study author contacted: yes, no reply from author. Trial registry record or protocol available: clinical trials number NCT1209035 Funding source/declaration of interest: none reported Adverse events: none reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Assessor‐reported outcomes: RSA analysis Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | 2 groups: surgery with a tourniquet; surgery performed without a tourniquet | |
| Participants | 200 participants randomised Duration of illness: unspecified | |
| Interventions | In the tourniquet group, surgery was performed with a tourniquet inflated at 225 mmHg or 300 mmHg depending on surgeon preference. In the non‐tourniquet group, surgery was performed without a tourniquet | |
| Outcomes |
| |
| Identification | Contact information: Rahul Goel, Department of Orthopaedic Surgery, Emory University, Atlanta, Georgia, USA | |
| Notes | Country: USA 1 person in the tourniquet group had a wound complication requiring antibiotics 1 patient in each group had postoperative wound blistering 1 patient in the non‐tourniquet group developed symptomatic DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number generator |
| Allocation concealment (selection bias) | Low risk | Envelopes with allocation opened before incision |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; surgeons not blinded |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: pain, satisfaction, SF‐12, KOOS Patients blinded |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Outcome assessors blinded Assessor‐reported scores: blood loss, duration of surgery, range of motion, stair climb test, time to up and go |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 48 hours Study design: single‐centre randomised controlled trial | |
| Participants | 64 participants in total Male:Female: 17:15; 18:14 Age, years (SD): 68 (8.0); 66 (8) BMI (SD): 27.4; 28.4 Inclusion criteria: ASA I to III, able to understand given information, 45 to 85 years of age Exclusion criteria: previous major knee surgery to the same knee, preoperative inability to flex the knee > 90 degrees, rheumatoid arthritis, allergy to any of the drugs used in the study Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 32) Group B: surgery performed without a tourniquet (n = 32) As premedication, all patients received oral celecoxib 400 mg and acetaminophen 1 g; thereafter 12‐hourly (celecoxib 200 mg) and 6‐hourly (acetaminophen 1 g). No subjects received an indwelling urinary catheter, and no drains were used. A low‐volume fluid regimen was used with 2000 mL of Ringer's solution during the first 24 hours. All subjects were given 1 g of tranexamic acid i.v. Oxycodone 5 mg i.v. was used as postoperative rescue pain medication. No femoral nerve blocks were used. All patients were anaesthetized using intrathecal administration of hyperbaric bupivacaine 0.5%, 3 mL. An infusion of propofol 10 mg mL−1 was given to induce light sedation during surgery. All patients breathed spontaneously with supplemental oxygen 2 L min−1 | |
| Outcomes |
| |
| Identification | A. Harsten, Department of Anaesthesiology, Hassleholm Hospital, Box 351, 281 25 Hassleholm, Sweden, telephone +46451298848, [email protected] | |
| Notes | Country: Sweden Language: English Study author contacted: author not contacted Trial registry record or protocol available: clinical trials number NCT01808859 Funding source/declaration of interest: study was supported by institutional grants Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | High risk | Patients and surgeons not blinded |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | High risk | Self‐reported outcomes: pain and nausea Patients not blinded; therefore high risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: knee extension strength, swelling, duration of surgery Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Three groups: surgery with a tourniquet as well as multiple doses of intravenous tranexamic acid (TXA); surgery with no tourniquet and multiple doses of TXA; surgery with a tourniquet and no TXA Follow‐up: 6 months Study design: single‐centre randomised controlled trial | |
| Participants | 150 participants in total Male:Female: 18:32; 16:34 Age, years (SD): 66.2 (8.3); 65.1 (6.8) BMI (SD): 25.1 (1.5); 24.4 (1.5) Inclusion criteria: patients older than 18 scheduled for primary total knee arthroplasty for end stage‐osteoarthritis Exclusion criteria: revision procedures, bilateral procedures, previous knee surgery, flexion deformity > 30 degrees, anaemia, contraindication for TXA, ASA grade IV, coagulation disorder Duration of illness: unspecified | |
| Interventions | Group A: surgery with a tourniquet and multiple doses of TXA (n = 50) Group B: surgery without a tourniquet and multiple doses of TXA (n = 50) Group C: surgery with a tourniquet and no TXA (n = 50) A surgeon‐selected cemented posterior‐stabilised prosthetic design was used. Vacuum wound drainage was used for every patient and was removed the next morning. An intraoperative periarticular injection of ropivacaine and postoperative oral diclofenac sodium (Voltaren; 50 mg twice daily) were administered for pain. On the day of the surgery and 3 times daily thereafter until hospital discharge, all patients were evaluated by a physical therapist and began walking with partial weight‐bearing and wearing a knee brace to protect the surgical site | |
| Outcomes |
| |
| Identification | Contact information: ZeYu Huang, MD, PhD, Department of Orthopaedic Surgery, West China Hospital, West China Medical School, Sichuan University, Cheng Du, Sichuan Province, People's Republic of China | |
| Notes | Country: China Language: English Study author contacted: no Trial registry record or protocol available: registered on Chinese clinical trials registry ChiCTR‐INR‐16009762 Funding source/declaration of interest: none reported Adverse events: Group A: 1 patient had a superficial wound infection Group B: no adverse events were reported Group C: 3 patients developed a superficial wound infection and 3 patients reported blistering We reported groups A and B in our analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded; surgeons blinded only until the morning of the operation However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: HSS score, patient satisfaction, DVT, PE Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: duration of surgery, intraoperative blood loss, total blood loss, transfusion rate, length of hospital stay, swelling Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 60 minutes postoperatively Study design: single‐centre randomised controlled trial | |
| Participants | 34 participants in total Male:Female: 8:15; 8:15 Age, years (SD): 70.6 (7); 70.6 (6) BMI (SD): 32.1 (5); 33.8 (5) Inclusion criteria: 55 to 85 years of age, osteoarthritis of the knee joint (degree III or IV), physical status (ASA I or II), BMI < 45, able to provide written informed consent Exclusion criteria: < 55 or > 85 years of age, osteoarthritis of the knee joint (degree I or II), ASA physical status III or IV, BMI > 45, unable to provide written consent, malignant disease, rheumatoid disease, infectious disease, coagulation disorder, history of deep vein thrombosis or pulmonary embolism, peripheral arterial disease, immune deficiency, medication (glucocorticoid, aspirin, heparin, cumarin, warfarin), neurological dysfunction, liver insufficiency, coronary heart disease, immobility Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 17) Group B: surgery performed without a tourniquet (n = 17) After accomplishment of the anaesthesia, a pneumatic tourniquet was applied at the proximal third of the thigh. Thereafter, a standard sterilisation procedure was performed. The tourniquet was inflated (380 mmHg) immediately before the skin incision. The standard medial parapatellar approach was performed in all cases. For each of these groups, 2 biopsies were taken from the vastus medialis. The first muscle biopsy was obtained immediately after surgery was performed; this was followed by the second muscle biopsy exactly 60 minutes later (before tourniquet deflation). Biopsy volume was set to be 5 × 5 × 5 mm (125 mm³) with distance between biopsies > 10 mm. Muscle extracts were frozen in liquid nitrogen until further analyses | |
| Outcomes | Primary outcome: Measurement of intracellular proteolytic activity: the total ubiquitination, as a result of total ubiquitin‐protein ligase activity (tUbPL), was determined as biotinylated ubiquitin incorporation into the sum of the cytosolic proteins. The ubiquitination was expressed in katal, which is defined as 1 mol biotinylated ubiquitin incorporated into cytosolic proteins per second Secondary outcomes:
| |
| Identification | Contact information: Ahmed Jawhar, Department of Orthopaedics and Trauma Surgery, University Medical Centre Mannheim of University Heidelberg, Theodor‐Kutzer‐Ufer 1‐3, 68167 Mannheim, Germany, [email protected] No conflicts of interest | |
| Notes | Country: Germany Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: clinical trials ID NCT02475603 Funding source/declaration of interest: no sources of funding stated Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | Outcomes in registration/protocol not reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | 2 groups: surgery performed with a tourniquet; surgery performed without a tourniquet | |
| Participants | 99 participants included in this study | |
| Interventions | All TKAs were performed at the Department of Orthopaedics and Trauma Surgery according to institutional standard operating procedure. Medial parapatellar approach and femur first surgical technique were performed to implant a cemented prosthesis design (SmartSet Bone cement, DePuy Synthes, Warsaw, IN, USA) (PFC® SIGMA®). After introduction of anaesthesia, a pneumatic tourniquet (Balbina™, Ulrich Medical, Ulm, Germany) was placed on the proximal thigh and was inflated only in the tourniquet group to 360 mmHg, immediately before skin incision. The tourniquet was released on completion of wound closure and after application of an elastic–compressive bandage. In the non‐tourniquet group, the same surgery was performed without a tourniquet | |
| Outcomes |
| |
| Identification | Ahmed Jawhar, [email protected], Department of Orthopaedics and Trauma Surgery, University Medical Center Mannheim of University Heidelberg, Theodor‐Kutzer‐Ufer 1‐3, 68167 Mannheim, Germany | |
| Notes | Country: Germany | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources identified |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: 2 days Study design: single‐centre randomised controlled trial | |
| Participants | 30 participants in total Male:Female: 7:9; 4:10 Age, years (range): 69 (52 to 89); 64 (46 to 86) BMI (SD): not reported Inclusion criteria: patients undergoing primary cemented TKR Exclusion criteria: patients younger than 18 years, recent (< 6 months) myocardial infarction, unstable angina, severe aortic or mitral valve stenosis, previous stroke, unmedicated hypertension, treatment with beta‐antagonist or anticoagulant Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with epidural anaesthesia without a tourniquet (n = 14) Group B: surgery performed with spinal anaesthesia and a tourniquet (n = 16) In all cases, the leg planned for operation was exsanguinated with an Esmarch bandage before the tourniquet was inflated around the upper femur; tourniquet inflation pressure was maintained at 350 to 400 mmHg during the operation. At the end of surgery, the surgeon deflated the tourniquet to enable establishment of haemostasis; during surgery, sedation was adjusted to a level where communication was possible. Oxygen was delivered at 3L/min on a nasal catheter. All patients had urine output monitored via a urinary bladder catheter | |
| Outcomes |
| |
| Identification | Contact information: Palle Juelsgaard, MD, Tokkerbakken 20, DK‐8240, Risskov, Denmark, [email protected] No source of funding stated | |
| Notes | Country: Denmark Language: English Study author contacted: not contacted Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: no sources of funding stated Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded; insufficient information on whether surgeons were blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | High risk | Had potential source of bias related to specific study design (different methods of anaesthesia) |
| Study characteristics | ||
| Methods | Two groups: surgery with a tourniquet; surgery without a tourniquet Follow‐up: no follow‐up beyond operation Study design: single‐centre randomised controlled trial | |
| Participants | 46 participants in total Male:Female: 2:20; 2:23 Age, years (SD): 65 (10); 63 (8) BMI (SD): not reported Inclusion criteria: patients due to undergo primary total knee replacement surgery Exclusion criteria: none stated Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 22) Group B: surgery performed without a tourniquet (n = 24) Anaesthesia was induced with intravenous propofol (1.5 mg/kg) and fentanyl citrate (0.1 mg). Tracheal intubation was facilitated with vecuronium bromide (0.1 mg/kg); anaesthesia was maintained with 66% nitrous oxide in 33% oxygen and sevoflurane. During the operation, all patients were ventilated with a tidal volume of 10 mL/kg and had a respiratory rate of 10 breaths/min. In the tourniquet group, the involved limb was exsanguinated by elevation and an Esmarch bandage, after which a pneumatic thigh tourniquet was applied to the limb and was inflated to pressure of 350 mmHg. No tourniquet was applied to the legs of control patients. The same surgeon performed all procedures; a similar surgical technique was used in each patient | |
| Outcomes |
| |
| Identification | Contact information: Dr. Kato, Department of Anesthesia, Chiba Hokusoh Hospital, Nippon Medical School, 1715 Kamakari, Inba‐mura, Inba‐gun, Chiba 270‐1694, Japan, n‐[email protected] No sources of funding mentioned for this study | |
| Notes | Country: Japan Language: English Study author contacted: yes, no reply from the author Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: no sources of funding stated Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors are blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery with epinephrine‐augmented hypotensive anaesthesia without use of a tourniquet; normotensive epidural anaesthesia with a tourniquet Follow‐up: 6 days Study design: single‐centre randomised controlled trial | |
| Participants | 100 participants in total Male:Female: 13:36; 10:41 Age, years (SD): 74.7 (7.4); 72.6 (7.1) BMI (SD): 28.5 (3.3); 28.8 (3.9) Inclusion criteria: patients listed for total knee replacement surgery, ASA grade I and II Exclusion criteria: patients with history of myocardial infarction with angina, severe aortic or mitral valve stenosis, untreated hypertension, renal disease, preoperative bleeding disorders Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with epinephrine‐augmented hypotensive epidural anaesthesia and no tourniquet group (n = 49) Group B: surgery performed with normotensive epidural anaesthesia with tourniquet group (n = 51) All patients were given oral premedication of 7.5 mg midazolam. Arterial blood pressure was monitored by inserting a 20‐gauge cannula into the radial artery. In all patients, a central venous catheter was placed into the right internal jugular vein to measure central venous pressure. The catheter also was used to administer the epinephrine infusion to the group of patients who received EAHEA (Group A) In Group B, patients received 500 mL Ringer’s solution (Fresenius Kabi Austria GmbH) before the epidural dose as fluid preload. Epidural anaesthesia was administered at the L3‐L4 and L4‐L5 interspaces through a paramedian approach with ropivacaine 1% (15 mL) and fentanyl (50 micrograms) | |
| Outcomes |
| |
| Identification | Contact information: Martin Raffl, MD, Clinic for Anesthesiology and Intensive Care, Paracelsus Medical Private School, Salzburg, Muellner Hauptstrasse 48, A‐5020, Salzburg, Austria Phone 0043‐662‐4482‐2701; Fax 0043‐662‐4482‐2703; [email protected] | |
| Notes | Country: Austria Language: English Study author contacted: yes, no reply from the author Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: no sources of funding stated Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgment |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: change in haemoglobin, blood transfusion rate, duration of surgery Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | High risk | Had potential source of bias related to specific study design |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 6 weeks Study design: single‐centre randomised controlled trial | |
| Participants | 30 participants in total Male:Female: 9:21 Age, years (range): 58 (45 to 69) BMI (SD): not reported Inclusion criteria: patients undergoing bilateral total knee replacement Exclusion criteria: patients with severe cardiac comorbidities or neurological problems Duration of illness: unspecified | |
| Interventions | 30 patients undergoing bilateral knee replacement surgery Group A: surgery performed with a tourniquet (n = 30) Group B: surgery performed without a tourniquet (n = 30) All surgeries were performed by the same surgical team with standard technique. Epidural anaesthesia was used in all patients given with epidural morphine 50 mg/kg along with 0.1% bupivacaine in 10 mL normal saline. Along with that, IV diclofenac sodium was used twice daily for 5 days postoperatively and then was shifted to oral formulation accordingly. Both knees were prepared at the same time, and a single set of instruments were used. One knee was operated first and then the other by senior author [CSY]. All patients received perioperative antibiotics (amoxicillin‐clavulanic acid 1.2 grams intravenously). | |
| Outcomes |
| |
| Identification | Corresponding author: c/o Bipin Kumar, Sector‐4/D, Quarter No. 1038, Bokarosteel City, Jharkhand, India, [email protected] (N. Kumar) | |
| Notes | Country: India Language: English Study author contacted: no Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: no sources of funding stated Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin toss |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Self‐reported outcome: pain Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: none |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 2 years Study design: single centre randomised controlled trial | |
| Participants | 50 participants in total Male:Female: 10:15; 9:14 Age, years (SD): 70 (8); 71 (6) BMI (SD): 29 (4.8); 28 (4.8) Inclusion criteria: patients on the waiting list for elective primary total knee surgery due to arthritis, ASA I or II Exclusion criteria: Inability to give informed consent, rheumatic arthritis, malignancy, coagulation disorder or medical treatment influencing coagulation, liver disease, severe heart disease, bilateral operation Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 25) Group B: surgery performed without a tourniquet (n = 25) All operations were done under spinal anaesthesia. The Nexgen CR all‐poly tibia knee prosthesis (Zimmer) was inserted after pulsed lavage and was cemented with Palacos R + G (Heraeus Medical Nordic, Sollentuna, Sweden) (40 g Palacos and 0.5 g gentamicin). 2 g cloxacillin was given intravenously just before and 3 times after the operation Low–molecular‐weight heparin (Innohep, 4500 IE subcutaneously) was used for the first 14 postoperative days | |
| Outcomes |
| |
| Identification | Contact information: Department of Orthopedics, Aleris Specialist Care Motala; Department of Orthopedics, Linköping University Hospital, Linköping; Department of Orthopedics, Oskarshamn Hospital, Oskarshamn, Sweden, [email protected] | |
| Notes | Country: Sweden Language: English Study author contacted: no Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: the study was funded by Swedish Research Council (VR‐2009‐6725) Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcome: pain Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: MTPM, blood loss, range of motion Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 7 days Study design: single‐centre randomised controlled trial | |
| Participants | 60 participants in total Male:Female: 9:21; 10:20 Age, years (SD): 71 (7); 70 (7) BMI (SD): 24 (5); 24 (5) Inclusion criteria: patients with initial unilateral TKA osteoarthritis (OA), rheumatoid arthritis (RA) Exclusion criteria: patients with diabetes, haemorrhagic haematological disease, haemoglobin (Hb) < 100 g/L, peripheral neurovascular disease, malignant tumour, history of vascular embolism, history of infection in the affected lower limb Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 30) Group B: surgery performed without a tourniquet (n = 30) All surgical prostheses were replaced with posterior cruciate ligament instead of a cemented artificial knee joint (GENES II, Smith & Nephew, Andover, MA, USA). Surgery was performed by the same group of physicians using the midvastus route. 3 g of haemostatic powder was sprayed on the surface of the joint cavity and soft tissue before the incision was sutured. Tourniquet pressure in the tourniquet group was the patient's own arterial systolic pressure + 100 mmHg (1 mmHg = 0.133 kPa); the wound was closed, and the tourniquet was loosened with standard dressing. Operation of the non‐haemostatic group was the same as above, but the anaesthetist reduced basal blood pressure by 30 to 40 mmHg during the period from osteotomy to the bone cement‐covered bone bed to reduce bleeding and found that the active bleeding point was electrocoagulated in time. All patients had no drainage in the incision [6]. Low‐molecular‐weight heparin calcium and plantar pump were routinely used postoperatively for anti‐deep vein thrombosis. The wound dressing was replaced when it was oozing out or when the dressing of the incision was slightly tight to prevent distal blood flow. The knee joint was not passively exercised after CPM, and the affected limb was lifted higher than the heart plane | |
| Outcomes |
| |
| Identification | Contact information: 200003 Shanghai, Changzheng Hospital, Second Military Medical University, Department of Orthopedics Corresponding author: Qian Qi Rong, qianqr @ 163. corn No funding mentioned for this study | |
| Notes | Country: China Language: Chinese Study author contacted: yes, no reply from study author Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: none stated or identified Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: pain, adverse events Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: change in haemoglobin and haematocrit, duration of surgery, blood loss, periarticular circumference Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 14 days Study design: single‐centre randomised controlled trial | |
| Participants | 80 participants in total Male:Female: 11:29; 13:27 Age, years (SD): 71 (6); 70 (7) BMI (SD): 27.3 (6.3); 26.8 (5.1) Inclusion criteria: patients with primary osteoarthritis or rheumatoid arthritis undergoing primary total knee replacement Exclusion criteria: bilateral total knee replacement either simultaneously or staged at less than 3‐month intervals, diabetes, haemostatic defect, history of peripheral vascular disease, presence of malignant tumour, preoperative level of Hb < 10 g/L, previous thromboembolism Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 40) Group B: surgery performed without a tourniquet (n = 40) All procedures were performed by 4 similar staff surgeons. The implant used was the type of posterior cruciate ligament substituting total knee prosthetic components (Genesis II, Smith & Nephew, Memphis, TN, USA). In group A, the tourniquet was inflated to 100 mmHg above systolic blood pressure after the leg was elevated and exsanguinated, and deflation was performed after the wound was closed and the compressive dressing applied. The tourniquet was not used in group B, and active bleeding points were promptly sealed with electrical coagulation. A uniform perioperative regimen was used in all cases. Antibiotic treatment with second‐generation cephalosporin was infused intravenously (1 dose preoperatively and for the next 2 days). The quantity of saline transfused intravenously within 24 hours postoperatively was 2500 to 3000 mL | |
| Outcomes |
| |
| Identification | Contact details: B Li: H Wu; Q Qian; X Lin; H Zhao, Department of Orthopaedic Surgery, Arthritis Institute, Changzheng Hospital, Second Military Medical University, Shanghai 200003, People’s Republic of China, [email protected] | |
| Notes | Country: China Language: English Study author contacted: yes, no reply from study author Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: none stated or identified Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number list was used |
| Allocation concealment (selection bias) | High risk | Open random allocation schedule |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: number of people conducting straight leg raise Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: range of motion, circumference of knee, duration of surgery, intraoperative blood loss, postoperative blood loss, total blood loss Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 12 months Study design: single‐centre randomised controlled trial | |
| Participants | 20 participants in total Male:Female: 7:3; 9:1 Age, years (SD): 67; 70 BMI (SD): 25.6; 27.1 Inclusion criteria: patients undergoing total knee replacement for osteoarthritis Exclusion criteria: patients with symptomatic peripheral vascular disease or contraindication to tourniquet use Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 10) Group B: surgery performed without a tourniquet (n = 10) All patients underwent TKA by the senior surgeon (Liu) through a standardised technique and prosthesis. All patients received a general anaesthetic without regional blocks or local anaesthesia, in an effort to minimise confounding variables that may influence pain scores. A medial parapatellar approach was used with eversion of the patella. An intra‐articular drain on low suction was inserted before wound closure and was removed day 1 postoperatively. All patients received patient‐controlled analgesia with morphine sulphate for the first 24 hours. Patients were mobilised day 1 postoperatively and were discharged home when mobilising safely. The same standardised physiotherapy protocol was undertaken in all patients postoperatively. Active and passive range of motion was encouraged without the use of continuous passive motion | |
| Outcomes |
| |
| Identification | Contact details: David Liu, FRACS Gold Coast Centre for Bone and Joint Surgery, John Flynn Private Hospital, Suite 8A, Fred McKay House, 42 Inland Dr, Tugun Queensland 4224, Australia Tel: +61‐7‐5598‐0205, fax: +61‐7‐5598‐0205, [email protected] Smith & Nephew Australia provided financial support | |
| Notes | Country: Australia Language: English Study author contacted: yes, no reply from study author Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: Smith & Nephew Australia provided financial support Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; insufficient information on whether surgeons were blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: pain, OKS Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 12 months Study design: single‐centre randomised controlled trial | |
| Participants | 56 participants in total Male:Female: 16:36 Age, years (SD): 67 (8) BMI (SD): 28.1 (5.5) Inclusion criteria: bilateral severe osteoarthritis with pain, accompanied with or without significant deformity, failure of conservative treatment Exclusion criteria: recent or current knee sepsis, extensor mechanism discontinuity or severe dysfunction, age > 70 years, coagulation disorder or treatment with drugs known to influence coagulation, diabetes, renal or liver disease, severe cardiovascular problems, lung disease, neurological disorders, cancer Duration of illness: unspecified | |
| Interventions | Patients undergoing bilateral knee replacement surgery Group A: surgery performed with a tourniquet (n = 56) Group B: surgery performed without a tourniquet (n = 56) The tourniquet was applied on a layer of cotton wool padding applied over the thigh. Both right and left thighs were prepared with a tourniquet before surgery; only the limb of the TG side was elevated and exsanguinated with a rubber limb Eschmarch’s bandage. The tourniquet was then inflated to pressure of 125 mmHg above systolic blood pressure (SBP) just before the incision. Longitudinal incisions were made at the midline with the knee positioned in 90 degrees flexion, from 4 cm proximal to the upper end of the patella up to the tibial tuberosity. The tourniquet was inflated for less than 120 minutes until wound closure was done and compressive dressing was applied. For NG knees, SBP was maintained at a level of approximately 100 mmHg at the time of cementation with antihypertensive drugs. Posterior stabilised knee prostheses (26 GENESIS II, Smith & Nephew, Memphis, TN, USA; 26 Vanguard, Biomet, Warsaw, IN, USA) were used in the surgery. Periarticular injection of ropivacaine (200 mg), adrenaline hydrochloride (0.1 mg), and morphine (5 mg) was administered just before skin closure Intravenous patient‐controlled analgesia (PCA) with morphine was started postoperatively. All patients received rivaroxaban (10 mg once a day) from the first postoperative day, for 2 weeks, as prophylaxis against thromboembolic complications | |
| Outcomes |
| |
| Identification | Contact details: Pei‐lai Liu, PhD, Department of Orthopaedics, Qilu Hospital, Shandong University, 107 Wenhua West Road, Jinan, China 250012 Tel: 0086‐531‐82166542; fax: 0086‐531‐86927544; [email protected] No source of funding for this study identified | |
| Notes | Country: China Language: English Study author contacted: no Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: no source of funding identified for this study Adverse events: In the tourniquet group: 4 knees had symptomatic DVT In the non‐tourniquet group: 4 knees had symptomatic DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Surgeon not blinded ‐ always performed the non‐tourniquet side first; not stated if patients blinded |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: duration of surgery, range of motion, swelling, wound healing Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 12 months Study design: single‐centre randomised controlled trial | |
| Participants | 26 participants in total Male:Female: 8:18 Age, years (SD): 65.8 (9.2) BMI (SD): 28.2 (5.6) Inclusion criteria: patients undergoing bilateral total knee replacement surgery Exclusion criteria: history of coagulation disorder or medications likely to influence coagulation; diabetes; renal or liver disease; severe cardiovascular problems and lung disease; nerve disorder; cancer; skin disease; history of a previous surgical procedure of the knee other than arthroscopy; apparent keloid constitution Duration of illness: unspecified | |
| Interventions | Patients undergoing bilateral knee replacement surgery Group A: surgery performed with a tourniquet (n = 26) Group B: surgery performed without a tourniquet (n = 26) As per the surgeon’s usual practice, TKA with or without tourniquet placement was first completed on the left knee, then was performed on the right side after closure. All surgeries were performed under general anaesthesia by the same team of surgeons. A layer of cotton wool padding was applied over both thighs, above which the tourniquet was applied. The tourniquet size was 105 cm × 7 cm. Further, the lower limb on the side assigned to the TP group was elevated, and a rubber limb exsanguinator was inflated to pressure of 125 mmHg above systolic blood pressure immediately before the incision was made. Intravenous morphine for patient‐controlled analgesia (PCA) was started postoperatively. All patients received rivaroxaban (10 mg 2 times daily) for 2 weeks from the first day of the operation as prophylaxis against thromboembolic complications | |
| Outcomes |
| |
| Identification | Contact details: Pei‐lai Liu, PhD, Department of Orthopaedics, Qilu Hospital, Shandong University, 107 Wenhua West Road, Jinan, China 250012 Tel: 0086‐531‐82166542; fax: 0086‐531‐86927544; [email protected] | |
| Notes | Country: China Language: English Study author contacted: yes, no reply from study author Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: no source of funding identified for this study Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: duration of surgery, wound healing, mean change in suprapatellar girth, range of motion, wound length, revision rate, MSS score Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 48 hours Study design: single‐centre randomised controlled trial | |
| Participants | 20 participants in total Male:Female: 2:8; 3:7 Age, years (range): 72.4 (64 to 83); 76.6 (65 to 84) BMI (range): 28.3 (19.3 to 35.5); 29.5 (24 to 43.8) Inclusion criteria: patients listed for primary total knee replacement surgery Exclusion criteria: diabetes mellitus, presence of peripheral arterial occlusive disease (pAOD) or cardiopulmonary diseases ruled out by clinical examination and if necessary duplex ultrasound, medical history of thrombosis or embolism and renal insufficiency (creatinine mmol/L). Patients with severe varus or valgus deformity > 15" or flexion contracture > 20" were excluded from the study to minimise bias due to different soft tissue trauma by preparation and postoperative tension of the skin Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 10) Group B: surgery performed without a tourniquet (n = 10) In all cases, a cemented PFC Sigma total knee replacement (DePuy, Warsaw, IN, USA) without resurfacing of the patella was implanted by the same surgeon (GM) through a midline incision and a medial parapatellar approach | |
| Outcomes |
| |
| Identification | Contact information: G Matziolis, Centre for Musculoskeletal Surgery, Charite University Hospital, Schumannsir, 20‐21, 10117, Berlin, Germany, [email protected] | |
| Notes | Country: Germany Language: English Study author contacted: yes, no reply from study author Trial registry record or protocol available: none stated or identified Funding source/declaration of interest: no financial affiliation has been paid by third parties others than the Center for Musculoskeletal Surgery of the Charite University Hospital Adverse events: In the tourniquet group: 1 patient had a wound healing disorder that required revision surgery In the non‐tourniquet group: 1 patient had nerve injury | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | High risk | Open random allocation schedule |
| Blinding of participants and personnel (performance bias) | Unclear risk | Participants blinded; insufficient information to specify if surgeons were blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcome: nerve injury Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 2 years Study design: single‐centre randomised controlled trial | |
| Participants | 60 participants in total Male:Female: 16:14; 16:14 Age, years (SD): 70 (7); 67 (9) BMI (SD): 28 (3); 28 (3) Inclusion criteria: patients suffering exclusively from OA, stages II to V; patients requiring knee prosthesis suitable for the use of a triathlon knee system; patients understanding the conditions of the study and willing and able to comply with scheduled postoperative clinical and radiographic evaluations and prescribed rehabilitation; patients who signed the Ethics Committee approved informed consent form before surgery Exclusion criteria: previous major knee arthroplasty; significant disabling problems from the muscular‐skeletal system other than the knees; obese patients with obesity severe enough to affect their ability to perform activities of daily living (BMI > 35); patients with active or suspected infection; patients with active malignancy; patients with severe osteoporosis, Paget's disease, renal osteodystrophy; patients immunologically suppressed or receiving steroids in excess of physiological dose requirements; patients with a neuromuscular or neurosensory deficit that would limit their ability to assess performance of the device or that interferes with the patient's ability to limit weight‐bearing or places an extreme load on the implant during the healing period; pregnancy; systemic or metabolic disorders leading to progressive bone deterioration; concurrent illness such as sickle cell anaemia, SLE, or renal disease requiring dialysis Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 30) Group B: surgery performed without a tourniquet (n = 30) Each patient was given preoperative antibiotics and tranexamic acid. Surgeries were performed via a midline incision with a parapatellar medial entrance to the joint using appropriate guide instruments according to the surgical technique supplied to the knee system. In both groups, the bony surfaces were prepared in the same manner, were cleansed by saline pulse lavage, and were kept clean of blood by saline‐prepared medical pads. At the time of surgery, 8 tantalum markers (0.8 mm diameter; RSA Biomedical, Umeå, Sweden) were inserted into the proximal tibial metaphysis and 5 markers were inserted into the polyethylene tibial insert. The tourniquet was applied during dressing and was inflated to 300 mmHg just before the start of surgery and was not deflated until the leg was sutured and dressed. The group operated on without the use of a tourniquet was operated on with the same surgical and cementing technique. The cement used was Refobacin® Bone Cement R (Biomet Inc., Warsaw, IN, USA). No patellar components were used in either group. Postoperatively, low‐molecular‐weight heparin was used for thromboembolic prophylaxis. Early full weight‐bearing and mobilisation were similar for both groups | |
| Outcomes |
| |
| Identification | Contact information: Mats Molt, Department of Orthopaedics Hässleholm‐Kristianstad Ystad, Hässleholm, Sjukhusorganisation, Box 351, S‐281, 25 Hässleholm, Sweden Tel.: +46 451298707, [email protected] (M. Molt) | |
| Notes | Country: Sweden Language: English Study author contacted: no Trial registry record or protocol available: clinical trial ID: NCT01604382 Funding source/declaration of interest: no funding for this study Adverse events: In the tourniquet group: 2 patients required re‐operation; 1 patient reported instability in the index knee at 2 years' follow‐up In the non‐tourniquet group: 1 patient died due to postoperative septicaemia; 1 patient had a stroke; 1 patient had DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 7 days Study design: single‐centre randomised controlled trial | |
| Participants | 103 participants in total Male:Female: 6:45; 9:43 Age, years (SD): 72.8 (7.3); 74.6 (7.6) BMI (SD): 27.7 (3.4); 29.2 (3.9) Inclusion criteria: patients undergoing total knee replacement surgery Exclusion criteria: patients showing preoperative DVT, coagulation disorder, abnormal coagulation test values, or receiving anticoagulants; patients who received anticoagulant therapy postoperatively according to the judgement of the physician Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 51) Group B: surgery performed without a tourniquet (n = 52) All operations were conducted by a single surgeon (S.K.). All patients received spinal and epidural anaesthesia in conjunction with general anaesthesia, and the tube for the epidural continuation was removed on the second postoperative day. Exposure of the knee was through a midline skin incision (approximately 8 to 10 cm) and a mid‐vastus approach. All patients had a Scorpio non‐restrictive geometry posterior‐stabilised system (Stryker Howmedica Osteonics, Allendale, NJ, USA) cemented arthroplasty. An extramedullary guide was used for the tibia, and an intramedullary osteotomy guide was used for the distal cut of the femur. The patella was resurfaced in all patients | |
| Outcomes |
| |
| Identification | Contact information: Noriaki Mori, Department of Orthopaedic Surgery, Wajo Eniwa Hospital, Koganechuo 2‐1‐1, Eniwa City 061‐1449, Japan Tel.: +81 123 33 2333; fax: +81 123 335108; [email protected] (N.Mori) | |
| Notes | Country: Japan Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study Adverse events: no additional adverse events reported in the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Three groups: surgery performed with a tourniquet for the entire procedure; surgery performed with a tourniquet for cementing only; surgery performed without a tourniquet Follow‐up: 6 weeks Study design: single‐centre randomised controlled trial | |
| Participants | 69 participants in total Male:Female: not stated Age, years (range): 65.05 (52 to 81) BMI (SD): not stated Inclusion criteria: patients diagnosed with arthritis refractory to conservative treatment and identified as TKA candidates Exclusion criteria: secondary arthritis, extreme deformity, previous cardiovascular disease Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet for the entire procedure (n = 24) Group B: surgery performed with a tourniquet for cementing only (n = 20) Group C: surgery performed without a tourniquet (n = 25) Posterior cruciate retaining Genesis II (Smith & Nephew, Memphis, TN, USA) cemented knee system and OrCem 3 low‐viscosity polymethylmethacrylate (PMMA) bone cement (European Medical Contract Manufacturing, Nijmegen, Netherlands) were used in all patients. All patients were operated under general anaesthesia with propofol and desfluran | |
| Outcomes |
| |
| Identification | Contact information: Okan Ozkunt, Department of Orthopedics and Traumatology, Acibadem University Atakent Hospital, Halkali/Kucukcekmece, Istanbul, 34303, Turkey, [email protected] | |
| Notes | Country: Turkey Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study Adverse events: no additional adverse events reported in the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 4 days Study design: single‐centre randomised controlled trial | |
| Participants | 90 participants in total Male:Female: 21:24; 11:34 Age, years (range): 69 (47 to 85); 70.5 (50 to 90) BMI (range): 27.8 (18.5 to 38.1); 26 (18.5 to 33.9) Inclusion criteria: patients with primary end‐stage osteoarthritis receiving unilateral total knee arthroplasty (TKA) Exclusion criteria: patients receiving any anticoagulation before surgery (e.g. acetylsalicylic acid, phenprocoumon, warfarin, clopidogrel, dabigatran, rivaroxaban, low‐molecular‐weight heparin) with the diagnosis of liver dysfunction/coagulation dysfunction or a history of peripheral arterial obstructive disease or thromboembolic events Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 45) Group B: surgery performed without a tourniquet (n = 45) All patients received a cemented, posterior‐stabilised primary TKA (Nexgen LPS Flex, Zimmer, Warsaw, IN, USA) with a fixed bearing design without patellar resurfacing. A total of 40 g of bone cement (Palacos R®, Heraeus, Hanau, Germany) was used with a fourth‐generation cementing technique including pulsatile lavage, vacuum mixture, double‐cementing technique, and cement gun pressurisation. Every patient received the same standardised postoperative pain medication protocol | |
| Outcomes |
| |
| Identification | Contact information: T Pfitzner, P von Roth, C Perka, Orthopaedic Department, Center for Musculoskeletal Surgery, Charité – Universitätsmedizin Berlin, Charitéplatz 1, 10117 Berlin, Germany, [email protected] | |
| Notes | Country: Germany Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study Adverse events: no additional adverse events reported in the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 4 days Study design: single‐centre randomised controlled trial | |
| Participants | 72 participants in total Male:Female: 9:27; 8:28 Age, years (SD): 72.1 (6.9); 71.5 (6.8) BMI (SD): 28.6 (4.5); 27.9 (4.2) Inclusion criteria: patients undergoing total knee replacement surgery for osteoarthritis Exclusion criteria: rheumatoid arthritis, coagulopathy, uncontrolled hypertension, peripheral vascular disease Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 36) Group B: surgery performed without a tourniquet (n = 36) All patients underwent primary total knee arthroplasty with minimally invasive techniques and cemented prostheses (Genesis II Total Knee System, Smith & Nephew, Memphis, TN, USA; or U2 Knee System, United Orthopedic, Taipei, Taiwan). All operations were performed through the medial parapatellar approach by experienced knee surgeons. An intramedullary guide was used for both tibial and femoral cuts. No drainage system was used postoperatively for any patient | |
| Outcomes |
| |
| Identification | Contact information: Ta‐Wei Tai, Department of Orthopaedics, National Cheng Kung University Hospital, College of Medicine, National Cheng Kung University, Tainan, Taiwan No funding for this study | |
| Notes | Country: Taiwan Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study Adverse events: no additional adverse events reported in the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcome: pain Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: intraoperative blood loss; overall blood loss; blood transfusion rate; change in haemoglobin; change in haematocrit, serum creatinine phosphokinase, myoglobin, lactate dehydrogenase, CRP, duration of surgery Outcome assessors blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 10 days Study design: single‐centre randomised controlled trial | |
| Participants | 63 participants in total Male:Female: 15:18; 11:19 Age, years (SD): 69.8 (6.7); 69.8 (9.0) BMI (SD): 28.6 (4.5); 27.9 (4.2) Inclusion criteria: patients undergoing total knee replacement for osteoarthritis or rheumatoid arthritis Exclusion criteria: bilateral TKA required simultaneously or staged at less than 3‐month intervals; history of bleeding diathesis; revision TKA; history of musculoskeletal infection of the affected limb; history of peripheral vascular disease Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 33) Group B: surgery performed without a tourniquet (n = 30) Groups were similar with respect to operative procedure. Lateral release was performed in 22 patients in the tourniquet group and in 20 in the non‐tourniquet group (Table 1). One synovectomy was performed in each group. All patients received a primary cemented total knee replacement with a cemented polyethylene patellar replacement. In the tourniquet group, the leg was elevated and exsanguinated (without use of an Esmarch bandage) before tourniquet inflation. The tourniquet was set at 125 to 150 mmHg above systolic blood pressure, up to a maximum value of 300 mmHg. The tourniquet was deflated after the bone cement had set, and only then was electrocautery used for haemostasis. In the non‐tourniquet group, electrocautery was used as necessary throughout the procedure | |
| Outcomes |
| |
| Identification | Contact information: Dr John F Rudan, Department of Surgery, Queen's University, Kingston ON K7L 3N6 Fax 613 549‐2529, [email protected] | |
| Notes | Country: Kingston Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study Adverse events: In the tourniquet group: 4 wound infections In the non‐tourniquet group: 1 wound infection, 1 gastrointestinal haemorrhage | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Patients 'blindly' randomised; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: DVT, infection Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | High risk | Assessor‐reported outcomes:intraoperative blood loss, blood transfusion rate, duration of surgery, haemoglobin level, postoperative blood loss, overall blood loss Outcome assessors were not blinded; therefore high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of blinding identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 3 months Study design: single‐centre randomised controlled trial | |
| Participants | 80 participants in total Male:Female: 9:31; 16:24 Age, years (range): 73.65 (52 to 110); 80.25 (50 to 110) BMI (SD): not reported Inclusion criteria: patients undergoing primary total knee replacement surgery for osteoarthritis Exclusion criteria: patients with diabetes, haemostasis defect, rheumatoid arthritis, previous thromboembolism, abnormal vascular supply to the leg, previous open knee surgery, bilateral TKA Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 40) Group B: surgery performed without a tourniquet (n = 40) All operations were performed under general anaesthesia by a single surgeon or by house staff using a standardised technique. Cefamandol 1500 mg was given intravenously at induction of anaesthesia, and 4 further doses of 750 mg were given postoperatively. Standard anticoagulant prophylaxis using enoxaparin was started the evening before surgery and continued until the patient was fully mobile. In group A, the limb was first exsanguinated by elevation for 2 minutes, then the tourniquet was inflated to 350 mmHg. If the duration of tourniquet use exceeded 90 minutes, the tourniquet was released intraoperatively and haemostasis was completed. After 10 minutes of release, a new exsanguination was instituted. The tourniquet was not released until after the wound was closed and the compressive dressing was applied | |
| Outcomes |
| |
| Identification | Contact information: E Vandenbussche, L‐D Duranthon, B Augereau, Department of Orthopaedic Surgery, Hôpital Européen Georges Pompidou, 20 Rue Louis Blanc, 75908 Paris Cedex 15, France, [email protected]‐hop‐paris.fr | |
| Notes | Country: France Language: English Study author contacted: yes, no reply from author Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study Adverse events: In the tourniquet group: 1 patient had DVT In the non‐tourniquet group: 2 patients had DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Patients blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: pain, complications, time to achieve straight leg raise Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: duration of surgery, change in haemoglobin, change in haematocrit, overall blood loss, implant loosening, range of knee flexion Outcome assessors were blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 2 days Study design: single‐centre randomised controlled trial | |
| Participants | 40 participants in total Male:Female: 10:10; 11:9 Age, years (SD): 67.85 (6.91); 65.65 (8.54) BMI (SD): 30.43 (5.07); 31 (5.31) Inclusion criteria: Patients with end stage knee osteoarthritis who have failed non‐operative management and are being listed for a primary total knee replacement. Exclusion criteria: history of peripheral vascular disease that precluded tourniquet use or required a semi‐constrained prosthesis due to ligament instability necessitating a fixed bearing tibial component with tibial stem Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 20) Group B: surgery performed without a tourniquet (n = 20) In both groups, the knee replacement procedure was commenced with a tourniquet applied but without inflation. The patient’s blood pressure was maintained hypotensive if no contraindications were applied, else normotensive. Proximal tibial osteotomy was undertaken via a computerised navigation system, with all resections at 90 to the tibial long axis, removing a planned 10‐mm resection of the lateral tibial plateau. After bone resection and preparation were undertaken, patients were randomised to group A or B. In group A, the leg was elevated for 1 minutes, then the tourniquet was inflated to 300 mmHg for the duration of the cementing procedure. In group B, the tourniquet was not inflated | |
| Outcomes |
| |
| Identification | Contact information: Christopher John Vertullo, Orthopaedic Surgery & Sports Medicine Centre, 8‐10 Carrara Street, Benowa, QLD, Australia, [email protected] | |
| Notes | Country: France Language: English Study author contacted: no Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: cement penetration depth Outcome assessors were blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 4 months Study design: single‐centre randomised controlled trial | |
| Participants | 77 participants in total Male:Female: 11:26; 14:26 Age, years (range): 72.5 (57 to 85); 71.8 (43 to 91) BMI (SD): not reported Inclusion criteria: patients undergoing primary total knee arthroplasty Exclusion criteria: patients with diabetes, rheumatoid arthritis, previous thromboembolism, active malignancy, those having 1‐stage bilateral surgery Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 37) Group B: surgery performed without a tourniquet (n = 40) All patients had identical anaesthesia, which included premedication with temazepam and general anaesthesia with fentanyl. No patient had spinal or epidural anaesthesia Postoperatively, all had ‘patient‐controlled analgesia’ (PCA) with an infusion of morphine sulphate. All patients received intravenous cefuroxime (1.5 g) after induction of anaesthesia and twice postoperatively. Those in group A had TKA under a tourniquet after the leg had been exsanguinated. Tourniquet pressure was twice the systolic blood pressure. Patients in group B did not have a tourniquet applied to the leg. Low‐dose warfarin was given to maintain the international normalised ratio between 1.3 and 2.0, and was continued until discharge from the hospital. Mobilisation began on removal of the drains, 48 hours after the operation | |
| Outcomes |
| |
| Identification | Contact information: JC D’Arcy, FRCS, Consultant Orthopaedic Surgeon, Department of Orthopaedics, Eastbourne District General Hospital, King’s Drive, Eastbourne, East Sussex BN21 2UD, UK | |
| Notes | Country: UK Language: English Study author contacted: no Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study Adverse events: In the tourniquet group: 6 patients required manipulation under anaesthesia, 1 patient had wound leakage, 1 patient died from unrelated causes In the non‐tourniquet group: 5 patients required manipulation under anaesthesia, 2 patients died from unrelated causes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 4 weeks Study design: single‐centre randomised controlled trial | |
| Participants | 37 participants in total Male:Female: not reported Age, years (SD): 63.2 (8.7); 61.4 (7.4) BMI (SD): not reported Inclusion criteria: patients undergoing primary total knee replacement surgery Duration of illness: unspecified | |
| Interventions | All patients under general anaesthesia using sevoflurane and propofol. Endotracheal intubation and mechanical ventilation were employed to maintain a constant end‐tidal CO₂ level. The fractional inspired O₂ concentration (33%) did not change during the operation. In the with tourniquet group, a tourniquet was applied at approximately 100 mmHg above systolic blood pressure. The operations were performed with autologous blood transfusion and used a postoperative blood conservation system. Blood losses in the 2 groups were measured and compared. Heparin at 5000 U/d was postoperatively administered to all patients for 3 weeks | |
| Outcomes |
| |
| Identification | Contact information: N Kato, R Ogawa, Department of Anesthesiology, Nippon Medical School, 1‐1‐5 Sendagi, Bunkyo‐ku, Tokyo, Japan | |
| Notes | Country: Japan Language: English Study author contacted: no Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no funding for this study reported Adverse events: In the tourniquet group: 1 patient had PE and 2 patients had DVT In the non‐tourniquet group: 0 patients had PE or DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 6 months Study design: single‐centre randomised controlled trial | |
| Participants | 100 participants in total Male:Female: 22:28; 19:31 Age, years (SD): 67.58 (4.61); 68.06 (3.16) BMI (SD): 24.10 (2.16); 23.87 (2.13) Inclusion criteria: patients undergoing primary total knee replacement surgery for osteoarthritis Exclusion criteria: patients < 18 years or > 85 years of age, rheumatoid arthritis, allergy to TXA, history of thrombosis, coagulation dysfunction, uncontrolled hypertension, infection, body mass index (BMI) > 35 Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 50) Group B: surgery performed without a tourniquet (n = 50) All patients were treated intravenously with 15 mg/kg TXA 10 minutes before skin incision; an additional 1 g TXA was used after 3 hours. All patients were treated by a senior orthopaedic surgeon under general anaesthesia. Drainage tubes were used in all patients and were removed 24 hours postoperatively if blood loss was < 300 mL. Otherwise, they continued to be used until the quantity was < 50 mL | |
| Outcomes |
| |
| Identification | Contact information: Yuangang Wu, Department of Orthopaedics, West China Hospital, Sichuan University, 37 # Guoxue Road, Chengdu, 610041, People's Republic of China, [email protected] | |
| Notes | Country: China Language: English Study author contacted: no Trial registry record or protocol available: researchregistry4423 Funding source/declaration of interest: study was funded by the Science and Technology Department of Sichuan Province (2017FZ0056 and No. 2018HH0141), and also by the Health Department of Sichuan Province (N0. 18ZD016) Adverse events: no adverse events reported between the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Low risk | Participants blinded; surgeons not blinded However surgeons would be able to influence/alter their performance only for intraoperative outcomes such as intraoperative blood loss, operative time, or quality of cementation. It is unlikely that surgeons would alter their performance to influence these outcomes for fear of damaging the overall quality and safety of the surgery |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: pain, DVT, wound‐related complications Patients blinded; therefore low risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | High risk | Assessor‐reported outcomes: total blood loss, intraoperative blood loss, hidden blood loss, drainage volume, transfusion requirements, maximum Hb drop, knee circumference, range of motion, length of stay Outcome assessors not blinded; therefore high risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Three groups: surgery performed with a tourniquet inflated until wound closure; surgery performed with a tourniquet inflated until components were inserted; surgery performed without a tourniquet Follow‐up: day 1 Study design: single‐centre randomised controlled trial | |
| Participants | 84 participants in total Male:Female: 6:16; 9:24; 7:22 Age, years (range): 68 (54 to 72); 64 (54 to 73); 66 (51 to 74) BMI (SD): not reported Inclusion criteria: patients with a diagnosis of severe primary osteoarthritis, insertion of bicompartmental prosthesis, absence of any known coagulation disorder Exclusion criteria: Patients undergoing a unicondylar knee replacement or a revision knee replacement. Presence of a known coagulation disorder or a patient who is routinely taking anticoagulant medication. Duration of illness: unspecified | |
| Interventions | Group 1: surgery performed without a tourniquet (n = 29) Group 2: surgery performed with a tourniquet inflated until components inserted (n = 33) Group 3: surgery performed with a tourniquet inflated until wound closure (n = 22) For all patients, suction drainage was used routinely and was removed after 24 hours. Low‐molecular‐weight heparin was administered to all patients, and no monitoring for INR was performed. Antibiotic prophylaxis was started just before the tourniquet was inflated with 1 g cefazolin, then was continued 3 times daily for 48 hours | |
| Outcomes |
| |
| Identification | Contact information: Alireza Yavarika, Department of Orthopaedics, Ward of Orthopaedics, Besat Hospital, Hamadan University of Medical Sciences, Hamadan, Iran, tel +959144122542 | |
| Notes | Country: Iran Language: English Study author contacted: yes, no reply from study author Trial registry record or protocol available: none reported or identified Funding source/ declaration of interest: no source of funding reported or identified Adverse events: no adverse events reported between the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Low risk | Self‐reported outcomes: none |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: not reported Study design: single‐centre randomised controlled trial | |
| Participants | 60 participants in total Male:Female: 8:22; 11:19 Age, years (SD): 72 (6); 71 (6) BMI (SD): 25 (4); 26 (4) Inclusion criteria: patients undergoing primary total knee replacement surgery for osteoarthritis or rheumatoid arthritis Exclusion criteria: patients with diabetes, haemorrhagic disease, Hb < 100 g/L, peripheral neurovascular disease, malignant tumour, history of vascular thrombosis, history of infection in the lower limb Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 30) Group B: surgery performed without a tourniquet (n = 30) Tourniquet pressure in the tourniquet group was based on the patient’s arterial systolic pressure + 100 mmHg (1 mmHg = 0.133 kPa). The wound was closed and dressed with standard wound dressings and compression before the tourniquet was loosening. Procedures applied in the control group were identical, but between osteotomy and bone cement insertion, the anaesthesiologist controlled blood pressure at 30 to 40 mmHg to reduce bleeding. Intraoperative active bleeding was coagulated electrically. Conventional low‐molecular‐weight heparin and a foot pump were used postoperatively to prevent deep vein thrombosis (DVT) | |
| Outcomes |
| |
| Identification | Contact information: Dr ZHANG Fu‐jiang, Department of Orthopaedics, Tianjin Hospital, Tianjin 300211, China No funding for this study | |
| Notes | Country: China Language: English Study author contacted: no Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no source of funding reported or identified Adverse events: no adverse events reported between the 2 groups | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | High risk | Open random allocation schedule |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Unclear risk | Insufficient reporting of attrition |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 22 months Study design: single‐centre randomised controlled trial | |
| Participants | 166 participants in total Male:Female: 12:72; 13:69 Age, years (range): 63.2 (46 to 80); 65.2 (46 to 83) BMI: 28.1; 28.8 Inclusion criteria: unilateral primary total knee arthroplasty for knee osteoarthritis; volunteers to participate in the study Exclusion criteria: severe medical disease, peripheral vascular disease or deep venous thrombosis of the lower extremities, abnormal coagulation function, severe internal and external valgus deformity of the knee joint Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 84) Group B: surgery performed without a tourniquet (n = 82) Surgery was performed under general anaesthesia (34 cases) or sciatic nerve combined with femoral nerve block anaesthesia (50 cases) in the tourniquet group. Surgery under general anaesthesia (29 cases) or sciatic nerve was combined with femoral nerve block anaesthesia (53 cases) in the non‐tourniquet group. Regular intravenous infusion of antibiotics (cefuroxime or ceftriaxone or vancomycin) from 30 mmn to 48 hours after surgery. Intraoperative routine infusion, ambulation, 2 U autologous blood or suspended red blood cells, fresh frozen plasma, blood transfusion treatment was given when the patient developed anaemia symptoms and Hb was lower than 80 g/L. Oral rivaroxaban 5 mg/d was given to prevent thrombosis within 28 days after surgery; was combined with non‐steroidal and opioid drugs to relieve pain. Drainage tube was removed on the first day after surgery and lower limb function training was started | |
| Outcomes |
| |
| Identification | Contact information: DONG Jiyuan, [email protected], Department of Orthopaedics, PLA General Hospital, Beijing, 100853 | |
| Notes | Country: China Language: English Study author contacted: yes, no reply from the author Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no source of funding reported or identified Adverse events: In the tourniquet group: 9 patients had DVT In the non‐tourniquet group: 2 patients had DVT | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: not reported Study design: single‐centre randomised controlled trial | |
| Participants | 39 participants in total Male:Female: 7:13; 5:14 Age, years (SD): 63.12 (6.79); 61.89 (7.93) BMI: not reported Inclusion criteria: patients undergoing total knee replacement surgery, able to provide informed consent and adhere to the study protocol Exclusion criteria: patients with a history of deep venous thrombosis of the lower extremities Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 20) Group B: surgery performed without a tourniquet (n = 19) | |
| Outcomes |
| |
| Identification | Contact information: Zhou Wei, Master attending physician, Department of Orthopaedics, First People's Hospital of Pingdingshan, Pingdingshan 467000, Henan Province, China, [email protected] No funding for this study | |
| Notes | Country: China Language: English Study author contacted: yes, no reply from the author Trial registry record or protocol available: none reported or identified Funding source/declaration of interest: no source of funding reported or identified Adverse events: no adverse events reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random number generator |
| Allocation concealment (selection bias) | Low risk | Sealed envelope |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Low risk | Assessor‐reported outcomes: change in haematocrit, D‐dimer, fibrinogen, plasma viscosity, antithrombin III Outcome assessors were blinded; therefore low risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement No protocol reported or identified |
| Other bias | Low risk | No other sources of bias identified |
| Study characteristics | ||
| Methods | Two groups: surgery performed with a tourniquet; surgery performed without a tourniquet Follow‐up: 6 months Study design: single‐centre randomised controlled trial | |
| Participants | 140 participants in total Male:Female: 13:59; 7:61 Age, years (SD): 66.8 (8.6); 69.1 (7.6) BMI: 25.7 (3.4); 26.1 (4.1) Inclusion criteria: patients with end‐stage osteoarthritis or rheumatoid arthritis scheduled for unilateral total knee arthroplasty Exclusion criteria: patients with prior surgery involving the femur or tibia, prior lower extremity fracture, coagulopathy, uncontrolled hypertension Duration of illness: unspecified | |
| Interventions | Group A: surgery performed with a tourniquet (n = 72) Group B: surgery performed without a tourniquet (n = 68) All operations were performed by the same surgeon using a Sigma fixed or rotating plant posterior‐stabilised total knee prosthesis (PFC, Johnson & Johnson/DePuy, Warsaw, IN, USA). All patients with controlled hypotension received a general anaesthetic. Each patient received the same perioperative treatment strategies: tranexamic acid (TXA), pain control, rehabilitation. TXA was given at initiation of surgery and just before closure | |
| Outcomes |
| |
| Identification | Contact information: correspondence: [email protected], Department of Orthopaedics, West China Hospital of Sichuan University, Chengdu 610041, China | |
| Notes | Country: China Language: English Study author contacted: no Trial registry record or protocol available: this study was funded by Health Industry Special Scientific Research Projects of China ‐ the safety and effectiveness evaluation of arthroplasty (grant number 201302007) Funding source/declaration of interest: Chinese clinical trials registry number ChicTR‐IOR‐16007851 Adverse events: In the tourniquet group: 5 patients developed infection and 2 had DVT In the non‐tourniquet group: 3 patients developed infection | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment ‐ self‐reported outcomes (detection bias) | Unclear risk | Not stated if patients were blinded; therefore unclear risk of bias |
| Blinding of outcome assessment ‐ assessor reported outcomes (detection bias) | Unclear risk | Not stated if outcome assessors were blinded; therefore unclear risk of bias |
| Incomplete outcome data (attrition bias) | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | All outcome measures reported |
| Other bias | Low risk | No other sources of bias |
APTT: activated partial thromboplastin time.
ASA: American Society of Anesthesiologists.
BMI: body mass index.
CRP: C‐reactive protein.
DVT: deep venous thrombosis.
EDTA: ethylene diamine tetra‐acetic acid.
EQ‐5D: EuroQoL Group Quality of Life Questionnaire based on five dimensions.
GFR: glomerular filtration rate.
HSS: Hospital for Special Surgery.
NYHA: New York Heart Association.
OA: osteoarthritis.
PE: pulmonary embolism.
RA: rheumatic arthritis.
ROM: range of motion.
RSA: radiostereometric analysis.
SD: standard deviation.
SF‐12: 12‐Item Short Form Survey.
TKA: total knee arthroplasty.
TXA: tranexamic acid.
VAS: visual analogue scale.
WOMAC: Western Ontario McMaster Arthritis Index.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
| Non‐randomised study with fewer than 1000 participants | |
| Non‐randomised study with fewer than 1000 participants | |
| Non‐randomised study with fewer than 1000 participants | |
| Wrong comparator | |
| Non‐randomised study with fewer than 1000 participants | |
| Wrong comparator | |
| Commentary piece | |
| Non‐randomised study with fewer than 1000 participants | |
| Wrong comparator | |
| Wrong study design | |
| Non‐randomised study with fewer than 1000 participants | |
| Wrong study design | |
| Wrong comparator | |
| Non‐randomised study with fewer than 1000 participants | |
| Non‐randomised study with fewer than 1000 participants | |
| Non‐randomised study with fewer than 1000 participants | |
| Supplementary piece | |
| Non‐randomised study with fewer than 1000 participants | |
| Non‐randomised study with fewer than 1000 participants | |
| Wrong study design | |
| Wrong comparator | |
| Non‐randomised study with fewer than 1000 participants | |
| Wrong comparator | |
| Non‐randomised study with fewer than 1000 participants | |
| Non‐randomised study with fewer than 1000 participants | |
| Non‐randomised study with fewer than 1000 participants | |
| Non‐randomised study with fewer than 1000 participants |
Characteristics of ongoing studies [ordered by study ID]
| Study name | Total Knee Replacement With Tourniquet or Aquamantys |
| Methods | Double‐blind randomised controlled trial |
| Participants | Participants aged 18 to 100 Inclusion criteria: primary total knee arthroplasty Exclusion criteria: repeat knee replacement (revision arthroplasty), bilateral knee replacements on the same day, partial knee replacements, health or social limitations that do not allow the participant to be discharged to home on the same day or on the day after surgery |
| Interventions | Control: surgery performed with tourniquet inflated to control bleeding Intervention: the Aquamantys bipolar sealer is a device used during surgery to help reduce bleeding in the joint. The system uses radiofrequency energy and sterile saline (salt water) to close small blood vessels in the knee to help reduce bleeding |
| Outcomes | Primary outcome: Isometric quadriceps strength [Time Frame: 2 weeks] Secondary outcomes: 1. Pain (VAS) [Time Frame: preoperative, 2 weeks, 6 weeks, 12 weeks] 2. Knee Injury Osteoarthritis Outcome Score, Joint Replacement (KOOS, JR) patient‐reported outcome score 3. Emotional health (VR‐12 MCS). The Veterans Rand‐12 Mental Component Score will be used to quantify the impact of participants' emotional health on their daily activities. The VR‐12 consists of 12 questions and is scored from 0 to 100, with lower scores indicating that emotional health has a more dramatic impact on the participant's daily life 4. Knee function questionnaire [Time Frame: preoperative, 2 weeks, 6 weeks, 12 weeks] 5. Sit to stand test [Time Frame: 6 weeks, 12 weeks] 6. Opioid use [Time Frame: preoperative, 2 weeks, 6 weeks, 12 weeks] 7. Isometric quadriceps strength [Time Frame: 6 weeks, 12 weeks] |
| Starting date | 15/08/2019 |
| Contact information | Stephen Duncan University of Kentucky Lexington, Kentucky, USA 40536 859‐323‐5533; [email protected] |
| Notes | ClinicalTrials.gov Identifier NCT04016285 |
| Study name | The Effects of a Tourniquet in Total Knee Arthroplasty |
| Methods | Triple‐blinded randomised controlled trial |
| Participants | Participants aged 18 and older Inclusion criteria: knee osteoarthrosis qualifying for total knee arthroplasty Exclusion criteria: coagulation disease, rheumatoid arthritis, peripheral vascular disease, malignant disease, pregnancy, ongoing infection, not able to understand written and oral information in Norwegian |
| Interventions | No use of tourniquet during surgery vs use of tourniquet during surgery where cuff will be inflated to 300 mmHg |
| Outcomes | Primary outcome measures: 1. Mmax [Time Frame: (1) change from preoperative (baseline) to day 2 postoperative, (2) change from preoperative to 8 weeks postoperative, (3) change from preoperative to 1 year postoperative]. EMG recordings are made using 10 mm electrodes (Ag‐AgCl) attached in a bipolar configuration over the vastus lateralis and rectus femoris Secondary outcome measures: 1. Maximal leg strength [Time Frame: (1) change from preoperative (baseline) to day 2 postoperative, (2) change from preoperative to 8 weeks postoperative, (3) change from preoperative to 1 year postoperative]. 1 RM leg strength is measured using a leg press ergometer with the participant in a supine position (Steens Physical, Ring Mekanikk, Moelv, Norway) |
| Starting date | 12/09/2018 |
| Contact information | Vigdis Schnell Husby, PhD, +4773412312, [email protected]; Siri Bjorgen Winther, PhD, +4772573669, [email protected] |
| Notes | Sponsors and Collaborators Norwegian University of Science and Technology, Zimmer Biomet, University of British Columbia, Karolinska University Hospital, St Olav's Hospital, University Hospital in Trondheim, Kristiansund Hospital NCT03666598 |
| Study name | A Single‐Centre, Parallel‐Arm, Double‐Blind Randomised Trial Evaluating the Effects of Tourniquet Use in Total Knee Arthroplasty on Intraoperative and Postoperative Outcomes |
| Methods | Randomised controlled trial |
| Participants | 90 participants with osteoarthritis Inclusion criteria: undergoing primary total knee replacement for primary osteoarthritis; > 18 years of age; willing, able, and mentally competent to provide informed consent |
| Interventions | Surgery with a tourniquet vs surgery without a tourniquet |
| Outcomes | Primary outcome: Isometric quadriceps strength will be measured in Newtons Secondary outcomes: 1. Analgesic requirements will be determined from patients; hospital medication charts and average morphine equivalent daily dose calculated (mg) |
| Starting date | 1/10/2014 |
| Contact information | Name: Dr Stephen Gill Study registered with the Australian New Zealand Clinical Trials Registry: ACTRN12618000425291 |
| Notes | Funded by Barwon Health |
| Study name | The Efficacy of Oral Tranexamic Acid on Blood Loss in Primary Total Knee Arthroplasty With or Without Tourniquet: A Prospective, Randomized, Controlled Trial |
| Methods | Randomised controlled trial |
| Participants | 60 participants in total: 30 with a tourniquet vs 30 without a tourniquet Inclusion criteria: patients with osteoarthritis of the knee |
| Interventions | Surgery with a tourniquet vs surgery without a tourniquet |
| Outcomes | 1. Blood loss 2. Range of motion 3. Pain 4. Swelling |
| Starting date | 25/10/2017 |
| Contact information | Name: Kang Pengde |
| Notes | Funded by the National Health and Family Planning Commission of China |
| Study name | Effect of Tourniquet on UKA |
| Methods | Triple‐blinded randomised controlled trial |
| Participants | 30 participants Inclusion criteria: waiting list for unicondylar knee arthroplasty Exclusion criteria: failed upper tibial osteotomy, insufficiency of collateral or anterior cruciate ligaments, fixed varus or valgus deformity (not passively correctable) above 15°, flexion deformity > 15°, rheumatoid arthritis, intake of medicinal anticoagulation before surgery, liver dysfunction/coagulation dysfunction, peripheral arterial occlusive disease |
| Interventions | UKA surgery with tourniquet vs UKA surgery without tourniquet |
| Outcomes | Primary outcome measure: Cement mantle thickness [Time Frame: 1 week] |
| Starting date | 08/06/2015 |
| Contact information | Michael Liebensteiner, +4351250480547, Michael.liebensteiner@i‐med.ac.at |
| Notes | Not yet recruiting ID: NCT02465684 |
| Study name | Tourniquet Versus No Tourniquet on Early Rehabilitation and Cement Mantle After Primary Total Knee Arthroplasty Using a Multimodal Blood Management Protocol: A Randomized Controlled Trial |
| Methods | Randomised controlled trial |
| Participants | 60 participants in total Inclusion criteria: patients aged 18 years and older, scheduled for primary TKA because of end‐stage osteoarthritis |
| Interventions | Surgery with a tourniquet vs surgery without a tourniquet |
| Outcomes | 1. Total blood loss 2. Bone cement mantle interface 3. Pain score 4. Swelling 5. Change in Hb 6. CRP 7. IL‐6 8. Transfusion rate 9. Patient satisfaction |
| Starting date | 07/11/2016 |
| Contact information | Name: Fuxing Pei |
| Notes | Funded by China National Health and Family Planning Commission |
| Study name | Is Tourniquet Really Necessary When Multiple Uses of Intravenous and Topical Tranexamic Acid Are Applied in Primary Total Knee Arthroplasty? A Prospective Randomised Controlled Trial |
| Methods | Randomised controlled trial |
| Participants | 150 participants Inclusion criteria: aged 18 years and older, scheduled for primary TKA because of end‐stage osteoarthritis Exclusion criteria: revisions, bilateral procedures, previous knee surgery history, flexion deformity 30°, varus/valgus deformity 30°, anaemia (< 120 g/L for female, < 130 g/L for male), contraindications for use of TXA, coagulation disorder Age minimum: 18 Age maximum: 80 Gender: both |
| Interventions | Group A: tourniquet + 20 mg/kg IV TXA administered 5 to 10 minutes before skin incision and 10 mg/kg TXA administered 3, 6, 12, and 24 hours later Group B: 20 mg/kg IV TXA administered 5 to 10 minutes before skin incision and 10 mg/kg TXA administered 3, 6, 12, and 24 hours later Group C: only tourniquet used during surgery |
| Outcomes | Primary outcomes: 1. Hidden blood loss 2. Maximum Hb change 3. CRP 4. IL‐6 Secondary outcomes: 1. Lower limb swelling ratio 2. VAS pain score 3. Length of hospital stay 4. Transfusion rate 5. Patient satisfaction 6. Complications |
| Starting date | 01/07/2016 |
| Contact information | Fuxing Pei37 Guoxuexiang, Chengdu, China 610041, +8613551068719, [email protected], West China Hospital, Sichuan University |
| Notes | Currently recruiting ID: ChiCTR‐INR‐16008762 |
| Study name | Effects of Postoperative Limb Positions on Blood Loss and Range of Motion in Total Knee Arthroplasty Without Tourniquet: A Randomized Controlled Trial |
| Methods | Randomised controlled trial |
| Participants | 100 participants with osteoarthritis undergoing total knee replacement surgery Inclusion criteria: with total knee replacement |
| Interventions | Surgery with a tourniquet vs surgery without a tourniquet |
| Outcomes | 1. Blood loss 2. Range of motion |
| Starting date | 13/02/2018 |
| Contact information | Name: Bin Shen Registration: ChiCTR1800014896 |
| Notes | No source of funding |
| Study name | Randomized Controlled Trial for Comparision of Functional Outcome in Total Knee Replacement With Tourniquet and Without Tourniquet |
| Methods | Randomised controlled trial |
| Participants | 60 participants diagnosed with osteoarthritis undergoing primary total knee replacement surgery Inclusion criteria: diagnosed with osteoarthritis, scheduled for unilateral cemented TKA, either sex, < 80 years of age |
| Interventions | Surgery with a tourniquet vs surgery without a tourniquet |
| Outcomes | 1. Postoperative limb pain 2. Blood loss 3. Range of motion 4. Deep vein thrombosis 5. Radiolucency at bone cement interface |
| Starting date | 01/04/2013 |
| Contact information | Name: Swapnil Singh |
| Notes | Funded by Orthopedics Unit 2, AIIMS New Delhi 110029 |
| Study name | After Surgery Acute Renal Failure Incidence in Total Knee Arthroplasty With and Without Tourniquet |
| Methods | Double‐blinded randomised controlled trial |
| Participants | 100 participants Inclusion criteria: knee arthrosis, requiring surgical treatment with total knee arthroplasty Exclusion criteria: not acceptable to be in study, no signed consent form, not having blood sample for creatinine measure |
| Interventions | Total knee arthroplasty and use of tourniquet limb cuff at 270 mmHg vs total knee arthroplasty with intra‐articular lidocaine |
| Outcomes | Primary outcome measures:
|
| Starting date | 08/01/2019 |
| Contact information | Avelino Colin Vazquez, MD Instituto Mexicano del Seguro Social |
| Notes | ID: NCT03795805 |
| Study name | Safety and Feasibility Evaluation of Tourniquets for Total Knee Replacement Study |
| Methods | Randomised controlled trial |
| Participants | 50 participants undergoing total knee replacement surgery Inclusion criteria: aged 18 years and over, undergoing primary unilateral knee replacement, able to give written informed consent and to participate fully in trial interventions and follow‐up procedures Exclusion criteria: patients for whom magnetic resonance (MR) imaging is contraindicated due to non‐compliant heart pacemaker or defibrillator, non‐compliant metallic foreign body (e.g. in one or both eyes, aneurysm clips in the brain), claustrophobia (e.g. difficulty in an elevator or telephone box); not suitable for a thigh tourniquet (e.g. significant peripheral vascular disease); previous participation in the SAFE‐TKR trial |
| Interventions | Surgery with a tourniquet vs surgery without a tourniquet |
| Outcomes | Primary outcome: 1. Total volume of acute brain lesions detected on magnetic resonance (MR) brain imaging per patient, day 1 or 2 postoperatively Secondary outcomes: 1. Montreal Cognitive Assessment (MoCA) preoperatively; on days 1, 2, and 7 postoperatively; and at 6 and 12 months postoperatively |
| Starting date | 17/02/2016 |
| Contact information | Peter Wall University of Warwick |
| Notes | Funded by National Institute of Health Research ID: ISRCTN20873088 |
| Study name | Tourniquet Versus No Tourniquet on Rehabilitation After Fast‐Track Total Knee Arthroplasty |
| Methods | Randomised controlled trial |
| Participants | 60 participants (30 in each group) Inclusion criteria: adult patients who plan to undergo primary TKA on simultaneous bilateral knee joints with diagnosis of osteoarthritis but not of rheumatoid arthritis |
| Interventions | Surgery with a tourniquet vs surgery without a tourniquet |
| Outcomes | 1. Quadriceps strength 2. Pain score 3. Postoperative knee flexion 4. Postoperative knee swelling 5. Intraoperative bleeding 6. Patient satisfaction |
| Starting date | 01/10/2015 |
| Contact information | Name: Gang Wang |
| Notes | Funded by the Boosting Academic Program of Xijing Hospital |
BMI: body mass index.
CGRP: calcitonin gene‐related peptide.
eGFR: estimated glomerular filtration rate.
EQ‐5D‐5L: EuroQoL Group Quality of Life Questionnaire based on five‐level scale.
IL: interleukin.
NGF: nerve growth factor.
NRS: numerical rating scale.
RT‐PCR: reverse transcriptase polymerase chain reaction.
TKA: total knee arthroplasty.
TNF: tumour necrosis factor.
UKA: unicompartmental knee arthroplasty.
VEGF: vascular endothelial growth factor.
VTE: venous thromboembolism.
Data and analyses
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain at different postoperative days (visual analogue scale 0 to 10, lower is better) Show forest plot | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| Analysis 1.1  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 1: Pain at different postoperative days (visual analogue scale 0 to 10, lower is better) | ||||
| 1.1.1 Pain: day 1 | 8 | 577 | Mean Difference (IV, Random, 95% CI) | 1.25 [0.32, 2.19] |
| 1.1.2 Pain: day 2 | 6 | 394 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.03, 0.76] |
| 1.1.3 Pain: day 3 | 10 | 807 | Mean Difference (IV, Random, 95% CI) | 0.78 [0.34, 1.23] |
| 1.1.4 Pain: week 2 | 6 | 562 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.53] |
| 1.1.5 Pain: week 6 | 6 | 637 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.48, 1.23] |
| 1.2 Function: patient‐reported knee function at 3 months (scale 0 to 100, higher is better) Show forest plot | 4 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.52, 0.25] |
| Analysis 1.2  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 2: Function: patient‐reported knee function at 3 months (scale 0 to 100, higher is better) | ||||
| 1.3 Function: patient‐reported knee function at 12 months (scale 0 to 100, higher is better) Show forest plot | 5 | 611 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| Analysis 1.3  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 3: Function: patient‐reported knee function at 12 months (scale 0 to 100, higher is better) | ||||
| 1.4 Global assessment of success: participant‐reported satisfaction at 3 months (based on number of participants, higher is better) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.92, 1.14] |
| Analysis 1.4  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 4: Global assessment of success: participant‐reported satisfaction at 3 months (based on number of participants, higher is better) | ||||
| 1.5 Global assessment of success: participant‐reported satisfaction at 6 months (based on number of participants, higher is better) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.10] |
| Analysis 1.5  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 5: Global assessment of success: participant‐reported satisfaction at 6 months (based on number of participants, higher is better) | ||||
| 1.6 Health‐related quality of life: SF‐12 mental component at 6 weeks (0 to 100, higher is better) Show forest plot | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐0.09, 5.25] |
| Analysis 1.6  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 6: Health‐related quality of life: SF‐12 mental component at 6 weeks (0 to 100, higher is better) | ||||
| 1.7 Health‐related quality of life: SF‐12 mental component at 6 months (0 to 100, higher is better) Show forest plot | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 1.53 [‐0.85, 3.91] |
| Analysis 1.7  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 7: Health‐related quality of life: SF‐12 mental component at 6 months (0 to 100, higher is better) | ||||
| 1.8 Serious adverse events Show forest plot | 21 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.10, 2.73] |
| Analysis 1.8  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 8: Serious adverse events | ||||
| 1.9 Serious adverse event: venous thromboembolic event (VTE) Show forest plot | 17 | 1575 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.99, 3.82] |
| Analysis 1.9  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 9: Serious adverse event: venous thromboembolic event (VTE) | ||||
| 1.10 Serious adverse event: deep vein thrombosis (DVT) Show forest plot | 17 | 1602 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.35, 3.13] |
| Analysis 1.10  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 10: Serious adverse event: deep vein thrombosis (DVT) | ||||
| 1.10.1 Symptomatic DVT | 16 | 1499 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.92, 3.65] |
| 1.10.2 Asymptomatic DVT | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [1.29, 3.74] |
| 1.11 Serious adverse event: pulmonary embolism (PE) Show forest plot | 5 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 4.51 [0.49, 41.81] |
| Analysis 1.11  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 11: Serious adverse event: pulmonary embolism (PE) | ||||
| 1.12 Serious adverse event: infection Show forest plot | 9 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [1.15, 6.42] |
| Analysis 1.12  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 12: Serious adverse event: infection | ||||
| 1.13 Serious adverse event: re‐operation Show forest plot | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.61, 4.34] |
| Analysis 1.13  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 13: Serious adverse event: re‐operation | ||||
| 1.14 Survival of the implant: risk of revision up to 2 years Show forest plot | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.23, 8.92] |
| Analysis 1.14  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 14: Survival of the implant: risk of revision up to 2 years | ||||
| 1.15 Blood loss: postoperative transfusion risk (lower is better) Show forest plot | 18 | 1286 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.86, 1.67] |
| Analysis 1.15  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 15: Blood loss: postoperative transfusion risk (lower is better) | ||||
| 1.16 Blood loss: intraoperative (mL, lower is better) Show forest plot | 15 | 1187 | Mean Difference (IV, Random, 95% CI) | ‐147.05 [‐190.97, ‐103.12] |
| Analysis 1.16  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 16: Blood loss: intraoperative (mL, lower is better) | ||||
| 1.17 Blood loss: postoperative (mL, lower is better) Show forest plot | 12 | 776 | Mean Difference (IV, Random, 95% CI) | 57.72 [13.58, 101.87] |
| Analysis 1.17  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 17: Blood loss: postoperative (mL, lower is better) | ||||
| 1.18 Blood loss: overall blood loss (mL, lower is better) Show forest plot | 18 | 1500 | Mean Difference (IV, Random, 95% CI) | 8.61 [‐83.76, 100.97] |
| Analysis 1.18  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 18: Blood loss: overall blood loss (mL, lower is better) | ||||
| 1.19 Blood loss: change in haemoglobin (g/dL, lower is better) Show forest plot | 9 | 713 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.48, 0.19] |
| Analysis 1.19  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 19: Blood loss: change in haemoglobin (g/dL, lower is better) | ||||
| 1.20 Economic: length of hospital stay (days, lower is better) Show forest plot | 12 | 995 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.03, 0.64] |
| Analysis 1.20  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 20: Economic: length of hospital stay (days, lower is better) | ||||
| 1.21 Economic: duration of surgery (minutes, lower is better) Show forest plot | 27 | 2070 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐5.53, ‐1.87] |
| Analysis 1.21  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 21: Economic: duration of surgery (minutes, lower is better) | ||||
| 1.22 Implant stability: maximum total point motion at 8 weeks (mm, lower is better) Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.13, 0.01] |
| Analysis 1.22  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 22: Implant stability: maximum total point motion at 8 weeks (mm, lower is better) | ||||
| 1.23 Implant stability: maximum total point motion at 1 year (mm, lower is better) Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.09, 0.18] |
| Analysis 1.23  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 23: Implant stability: maximum total point motion at 1 year (mm, lower is better) | ||||
| 1.24 Implant stability: maximum total point motion at 2 years (mm, lower is better) Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.08, 0.19] |
| Analysis 1.24  Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 24: Implant stability: maximum total point motion at 2 years (mm, lower is better) | ||||
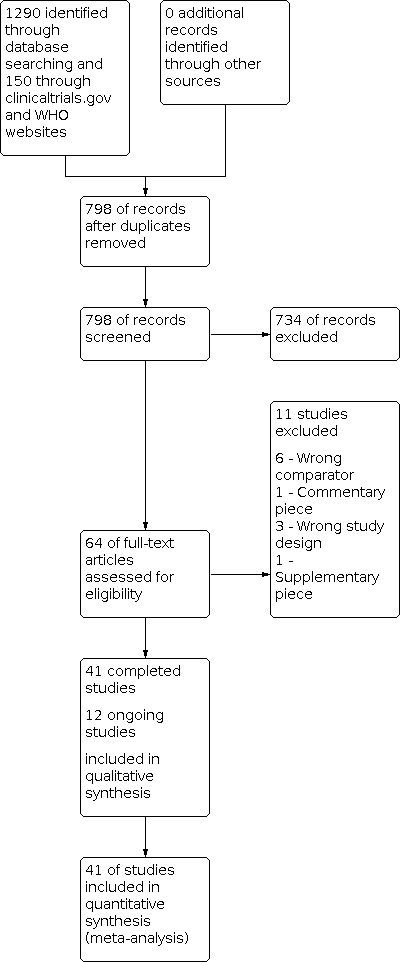
Study flow diagram: search for randomised controlled trials.

Study flow diagram: search for non‐randomised studies.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 1: Pain at different postoperative days (visual analogue scale 0 to 10, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 2: Function: patient‐reported knee function at 3 months (scale 0 to 100, higher is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 3: Function: patient‐reported knee function at 12 months (scale 0 to 100, higher is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 4: Global assessment of success: participant‐reported satisfaction at 3 months (based on number of participants, higher is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 5: Global assessment of success: participant‐reported satisfaction at 6 months (based on number of participants, higher is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 6: Health‐related quality of life: SF‐12 mental component at 6 weeks (0 to 100, higher is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 7: Health‐related quality of life: SF‐12 mental component at 6 months (0 to 100, higher is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 8: Serious adverse events

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 9: Serious adverse event: venous thromboembolic event (VTE)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 10: Serious adverse event: deep vein thrombosis (DVT)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 11: Serious adverse event: pulmonary embolism (PE)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 12: Serious adverse event: infection

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 13: Serious adverse event: re‐operation

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 14: Survival of the implant: risk of revision up to 2 years

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 15: Blood loss: postoperative transfusion risk (lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 16: Blood loss: intraoperative (mL, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 17: Blood loss: postoperative (mL, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 18: Blood loss: overall blood loss (mL, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 19: Blood loss: change in haemoglobin (g/dL, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 20: Economic: length of hospital stay (days, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 21: Economic: duration of surgery (minutes, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 22: Implant stability: maximum total point motion at 8 weeks (mm, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 23: Implant stability: maximum total point motion at 1 year (mm, lower is better)

Comparison 1: Surgery with a tourniquet vs surgery without a tourniquet, Outcome 24: Implant stability: maximum total point motion at 2 years (mm, lower is better)
| Participants: patients undergoing knee replacement surgery Settings: hospitals around the world performing knee replacement surgery Intervention: surgery performed with a tourniquet for all or part of the procedure Comparator: surgery performed without a tourniquet | ||||||
|---|---|---|---|---|---|---|
| Outcomes | Anticipated absolute effects* | Relative effect (95% CI) | No. of participants | Certainty of the evidence | Comments | |
| Risk without tourniquet | Risk with tourniquet | |||||
| Pain Visual analogue scale (VAS) for pain from zero to 10 (higher scores indicate more pain) Follow‐up day 1 postoperative pain scores | Mean pain was 4.56 | MD 1.25 worse pain | ‐ | 577 (8 RCTs) | ⊕⊕⊕⊝ | Knee replacement with a tourniquet led to higher postoperative pain scores at day 1, although this difference may or may not be noticeable to patients b Absolute difference 12.5% worse (3.2% worse to 21.9% worse) Relative difference 19% worse (3.4% worse to 49% worse)c |
| Function Similar 0 to 100 scales (100 is best) were used to measure the same conceptual functional outcome: Knee Injury and Osteoarthritis Outcome Score Activities of Daily Living (KOOS‐ADL); Knee Society Score (KSS); Hospital for Special Surgery Score (HSS) Follow‐up 12 months | Mean function was 90.03 | MD 0.29 worse function (1.06 worse to 0.48 better)d | ‐ | 611 (5 RCTs) | ⊕⊕⊕⊝ MODERATEa | Knee replacement with tourniquet probably has little or no meaningful effect on function b Absolute difference 0.29% worse (1.06% worse to 0.48% better) Relative difference 0.57% worse (2.07% worse to 0.94% better)c |
| Global assessment of success Participants reporting overall successful treatment and satisfactione Follow‐up 6 months | 940 per 1000 | 940 per 1000 (855 to 1034) | RR 1.0 (0.91 to 1.10) | 100 (1 RCT) | ⊕⊕⊝ LOWa,f | Number of participants reporting success may not differ Absolute difference 0% (8.5% worse to 9.4% better) Relative difference 0% (9% worse to 10% better) |
| Health‐related quality of life SF‐12 mental component from zero to 100 (100 is best) Follow‐up 6 months | Mean health‐related quality of life was 54.64 | MD 1.53 better (0.85 worse to 3.91 better) | 199 | ⊕⊕⊝ | Knee replacement with tourniquet may have little or no meaningful effect on health‐related quality of lifeb Absolute difference 1.53% better (0.85% worse to 3.91% better) Relative difference 3% better (2% worse to 7% better)c | |
| Serious adverse events | 29 per 1000 | 59 per 1000 (32 to 79) | RR 1.73 | 1799 | ⊕⊕⊕⊝ | Knee replacement with tourniquet probably has a meaningful effect on risk of serious adverse events Absolute difference 2.1% more (0.29% more to 5.00% more)g Relative difference 73% (10% more to 173% more) Number needed to harm (NNTH) is 48 (20 to 345) participants to have surgery with a tourniquet for 1 serious adverse event (venous thromboembolism, infection, or re‐operation) |
| Cognitive function | ‐ | ‐ | ‐ | ‐ | ‐ | No studies with adequate data |
| Survival of the implant Risk of revision At 1 year | 9 per 1000 | 13 per 1000 (2 to 83) | RR 1.44 (0.23 to 8.92) | 214 | ⊕⊕⊕⊝ | It is uncertain if knee replacement has an effect on survival of implant at 1 year Absolute difference 0.4% more (0.7% less to 7% more) in the surgery with a tourniquet group Relative difference 44% more (77% lower to 892% more) in the surgery with a tourniquet group |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| GRADE Working Group grades of evidence. | ||||||
| aDowngraded by one level due to risk of bias. Many studies had unclear risk of allocation concealment and unclear risk of participant blinding. bWe assumed that clinically important improvement was 1 point or 10% absolute improvement for pain on a VAS (0 to 10) (Dworkin 2008; Kelly 2001; Wall 2017); 5.3 points or 5.3% absolute improvement in KSS for function (Chean Lee 2017), and 10 points or 10% absolute improvement for health‐related quality of life. cRelative changes calculated relative to baseline in the surgery with a tourniquet group (i.e. absolute change (mean difference) divided by mean at baseline in the surgery without a tourniquet group from Liu 2017 b (values were 6.54 points on a 0 to 10 point VAS scale for pain and 51.3 on a 0 to 100 point KSS scale for function) and Goel 2019 (values were 54.64 on a 0 to 100 point SF‐12 mental component score for continuous outcomes). dThe mean difference was calculated by multiplying the SMD by the baseline SD (4.8) of the control group (Liu 2017 b). eParticipant satisfaction was derived from one study (Huang 2017). Satisfaction was defined as the number of participants who were 'extremely' or 'very' satisfied with their treatment. fDowngraded by one level due to imprecision. Small total number of participants. Not enough information to calculate effect estimate precisely. gConfidence intervals around absolute risk demonstrated an effect equal to or greater than 0.29%, which was deemed to be highly clinically relevant given the seriousness of the outcome. The total number of events was low; however, this was expected, and we did not downgrade for imprecision, as this was is in line with previous literature on SAEs (Benjamin 2016), which reported an incidence of VTE of 2.4% in patients undergoing TKR. Our results therefore do not indicate a 'low' total number of events for this outcome of interest. hDowngraded again due to very serious imprecision (only three events reported across the studies). | ||||||
| Author | Number of participants | Number in tourniquet group | Number in control group | Mean age in tourniquet group (SD) | Mean age in control group (SD) | Proportion of males in tourniquet group, % | Proportion of males in control group, % | BMI in tourniquet group (SD) | BMI in control group (SD) |
| Abdel‐Salem 1995 | 80 | 40 | 40 | 73 | 73 | ||||
| Aglietti 2000 | 20 | 10 | 10 | 70 (8) | 68 (4.5) | 30 | 40 | 27.9 | 27.3 |
| Alexandersson 2018 | 81 | 38 | 43 | 68 (7.4) | 69.7 (6.4) | 47 | 51 | 28.6 (3.4) | 27.9 (3.5) |
| Ayik 2020 | 65 | 32 | 33 | 65.39 (7.25) | 64.90 (6.58) | 44 | 42 | 31.38 (4.72) | 30.3 (7.1) |
| Clarke 2001 | 31 | 21 | 10 | ||||||
| Dong 2019 | 122 | 58 | 64 | 68.2 (17.1) | 69.5 (15.9) | 34 | 35 | ||
| Ejaz 2014 | 64 | 33 | 31 | 68 (8.4) | 68 (7.4) | 55 | 55 | 25 (2) | 25 (2.5) |
| Ejaz 2015 | 62 | 31 | 31 | 68 (6.3) | 68.2 (7.2) | 52 | 55 | 25.1 (2) | 25.2 (2.5) |
| Ejaz 2015 b | 57 | 29 | 28 | 68.3 (8.4) | 68.2 (7.8) | 45 | 54 | 25.1 (2) | 25.2 (2.5) |
| Goel 2019 | 199 | 100 | 99 | 66.0 (7.0) | 65.5 (7.8) | 50 | 48 | 30.9 (4.6) | 31.3 (4.5) |
| Harston 2015 | 64 | 32 | 32 | 68 (8) | 66 (8) | 27.4 | 28.4 | ||
| Huang 2017 | 100 | 50 | 50 | 66.2 (8.3) | 65.1 (8.1) | 36 | 32 | 25.1 (1.5) | 24.2 (1.5) |
| Jawhar 2015 | 34 | 17 | 17 | 70.6 (6) | 70.6 (6) | 53 | 53 | 32.1 (5) | 33.8 (5) |
| Jawhar 2019 | 99 | 50 | 49 | 69.3 (7.4) | 68.3 ± 7.8 | 34 | 39 | 31.9 (6) | 31.4 (5.5) |
| Juelsgaard 2001 | 30 | 16 | 14 | 69 | 64 | 44 | 29 | ||
| Kato 2002 | 46 | 22 | 24 | 65 | 63 | ||||
| Kiss 2015 | 100 | 51 | 49 | 72.6 (7.1) | 74.7 (7.4) | 20 | 27 | 28.8 (3.9) | 28.5 (3.3) |
| Kumar 2015 | 30 | 30 | 30 | 58 | 58 | 30 | 30 | ||
| Ledin 2012 | 50 | 25 | 25 | 70 (8) | 71 (6) | 67 | 39 | 29 (4.8) | 28 (4.8) |
| Li 2008 | 60 | 30 | 30 | 71 (7) | 70 (7) | 24 (5) | 24 (5) | ||
| Li 2009 | 80 | 40 | 40 | 71 (6) | 70 (7) | 28 | 33 | 27.3 (6.3) | 26.8 (5.1) |
| Liu 2014 | 20 | 10 | 10 | 67 | 60 | 70 | 90 | 25.5 | 28.7 |
| Liu 2017 | 52 | 52 | 52 | 67 (8) | 67 (8) | 28.1 (5.5) | 28.1 (5.5) | ||
| Liu 2017 b | 26 | 26 | 26 | 65.8 (9.2) | 65.8 (9.2) | 35 | 35 | 28.2 (5.6) | 28.2 (5.6) |
| Matziolis 2015 | 20 | 10 | 10 | 72.4 | 76.6 | 80 | 70 | 28.3 | 29.5 |
| Molt 2014 | 60 | 30 | 30 | 70 (7) | 67 (9) | 53 | 53 | 28 (3) | 28 (3) |
| Mori 2016 | 103 | 51 | 52 | 72.8 (7.3) | 74.6 (7.6) | 12 | 17 | 27.7 (3.4) | 29.2 (3.9) |
| Ozkunt 018 | 49 | 24 | 25 | 65.05 | 65.05 | ||||
| Pfitzner 2014 | 90 | 45 | 45 | 69.3 | 70.5 | 47 | 24 | 27.8 | 26 |
| Tai 2012 | 72 | 36 | 36 | 72.1 (6.9) | 71.5 (6.8) | 28.6 (4.5) | 27.9 (4.2) | ||
| Tetro 2001 | 63 | 33 | 30 | 69.8 (6.7) | 69.8 (9) | 45 | 37 | ||
| Vandenbussche 2001 | 80 | 40 | 40 | 72.5 | 68.5 | 22.5 | 40 | ||
| Vertullo 2017 | 40 | 20 | 20 | 67.85 (6.91) | 65.65 (8.54) | 50 | 55 | 30.43 (5.07) | 31 (5.31) |
| Wakankar 1999 | 77 | 37 | 40 | 72.5 | 71.8 | 30 | 35 | ||
| Wauke 2002 | 37 | 19 | 18 | 63.2 (8.7) | 61.4 (7.4) | ||||
| Wu 2018 | 100 | 50 | 50 | 68.06 (3.16) | 67.58 (4.61) | 38 | 44 | 23.87 (2.13) | 24.10 (2.16) |
| Yavarikia 2010 | 51 | 22 | 29 | 68 | 66 | 27 | 24 | ||
| Zhang 2010 | 60 | 30 | 30 | 72 (6) | 71 (6) | 27 | 37 | 25 (4) | 26 (4) |
| Zhang 2016 | 166 | 84 | 82 | 84 | 82 | ||||
| Zhou 2011 | 39 | 20 | 19 | 63.12 (6.79) | 61.89 (7.93) | 35 | 26 | ||
| Zhou 2017 | 140 | 72 | 68 | 72 | 68 | 18 | 10 | 26.1 (4.1) | 25.7 (3.4) |
| Outcome | Bias estimate (standard error) | P value |
| Pain | 3.875 (2.168) | 0.097 |
| Intraoperative blood loss | ‐8.732 (2.596) | 0.005 |
| Overall blood loss | 5.585 (3.968) | 0.178 |
| Postoperative blood loss | ‐0.049 (3.420) | 0.989 |
| Transfusion rate | 0.47 (0.63) | 0.468 |
| Length of stay | 0.219 (2.182) | 0.922 |
| Duration of surgery | ‐2.947 (1.113) | 0.014 |
| Serious adverse events | 0.567 (0.552) | 0.318 |
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
| 1.1 Pain at different postoperative days (visual analogue scale 0 to 10, lower is better) Show forest plot | 14 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1.1 Pain: day 1 | 8 | 577 | Mean Difference (IV, Random, 95% CI) | 1.25 [0.32, 2.19] |
| 1.1.2 Pain: day 2 | 6 | 394 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.03, 0.76] |
| 1.1.3 Pain: day 3 | 10 | 807 | Mean Difference (IV, Random, 95% CI) | 0.78 [0.34, 1.23] |
| 1.1.4 Pain: week 2 | 6 | 562 | Mean Difference (IV, Random, 95% CI) | 0.32 [0.12, 0.53] |
| 1.1.5 Pain: week 6 | 6 | 637 | Mean Difference (IV, Random, 95% CI) | 0.38 [‐0.48, 1.23] |
| 1.2 Function: patient‐reported knee function at 3 months (scale 0 to 100, higher is better) Show forest plot | 4 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.52, 0.25] |
| 1.3 Function: patient‐reported knee function at 12 months (scale 0 to 100, higher is better) Show forest plot | 5 | 611 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.22, 0.10] |
| 1.4 Global assessment of success: participant‐reported satisfaction at 3 months (based on number of participants, higher is better) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.92, 1.14] |
| 1.5 Global assessment of success: participant‐reported satisfaction at 6 months (based on number of participants, higher is better) Show forest plot | 1 | 100 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.91, 1.10] |
| 1.6 Health‐related quality of life: SF‐12 mental component at 6 weeks (0 to 100, higher is better) Show forest plot | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 2.58 [‐0.09, 5.25] |
| 1.7 Health‐related quality of life: SF‐12 mental component at 6 months (0 to 100, higher is better) Show forest plot | 1 | 199 | Mean Difference (IV, Random, 95% CI) | 1.53 [‐0.85, 3.91] |
| 1.8 Serious adverse events Show forest plot | 21 | 1799 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [1.10, 2.73] |
| 1.9 Serious adverse event: venous thromboembolic event (VTE) Show forest plot | 17 | 1575 | Risk Ratio (M‐H, Random, 95% CI) | 1.95 [0.99, 3.82] |
| 1.10 Serious adverse event: deep vein thrombosis (DVT) Show forest plot | 17 | 1602 | Risk Ratio (M‐H, Random, 95% CI) | 2.05 [1.35, 3.13] |
| 1.10.1 Symptomatic DVT | 16 | 1499 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.92, 3.65] |
| 1.10.2 Asymptomatic DVT | 1 | 103 | Risk Ratio (M‐H, Random, 95% CI) | 2.20 [1.29, 3.74] |
| 1.11 Serious adverse event: pulmonary embolism (PE) Show forest plot | 5 | 416 | Risk Ratio (M‐H, Random, 95% CI) | 4.51 [0.49, 41.81] |
| 1.12 Serious adverse event: infection Show forest plot | 9 | 846 | Risk Ratio (M‐H, Random, 95% CI) | 2.72 [1.15, 6.42] |
| 1.13 Serious adverse event: re‐operation Show forest plot | 3 | 157 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.61, 4.34] |
| 1.14 Survival of the implant: risk of revision up to 2 years Show forest plot | 3 | 214 | Risk Ratio (M‐H, Random, 95% CI) | 1.44 [0.23, 8.92] |
| 1.15 Blood loss: postoperative transfusion risk (lower is better) Show forest plot | 18 | 1286 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.86, 1.67] |
| 1.16 Blood loss: intraoperative (mL, lower is better) Show forest plot | 15 | 1187 | Mean Difference (IV, Random, 95% CI) | ‐147.05 [‐190.97, ‐103.12] |
| 1.17 Blood loss: postoperative (mL, lower is better) Show forest plot | 12 | 776 | Mean Difference (IV, Random, 95% CI) | 57.72 [13.58, 101.87] |
| 1.18 Blood loss: overall blood loss (mL, lower is better) Show forest plot | 18 | 1500 | Mean Difference (IV, Random, 95% CI) | 8.61 [‐83.76, 100.97] |
| 1.19 Blood loss: change in haemoglobin (g/dL, lower is better) Show forest plot | 9 | 713 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.48, 0.19] |
| 1.20 Economic: length of hospital stay (days, lower is better) Show forest plot | 12 | 995 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.03, 0.64] |
| 1.21 Economic: duration of surgery (minutes, lower is better) Show forest plot | 27 | 2070 | Mean Difference (IV, Random, 95% CI) | ‐3.70 [‐5.53, ‐1.87] |
| 1.22 Implant stability: maximum total point motion at 8 weeks (mm, lower is better) Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.13, 0.01] |
| 1.23 Implant stability: maximum total point motion at 1 year (mm, lower is better) Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.09, 0.18] |
| 1.24 Implant stability: maximum total point motion at 2 years (mm, lower is better) Show forest plot | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.08, 0.19] |

